Abstract
Preterm birth impacts brain development and leads to chronic deficits including cognitive delay, behavioral problems, and epilepsy. Premature loss of the subplate, a transient subcortical layer that guides development of the cerebral cortex and axonal refinement, has been implicated in these neurological disorders. Subplate neurons influence postnatal upregulation of the potassium chloride co-transporter KCC2 and maturation of γ-amino-butyric acid A receptor (GABAAR) subunits. We hypothesized that prenatal transient systemic hypoxia–ischemia (TSHI) in Sprague–Dawley rats that mimic brain injury from extreme prematurity in humans would cause premature subplate loss and affect cortical layer IV development. Further, we predicted that the neuroprotective agent erythropoietin (EPO) could attenuate the injury. Prenatal TSHI induced subplate neuronal loss via apoptosis. TSHI impaired cortical layer IV postnatal upregulation of KCC2 and GABAAR subunits, and postnatal EPO treatment mitigated the loss (n ≥ 8). To specifically address how subplate loss affects cortical development, we used in vitro mechanical subplate ablation in slice cultures (n ≥ 3) and found EPO treatment attenuates KCC2 loss. Together, these results show that subplate loss contributes to impaired cerebral development, and EPO treatment diminishes the damage. Limitation of premature subplate loss and the resultant impaired cortical development may minimize cerebral deficits suffered by extremely preterm infants.
Keywords: development, GABA receptor, KCC2, prematurity, subplate, thalamocortical, white matter
Introduction
Premature birth remains a leading cause of lifelong chronic neurological deficits including cerebral palsy (Hack and Costello 2008), epilepsy (Crump et al. 2011) and cortical visual impairment (Fazzi et al. 2009). The most common and debilitating deficits are the cognitive (Orchinik et al. 2011; Marret et al. 2013) and behavioral abnormalities such as autism (Limperopoulos et al. 2008), anxiety, hyperactivity and inattention (Scott et al. 2012), which are all critically dependent on adequate function of cerebral cortical circuits (Volpe 2009a; Kostovic et al. 2012). Infants born extremely preterm are also more likely to experience cumulative deficits contributing to the life-long burden of disability. A better understanding of the mechanisms of abnormal cerebral circuit formation in the developing central nervous system (CNS) in the context of preterm birth and prenatal brain injury is imperative to devise rational use of existing and emerging therapeutic interventions.
The subplate is a transient, highly dynamic and complex cerebral cortical layer between the underlying white matter anlage and the overlying cortex and is integral to cerebral circuit formation (Kostovic and Rakic 1980; Ghosh and Shatz 1993; Kanold and Luhmann 2010). The relative prominence of the subplate in monkeys and humans compared with other species supports the importance of the subplate in cognition and behavior (Molnar and Clowry 2012). In humans, the subplate is maximal at ∼25-week gestation and recedes by 6 months postnatally (Kostovic and Rakic 1990; Kostovic et al. 2012), corresponding to the time when infants born very preterm are most vulnerable to CNS injury from late gestation, peripartum, and neonatal complications (Volpe 1996). The subplate is comprised of axons, glia, extracellular matrix, migrating neurons, and subplate neurons (Vasung et al. 2010).
Subplate neurons guide the development of the overlying cortex and refinement of white matter axonal pathways (Kanold and Shatz 2006; Kostovic et al. 2012). They are lost with brain injury from very preterm birth in human infants (Kinney et al. 2012), and this loss has been proposed to contribute to encephalopathy of prematurity (Volpe 1996; Leviton and Gressens 2007; Miller and Ferriero 2009; Kostovic et al. 2012). Following extremely preterm birth, loss of subplate neurons in the frontal lobe especially, where the subplate has the most prominent and prolonged existence in humans (Kostovic and Judas 2010; Kostovic et al. 2012), likely contributes to impaired cerebral function, including impaired cognition and behavior. These neurons are also central to the development of initial inhibitory circuits in the developing visual cortex as the brain begins to respond to environmental stimuli (Aboitiz et al. 2005; Kanold and Shatz 2006; Kanold 2009) and guide cortical circuit development by promoting the upregulation of layer IV potassium chloride co-transporter 2 (KCC2) (Kanold and Shatz 2006). Subplate neurons excite cortical layer IV neurons, and the developmental increase in KCC2 expression is likely driven by neuronal activity (Ludwig et al. 2003; Rivera et al. 2004). As functional KCC2 expression increases in the early postnatal period, chloride is extruded (Rivera et al. 1999), and the local microdomain of the neuronal membrane is hyperpolarized (Chamma et al. 2013). This presence of KCC2 in cortical layer IV facilitates the switch of responses to GABA from excitatory to inhibitory, resulting in fast inhibition of pyramidal neurons, and refinement of cortical inhibitory circuits (Daw et al. 2007).
KCC2 upregulation with development is also closely integrated with the maturation of GABAA receptor subunits (Kanold and Shatz 2006; Huang et al. 2013). As a consequence of their molecular heterogeneity and subunit specificity, synaptic and extrasynaptic GABAARs mediate phasic and tonic inhibition. Different subunit compositions allow diverse channel kinetics and agonist affinities and facilitate membrane targeting and regulation (Farrant and Nusser 2005; Balia et al. 2015). Most synaptic GABAARs in neurons are assembled from a combination of α- and β-subunit variants with the γ2 subunit; γ2 is essential for clustering of the functional receptor at the postsynaptic site (Essrich et al. 1998; Schweizer et al. 2003; Balia et al. 2015). GABAAR subunits α1, α2 and γ2 are the predominant developmentally regulated subunits on cortical interneurons in layer IV (Kanold and Shatz 2006). It has been postulated, but not shown, that the subplate is similarly responsible for guiding cortical interconnectivity in the frontal and central cortical regions as well as the visual cortex (Volpe 1996; Kostovic et al. 2012).
Premature loss of subplate neuronal input into the developing cortex from brain injury associated with preterm birth may contribute to the cerebral dysfunction in preterm infants. However, this hypothesis has not yet been tested in a clinically relevant model of extreme preterm birth, equivalent to birth before 28 weeks of gestation. Postmortem studies of human infants born preterm show that loss of KCC2 expression occurs with pathological evidence of white matter injury, but not in aged-matched uninjured controls (Robinson et al. 2010). Further, toxic ablation of subplate neurons causes loss of targeting of thalamocortical neurons (Ghosh et al. 1990) and cortical layer IV KCC2 and impaired maturation of GABAAR in visual cortex (Kanold and Shatz 2006). Perinatal brain injury from unilateral carotid ligation plus hypoxia at postnatal day 1–2 demonstrated that subplate neurons are especially vulnerable to hypoxic–ischemic injury (McQuillen et al. 2003) and that this insult impairs visual cortical plasticity (Failor et al. 2010). Here, using an established model of transient uterine artery occlusion on embryonic day 18 (E18) in Sprague–Dawley rats that mimic the pathological changes observed in encephalopathy from extremely preterm birth and cause sustained functional deficits in adult rats including a lower seizure threshold and impaired motor performance (Robinson et al. 2005; Mazur et al. 2010), we tested whether loss of subplate neurons from prenatal injury disrupts postnatal upregulation of functional membrane-bound KCC2 oligomers and maturation of GABAAR subunits. We demonstrate that prenatal transient systemic hypoxia–ischemia (TSHI) induces in vivo loss of the subplate with elevated apoptotic cell death in subplate neurons, disrupts the cortical layer IV developmental upregulation of KCC2, maturation of developmentally regulated GABAAR subunits, and induces significant functional impairment. Mechanical ablation of the subplate and closely adjacent white matter in slice cultures caused similar loss of layer IV KCC2 expression. Moreover, treatment with the neuroprotective agent erythropoietin (EPO) in a clinically relevant postnatal dosing paradigm restores KCC2 expression both in vitro and in vivo, mitigates GABAAR developmental subunit dysregulation, and reverses motor deficits. These results suggest that altered expression of KCC2 and GABAAR subunit maturation is responsive to postnatal interventions and supports the premise that EPO may be an effective therapy for brain injury associated with very early preterm birth.
Materials and Methods
Transient Systemic Hypoxia–Ischemia
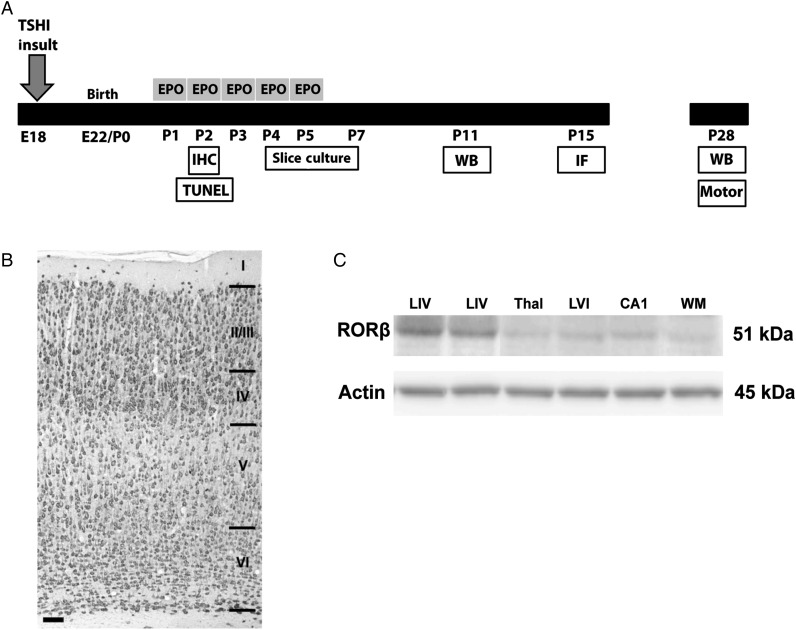
A summary of the experimental design is presented in Figure 1A. Prenatal TSHI was performed on embryonic day 18 (E18, equivalent to ∼25 weeks of human gestation), which is after thalamic axons arrive in the subplate on E16 (Kostovic and Judas 2010), but before subplate neurons stimulate cortical layer IV on E21 (Kanold and Luhmann 2010). All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee at Boston Children's Hospital. Under isoflurane anesthesia, a laparotomy was performed on pregnant Sprague–Dawley rats on E18 (Robinson et al. 2005). Uterine arteries were transiently occluded for 60 min. After 60 min of anesthesia, the laparotomy was closed. For sham controls, the laparotomy was performed and uterine horns were exposed for 60 min, but the arteries were not clamped. Thus, all dams experienced an equivalent time of anesthesia and laparotomy. Pups were born at term (E22), after subplate neurons have innervated cortical layer IV on E21 (Kostovic and Judas 2010), and pups matured with their respective dams until tissue was collected at the ages noted for each experiment. Fetal loss was 23% and 0% after TSHI and sham controls, respectively. Both male and female pups were used in the study, and gender and weights were recorded at P28, the time when the majority of the subplate has receded in rats (Robertson et al. 2000). No gender differences were observed in any of the investigated parameters.
Figure 1.
Experimental design and cortical layering. (A) Experimental design showing induction of in utero insult on embryonic day 18 (E18), subsequent delivery of pups at term (E22), and postnatal administration of EPO (2000 U/kg/day) from P1 to P5. Experimental endpoints are indicated in white boxes. (B) NeuN immunostaining showing cortical layering and the entire cortical plate in P15 Sprague–Dawley rat (scale bar = 100 μm). (C) Western blot depicting specificity of cortical layer IV microdissections. RORβ is a transcription factor expressed in layer IV cortical neurons, with minimal expression in other regions of the developing rat brain.
Erythropoietin Administration
Each TSHI litter was randomly divided into half, and pups received either EPO (2000 U/kg/dose, Tissue Grade, R&D) or vehicle (sterile saline) intraperitoneally once a day in a clinically relevant, delayed neonatal dosing paradigm from postnatal day 1 (P1) to P5 (Mazur et al. 2010). Previous work demonstrated that sham-EPO-treated pups were similar to sham-vehicle (Mazur et al. 2010).
Immunohistochemistry
Serial 20-μm coronal brain sections were cut on a cryostat (Leica) from the anterior frontal lobes through the posterior extent of the dorsal hippocampus. Every 8th section was collected and mounted on slides. Sections were incubated in a blocking solution containing 10% Normal Goat Serum. The transcription factor Nurr1 (nuclear receptor-related 1) labels the subplate in the perinatal period in rodents and humans (Arimatsu et al. 2003; Hoerder-Suabedissen et al. 2009). For P2 Nurr1 immunolabeling (sham: n = 6, TSHI: n = 5), after blocking, anti-Nurr 1 antibodies (1:150, Santa Cruz) were incubated overnight on sections at 4°C. The following day, sections were washed, incubated sequentially with appropriate secondary antibodies, Vectastain (Vector), and diaminobenzidine, and mounted with Permount. For P15 KCC2 immunolabeling (sham: n = 5, TSHI: n = 6, TSHI + EPO: n = 4), sections were incubated with anti-KCC2 antibodies (1:500, Millipore) overnight. The following day, sections were washed and incubated with biotinylated anti-rabbit secondary antibodies (1:200, 1 h RT, Vector), followed by fluorescein-conjugated avidin (1:2000, 1 h RT, Vector). Sections were then washed and coverslipped in an aqueous, DAPI-containing mounting medium. Images were acquired on a Zeiss LMS510 META 2-Photon Confocal microscope and a Leica DM500B Fluorescent microscope using a 40× objective.
Stereological Estimates
All sections were coded prior to analysis to blind the observer performing the counting. A Leica microscope with a motorized stage and electronic microcator was used with Stereologer software (Stereology Resource Center) to perform the analyses. Estimates of the number of subplate cells were obtained on P2 (sham: n = 6, TSHI: n = 5) 20-µm coronal sections using a thin section modification of the optical fractionator method to determine cell load using object area fraction and region point counting probes (Gundersen et al. 1988; Mouton et al. 2002). Briefly, the subplate was defined for this analysis as the band of cells between layer VI of the cortex and the white matter (Fig. 1B). The medial border of the region of interest (ROI) was congruent with the junction of M1 and M2 of the motor cortex. The lateral border was defined as the primary somatosensory cortex junction between the forelimb and the upper lip. The anterior margin was congruent with the fusion of the intermediate lateral septal nuclei (Bregma 1.2 mm), and the posterior margin was the anterior boarder of the internal capsule (Bregma 0.0 mm). The volume of the ROI was calculated using the Cavalieri method. On every 8th section, the software superimposed a lattice of regularly spaced plus-signs over the ROI, and the ROI was outlined. Then, under high magnification, the number of cells within systematically spaced unbiased sampling frame was counted. At the completion of the stereological analyses, the samples were decoded, and mean and standard error of the mean (SEM) of the subplate volume and the number of subplate cells were calculated. Multiple unbiased sampling frames were counted on an average of four sections containing the ROI/group. All coefficients of error values for the stereological estimates were <10%.
TUNEL Staining and Neuronal Labeling
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed as per the manufacturer's specifications using a TMR-Red In Situ Cell Death Detection Kit (Roche) (sham: n = 3, TSHI: n = 4). Briefly, P2 sections were washed and blocked as described earlier and incubated with anti-NeuN antibodies (1:100, Millipore) overnight in a cold room. NeuN is a specific marker of neuronal nuclei. The following day, sections were washed and species appropriate secondary antibodies (anti-mouse IgG Alexa-Fluor 488) were applied for 1 h. Following washes, sections were incubated in cold sodium citrate buffer for 2 min and washed, after which TUNEL enzyme solution was applied and incubated at 37°C for 1 h. Sections were then coverslipped with a mounting media containing DAPI before imaging.
ImageJ Quantification of KCC2 Immunolabeling
All sections were coded prior to analysis to blind the observer performing the imaging. Ten images of P15 cortical layer IV cortex were taken per animal on a Leica DM5000B microscope at 40× magnification (sham: n = 5, TSHI: n = 6, TSHI + EPO: n = 4). Images were recorded at the same exposure and contrast levels by a single observer. Using ImageJ Version 1.46r, the immunolabeling was framed using a rectangular selection. Mean pixel intensity was determined using the Measure tool. Pixel intensity was then divided by the area of the rectangular selection to determine intensity density (area of the rectangular selection did not vary with group). Intensity density was averaged across groups.
Western Blots
To quantify oligomeric KCC2 and GABAAR subunit expression following prenatal brain injury, cortical layer IV samples at P11 and P28 were microdissected under a dissecting microscope and membrane fractions isolated and assayed with western blots (n > 6 per group). Layer IV was identified visually and in reference to the distance from the pial surface (Paxinos and Watson 1998; Takayama and Inoue 2010) (Fig. 1B). For P11 brains, layer IV samples from two pups were pooled, due to the limited tissue available from layer IV in each animal. After sonication, membrane and whole cell proteins were isolated using differential centrifugation in sucrose containing buffers and a Bradford protein assay was performed. Thirty micrograms of protein was loaded on 4–20% precast Tris–HCl (BioRad) gels. Following transfer to PVDF membranes, membranes were blocked and incubated overnight at 4°C in anti-KCC2 (1:1000, Millipore), anti-GABAARα1 (1:1500, Millipore), anti-GABAARα2 (1:500, Millipore), GABAARγ2 (1:750, Millipore), or anti-β-Tubulin (1:15,000, Covance) antibodies. Following washes and incubation with species-appropriate HRP-conjugated secondary antibodies, membranes were washed and incubated in Femto-West ECL (Thermo) and developed on a GE-LAS 4000 Digital image reader. Resultant bands were quantified using GE ImageQuant software and standardized to β-tubulin to correct for equal loading among lanes. To confirm specificity of Layer IV microdissections, western blots for RORβ (1:1000, Diagenode), a specific transcription factor expressed in Layer IV cortical neurons, were performed (Fig. 1C).
Slice Culture and In Vitro Subplate Ablation
Coronal 350-µm slices were collected using a vibratome (Leica) from P4 sham and TSHI brains. Each experiment was performed a minimum of 3 times using a total of eight brains per condition and 3 slices per insert. Slices were placed on PTFE inserts (0.4 µm pore, 30 mm diameter, Millipore) and where indicated, mechanically ablated under a dissecting microscope just dorsal to the periventricular white matter using Dumont superfine #5 forceps. Ablation was successful 100% of the time as judged by complete severance of the subplate neuronal layer and visual confirmation of the PTFE insert underneath. We specifically performed ablation in slices collected from the anterior half of the developing rat brain with prominent subplate corresponding to Bregma 2.28 to Bregma −3.34. Mechanical ablation mimics not only the loss of subplate neurons but also the alterations in axons, migrating neurons, glia, and extracellular matrix that occurs in vivo, similar to the structural loss observed in humans following extremely preterm birth (Kostovic et al. 2012). Intact and ablated slices were then incubated overnight in basal media with 15% fetal calf serum to equilibrate. On Day 1 in vitro (DIV) 1, media was changed to serum free with brain-derived growth factor (BDNF) (100 ng/mL, Prospec), K252a (0.2 µm, Tocris), EPO (10 U/mL, R&D Tissue Grade), polyclonal Erythropoietin receptor (EPOR)-neutralizing antibodies (1:50, SantaCruz) or polyclonal isotype control antibodies (1:50, Santa Cruz). Microdissected Layer IV was then collected 48 h following the administration of pharmacological agents and snap-frozen (DIV 3, P7 equivalent).
Motor Function
On P28 the ambulatory gait of sham control (n = 18), TSHI (n = 14), and TSHI + EPO (n = 12) rats were quantified using the DigiGait™ Imaging System (Mouse Specifics, Inc.). Rats were placed on a motorized transparent treadmill that was rapidly accelerated to 30 cm/s. A video camera mounted below the belt captured the ventral side of each rat while walking. Total time of recording was ∼1 min. The recorded output of stride length and step frequency was analyzed using the DigiGait analysis software v.12.2 (Mouse Specifics, Inc., Amende et al. 2005). All DigiGait testing was performed in a blinded fashion, and all settings, including camera, lighting, shutter and belt speed, were optimized before experimental testing. Data were taken as the average measurement taken across the respective hind left and right limbs (Paumier et al. 2013; Hansen and Pulst 2013).
Quantification and Statistical Analysis
Data are presented as mean with SEM. Statistical analyses comparing TSHI and sham groups were done using a two-sample two-tailed student t-test assuming unequal variance, with a significance level of P < 0.05. Differences among treatment groups were compared using two-way ANOVA with Bonferroni's correction for multiple pairwise comparisons using SPSS 21 (IBM), with P < 0.05 considered significant.
Results
Prenatal TSHI Induces Loss of Subplate Neurons via Apoptosis
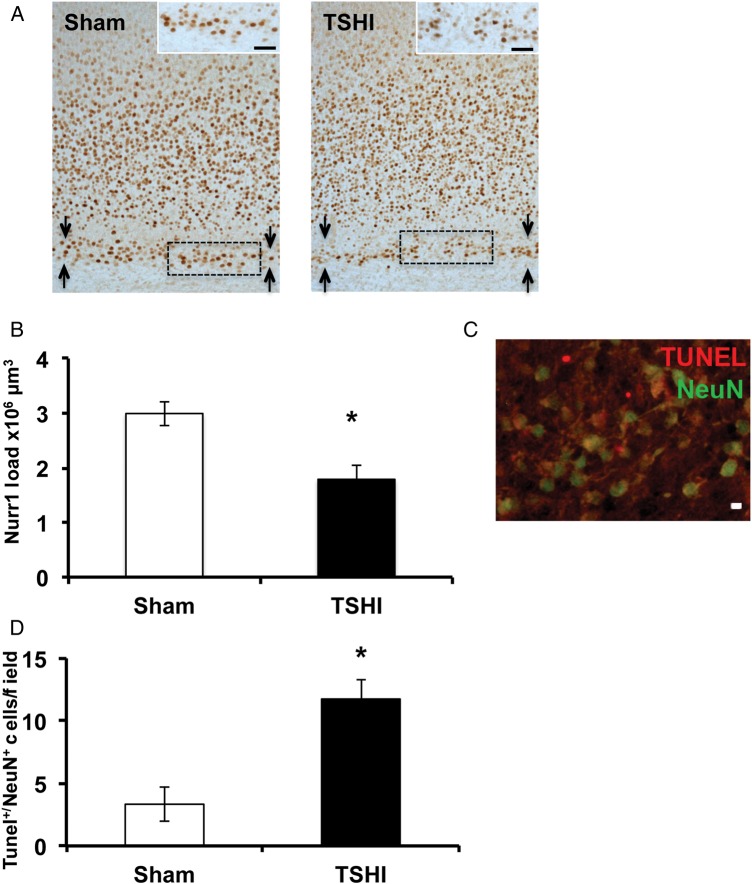
Using stereological techniques, we estimated the load of Nurr1 immunolabeling on postnatal day 2 (P2). Prenatal TSHI induces a 45% reduction of Nurr1+ load within the subplate (n = 5), compared with sham controls (n = 6, P = 0.012) (Fig. 2A,B). Additionally, the number of NeuN+/TUNEL+ cells within the subplate at P2 is markedly elevated following prenatal TSHI (n = 4), compared with shams (n = 3, P = 0.019) (Fig. 2C,D), indicating that prenatal TSHI causes premature loss of the subplate, including excess apoptotic cell death of subplate neurons.
Figure 2.
Prenatal TSHI induces premature loss of subplate. (A) Nurr1 immunoreactivity is diminished and subplate loss is observed on P2 following TSHI. Bar = 100 μm. Subplate layer is delineated between arrows. (B) Stereological estimates of Nurr1 load indicate that the amount of Nurr1-immunolabeled cells in the subplate is decreased following TSHI (n = 5) compared with sham (n = 6, P = 0.012). (C) Representative photomicrograph showing co-localization of TUNEL in NeuN+ subplate neurons at P2 following TSHI reflecting increased subplate neuron apoptosis following TSHI. Bar = 10 μm. (D) The proportion of TUNEL+/NeuN+ cells in the subplate is increased at P2 following TSHI (n = 4) compared with sham (n = 3, P = 0.019) (*P < 0.05).
EPO Mitigates Layer IV KCC2 Loss Following Prenatal TSHI and Subplate Damage
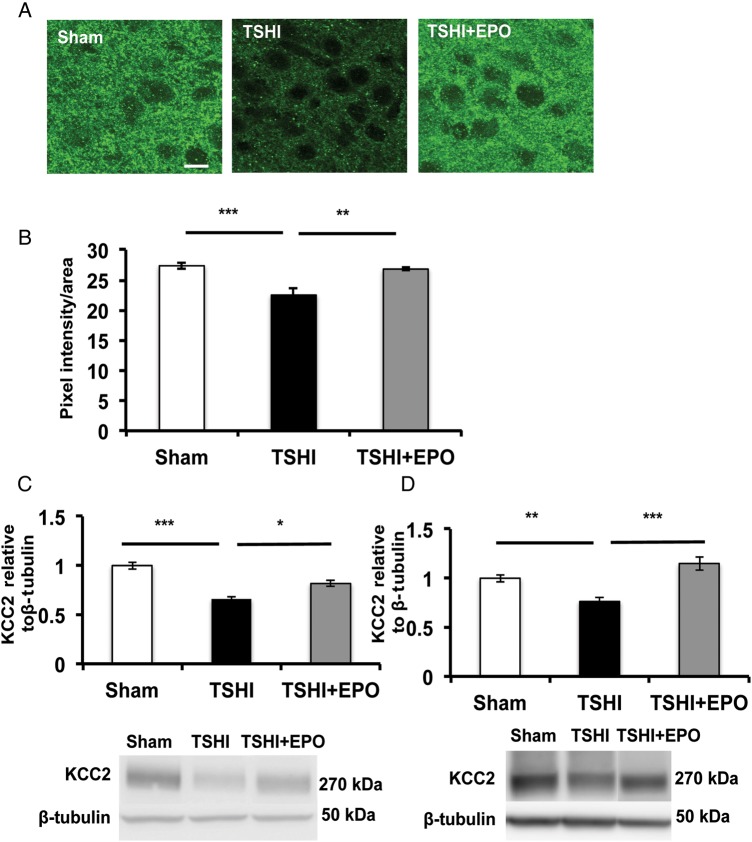
To investigate the implications of subplate loss following prenatal TSHI on cortical development in vivo, we examined the postnatal expression of KCC2 and GABAAR subunits in cortical layer IV. Brains from prenatal TSHI pups treated with vehicle (TSHI-veh) show limited expression of KCC2 in cortical layer IV compared with shams (Fig. 3A). In contrast, brains from prenatal TSHI pups treated with EPO (TSHI-EPO) had restoration of transporter puncta on cell membranes. Quantification revealed a marked decrease in KCC2 immunoreactivity in cortical layer IV of TSHI-veh brains (n = 6, 22.5 ± 1.1 arbitrary units) compared with shams (n = 5, 27.4 ± 0.5, two-way ANOVA, P = 0.001), whereas TSHI-EPO pups showed increased KCC2 immunoreactivity (n = 4, 26.8 ± 0.2 arbitrary units) compared with TSHI-veh pups (P = 0.006), and levels similar to shams (Fig. 3B).
Figure 3.
Erythropoietin treatment attenuates KCC2 loss on layer IV cortical neurons following prenatal TSHI. (A) Representative confocal images showing TSHI-veh pups have reduced KCC2 immunoreactivity on layer IV cortical neurons at P15 compared with both sham and TSHI-EPO pups. (B) Quantitation of KCC2 intensity density confirms reductions in KCC2 immunolabeling following TSHI-veh (n = 6) compared with shams (n = 5, P = 0.001) and that postnatal EPO administration attenuates this loss (n = 4, P = 0.006). (C) Western blots of layer IV microdissected membrane fractions showing oligomeric KCC2 expression is lost in TSHI-veh animals at P11 (n = 28) compared with sham KCC2 expression (n = 28, P < 0.001), and postnatal treatment with EPO mitigates the loss of KCC2 (n = 8, P = 0.037). (D) TSHI induces sustained loss of oligomeric KCC2 protein expression through development because at P28, TSHI-veh KCC2 is still reduced (n = 14) compared with sham (n = 18, P = 0.008) and TSHI-EPO pups (n = 6, P < 0.0001). Measurements represent values relative to sham (*P < 0.05. **P < 0.01, ***P < 0.001).
Functional KCC2 activity has been suggested to correlate with membrane-bound oligomer expression (Blaesse et al. 2006). Consistent with the critical period of inhibitory circuit development and our pilot studies, the most prominent difference in layer IV oligomer KCC2 expression between TSHI-injured and sham controls was observed at P11. After TSHI-veh, KCC2 cortical layer IV oligomer levels on P11 were reduced by 33% (n = 28, 0.66 ± 0.02) compared with shams (n = 28, 1.02 ± 0.04, P < 0.001, Fig. 3C). Postnatal EPO treatment elevated KCC2 oligomer expression following TSHI, compared with TSHI-veh brains (n = 8, P = 0.037). At P28, cortical layer IV KCC2 oligomer levels in TSHI-veh brains were still reduced by 24% (n = 14, 0.76 ± 0.037), compared with shams (n = 18, 1.00 ± 0.04, P = 0.008, Fig. 3D). In contrast, cortical layer IV KCC2 oligomer levels in TSHI-EPO pups (n = 6, 1.15 ± 0.07) were similar to control levels, and markedly higher than TSHI-veh pups (P < 0.0001). These data show that prenatal TSHI on E18, after thalamic afferents have arrived in the subplate on E16 but before the innervation of cortical layer IV on E21, delays the upregulation of cortical layer IV KCC2 expression throughout the neonatal period up to P28, approximately the time the subplate recedes in rats (Robertson et al. 2000). Furthermore, early postnatal treatment with EPO following prenatal TSHI mitigates impaired KCC2 expression, consistent with the concept that the cortical development is a highly dynamic process during this critical period.
Layer IV GABAAR Subunit Maturation is Impaired Following Prenatal TSHI
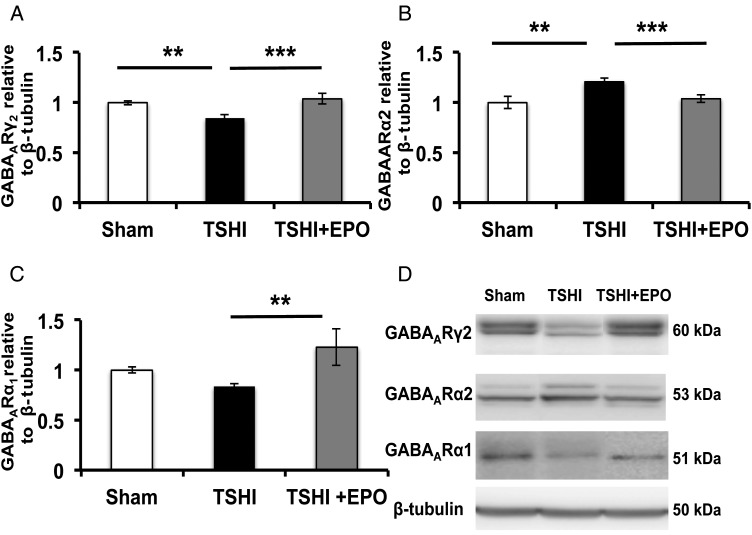
KCC2 expression is closely integrated with GABAAR subunit maturation (Kanold and Shatz 2006; Huang et al. 2013). During postnatal development, GABAARγ2 and α1 subunit levels rise postnatally with age, whereas α2 levels decrease (Jansen et al. 2010; Balia et al. 2015). We assayed GABAAR subunit levels at P11, near the midpoint of the period of GABA subunit maturation when TSHI-induced loss of cortical layer IV KCC2 oligomers is most prominent, and at P28 after the completion of GABAergic signaling development and subplate recession. At P11, GABAARγ2 levels were reduced in TSHI-veh layer IV (n = 28, 0.85 ± 0.03) compared with shams (n = 26, 1.0 ± 0.02, P = 0.002, Fig. 4A,D). Treatment with EPO elevated TSHI GABAARγ2 levels (n = 18, 1.04 ± 0.05) compared with TSHI-veh pups (P < 0.001), and TSHI-EPO levels were similar to sham controls. At P28, the injury-induced changes in GABAARγ2 had resolved. These data suggest that although transient, GABAARγ2 maturation is responsive to mechanisms of injury and restoration by neuroprotective agents.
Figure 4.
Transient systemic hypoxia–ischemia alters GABAA receptor subunit maturation in layer IV cortical neurons. (A) GABAARγ2 subunit expression is decreased in the membrane fraction isolated from microdissected layer IV at P11 from TSHI-veh brains (n = 26), compared with sham (n = 26, P = 0.002). EPO treatment following TSHI attenuates the loss of GABAARγ2 (n = 14, P < 0.001). (B) At P11, GABAARα2 expression levels are increased in layer IV membrane fractions from TSHI-veh brains (n = 28) compared with both sham (n = 26, P = 0.002) and TSHI-EPO animals (n = 18, P < 0.001), which indicates deviation from the normal developmental expression pattern. (C) At P28, TSHI-EPO pups have elevated GABAARα1 expression reflective of a normalization of subunit expression in layer IV, compared with TSHI-veh (P = 0.003). (D) Representative immunoblots of GABAAR subunits and the deviations in cortical layer IV expression pattern following TSHI-veh, and elevated expression in TSHI-EPO. Measurements represent values relative to sham (**P < 0.01, ***P < 0.001).
In contrast to GABAARγ2 levels that rise postnatally, GABAARα2 levels decline with maturation. At P11, layer IV GABAARα2 levels remained higher in TSHI-veh pups (n = 16, 1.21 ± 0.04) compared with sham controls (n = 20, 1 ± 0.06, P = 0.002, Fig. 4B,D), indicative of delayed subunit maturation. EPO treatment following prenatal TSHI normalized GABAARα2 levels (n = 16, 1.04 ± 0.04) compared with TSHI-veh pups (P < 0.001, Fig. 4B,D). By P28, injury-induced differences in GABAARα2 levels had resolved, similar to the GABAARγ2 subunit levels, suggesting that the prenatal injury transiently delays the maturation of the GABAAR subunits. At P11, no difference in expression of cortical layer IV GABAARα1 was observed between TSHI-veh and shams. However, at P28, GABAARα1 levels were diminished by 16% in TSHI-veh pups (n = 15, 0.84 ± 0.03) compared with shams (n = 11, 1.0 ± 0.03 Fig. 4C,D), although this reduction failed to reach statistical significance. Postnatal EPO treatment following prenatal TSHI restored cortical layer IV GABAARα1 levels (n = 5, 1.23 ± 0.18) compared with TSHI-veh (P = 0.003), supporting the γ2 and α2 findings that EPO-treatment influences GABA subunit maturation after prenatal injury and can restore the normal GABA subunit developmental program.
Subplate Loss Directly Impacts Cortical Layer IV KCC2 Expression
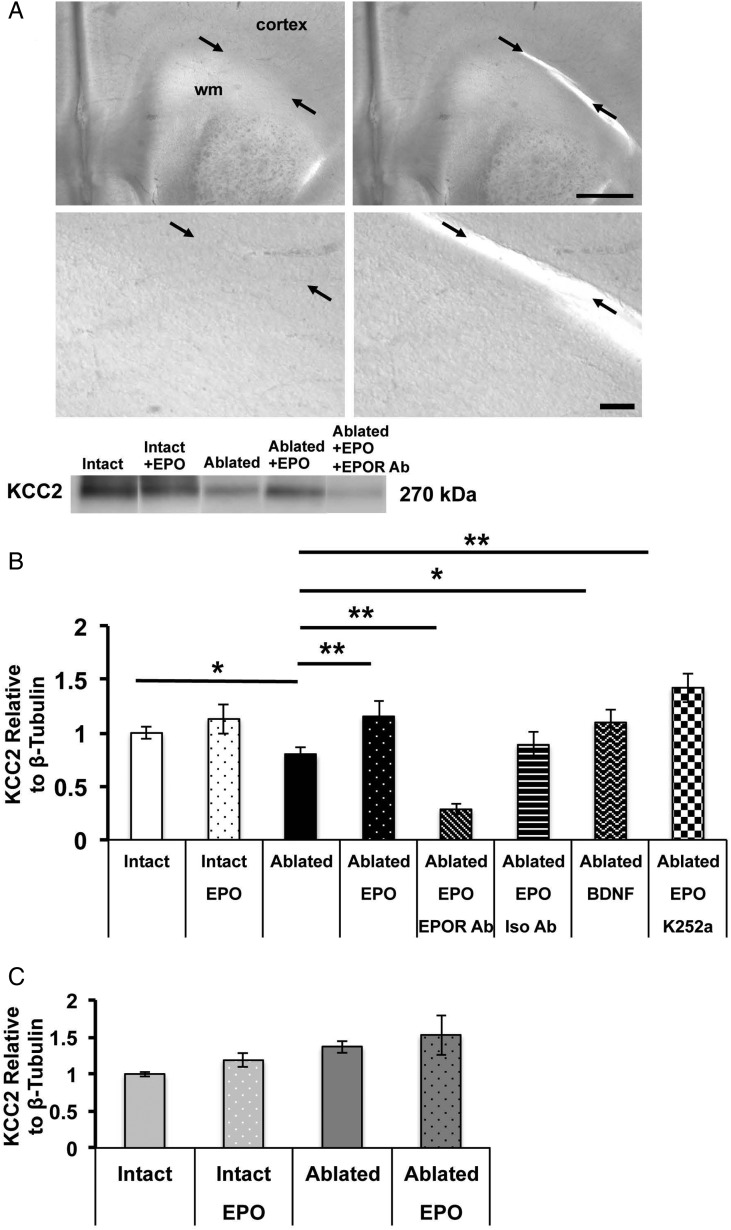
To specifically test the impact of premature subplate loss, we mechanically ablated the subplate and closely adjacent white matter on P4 (Fig. 5A). In sham slices, subplate ablation caused a 22% reduction in cortical layer IV KCC2 oligomer expression (0.78 ± 0.08, n = 8 samples pooled from three slices per animal), compared with intact slices (1.0 ± 0.06, n = 9, one-way ANOVA, P = 0.048, Fig. 5B). To determine whether EPO treatment altered KCC2 expression in vitro, EPO (10 U/mL) was added to cultures. Addition of EPO to intact slices produced only a mild (13%) increase in cortical layer IV KCC2 oligomer expression (1.13 ± 0.14, n = 5). However, addition of EPO to sham slices with subplate ablation showed a marked increase in cortical layer IV KCC2 expression compared with ablated sham slices without EPO (1.16 ± 0.14, n = 7, P = 0.001). To test the specificity of the EPO signaling on layer IV KCC2 levels, neutralizing EPO receptor antibodies were added. In sham slices with ablated subplate treated with EPO, KCC2 oligomer levels were reduced in the presence of EPOR antibodies (0.29 ± 0.05, n = 5) compared with slices with similar conditions without the EPOR antibodies (P = 0.001). In contrast, addition of isotype control antibodies did not impact KCC2 oligomer levels (0.89 ± 0.12, n = 3). As BDNF has been previously reported to influence KCC2 levels (Rivera et al. 2002), we applied BDNF to our slice cultures. During development, BDNF can increase KCC2 expression (Ludwig et al. 2011), whereas after injury in the mature CNS, BDNF reduces KCC2 expression (Rivera et al. 2002; Boulenguez et al. 2010). In sham slices with subplate ablation, addition of BDNF (100 ng/mL) increased KCC2 oligomer expression to near the levels observed in intact slices (1.10 ± 0.12, n = 6, P = 0.03). Addition of K252a (0.2 µm), a tyrosine kinase inhibitor and indirect TrkB receptor blocker, inhibited the BDNF-induced elevation of KCC2 oligomer expression. In contrast, addition of K252a to ablated sham slices treated with EPO had no impact on KCC2 oligomer expression (1.42 ± 0.14, n = 3) compared with sham slices with similar subplate ablation and EPO alone, suggesting that the EPO treatment effect on KCC2 level restoration is mediated by a BDNF-independent mechanism.
Figure 5.
Mechanical subplate ablation in vitro induces KCC2 loss in cortical layer IV. (A) Representative photomicrographs of acute sham brain slices before and after mechanical subplate ablation. Arrows indicate subplate lying between white matter anlage and overlying cortex, with both white matter and cortex identified. Bar = 1mm (upper) and 100 μm (lower). (B) Western blots performed on layer IV microdissected from acute sham slices show that oligomeric KCC2 expression is decreased following mechanical subplate ablation (n = 9), compared with intact slices (n = 8, P = 0.048) and that application of EPO to ablated slices attenuates this loss (n = 7, P = 0.001). Co-incubation of EPO receptor-neutralizing antibodies with EPO prevents the restoration of KCC2 expression following mechanical ablation (P = 0.001), whereas co-incubation with isotype control antibodies does not (n = 3). Addition of BDNF confirms that KCC2 loss following ablation can be mitigated by growth factors (n = 6, P = 0.03). EPO is unlikely to be protective through a TrkB-dependent mechanism as co-application of EPO with K252a (n = 3), a protein kinase inhibitor that acts immediately downstream of BDNF binding to its receptor TrkB, does not attenuate EPO's protective effects on KCC2 levels. Measurements represent values relative to intact slice cultures. (C) Western blots performed on microdissected layer IV indicate that KCC2 levels are unchanged following mechanical subplate ablation in TSHI slices (n = 5), compared with intact slices from TSHI brains (n = 7) (*P < 0.05, **P < 0.01).
Similar to our findings of cortical layer IV KCC2 loss in vivo following TSHI on E18, culture of slices from TSHI brains showed a 33% reduction in cortical layer IV KCC2 oligomer expression (0.68 ± 0.13, n = 7) compared with slices from sham controls in similar conditions (P < 0.01). In slices from TSHI brains, mechanical subplate ablation in vitro at P4 did not alter layer IV KCC2 expression at P7 equivalent (1.19 ± 0.09, n = 5) compared with intact TSHI slices (1.0 ± 0.03, n = 7, P > 0.05), unlike the sham slices with subplate ablation described earlier. Interestingly, addition of EPO (10 U/mL) to cultures from TSHI pups increased cortical layer IV KCC2 oligomer expression in both intact slices (1.37 ± 0.08, n = 3) and in slices with mechanical subplate ablation (1.53 ± 0.27, n = 3), when compared with intact TSHI slices without EPO (1.0 ± 0.03). These results suggest that prenatal TSHI induces alterations in the developing brain that are mimicked by mechanical subplate ablation.
EPO Restores Functional Gait Impairment Following Prenatal TSHI
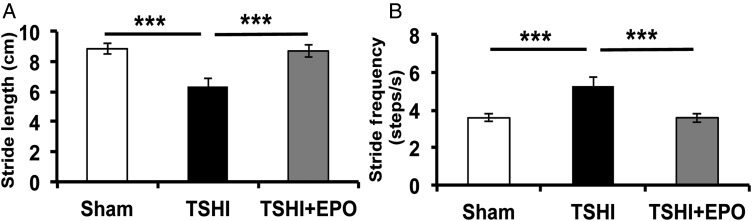
To assess EPO's putative ability to reverse functional deficits, we investigated whether prenatal TSHI caused gait abnormalities, common deficits observed in children born preterm. At P28, TSHI-veh animals had decreased stride length (6.35 ± 0.51 cm, n = 14) compared with sham controls (8.86 ± 0.35, n = 18, P < 0.001, Fig. 6A), concomitant with increased stepping frequency (5.28 ± 0.49 steps/s vs. 3.58 ± 0.19, P < 0.001, Fig. 6B). Importantly, TSHI-EPO rats had stride length (8.71 ± 0.44 cm, n = 12) and step frequency (3.59 ± 0.24 steps/s) indistinguishable from shams. Together, these data indicate that in addition to restoring biochemical changes associated with TSHI, EPO also reverses chronic deficits in motor behavior.
Figure 6.
EPO restores gait deficits and attenuates abnormal motor behavior. (A) TSHI-veh rats have significantly decreased stride length on P28 (6.35 ± 0.51 cm, n = 18) compared with shams (8.86 ± 0.35, n = 14, P < 0.001), whereas postnatal administration of EPO reverses abnormalities in stride length (8.71 ± 0.44, P < 0.001, n = 12). (B) TSHI-veh animals have abnormal cadence compared with sham animals (5.28 ± 0.49 vs. 3.58 ± 0.19, P < 0.001), and postnatal administration of EPO reverses abnormalities in stride frequency (3.59 ± 0.24, P < 0.001) ***P < 0.001.
Discussion
Neurologic and neuropsychiatric disorders associated with brain injury from preterm birth, including cerebral palsy, epilepsy, autism, cognitive and behavioral deficits, can be characterized by abnormal neuronal activity and hyperexcitability due to impaired cortical inhibition (Mathern et al. 2000; Lewis and Levitt 2002; Robinson et al. 2006; Kanold and Luhmann 2010). Together with the data shown here, we propose that impaired cortical inhibition likely arises from altered excitatory and inhibitory circuit development and modified critical period plasticity secondary to injury to the subplate during a vulnerable developmental window. Subplate neurons form the first cortical circuits and enable functional circuit maturation (Kostovic and Rakic 1980; Kanold and Shatz 2006; Miller and Ferriero 2009). In humans, subplate neurons comprise up to half of the cortical neurons in the second trimester and gradually recede through the first 6 months of life (Kanold and Luhmann 2010; Kostovic and Judas 2010), a critical neurodevelopmental period that coincides with the period of vulnerability of brain injury from preterm birth. Studies in visual cortex show that subplate neurons receive thalamic inputs and project into the developing cortical plate, predominantly to layer IV (Friauf and Shatz 1991). After axons grow into layer IV, thalamocortical synapses and GABAergic circuits in layer IV are refined (Kanold and Shatz 2006), and in the absence of subplate neurons, cortical connections are unrefined and immature (Kanold and Shatz 2006; Kanold and Luhmann 2010). Interestingly, recent advances in human functional connectivity magnetic resonance imaging (fcMRI) show expanded fcMRI in somatosensory areas of the parietal cortex in children with spastic diplegia from prematurity compared with healthy age-matched controls, suggesting a similar lack of refinement in cortical networks (Burton et al. 2009).
In this study, we demonstrate for the first time in a preclinical rat model that prenatal TSHI that mimics brain injury from human extremely preterm birth significantly reduces subplate Nurr1+ immunolabeling, a marker of subplate cells in humans and rodents (Wang et al. 2010), and increases subplate neuronal apoptosis. We show that subplate loss occurs coincident with sustained loss of expression of KCC2 chloride transporters in cortical layer IV, beginning during the critical period of inhibitory circuit formation and extending through the childhood equivalent age of P28, together with disruption in the developmental maturation of GABAARα1, α2 and γ2 subunits and functional gait abnormalities. After birth, postsynaptic maturation of excitation and inhibition occurs through changes in the intracellular Cl− concentration mediated by the co-transporter KCC2 (Rivera et al. 1999), and through coincident changes in membrane GABAAR subunit composition. KCC2 expression rises rapidly in the postnatal period in a regional pattern, primarily from caudal to rostral and ventral to dorsal (Kovacs et al. 2013). Prior studies indicate that ablation of subplate neurons at early ages, when inhibition is immature, prevents essential developmental increases in expression of KCC2 and a mature complement of GABAAR subunits (Kanold and Shatz 2006). Together, these molecular and cellular changes manifest as electrophysiological abnormalities where GABAergic circuits remain depolarizing, are unrefined, and do not switch appropriately to the mature, hyperpolarizing state (Kanold and Shatz 2006). Our data are consistent with these reports, as we find sustained loss of KCC2 and dysregulation of GABAAR subunit expression of γ2, α2 and α1 following prenatal brain injury. We show that cortical layer IV KCC2 and GABAAR mature subunit levels remain reduced through P28, whereas expression of immature GABAAR subunits is prolonged, likely reflecting impaired GABAergic transmission and diminished facilitation of inhibition in the developing brain following in utero hypoxic–ischemic injury. Our data indicate that prenatal global hypoxia–ischemia induces a significant decrease in γ2 at P11, a deficit in protein expression that resolves by P28. While the loss of expression levels may be transient, the decreased expression at P11 coincides with decreased oligomeric KCC2 expression and is the midpoint of the critical period of inhibitory circuit development. Concomitant with this deficit in γ2 subunit levels, we found an abnormal persistent increase in α2 expression, suggesting deviation of the normal developmental program of GABAergic receptor maturation. Notably, a significant loss of expression of cortical and subcortical GABA and GABA receptors also occurs in neonatal preterm human brains with white matter lesions, in addition to oligodendrocyte loss, axonal disruption, and excess apoptosis (Robinson et al. 2006), and may exacerbate abnormalities in cortical development due to subplate loss. Our data suggest that KCC2 expression and GABAAR subunit maturation are altered by prenatal injury, and more significantly, responsive to restoration by a neuroprotective agent. This response to EPO is the first time to our knowledge that KCC2 and GABAAR subunit levels have been modulated during postnatal development after injury in a preclinical rat model using a clinically relevant intervention, together with substantial improvement in motor behavior.
Many theories have been proposed for the enhanced vulnerability of subplate cells, including early maturation, higher metabolic rate, susceptible intracellular matrix, and microenvironment (Kanold and Luhmann 2010; Kostovic and Judas 2010). Subplate neurons have several properties that allow them to influence neuronal network activity and to assemble neighboring neurons into a local cluster by gap junctions (Dupont et al. 2006). As mentioned earlier, damage to subplate neurons disrupts essential trophic support to GABAergic neurons and their migration to the cortical plate, and the development of inhibitory synapses in cortical layer IV. Further, damage to the individual subplate cells themselves impairs cell–cell and columnar communication through gap junctions and negatively impacts electrical and chemical coupling resulting in impaired synchronized oscillations (Dupont et al. 2006). Our data indicate that mechanical ablation of subplate neurons and adjacent underlying white matter in acute brain slices significantly reduced layer IV KCC2 expression. This loss of KCC2 expression in vitro, similar to the loss observed following TSHI in vivo, was attenuated by postnatal administration of EPO, an effect blocked by co-incubation with EPOR-neutralizing antibodies but not nonspecific isotype controls. These data indicate failure of layer IV chloride co-transporter maturation following mechanical subplate ablation in acute slices from the developing brain and are consistent with prior findings that deletion or ablation of the subplate with toxin (Ghosh and Shatz 1992; Kanold and Shatz 2006) or its improper differentiation (Zhou et al. 1999) causes failure of inhibitory synapses maturation, inappropriate thalamocortical innervation, and prevents accurate formation of layer IV (Zhou et al. 1999; Hanganu et al. 2002; Kanold and Shatz 2006).
Subplate neurons may be especially vulnerable to hypoxia–ischemia (Csillik et al. 2002; McQuillen et al. 2003; Albrecht et al. 2005), and this vulnerability may be more pronounced in infants born prematurely (Volpe 1996; Volpe 2000; McQuillen et al. 2003; McQuillen and Ferriero 2005; Robinson 2005; Kostovic et al. 2012). Subplate neurons are more prominent in species with increased radial and tangential cortical connectivity, including cats, monkeys, and humans, suggesting a role for these cells in the establishment of complex, higher-order processing capabilities and executive function (Mrzljak et al. 1988; Kostovic and Rakic 1990; Kostovic et al. 2002; Kostovic and Judas 2006). In this context, the importance of the subplate to cerebral development in infants born preterm is supported by: (1) the subplate is present throughout the entire mid-fetal period through early infancy, (2) the subplate is the thickest cortical layer reaching its peak between 22 and 34 weeks of gestation, and (3) changes of the subplate and its neurons are major events during the perinatal and early postnatal reorganization of the developing cortex (Kostovic and Rakic 1980; Kanold and Luhmann 2010; Kostovic et al. 2012). The data presented here show that in addition to the well-recognized white matter injury that occurs in preterm infants (Kuban and Leviton 1994; Back 2001; Back et al. 2005; Volpe 2009b), late gestation insults also cause injury to molecules essential for the development of cerebral cortical synaptic circuitry and organization such as KCC2 and GABAAR subunits.
The role of subplate damage following preterm birth has numerous implications for understanding pathophysiology of neurodevelopmental disorders and for rational design of clinically effective, age-appropriate therapies. In addition to cognitive deficits, preterm infants often suffer from spasticity, and recently, it has been proposed that prenatal subplate injury may contribute to motor deficits (Thomas et al. 2005; Burton et al. 2009; Hoon et al. 2009). Specifically, subplate injury may render the developing brain more reliant on intracortical instead of subcortical connections (Burton et al. 2009). A breakdown in the topography within and between somatosensory and motor areas may contribute to motor deficits and reflect unregulated network expansions within and between brain regions (Burton et al. 2009). More extensive damage to the subplate might be greater in individuals with more severe motor deficits as a result of poorer functional connectivity (McQuillen and Ferriero 2005; Burton et al. 2009). More severe motor symptoms are often concomitant with cognitive deficits (Bax et al. 2005), suggesting that cognitive deficits may result similarly from abnormal functional connectivity that disrupts defined links between cortical areas need for effective learning, attention, and executive function. Indeed, at P28, TSHI rats had significant gait deficits and motor impairment characterized by decreased stride length and increased step frequency, compared with sham controls. Moreover, treatment with EPO reversed these gait abnormalities in stride and cadence. Together, these results show that postnatal EPO treatment mitigated these biochemical and functional deficits, which suggest epoetin alpha may be a viable therapeutic strategy in this patient population. Furthermore, these findings suggest that cortical development is malleable and can be re-directed toward a more normal trajectory.
In conclusion, the mammalian cerebral cortex is comprised of morphologically and functionally distinct areas, each of which is reciprocally interconnected. Thus, understanding the organization and development of cerebral connections and network formation is fundamental to elucidating higher cortical processing, including cognitive functions. The data presented here suggest that loss of expression of KCC2 is malleable, at least during a critical developmental window and that prenatal TSHI induces alterations in the developing in vivo brain that are mimicked by mechanical subplate ablation in slice culture. The putative clinical utility of EPO for neuroprotection in preterm neonates and other infants who suffer HI injury is high, in part because of EPO's multiple mechanisms of action. While it has previously been reported that EPO protects the CNS after injury (Viviani et al. 2005; Mazur et al. 2010; Hirano et al. 2012; Jantzie et al. 2013), EPO's role in attenuating loss of KCC2 chloride transporter expression and GABAAR subunit maturation along with reversal of gait abnormalities has not previously been described to our knowledge. High doses of EPO are approved under Investigational New Drug applications from the FDA for clinical trials in very preterm infants, term infants with HI encephalopathy, and infants 1–12 months old with acute severe brain injury. In adult humans, EPO improves cognition, executive functioning, and memory retrieval (Miskowiak et al. 2007; Miskowiak et al. 2008), and EPO has an uncommon track record in that safety has already been tested in young, medically fragile neonates (Fauchere et al. 2008; Juul et al. 2008; Elmahdy et al. 2010; McPherson and Juul 2010; McAdams et al. 2012; Wu et al. 2012). Phase I trials have shown that high EPO dosages are safe for preterm (Fauchere et al. 2008; Juul et al. 2008; McAdams et al. 2012) and term infants with brain injury (Elmahdy et al. 2010; Wu et al. 2012). A Phase III trial to examine the neurodevelopmental impact of EPO treatment with respect to impact on death, disability (cerebral palsy and cognitive deficits), and MRI abnormalities in extremely preterm infants is underway (NCT01378273). In future studies, we will address the impact of prenatal injury on other subplate components, including axonal refinement, and different subplate neuronal populations that likely contribute to the balance of excitation and inhibition in the developing brain. While KCC2 loss and impaired GABAA receptor maturation are not the only factors that influence this balance, they are critical to disordered inhibition in the injured developing brain and appear responsive to neuroprotective strategies.
Funding
This work was supported by the National Institutes of Neurological Disorders and Stroke (NINDS) at the National Institutes of Health (NIH) (RO1 NS060765 to S.R.).
Notes
The authors are appreciative of the Boston Children's Hospital Intellectual and Developmental Disabilities Research Center (BCH IDDRC) Cellular Imaging Core (P30 HD18655). Conflict of Interest: None declared.
References
- Aboitiz F, Montiel J, Garcia RR. 2005. Ancestry of the mammalian preplate and its derivatives: evolutionary relicts or embryonic adaptations? Rev Neurosci. 16:359–376. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Hanganu IL, Heck N, Luhmann HJ. 2005. Oxygen and glucose deprivation induces major dysfunction in the somatosensory cortex of the newborn rat. Eur J Neurosci. 22:2295–2305. [DOI] [PubMed] [Google Scholar]
- Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. 2005. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neruoeng Rehabil. 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimatsu Y, Ishida M, Kaneko T, Ichinose S, Omori A. 2003. Organization and development of corticocortical associative neurons expressing the orphan nuclear receptor Nurr1. J Comp Neurol. 466:180–196. [DOI] [PubMed] [Google Scholar]
- Back S. 2001. Recent advances in human perinatal white matter injury. Prog Brain Res. 132:131–147. [DOI] [PubMed] [Google Scholar]
- Back S, Luo N, Mallinson R, O'Malley J, Wallen L, Frei B, Morrow J, Petito C, Roberts C, Murdoch G, et al. 2005. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 58:108–120. [DOI] [PubMed] [Google Scholar]
- Balia M, Velez-Fort M, Passlick S, Schafer C, Audinat E, Steinhauser C, Seifert G, Angulo MC. 2015. Postnatal down-regulation of the GABAA receptor gamma2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex. 25:1114–1123. [DOI] [PubMed] [Google Scholar]
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D. 2005. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 47:571–576. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, Friauf E, Nothwang H. 2006. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2641:10419–10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, et al. 2010. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 16:302–307. [DOI] [PubMed] [Google Scholar]
- Burton H, Dixit S, Litkowski P, Wingert JR. 2009. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens Mot Res. 26:90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamma I, Heubl M, Chevy Q, Renner M, Moutkine I, Eugene E, Poncer JC, Levi S. 2013. Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J Neurosci. 33:15488–15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C, Sundquist K, Winkleby MA, Sundquist J. 2011. Preterm birth and risk of epilepsy in Swedish adults. Neurology. 77:1376–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csillik A, Okuno E, Csillik B, Knylhar E, Vecsel L. 2002. Expression of kynureine aminotransferase in the subplate of the rat and its possible role in the regulation of programmed cell death. Cereb Cortex. 12:1193–1201. [DOI] [PubMed] [Google Scholar]
- Daw M, Ashby M, Issac J. 2007. Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nature Neurosci. 10:453–461. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. 2006. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 439:79–83. [DOI] [PubMed] [Google Scholar]
- Elmahdy H, El-Mashad A, Ell-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. 2010. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 125:e1135–e1142. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. 1998. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1:563–571. [DOI] [PubMed] [Google Scholar]
- Failor S, Nguyen V, Darcy D, Cang J, Wendland M, Stryker M, McQuillen P. 2010. Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex. J Neurosci. 30:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. 2005. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 6:215–229. [DOI] [PubMed] [Google Scholar]
- Fauchere J-C, Dame C, Vonthein R, Koller B, Arri S, Wolf M, Bucher H. 2008. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 122:375–382. [DOI] [PubMed] [Google Scholar]
- Fazzi E, Bova S, Giovenzana A, Signorini S, Uggetti C, Bianchi P. 2009. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev Med Child Neurol. 51:974–981. [DOI] [PubMed] [Google Scholar]
- Friauf E, Shatz C. 1991. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophys. 66:2059–2071. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell S, Shatz C. 1990. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 347:179–181. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz C. 1992. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 256:1441–1443. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz C. 1993. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 117:1031–1047. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. 1988. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 96:857–881. [DOI] [PubMed] [Google Scholar]
- Hack M, Costello DW. 2008. Trends in the rates of cerebral palsy associated with neonatal intensive care of preterm children. Clin Obstet Gynecol. 51:763–774. [DOI] [PubMed] [Google Scholar]
- Hanganu I, Kilb W, Luhmann H. 2002. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 22:7165–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ST, Pulst SM. 2013. Response to ethanol induced ataxia between C57BL/6J and 129X1/SyJ mouse strains using a treadmill based assay. Pharmacol Biochem Behav. 103:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Wagner K, Mark P, Pittermann E, Gabel R, Furlani D, Li W, Vollmar B, Yamada T, Steinhoff G, et al. 2012. Erythropoietin attenuates the sequels of ischaemic spinal cord injury with enhanced recruitment of CD34+ cells in mice. J Cell Mol Med. 16:1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, Davies KE, Goffinet AM, Rakic S, Parnavelas J, Reim K, Nicolic M, Paulsen O, et al. 2009. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 19:1738–1750. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Jr., Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV. 2009. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 51:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang J, Yung W. 2013. Coupling between GABA-A receptor and chloride transporter underlies ionic plasticity in cerebellar purkinje neurons. Cerebellum. 12:328–330. [DOI] [PubMed] [Google Scholar]
- Jansen LA, Peugh LD, Roden WH, Ojemann JG. 2010. Impaired maturation of cortical GABA(A) receptor expression in pediatric epilepsy. Epilepsia. 51:1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Miller RH, Robinson S. 2013. Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Pediatr Res. 74:658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul S, McPherson R, Bauer L, Ledbetter K, Gleason C, Mayock D. 2008. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 122:383–391. [DOI] [PubMed] [Google Scholar]
- Kanold P. 2009. Subplate neurons: crucial regulators of cortical development and plasticity. Front Neuroanat. 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold P, Shatz C. 2006. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 51:627–638. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. 2010. The subplate and early cortical circuits. Annu Rev Neurosci. 33:23–48. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Haynes RL, Xu G, Andiman SE, Folkerth RD, Sleeper LA, Volpe JJ. 2012. Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann Neurol. 71:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N, Rados M, Sedmak G, Benjak V, Kostovic-Srzentic M, Vasung L, Culjat M, Rados M, Huppi P, et al. 2012. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct. 219:231–253. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judas M, Rados M, Hrabac P. 2002. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 12:536–544. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. 2006. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol. 48:388–393. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. 2010. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 99:1119–1127. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1980. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 9:219–242. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1990. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 297:441–470. [DOI] [PubMed] [Google Scholar]
- Kovacs K, Basu K, Rouiller I, Sik A. 2013. Regional differences in the expression of K(+)-Cl (−) 2 cotransporter in the developing rat cortex. Brain Struct Funct. 219:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban K, Leviton A. 1994. Cerebral palsy. New Engl J Med. 330:188–195. [DOI] [PubMed] [Google Scholar]
- Leviton A, Gressens P. 2007. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 30:473–478. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. 2002. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 25:409–432. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Jr., Moore M, Ringer SA, Volpe JJ, du Plessis AJ. 2008. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 121:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Li H, Saarma M, Kaila K, Rivera C. 2003. Developmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmission. Eur J Neurosci. 18:3199–3206. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Uvarov P, Soni S, Thomas-Crusells J, Airaksinen M, Rivera C. 2011. Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J Neurosci. 31:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marret S, Marchand-Martin L, Picaud JC, Hascoet JM, Arnaud C, Roze JC, Truffert P, Larroque B, Kaminski M, Ancel PY, et al. 2013. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 8:e62683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Cepeda C, Hurst RS, Flores-Hernandez J, Mendoza D, Levine MS. 2000. Neurons recorded from pediatric epilepsy surgery patients with cortical dysplasia. Epilepsia. 41(Suppl 6):S162–S167. [DOI] [PubMed] [Google Scholar]
- Mazur M, Miller R, Robinson S. 2010. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. J Neurosurg Pediatrics. 6:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams R, McPherson R, Mayock D, Juul S. 2012. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 33:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson R, Juul S. 2010. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr Opin Pediatr. 22:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen P, Ferriero D. 2005. Perinatal subplate neuron injury: Implications for cortical development and plasticity. Brain Path. 15:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen P, Sheldon R, Shatz C, Ferriero D. 2003. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 23:3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, Ferriero DM. 2009. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 32:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K, Inkster B, Selvaraj S, Wise R, Goodwin GM, Harmer CJ. 2008. Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology. 33:611–618. [DOI] [PubMed] [Google Scholar]
- Miskowiak K, O'Sullivan U, Harmer C. 2007. Erythropoietin enhances hippocampal response during memory retrieval in humans. J Neurosci. 27:2788–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Clowry G. 2012. Cerebral cortical development in rodents and primates. Prog Brain Res. 195:45–70. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. 2002. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 956:30–35. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. 1988. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 271:355–386. [DOI] [PubMed] [Google Scholar]
- Orchinik LJ, Taylor HG, Espy KA, Minich N, Klein N, Sheffield T, Hack M. 2011. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc. 17:1067–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, Sukoff Rizzo SJ, Berger Z, Chen Y, Gonzales C, Kaftan E, Li L, Lotarski S, Monaghan M, Shen W, Stolyar P, Vasilyev D, Zaleska M, D Hirst W, Dunlop J. 2013. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease. PLoS One. 8:e70274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press. [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen J, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MV, Voipio J, Kaila K, Saarma M. 2002. BDNF-induced TrkB activation down-regulates the K+-CI− cotransporter KCC2 and impairs neuronal CI− extrusion. J Cell Biol. 159:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. 1999. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 397:251–255. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. 2004. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 24:4683–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R, Annis C, Baratta J, Haraldson S, Ingeman J, Kageyama G, Kimm E, Yu J. 2000. Do subplate neurons comprise a transient population of cells in developing neocortex of rats? J Comp Neurol. 426:632–650. [DOI] [PubMed] [Google Scholar]
- Robinson S. 2005. Systemic prenatal insults disrupt telencephalon development: Implications for treatment. Epilepsy Beh. 7:345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Li Q, DeChant A, Cohen M. 2006. Neonatal loss of gamma amino butyric acid pathway expression after human perinatal brain injury. J Neurosurgery: Pediatrics. 104:396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Mikolaenko I, Thompson I, Cohen M, Goyal M. 2010. Loss of cation-chloride cotransporter expression in preterm infants with white matter lesions: implications for the pathogenesis of epilepsy. J Neuropath Exp Neuro. 69:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Petelenz K, Li Q, Cohen M, Buczek M, Lust D, Miller R. 2005. Developmental changes induced by prenatal hypoxia-ischemia insult in rats models human perinatal brain injury. Neurobiol Dis. 18:568–581. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. 2003. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 24:442–450. [DOI] [PubMed] [Google Scholar]
- Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, Hack M. 2012. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. J Dev Behav Pediatr. 33:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama C, Inoue Y. 2010. Developmental localization of potassium chloride co-transporter 2 (KCC2), GABA and vesicular GABA transporter (VGAT) in the postnatal mouse somatosensory cortex. Neurosci Res. 67:137–148. [DOI] [PubMed] [Google Scholar]
- Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S. 2005. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain. 128:2562–2577. [DOI] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov-Milosevic N, Pletikos M, Mori S, Kostovic I. 2010. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat. 217:400–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, Galli C, Marinovich M. 2005. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 93:412–421. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 1996. Subplate neurons - Missing link in brain injury of the premature infant. Pediatrics. 97:112–113. [PubMed] [Google Scholar]
- Volpe JJ. 2009a. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2009b. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 16:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2000. Overview: normal and abnormal human brain development. Ment Retard Dev Disabil Res Rev. 6:1–5. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Hoerder-Suabedissen A, Oeschger FM, Bayatti N, Ip BK, Lindsay S, Supramaniam V, Srinivasan L, Rutherford M, Mollgard K, et al. 2010. Subplate in the developing cortex of mouse and human. J Anat. 217:368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Bauer L, Ballard R, Ferriero D, Glidden D, Mayock D, Chang T, Durand D, Song D, Bonifacio S, et al. 2012. Erythropoietin for neuroprotection in neonatal encephalopathy: Safety and pharmacokinetics. Pediatrics. 130:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. 1999. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 24:847–859. [DOI] [PubMed] [Google Scholar]