Abstract
One of the most important factors driving the development of memory during childhood is mnemonic control, or the capacity to initiate and maintain the processes that guide encoding and retrieval operations. The ability to selectively attend to and encode relevant stimuli is a particularly useful form of mnemonic control, and is one that undergoes marked improvement over childhood. We hypothesized that structural integrity of white matter tracts, in particular those connecting medial temporal lobe memory regions to other cortical areas, and/or those connecting frontal and parietal control regions, should contribute to successful mnemonic control. To test this hypothesis, we examined the relationship between structural integrity of selected white matter tracts and an experimental measure of mnemonic control, involving enhancement of memory by attention at encoding, in 116 children aged 7–11 and 25 young adults. We observed a positive relationship between integrity of uncinate fasciculus and mnemonic enhancement across age groups. In adults, but not in children, we also observed an association between mnemonic enhancement and integrity of ventral cingulum bundle and ventral fornix/fimbria. Integrity of fronto-parietal tracts, including dorsal cingulum and superior longitudinal fasciculus, was unrelated to mnemonic enhancement.
Keywords: connectivity, diffusion tensor imaging, fractional anisotropy, hippocampus, memory
Introduction
One of the most important factors driving the development of memory during childhood is mnemonic control, or the capacity to initiate and maintain the processes that guide encoding and retrieval operations (Schneider and Pressley 1997; Shing et al. 2010). Various mental operations can be characterized as mnemonic control: the use of overt encoding strategies (Olszewska and Ulatowska 2013), memory suppression (Depue et al. 2007), postretrieval monitoring (Ranganath et al. 2007; Hayama and Rugg 2009), and controlled retrieval (Badre et al. 2005) among others. The ability to selectively attend to and encode relevant stimuli, which emerges during childhood (Harnishfeger and Bjorklund 1994; Wendelken et al. 2011), may be a particularly critical form of mnemonic control, as it is this ability that allows us to enhance our memories of those aspects of our complex environment that ought to be retained based on current goals. This capacity for mnemonic enhancement may be particularly critical in formal learning settings, when individuals are expected to select and retain the most relevant material for later testing.
Research has begun to shed light on the neural underpinnings of mnemonic control, including the contribution of such regions as lateral prefrontal cortex (PFC) and posterior parietal cortex (PPC) (Simons and Spiers 2003; Wagner et al. 2005; Esterman et al. 2009). These regions may regulate the operations of other brain regions responsible for encoding and retrieving event representations, namely the medial temporal lobe (MTL), and the hippocampus in particular (Scoville and Milner 1957; Nadel and Moscovitch 1998; Norman and O'Reilly 2003; Eichenbaum et al. 2012).
Structural and functional changes within PFC, PPC, and/or MTL play an important role in driving the development of mnemonic control, but changes in the way these regions communicate may be just as critical (Ghetti and Bunge 2012). Increased coherence along relevant white matter tracts, enabling more efficient communication between regions, should lead to more effective mnemonic control. Indeed, increasing coherence of specific white matter tracts may be a key mechanism that underlies age-related improvements in mnemonic control. The overall goal of the present study is to elucidate the relationship between structural integrity of specific white matter tracts and mnemonic control, and to examine how these relationships differ as a function of tract and age. We considered 2 nonmutually exclusive hypotheses. First, we tested our primary hypothesis, that tracts connecting MTL to brain regions associated with cognitive control would support the development and operation of mnemonic control. Second, we tested the hypothesis that fronto-parietal white matter tracts, which support communication between regions most typically associated with cognitive control, could make a prominent contribution.
Given the central involvement of MTL in memory, there is good reason to suspect that communication between fronto-parietal control areas and MTL may be critical for mnemonic control. Indeed, in one recent study involving memory suppression, functional connectivity between hippocampus and PFC was related to mnemonic control (Benoit and Anderson 2012), while in another study of memory suppression, hippocampal connectivity with a network of regions, including most prominently PPC, was associated with increased mnemonic control (Paz-Alonso et al. 2013). Moreover, there is evidence that MTL, and the hippocampal formation in particular, changes into adolescence (DeMaster et al. 2013). Thus, the white matter tracts that connect to MTL and hippocampus may be particularly important for mnemonic control, and the maturation of these tracts may be critical for its development.
Tracts that are likely candidates for contributing to mnemonic control through their connection with MTL include the uncinate fasciculus (UF), the ventral cingulum bundle (CB), and the fornix. The UF connects the anterior temporal lobe to lateral orbitofrontal cortex through a direct, monosynaptic, bidirectional pathway. Although UF does not extend into the hippocampus (Von Der Heide et al. 2013), it is well-positioned to serve as a major conduit of communications between the hippocampus and lateral PFC, consistent with evidence of associations between UF and memory functioning (Niogi et al. 2008; Mabbott et al. 2009; Lockhart et al. 2012). The ventral CB connects parahippocampal gyrus to the posterior cingulate cortex and from there on to posterior parietal cortex (Jones et al. 2013). Thus, ventral CB is well-placed to subserve communication between MTL and control-related areas of parietal cortex. The fornix, which connects the hippocampus with subcortical structures including nucleus accumbens, thalamus, and the mammillary bodies, may also be relevant for mnemonic control. The subcortical structures to which the fornix projects are frequently associated with aspects of cognitive control, particularly motivation and gating of information (Delgado 2007; van Schouwenburg et al. 2010). Moreover, existing research points to a role for the fornix in memory; e.g., fornix volume has been linked to recall (Tsivilis et al. 2008), and structural integrity of the fornix has been associated with memory and processing speed (Sasson et al. 2013).
While the likely relevance of MTL-connected tracts for mnemonic operations is clear, it is possible that fronto-parietal tracts may be more important for mnemonic control. Studies examining regional contributions to cognitive control have emphasized the role of a fronto-parietal network that includes lateral PFC and PPC (Corbetta and Shulman 2002; Champod and Petrides 2007), and studies that have focused specifically on mnemonic control have highlighted the role of PFC (Badre et al. 2005; Ranganath et al. 2007; Hayama and Rugg 2009). Thus, white matter tracts connecting PFC and PPC might be particularly important for mnemonic control. Moreover, there is substantial evidence that the contribution of these regions changes during child development (Ofen et al. 2007; Wendelken et al. 2011), and so maturation of the relevant tracts may underlie developmental changes in mnemonic control. The tracts that connect PFC to parietal regions include the superior longitudinal fasciculus (SLF) and the dorsal CB. The SLF serves as the major conduit for communication between PFC and parietal cortex (Cavada and Goldman-Rakic 1991), and the dorsal CB connects anterior with posterior regions of the brain through fibers connecting the anterior cingulate and PFC to posterior cingulate cortex (Mufson and Pandya 1984; Jones et al. 2013). Initial evidence of associations between these tracts and various measures of executive function and memory on neuropsychological batteries have been reported for samples that include developmental and aging populations (Metzler-Baddeley et al. 2012; Tamnes et al. 2012; Chaddock-Heyman et al. 2013; Peters et al. 2013; Sasson et al. 2013).
Recent studies on the development of structural integrity of white matter tracts have shown substantial changes into late adolescence and/or early adulthood of a number of white matters tracts, including viable candidates for associations with mnemonic control (Lebel and Beaulieu 2011; Lebel et al. 2012). It is possible that the contribution of white matter integrity along these different tracts to mnemonic control remains stable over the course of development. Alternatively, the contributions of different tracts to mnemonic control may change over the course of development. Developmental differences in white matter tract-behavior associations, interesting in their own right, can also provide important insight into function in the mature brain.
To address our research goals, we collected behavioral and diffusion tensor imaging (DTI) data from 136 children and young adults. Behavioral data came from a task that was designed to probe attentional modulation of memory encoding. During a selective encoding phase, scenes were either actively attended to, passively viewed, or actively ignored; subsequently, during a surprise recognition phase, their memory for scenes from each condition—attended, passive, and ignored—was assessed through a recognition test. Recognition accuracy for Attended scenes minus average recognition accuracy for Unattended (Passive and Ignored) scenes was our primary measure of attentional modulation of memory, and this was related to fractional anisotropy (FA)—a measure of white matter integrity—in selected white matter tracts.
Materials and Methods
Participants
This investigation included 116 children, ranging in age from 7 to 11 (mean = 9.6 years, SD = 1.1 years; 59 females) as well as 25 young adults (18–22 years, mean = 19.0 years, SD = 0.7; 12 females). All participants were right-handed, with no diagnosis of an attention, learning, or sensory processing disorder, no history of neurological disease, and without ongoing or daily medications. An additional 15 children were excluded due to excessive motion artifacts in their DTI data. Specifically, an automated procedure flagged volumes (DTI directions) with striping (a characteristic motion artifact in DTI data), and subjects with >9 bad volumes (out of 64) were excluded. One additional child was excluded for having a memory modulation (MM) index (see below) >3 standard deviations away from other children (with 0% accuracy on one task condition). In addition to the measures reported here, participants completed a range of cognitive assessments, behavioral tasks, and a functional magnetic imaging (fMRI) task as part of their participation in a larger study examining the neural substrates of episodic memory.
Selective Encoding Task
Participants performed a selective encoding task designed to probe top-down control of mnemonic encoding. This computerized task was modified from a paradigm used in prior fMRI studies (Gazzaley et al. 2005), including an fMRI study comparing children and adults (Wendelken et al. 2011). The present version of the task included a selective encoding phase followed by a surprise recognition phase.
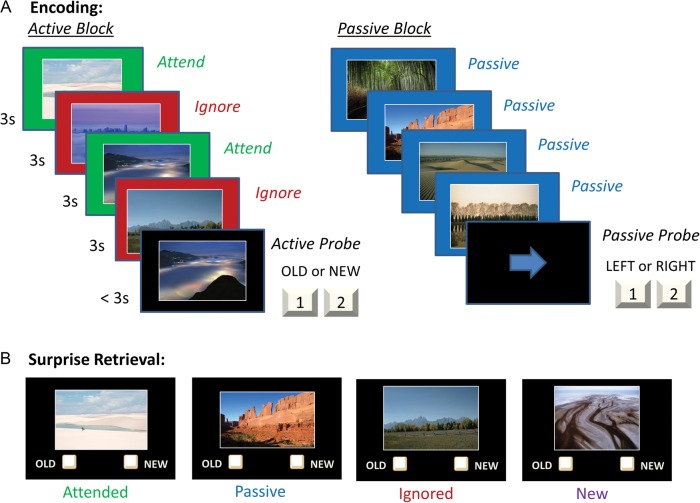
During the selective encoding phase, participants viewed sequences of 4 outdoor scenes followed by a probe, which required different responses as a function of encoding condition. (Fig. 1A). Scenes were presented in Active encoding blocks, which encouraged actively choosing whether or not to encode each scene, and also in Passive encoding blocks, which encouraged passive viewing of all scenes. In the Active encoding blocks, scenes were presented on either a green background or a red background (2 of each), and participants were instructed to attend to the scenes presented on green backgrounds and to ignore, while still viewing, the scenes presented on red backgrounds. Each scene was presented for 3 s. With the presentation of a probe scene on an Active block, participants would indicate via button press whether or not the probe scene was in the initial set of 4. The probe scene was presented for up to 3 s, until a response occurred. The probe scene was always either a previously attended scene (50%) or a novel scene (50%); ignored scenes were never included as a probe. In Passive encoding blocks, 4 scenes were presented in sequence on a blue background, and participants were instructed to passively view each scene. The passive block probe that followed the 4 scenes consisted of an arrow pointing to the left (50%) or right (50%), and participants were instructed to respond with a left or right button press. Participants completed a total of 16 Active blocks and 8 Passive blocks.
Figure 1.
(A) The selective encoding task started with an encoding phase, during which participants observed sequences of scenes. (B) This was followed by a surprise retrieval phase, during which participants viewed scenes and indicated which had been observed during the encoding phase.
Following the encoding blocks, participants were presented with a surprise recognition task (Fig. 1B), during which they viewed a sequence of scenes on a black background and indicated for each whether it was old or new—whether it had been presented in the encoding phase or not. Following the response to each probe, participants were presented with a 3-point confidence scale where they indicated low, medium, or high confidence in the accuracy of their response. The retrieval task was self-paced. Critically, the retrieval task included Attended scenes (20%), Ignored scenes (20%), and Passive scenes (20%), as well as Novel scenes (40%). No scene that had been previously used as a probe was included in this recognition task, so all old scenes had been observed exactly once.
The behavioral measures of interest for this task include hit rate (recollection of Attended, Passive, and Ignored scenes) and false alarm rate (false recollection of New scenes) at retrieval. In addition, we consider as our primary measure of interest an index of attentional modulation of memory that contrasts accuracy on Attended items with accuracy on Unattended (Passive or Ignored) items. This MM index is calculated as:
Neuroimaging Data Acquisition
Imaging data were acquired at the UC-Davis Imaging Research Center, on a 3T Siemens Trio Tim scanner, with a 32-channel head coil. The whole-brain anatomical scan was a high-resolution MPRAGE (voxel size = 0.7 mm isotropic, or 0.35 × 0.35 × 0.7 mm following k-space interpolation; Duration = 7.08 min; time repetition (TR) = 2500 ms; time echo (TE) = 3.24 ms; field of view (FOV) = 224 × 224 mm; GRAPPA with acceleration factor = 2). The DTI scan consisted in an echo-planar imaging scan designed to acquire diffusion data for 64 separate directions (duration = 8.32 min, voxel = 2.5 × 2.5 × 2.2 mm, FOV = 282 × 282 mm, TR = 7400 ms, TE = 81 ms, GRAPPA with acceleration factor = 2, Free diffusion mode with 2 diffusion weightings, B-value #1 = 0 s/mm2, B-value #2 = 1000 s/mm2).
DTI Analysis
FA is a widely used measure of white matter microstructure that is derived from DTI data. FA is a scalar measure that quantifies the directionality of diffusion; voxels containing coherently oriented, and well-myelinated, fibers will tend to have the highest FA values. We used average FA within selected tracts as a measure of white matter integrity.
DTI data were analyzed using the FMRIB Diffusion Toolbox (FDT) software tool (Behrens et al. 2003). First, eddy correction was run on the DTI images to correct for eddy current distortions. Next, brain extraction was performed to exclude nonbrain voxels from further analysis. Following these preliminary steps, a diffusion tensor model was fit to each voxel to calculate directions and magnitudes of diffusion. This procedure produces an FA image for each subject.
White matter ROIs were selected from the John Hopkins University (JHU) white matter tractography atlas included with FDT (Mori et al. 2005; Hua et al. 2008). These tracts were previously identified probabilistically by averaging results of deterministic tractography on 28 adults. Although there has been no systematic investigation of their suitability for child research, they have been used successfully in children before (Chaddock-Heyman et al. 2013), and our methods were designed to minimize potential registration errors (see below).
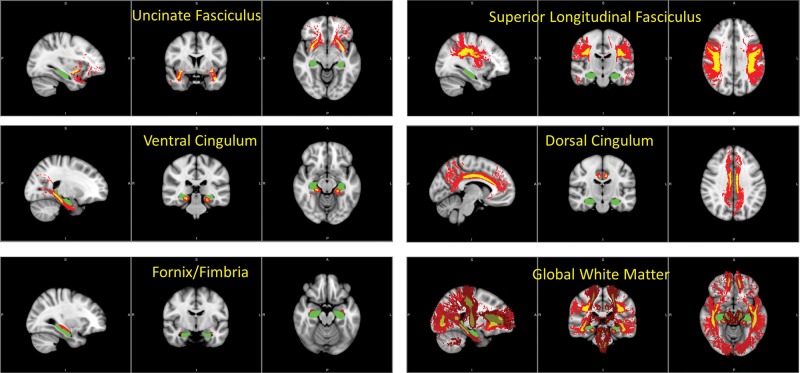
Of primary interest were the white matter tracts most reliably associated with hippocampus, including the UF, the fornix, and the CB. The full CB consists of a ventral part that primarily projects from MTL to posterior cingulate cortex, and also a dorsal part that primarily projects from posterior cingulate cortex forward through anterior cingulate cortex; we consider these separately, as ventral CB and dorsal CB. Similarly, fornix tracts were separated into a ventral part, the fimbria, which abuts the hippocampus and connects through to hypothalamus, and a dorso-medial part, the fornix body, which projects to the nucleus accumbens and is not considered here. We also examined the SLF, which connects prefrontal and parietal cortices most strongly associated with cognitive control. In addition, a global white matter control region of interest (ROI) was created by taking the union of white matter templates from the atlas. For each tract except the fornix, the atlas provides 2 versions created with different probabilistic thresholds: a version of the tract with maximal projection into cortex but with lower reliability of individual voxels, and a core version of the tract with reduced extent but greater reliability. We used the core version of each tract, in order to improve reliability of mapping each tract into subject space. These tract ROIs, overlaid on the more extensive versions of each tract for illustration, are shown in Figure 2.
Figure 2.
White matter region of interests (ROIs), from the JHU white matter tractography atlas. In each image, hippocampus is shown in green. For fornix/fimbria, the ROI is shown in red. For the other tracts, yellow indicates the ROI and red illustrates a more extensive version of the tract.
To extract subject-specific FA values for each ROI, it was necessary first to transform each participant's brain-extracted anatomical image, as well as their DTI image, into standard Montreal Neurological Institute (MNI) space. From these transformations, we obtained a mapping from standard MNI-space into subject DTI-space, and used this mapping to transform each template ROI into subject-specific DTI-space. All registrations were accomplished using the FMRIB Linear Registration Tool (FLIRT). For each subject DTI-space ROI, we extracted average FA across all voxels in the ROI, from the subject FA image.
Statistical Analyses
For the behavioral task, both accuracy and response times were examined with separate 2 × 4 analysis of variance (ANOVA) that included Group (Children or Adults) and Condition (Attended, Passive, Ignored, or New). Follow-up ANOVAS were conducted for adults and children separately. In all cases, post hoc pairwise t-tests (using the multiple comparison correction of Holm 1979) were conducted to examine specific effects identified in the ANOVAs. MM scores were computed for each participant (see above), and these were also submitted to ANOVA (examining effect of Group) and follow-up t-tests (examining effect for each group separately).
Age-related differences in FA were examined in 2 ways for each tract ROI (see Table 2). First, FA in adults was compared with children's FA via t-test. Second, correlations between age and FA were computed in the child sample. To assess the relationship between MM and FA, separately for each tract ROI and for each group, we computed correlation values and also conducted a multiple linear regression of MM on FA and age. In order to probe for differences between adults and children, in tracts that demonstrated significant effects for either group, we compared correlations values using a Fisher r-to-z transformation.
Table 2.
Age-related changes in FA in white matter tract ROIs
| Age-related changes in FA | FA: adults versus children |
FA–age in children |
|||
|---|---|---|---|---|---|
| Tract | T | q (FDR) | R | T | q (FDR) |
| Left UF | 3.6 | 0.002 | 0.26 | 2.8 | 0.05 |
| Right UF | 1.6 | 0.12 | 0.15 | 1.6 | ns |
| Left ventral CB | 2.4 | 0.03 | −0.08 | −0.8 | ns |
| Right ventral CB | 3.6 | 0.002 | −0.005 | −0.04 | ns |
| Left fornix/fimbria | 4.7 | 0.0002 | 0.03 | 0.37 | ns |
| Right fornix/fimbria | 3.8 | 0.002 | 0.01 | 0.14 | ns |
| Left SLF | 2.9 | 0.01 | 0.18 | 1.9 | ns |
| Right SLF | 2.2 | 0.05 | 0.07 | 0.70 | ns |
| Left dorsal CB | 1.6 | 0.12 | 0.07 | 0.80 | ns |
| Right dorsal CB | 1.5 | 0.14 | 0.02 | 0.26 | ns |
| Left global WM | 2.7 | NA | 0.18 | 2.0 | NA |
| Right global WM | 4.4 | NA | 0.16 | 1.7 | NA |
Statistics from testing for differences between children and adults (including T-value and FDR-corrected q-value) are shown in the left-hand columns. Statistics describing age-related differences in children (including correlation R-value, T-value, and FDR-corrected q-value) are shown in the right-hand columns.
False discovery rate (FDR) correction for multiple comparisons across tracts was applied to the 12 tests of our primary hypothesis of a relationship between MTL-connected tracts and mnemonic control, and separately to the 8 tests of our alternate hypothesis of a relationship between fronto-parietal tracts and mnemonic control.
Because the ability to detect a relationship between variables is dependent on the amount of variance in each variable, we calculated means and variances for each examined variable and report these in Table 1. We probed for differences in variance as a function of ROI set (MTL vs. non-MTL) for each age group, and as a function of age group (adults vs. children) for each ROI set, but observed no significant differences (all P's > 0.2).
Table 1.
Means and variances for each experimental measure
| Measure | Mean |
Variance |
||
|---|---|---|---|---|
| Adults | Children | Adults | Children | |
| Age | 19.0 | 9.6 | 0.55 | 1.3 |
| Memory modulation (MM) | 0.30 | 0.15 | 0.024 | 0.037 |
| FA: left UF | 0.41 | 0.37 | 0.003 | 0.004 |
| FA: right UF | 0.40 | 0.37 | 0.006 | 0.004 |
| FA: left ventral CB | 0.34 | 0.30 | 0.006 | 0.004 |
| FA: right ventral CB | 0.34 | 0.29 | 0.003 | 0.004 |
| FA: left fornix/fimbria | 0.49 | 0.44 | 0.002 | 0.004 |
| FA: right fornix/fimbria | 0.46 | 0.41 | 0.002 | 0.004 |
| FA: left dorsal CB | 0.39 | 0.36 | 0.008 | 0.008 |
| FA: right dorsal CB | 0.35 | 0.32 | 0.008 | 0.008 |
| FA: left SLF | 0.40 | 0.38 | 0.002 | 0.002 |
| FA: right SLF | 0.40 | 0.38 | 0.002 | 0.001 |
| FA: left global WM | 0.43 | 0.41 | 0.001 | 0.001 |
| FA: right global WM | 0.42 | 0.40 | 0.0005 | 0.001 |
Results
Age-Related Differences in Behavioral Performance
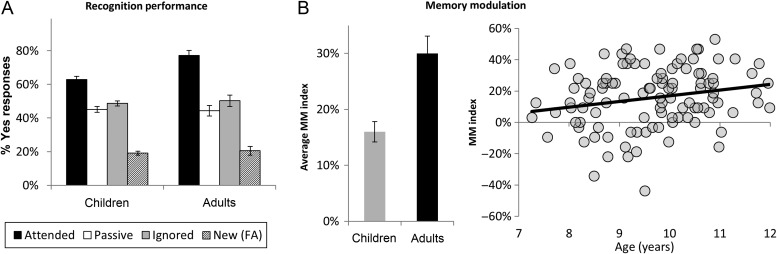
Response accuracy on the mnemonic control task was examined via 2 × 4 ANOVA that included Group (children or adults) and Condition (Attended, Passive, Ignored, and New). We analyzed rates of “Yes”/“Old” responses (i.e., hit rates for Attended, Passive, and Ignored scenes; false alarms to Novel scenes) and found a significant main effect of Condition (F = 155, P < 0.001), as well as a significant Group × Condition interaction (F = 4.8, P = 0.002; see Fig. 3A). No other effects reached significance.
Figure 3.
(A) Recognition performance for each of the 4 conditions. Note that the y-axis indicates the percentage of yes responses, so bars represent accuracy for Attended, Passive, and Ignored scenes but represent the false alarm rate for New scenes. (B) MM scores, for children versus adults and as a function of age in children.
Pairwise t-tests revealed differences between conditions. First, participants were more likely to recognize Attended, Passive, and Ignored scenes than New scenes (all P's < 0.001), indicating clear discrimination between studied and novel scenes across conditions. Both children and adults recognized old scenes significantly more often than new scenes, for Attended scenes (adults: t = 17.4, P < 0.001; children: t = 21, P < 0.001), Passive scenes (adults: t = 6.0, P < 0.001; children: t = 15.2, P < 0.001), and Ignored scenes (adults: t = 8.0, P < 0.001; children: t = 17.4, P < 0.001). Second, participants demonstrated a mnemonic enhancement effect, by recognizing Attended scenes more frequently than Passive or Ignored scenes (Attended > Passive: t = 11.1, P < 0.001; Attended > Ignored: t = 9.4, P < 0.001). Separate pairwise t-tests in children and adults revealed the same pattern in each both children and adults (all P's < 0.001). There was no difference between accuracy for Passive scenes and accuracy for Ignored scenes, for children or adults (all P's > 0.2); thus, we observed no effect of mnemonic suppression. In summary, both children and adults demonstrated attention-based mnemonic enhancement—better memory for attended versus unattended scenes.
A comparison of accuracy in children versus adults for each condition revealed significantly greater hit rates for Attended scenes in adults (t = 4.1, P < 0.001) but no other group differences. Similarly, hit rates for Attended scenes, but for no other scene type, were correlated with age among children (r = .33, P < 0.001). Thus, increased age was associated with improved recognition, but only for scenes that participants were cued to attend to during the encoding phase. To test for age-related changes in mnemonic enhancement, we created a MM index, calculated as accuracy on Attended scenes minus average accuracy on Passive and Ignored scenes (Fig. 3B). On this MM index, we observed both a significant difference between children and adults (t = 3.9, P < 0.001) and a significant age-related increase among children (r = 0.23, P = 0.01).
For response times, also examined with a Group × Condition ANOVA, there was a large main effect of Group (F1,132 = 19, P < 0.001), such that adults responded more quickly than children. In addition, average response time was negatively correlated with age among children (r = −0.28, P = 0.002). There was no main effect on response times of Condition, nor any interaction between Group and Condition. In summary, the key finding from the behavioral data is an age-related increase in attentional modulation of the accuracy of subsequent memory; these results led us to focus on accuracy and not response time in our analyses of brain–behavior relationships.
Relationships Between White Matter Coherence, Mnemonic Control, and Age
Our primary analysis examines the relationship between mnemonic control, here represented by the MM index, and white matter tract coherence, here represented by FA. For each tract ROI, we first describe the observed developmental changes in FA. Table 2 shows the complete set of results relating FA to age or group. We then report the relationship between MM and FA for each ROI, for adults and for children, and indicate where this effect differed between adults and children. Table 3 shows correlation values as well as results from regression analyses relating MM to FA.
Table 3.
Relation between MM and FA in white-matter tract ROIs, including correlation R-values as well as parameter estimates and FDR-corrected q-values from the multiple regression of MM on Age and FA (for each tract ROI)
| MM–FA | Adults |
Children |
||||
|---|---|---|---|---|---|---|
| Tract | R | PE | q (FDR) | R | PE | q (FDR) |
| Hippocampal | ||||||
| Left UF | 0.23 | 0.71 | n.s. | 0.28 | 0.68 | 0.06 |
| Right UF | 0.31 | 0.74 | 0.18 | 0.12 | 0.24 | ns |
| Left ventral CB | 0.63 | 1.28 | 0.005 | 0.09 | 0.33 | ns |
| Right ventral CB | 0.43 | 1.35 | 0.08 | 0.04 | 0.11 | ns |
| Left fornix/fimbria | 0.44 | 1.52 | 0.10 | 0.10 | 0.21 | ns |
| Right fornix/fimbria | 0.43 | 1.50 | 0.10 | −0.04 | −0.14 | ns |
| Frontoparietal | ||||||
| Left dorsal CB | 0.05 | 0.10 | ns | −0.01 | −0.07 | ns |
| Right dorsal CB | 0.12 | 0.24 | ns | 0.06 | 0.11 | ns |
| Left SLF | −0.15 | −0.50 | ns | 0.05 | −0.10 | ns |
| Right SLF | −0.17 | −0.63 | ns | −0.04 | −0.31 | ns |
| Control | ||||||
| Left global WM | 0.11 | 0.64 | ns | 0.18 | 0.67 | ns |
| Right global WM | 0.18 | 1.32 | ns | 0.10 | 0.28 | ns |
MTL-Connected Tracts
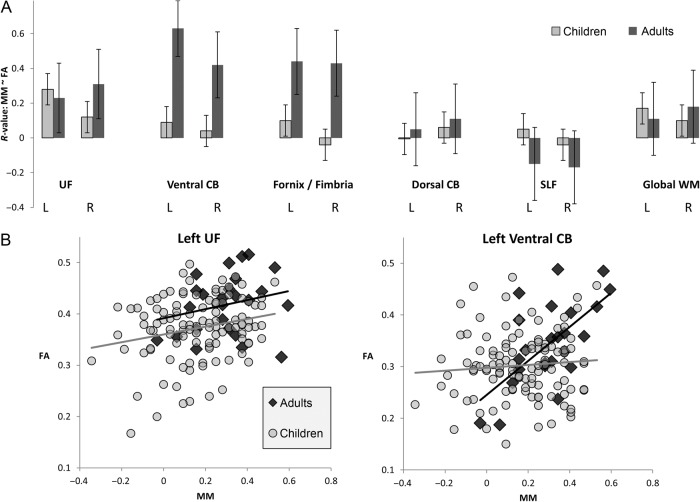
Within the UF, we observed significantly higher FA in young adults than in children; this effect was significant on the left side and marginal on the right side. Similarly, left UF demonstrated a significant age-related increase in FA in children. In children, there was a positive effect of FA on MM for left but not right UF; this effect was marginally significant after correction for multiple comparisons. For the smaller group of adults, the effect of FA on MM was not significant for left or right UF. However, the differences in correlation values between children and adults were not significant (left: z = 0.23, P > 0.2; right: z = 1.0, P > 0.2). In fact, with children and adults considered together, left UF demonstrated a statistically stronger effect of FA on MM (linear regression: t = 2.9, P = 0.004, qFDR = 0.03) than with children alone (overall correlation: r = 0.33; partial correlation, controlling for age: rAge = 0.24). In summary, we observed in left UF both age-related increases in FA and an effect of FA on MM across all participants.
Increased FA was observed in ventral CB for adults relative to children, bilaterally; however, there was no age-related increase among children for this tract. In adults, ventral CB FA was positively related to MM, strongly on the left and marginally on the right. In children, there was no such relationship between FA and MM. Indeed, the positive relationship observed in adults was significantly different than that observed in children (left: z = 2.8, P = 0.003; right: z = 1.9, P = 0.02).
In both left and right fornix/fimbria, FA was higher in adults than in children. Neither fornix ROI demonstrated age-related increases in children. In adults, a positive relation between FA and MM was observed in both left and right fornix; this effect was marginally significant after correction for multiple comparisons. In children, these effects were absent, and the correlations for left and right fornix were significantly lower than those observed in adults (left: z = 1.8, P = 0.03; right: z = 2.2, P = 0.01). Thus, effects in the fornix mirrored those observed in the CB, insofar as a positive relationship between FA and MM was observed in adults but not in children.
In a follow-up analysis, we examined separate correlations between FA and recognition accuracy for Attended and Ignored scenes, and observed a potentially important difference between ventral CB and fornix, particularly on the left: in fornix, the relationship between FA and MM was driven by a strong positive correlation between FA and recognition accuracy for Attended scenes (r = 0.52, P = 0.007) with no relation between FA and Ignored scene accuracy (r = −0.06, P > 0.2), while in ventral CB, this relationship was driven not only by a positive correlation between FA and Attended scene accuracy (r = 0.32, P = 0.1) but also by a negative correlation between FA and Ignored scene accuracy (r = −0.38, P = 0.06).
Non-MTL Tracts
In dorsal CB, there was a trend toward increased FA in adults relative to children, but there was no age-related increase in children. In contrast to ventral CB, there were no significant effects of FA on MM in dorsal CB in either children or adults. SLF demonstrated higher FA in adults relative to children on both the left and on the right. For SLF, however, there was no association between FA and MM. This was true for both left and right SLF, for children and adults. Finally, within the global white matter ROIs, there was a significant increase in FA for adults relative to children bilaterally, as well as marginal age-related increases in children. However, there was no significant effect of FA on MM for either group, after taking age into account. Thus, while age-related increases in FA appear to be characteristic of most white matter tracts, a relationship between FA and MM is not (Fig. 4).
Figure 4.
(A) Effects of FA on MM, in children and adults, across all 12 ROIs. Bars indicate correlation r-values, with standard error. (B) Scatter-plots of the relationship between MM and FA, for left UF (which showed an effect across the entire sample of participants) and for left ventral CB (which showed an effect in adults but not in children). In both graphs, adult data are dark gray and data from children are light gray.
Discussion
The main goal of our study was to examine the relation between the developing capacity for mnemonic control and the structural integrity of potentially relevant white matter tracts. We addressed this question with an experimental measure that gauged the extent to which attentional modulation during encoding affects long-term retrieval success. For this measure, summarized with our MM index, we were able to demonstrate a robust and steady pattern of age-related improvement across childhood. The effect of top-down control during encoding on subsequent retrieval success increased with age throughout middle childhood and increased further between middle childhood and adulthood. This pattern of results is consistent with prior investigations of developing mnemonic control (Barclay 1979; Paz-Alonso et al. 2013).
Our results demonstrated a clear effect of top-down enhancement—increased recall accuracy for attended versus unattended items. However, we did not observe top-down suppression, which would be apparent in differences in recall accuracy between passively viewed and ignored items. Thus, our findings of relationships between white matter integrity and mnemonic control relate specifically to attentional enhancement, and do not speak to possible mechanisms of suppression. While we demonstrate effects of attentional enhancement on memory, we did not examine other (nonmnemonic) effects of attention; thus, we cannot rule out the possibility that the relationships that we observed between attentional enhancement and white matter integrity might be present even for a nonmnemonic task. However, the pattern of results, as discussed below, weighs heavily in favor of specificity to memory.
Many studies have reported age-related changes in white matter coherence, at various stages of the lifespan (Giorgio et al. 2008; Imperati et al. 2011; Lebel et al. 2012). In a large-scale investigation of white matter changes across the lifespan, Lebel et al. (2012) reported increasing white matter integrity (FA) during childhood, and into early adulthood, across a large number of tracts as well as decreasing white matter integrity in older adults. More specifically, they reported a relatively early peak FA for fornix (at age 22), a somewhat later peak for SLF (age 29), and particularly late peaks for UF (age 35) and CB (age 43). Based on these findings, and the fact that our oldest young adult participant was 22, we would expect all of the tracts that we examined to show age-related increases in FA. Indeed, this is what we observed: all of the tracts that we examined demonstrated higher FA in adults relative to children. The absence of a correlation between age and FA in CB and fornix among children suggests a delayed and protracted developmental trajectory, in which increases in FA occur later than the age ranges examined in the present study. This result is consistent with the results of Lebel et al. (2012) with regard to CB, but not with regard to fornix, which in their study peaks earlier. This discrepancy may be accounted for differences in the ROI definition in these studies and will be monitored as we collect longitudinal data from the current sample.
White Matter Integrity and Mnemonic Control: Fronto-Parietal Versus MTL Tracts
We demonstrated both age-related improvements in mnemonic control as indicated by attentional enhancement of memory performance and developmental increases in white matter integrity. But our main question concerned whether, to what extent, and along which tracts, differences in white matter integrity relate to differences in the capacity for mnemonic control. We proposed 2 broad hypotheses. First, we posited that MTL-connected white matter tracts, and in particular those that connect MTL to prefrontal and parietal regions associated with cognitive control, should be more important for successful mnemonic control. Second, we considered the alternative hypothesis that fronto-parietal white matter tracts contribute to mnemonic control just as they contribute to other kinds of cognitive control. Our findings are consistent with the first hypothesis and inconsistent with the second. There was no relation between mnemonic control and FA in either of the fronto-parietal tracts—i.e., SLF or dorsal CB. On the other hand, all 3 MTL tracts that we examined, i.e., UF, ventral CB, and the fimbria, demonstrated a positive relationship between white matter integrity and mnemonic control in adults, and the first of these demonstrated a similar relationship in children.
UF Involvement in Memory and Cognitive Control
The UF, which connects MTL to PFC, contributed similarly to mnemonic control in children and young adults. This pattern of results suggests an already robust prefrontal contribution to mnemonic control in children that is carried into adulthood. This is perhaps surprising; given the fact that prefrontal cortex is known to undergo prolonged development during into adolescence, we might expect prefrontal-MTL communication along the UF to be relatively less important in children. However, the present evidence indicates that communication between MTL and PFC is important for mnemonic control even in childhood, when the PFC is not yet fully mature.
Though we are aware of no prior studies that have linked UF directly to mnemonic control, it is notable that several studies have reported an association between UF and memory (Niogi et al. 2008; Mabbott et al. 2009; Lockhart et al. 2012). Investigating typical adults as well as traumatic brain injury patients, Niogi et al. (2008) observed a specific positive association between memory performance and UF integrity (as measured with FA) while Lockhart et al. (2012) observed a negative association between memory performance UF injury (as measured by white matter hyperintensities). Moreover, Mabbott et al. (2009) reported a positive correlation between UF integrity and proficiency in a verbal-auditory memory task in 22 children aged 9–15. As some degree of mnemonic control is likely required in any memory task in which individuals must provide a deliberate response, it is entirely possible that a specific role for UF in communicating control signals between PFC and MTL could explain the associations seen for these various memory tasks. Conversely, a general role in memory would not easily explain the specific relation to attention that we observed here.
The link between UF and mnemonic control may reflect top-down, goal-oriented control with its origins in lateral PFC. However, UF does not connect directly to lateral PFC, but rather to orbitofrontal cortex (OFC). One theory posits 2 key roles for UF: (1) communicating MTL-supported mnemonic associations to OFC to influence decision-making, and (2) communicating OFC reward and punishment value signals to MTL to rapidly modify mnemonic representations (Von Der Heide et al. 2013). If this were the case, then we might interpret our observed association between UF integrity and mnemonic control as being due to communication not of the sort of goal-oriented signal (e.g., “attend to this scene” or “ignore this scene”) that might emerge from lateral PFC, but rather of lower-level reward associations (e.g., “green is good” or “red is bad”) that are thought to emerge from OFC. Whether mnemonic control is supported by lower-level reward information communicated from OFC, higher-level goal information communicated from lateral PFC, or some combination of these, the MTL-prefrontal connection is important for its operation in both children and young adults.
The Changing Contribution of the CB to Mnemonic Control
Patterns of developmental change of white matter tracts, in their relation to mnemonic control, can provide additional clues as to the nature of their contribution. In particular, differences in the contribution of a particular tract suggest a changing relevance of communication between its endpoints, and hints at developmental change in the role of these endpoints. The fact that ventral CB integrity predicted mnemonic control in adults, but not in children suggests that the parietal cortices, connected to MTL through the ventral CB, contribute to mnemonic control in adults to a greater extent than they do in children. Indeed, this increased contribution may be a source of improvements in the capacity for mnemonic control between childhood and adulthood.
The parietal cortices comprise a diverse set of functional subregions, many of which have been linked to memory in one form or another. Medial parietal regions, including retrosplenial cortex, precuneus, and posterior cingulate, are frequently activated in retrieval tasks (Levy 2012), and damage to these areas can produce anterograde amnesia (Aggleton 2010). To the extent that the observed relationship between ventral CB and mnemonic control reflects MTL communication with medial parietal regions, this relationship may not be driven by top-down control. However, if this were the case, then we would expect its integrity to correlate positively with memory regardless of control demand. In fact, ventral CB integrity was negatively related to accuracy for Ignored scenes, suggesting this tract may be a conduit for active mnemonic suppression. This finding lends anatomical support to a recent proposal that CB underlies patterns of functional connectivity observed during memory suppression (Paz-Alonso et al. 2009).
Lateral parietal cortex is frequently linked to top-down control, but the involvement of lateral parietal regions in memory is more subtle. Although damage to lateral parietal cortex has not been shown to affect memory, neuroimaging work has implicated it in various aspects of mnemonic processing. For example, evidence suggests that parietal activation scales with the mnemonic strength or expectation associated with retrieved items (Wagner et al. 2005; O'Connor et al. 2010); under this account, a changing parietal contribution to mnemonic control might reflect changes in the way that parietal mnemonic strength signals are created or used. This account is consistent with a recent fMRI investigation that demonstrated greater parietal sensitivity to memory strength in adults compared with children (DeMaster et al. 2013). Similarly, if parietal cortex provides a substrate for representation of memory contents (Vilberg and Rugg 2008; Shimamura 2011), then an increased contribution to mnemonic control of this region could indicate greater sensitivity to these contents at retrieval. On the other hand, if the parietal contribution to memory reflects attentional processes (Uncapher et al. 2006; Cabeza et al. 2008), then an increase in its contribution to mnemonic control might reflect an increase in the contribution of parietal attention mechanisms, during encoding and/or during retrieval.
Fornix and Mnemonic Control
As a tract that carries information between hippocampus and other subcortical structures, Fornix could be expected to play an important role in memory. Indeed, damage to fornix has been associated with memory impairment (Chang et al. 2010). However, the probable role of fornix in mnemonic control is more difficult to discern. As the fornix/fimbria is the white matter tract that is most closely and specifically linked to hippocampus, its relevance to mnemonic enhancement in adults indicates the importance of connectivity of this region, and suggests that adults improved capacity for attentional mnemonic enhancement may derive in part from interaction between hippocampus and other subcortical structures.
Conclusion
We set out to examine the relationship between structural integrity of white matter tracts and the capacity for attentional modulation of memory. We looked at MTL-connected tracts that are most likely to support memory performance (UF, ventral CB, and fornix) and fronto-parietal tracts that are most likely to be associated with cognitive control (SLF and dorsal CB). Our findings demonstrate that individual differences in mnemonic enhancement are related to variability in the structural integrity of MTL-connected tracts, but are unrelated to structural integrity of the fronto-parietal tracts. Most of the MTL-connected tracts that we examined were associated with mnemonic enhancement in adults, while only UF had this association in children. This pattern reflects the importance of prefrontal-MTL communication for mnemonic control across ages, and further suggests that the increased capacity for mnemonic control in adults may derive in part from increased communication between MTL and other brain regions including parietal cortex and subcortical areas.
Funding
This work was supported by a National Institutes of Health grant awarded to S.G. and S.A.B. (R01MH091109).
Notes
Conflict of Interest: None declared.
References
- Aggleton JP. 2010. Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia. 48(8):2328–2338. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. 2005. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 47(6):907–918. [DOI] [PubMed] [Google Scholar]
- Barclay CR. 1979. Executive control of mnemonic activity. J Exp Child Psychol. 27:262–276. [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. 2003. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 50(5):1077–1088. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Anderson MC. 2012. Opposing mechanisms support the voluntary forgetting of unwanted memories. Neuron. 76(2):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. 2008. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 9(8):613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. 1991. Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience. 42(3):683–696. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Voss MW, Powers JP, Knecht AM, Pontifex MB, Drollette ES, Moore RD, Raine LB, Scudder MR, et al. 2013. White matter microstructure is associated with cognitive control in children. Biol Psychol. 94(1):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M. 2007. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 104(37):14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Kim SH, Kim OL, Bai DS, Jang SH. 2010. The relation between fornix injury and memory impairment in patients with diffuse axonal injury: a diffusion tensor imaging study. NeuroRehabilitation. 26(4):347–353. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3(3):201–215. [DOI] [PubMed] [Google Scholar]
- Delgado MR. 2007. Reward-related responses in the human striatum. Ann N Y Acad Sci. 1104:70–88. [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Ghetti S. 2013. Development of memory for spatial context: hippocampal and cortical contributions. Neuropsychologia. 51(12):2415–2426. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. 2007. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 317(5835):215–219. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. 2012. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 36(7):1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Chiu YC, Tamber-Rosenau BJ, Yantis S. 2009. Decoding cognitive control in human parietal cortex. Proc. Natl. Acad. Sci. 106(42):17974–17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney J, McEvoy K, Knight R, D'Esposito M. 2005. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 17(3):507–517. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. 2012. Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2(4):381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins K, Douaud G, James A, James S, De S, Matthews P, Smith S, Johansen-Berg H. 2008. Changes in white matter microstructure during adolescence. Neuroimage. 39(1):52–61. [DOI] [PubMed] [Google Scholar]
- Harnishfeger KK, Bjorklund DF. 1994. A developmental perspective on individual differences in inhibition. Learn Individ Differ. 6(3):331–355. [Google Scholar]
- Hayama HR, Rugg MD. 2009. Right dorsolateral prefrontal cortex is engaged during post-retrieval processing of both episodic and semantic information. Neuropsychologia. 47(12):2409–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat. 6:65–70. [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S. 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 39(1):336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperati D, Colcombe S, Kelly C, Di M, Zhou J, Castellanos F, Milham M. 2011. Differential development of human brain white matter tracts. PLoS One. 6(8):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Christiansen K, Chapman R, Aggleton J. 2013. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 51(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 31(30):10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 60(1):340–352. [DOI] [PubMed] [Google Scholar]
- Levy DA. 2012. Towards an understanding of parietal mnemonic processes: some conceptual guideposts. Front n Integre Neurosci. 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SN, Mayda ABV, Roach AE, Fletcher E, Carmichael O, Maillard P, Schwarz CG, Yonelinas AP, Ranganath C, DeCarli C. 2012. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Human Neurosci. 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, Rockel C. 2009. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 1294:80–90. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O'Sullivan MJ. 2012. Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J Neurosci. 32(49):17612–17619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. 2005. MRI atlas of human white matter. Amsterdam: Elsevier. [Google Scholar]
- Mufson EJ, Pandya DN. 1984. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 225(1):31–43. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. 1998. Hippocampal contributions to cortical plasticity. Neuropharmacology. 37(4–5):431–439. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD. 2008. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 131(Pt 12):3209–3221. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. 2003. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 110(4):611–646. [DOI] [PubMed] [Google Scholar]
- O'Connor AR, Han S, Dobbins IG. 2010. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 30(8):2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. 2007. Development of the declarative memory system in the human brain. Nat Neurosci. 10(9):1198–1205. [DOI] [PubMed] [Google Scholar]
- Olszewska J, Ulatowska J. 2013. Encoding strategy affects false recall and recognition: Evidence from categorical study material. Advances in Cognitive Psychology/University of Finance and Management in Warsaw. 9(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso PM, Bunge SA, Anderson MC, Ghetti S. 2013. Strength of coupling within a mnemonic control network differentiates those who can and cannot suppress memory retrieval. J Neurosci. 33(11):5017–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso P, Ghetti S, Matlen BJ, Anderson MC, Bunge SA. 2009. Memory suppression is an active process that improves over childhood. Front Hum Neurosci. 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. 2013. Age-related differences in white matter tract microstructure are associated with cognitive performance from Childhood to Adulthood. Biol Psychiatry. 75(3):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller AS, Wilding EL. 2007. Dissociable correlates of two classes of retrieval processing in prefrontal cortex. Neuroimage. 35(4):1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. 2013. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci. 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Pressley M. 1997. Memory development between two and twenty. Greenwood: Praeger. [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 20(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. 2011. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 11(3):277–291. [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Brehmer Y, Müller V, Li SC, Lindenberger U. 2010. Episodic memory across the lifespan: The contributions of associative and strategic components. Neurosci Biobehav Rev. 34(7):1080–1091. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. 2003. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 4(8):637–648. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Ostby Y, Walhovd KB. 2012. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 32(3):972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. 2008. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 11(7):834–842. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. 2006. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 52(3):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HEM, Cools R. 2010. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 30(29):9910–9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. 2008. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 46(7):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. 2013. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 136(Pt 6):1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 9(9):445–453. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Baym CL, Gazzaley A, Bunge SA. 2011. Neural indices of improved attentional modulation over middle childhood. Dev Cogn Neurosci. 1(2):175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]