Abstract
The complex processing architecture underlying attentional control requires delineation of the functional role of different control-related brain networks. A key component is the cingulo-opercular (CO) network composed of anterior insula/operculum, dorsal anterior cingulate cortex, and thalamus. Its function has been particularly difficult to characterize due to the network's pervasive activity and frequent co-activation with other control-related networks. We previously suggested this network to underlie intrinsically maintained tonic alertness. Here, we tested this hypothesis by separately manipulating the demand for selective attention and for tonic alertness in a two-factorial, continuous pitch discrimination paradigm. The 2 factors had independent behavioral effects. Functional imaging revealed that activity as well as functional connectivity in the CO network increased when the task required more tonic alertness. Conversely, heightened selective attention to pitch increased activity in the dorsal attention (DAT) network but not in the CO network. Across participants, performance accuracy showed dissociable correlation patterns with activity in the CO, DAT, and fronto-parietal (FP) control networks. These results support tonic alertness as a fundamental function of the CO network. They further the characterization of this function as the effortful process of maintaining cognitive faculties available for current processing requirements.
Keywords: alertness, attention, cingulo-opercular network, fMRI, functional connectivity
Introduction
In functional imaging studies, a group of parietal, frontal, and subcortical regions commonly increase activity as a result of cognitive task engagement independent of the specific task (Duncan and Owen 2000; Dosenbach et al. 2006). In fact, the main effect of any cognitively engaging task against passive baseline (rather than at the commonly reported contrasts of interest) results in a core activation pattern that is quite similar across functional imaging experiments. In studies investigating intrinsic brain organization, the term “task-positive” has been proposed for this set of regions (Fox et al. 2005). The general task activation pattern suggests an involvement of these regions in foundational capacities such as attentional control common to cognitive performance in general.
The complex and multifaceted nature of these attentional control functions calls for a taxonomy to enable a neurobiological understanding. The cognitive architectures that have been proposed for such taxonomy commonly include a sustained and endogenously maintained type of top-down control process distinct from attentional control processes that are phasic in nature. This sustained function is referred to as “vigilance” (Mackworth 1948; Parasuraman 1998), “vigilant attention” (Robertson and Garavan 2004), “sustained attention” (Warm 1984), or “tonic alertness” (Posner 2008). In these accounts, this sustained function—henceforth called tonic alertness—is described as the mentally effortful, self-initiated (rather than externally driven) preparedness to process and to respond. Conversely, the phasic aspects of attentional control include “selective attention” to specific features such as color, pitch, or spatial location (Posner and Boies 1971; Driver 2001), and “phasic alertness” initiated by an external cue or stimulus (Posner 2008). How these dissociable cognitive functions are supported by specific brain regions and networks among the task-positive system is poorly understood. This holds especially true for tonic alertness, which has remained somewhat understudied in functional neuroimaging experiments. This contrasts with extensively studied cue-driven or bottom-up stimulus-driven attentional functions and feature-selective attention (Kanwisher and Wojciulik 2000; Kastner and Ungerleider 2000; Driver and Frackowiak 2001; Corbetta and Shulman 2002). The likely reason for this is that the study of tonic alertness requires sustained engagement in a particular task which in turn co-engages phasic alertness and selective attention specific to the task and stimulus content. Thus, it is difficult to study the neural substrate of tonic alertness independent of those underlying phasic alertness and selective attention.
Functional magnetic resonance imaging (fMRI) studies of intrinsic network organization have shown that the task-positive networks can also be identified during a task-free resting state. Such resting-state studies are based on correlations in spontaneous activity fluctuations across brain regions. This correlated activity suggests a functional organization and possibly cross-regional communication that exists independent of external processing demands (Sadaghiani and Kleinschmidt 2013). Thus, intrinsic functional connectivity may help to outline an anatomical dissociation of task-positive functional networks in the absence of the typical co-activation occurring during task settings. The most consistently observed intrinsic connectivity networks (ICNs) that involve task-positive regions include a cingulo-opercular (CO)/insular network, a lateral prefrontal-parietal or “fronto-parietal” (FP) network (Dosenbach et al. 2007; Seeley et al. 2007), and a dorsal parieto-frontal or “dorsal attention” (DAT) network (Fox et al. 2006).
The FP network is proposed to support phasic aspects of attentional control such as exogenously triggered initiation of control, adapting after errors (Dosenbach et al. 2007) and moment-to-moment adjustment of control as in repeated rapid task switching (Seeley et al. 2007). These functions are well matched with the aforementioned notion of phasic alertness (Sadaghiani et al. 2012). The DAT network is proposed to underlie selective attention especially in visual and spatial domains (Corbetta and Shulman 2002; Fox et al. 2006). Selective attention enhances processing of specific sensory input over other input (Driver 2001) in all likelihood by increasing activity gain of the neural populations encoding the attended stimulus or feature (Chawla et al. 1999; Kastner and Ungerleider 2000). The cognitive functions proposed for the CO network have been considerably more divergent than those associated with the FP and DAT networks. Dosenbach et al. (2006, 2007) suggested the CO network to underlie stable maintenance of task control and task goals referred to as “task-set maintenance.” In a different line OF research, Seeley et al. (2007) have proposed a quite different functional role for this network. In this view, visceral, autonomic, and sensory data are integrated by the CO network to assess the homeostatic relevance or “salience” of internal and external stimuli.
On the basis of our previous studies, we have proposed that at least one important function of the CO network is the maintenance of tonic alertness. Using fMRI and a continuous simple detection task strongly relying on tonic alertness, we previously observed a positive covariance of detection performance with prestimulus activity of the CO network (Sadaghiani, Hesselmann, et al. 2009). In this network, higher baseline activity predicted better detection. In accord with prior studies that explicitly probed tonic alertness and found effects in partly overlapping brain structures (Sturm et al. 2004), we suggested this network maintains tonic alertness. In a follow-up study, we argued that if this functional interpretation were correct, spontaneous fluctuations in the CO network during task-free rest (measured by fMRI) should correlate with fluctuations in the electrical signatures of tonic alertness (measured by simultaneous EEG). Indeed, we observed a very selective correspondence between neural activity in the CO network and global oscillation power in the upper α frequency band (∼10–12 Hz) the most consistent electrophysiological marker of tonic alertness (Sadaghiani et al. 2010). Importantly, this EEG-fMRI study also provided insight into a potential mechanism by which the CO network may support tonic alertness. α-Band oscillations are well known as an inhibitory rhythm (Klimesch et al. 2007). The CO network therefore may employ these oscillations across the cortex as a means to clear noisy information, suppress distraction, and keep cognitive faculties available for current processing demands (Sadaghiani et al. 2010).
This neurophysiologically rooted definition of tonic alertness is a substantial component of our functional understanding of the processes supported by the CO network. In this view, tonic alertness is a general and global neurophysiological mechanism. In contrast, selective attention and phasic aspects of alertness and executive control are content-specific, influencing task-specific processing (Sadaghiani et al. 2010, 2012). Note that although the role that we suggest for the CO network is closely related to “task-set maintenance” proposed by Dosenbach et al. (2006, 2007), there are functional differences. The concept of alertness extends beyond situations in which a known task-set is maintained, and includes alert states of high vigilance in which information about the environment, the potential sensory input and the need for action is lacking or sparse (such as in a dark unfamiliar environment with potential threats). While task-set maintenance involves the maintenance of specific information about the task, tonic alertness emphasizes the general mechanism of keeping cognitive faculties available for current processing demands and holding unwanted activity at bay. There are also marked differences between tonic alertness and salience (Seeley et al. 2007). While salience detection describes the monitoring and evaluation of homeostatic importance, tonic alertness maintenance is the sustained process of ensuring engagement (that may be a result of and action upon detection of homeostatically salient stimuli). In this view, tonic alertness scales with the need to effortfully engage. The higher the need for reducing other parallel (conscious or unconscious) brain processes to meet current processing demands, the more CO activity is expected. This increase in CO activity is predicted even in the absence of increase in homeostatic relevance, and even in tasks involving simple perceptual decisions that lack complex task-set. Thus, activation of the CO network is expected to varying degrees in all tasks in line with the common observation of this network in main effects of imaging experiments. But, its isolation from other activity requires dissociation of tonic alertness from content-specific task demands. Finally, note that the various suggested CO network functions—tonic alertness, task-set maintenance, and salience detection—are not mutually exclusive. We propose that maintenance of tonic alertness is at least one central function of the CO network while this network may also support other functions. It has also been proposed that different cognitive functions may be maintained by different subsystems within the CO network, and a more ventrally versus dorsally extended anatomical subdivision has been previously suggested (Power et al. 2011).
The current experimental design aims at activating the CO network in dissociation of other task-positive networks through targeted engagement of tonic alertness. As described before, one major challenge in understanding the function of the CO network has been the difficulty to find a functional manipulation that isolates its activity from other task-positive networks. Here, we hypothesize that, in modulating tonic alertness in isolation from other attentional control demands, the CO network can be selectively activated, thus confirming our functional interpretation of this network. In our above-described framework, one key feature that characterizes CO function in contradistinction to other attentional control networks is that it is not driven by specific stimulus content. We thus developed a paradigm that specifically orthogonalizes tonic alertness from phasic and feature-selective aspects of attentional control. We sought a factorial paradigm in which both factors effect behavior but in dissociable ways so as to modulate activity in the underlying networks separately. To this end, we used a continuous pitch discrimination task in a 2 × 2-factorial block design. The factor “selective attention demand” was manipulated using pitch intervals at 2 levels. Pitch height is “metaphorically” associated with spatial height (Maeda et al. 2004) and processed in parietal areas of the DAT network generally implicated in spatial processing (Sadaghiani, Maier, et al. 2009). We thus expected the factor selective attention to modulate activity in the DAT network. The second factor “tonic alertness demand” was controlled at 2 levels by using jittered versus regular interstimulus intervals (ISIs). In the context of this task, we expected jittering to increase demands on tonic alertness. Jittering makes stimuli unpredictable in time and adds a task-irrelevant dimension to stimulation, thus increasing task difficulty and intrinsic effort independent of pitch content. It furthermore initiates the need for active suppression of distracting information, a function we have linked to CO network activity (Sadaghiani et al. 2010). We hypothesized that this increase in intrinsically maintained tonic alertness would be selectively reflected in increased CO network activity, and possibly in increased functional connectivity within this network.
Materials and Methods
Participants and Data Acquisition
Twenty right-handed volunteers (10 females, average age 21.3 ± 1.8 years) with no history of neurologic or psychiatric conditions participated in the study. Informed consent was obtained in accordance with procedures approved by the Committees for Protection of Human Subjects at the University of California, Berkeley.
Imaging data were collected on a whole-body 3-T Siemens MAGNETOM Trio MRI scanner using a 12-channel head coil. Whole-brain functional images were acquired using the following parameters: T2*-weighted echo planar imaging sequence with TR = 2000 ms, TE = 28 ms, flip angle = 78°, 3 × 3 mm in-plane resolution, 210 × 210 mm field of view, 32 3-mm-thick oblique transversal slices with 0.3 mm interslice gap in descending contiguous order. Scalp fat signal was minimized using fat saturation. Structural images were acquired using a magnetization prepared rapid gradient echo T1-weighted sequence with the following parameters: TR = 2300 ms, TE = 2.98 ms, flip angle = 9°, 1 × 1 × 1 mm voxels).
The simple sinusoidal sound stimuli were presented using the Siemens default scanner air conduction headphones. After a brief auditory localizer run (110 s) participants performed 3 (n = 5) to 4 runs of a pitch discrimination task (11.5 min per run) followed by the structural image acquisition. Subsequently, an eye-closed resting-state run (6 min) was acquired. Finally, if time allowed (for 14 participants), a fourth run of the pitch discrimination task was recorded.
Paradigm
In a pitch discrimination task, participants pressed a response button with the right hand as soon as they perceived a predefined target pitch in a rapid stream of sounds. Eighty sounds were presented in each block at 20 s interblock intervals. The target sound was always identical (466.17 Hz corresponding to B♭ or si bémol) and the highest pitch used in the experiment. Each block contained 2 other sounds in addition to the target pitch. 10% of the sounds in the stream were targets. All sounds were pure sinusoidals of 200 ms duration and an on/off ramp of 30 ms. Before each block the target sound was played 3 times at 2 s intervals for rememorization of the (unchanged) target pitch.
Note that this paradigm is different from an oddball experiment in that the target is not “popping out” of the auditory stream. Here, the target pitch is quite close in frequency to the nontarget sounds, hence requiring active listening rather than attention capture in a bottom-up manner.
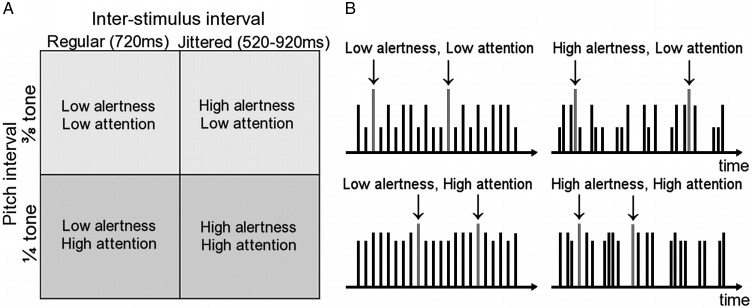
The experimental manipulations conformed to a 2 × 2 block design with the factors “alertness demand” and “attention demand” (Fig. 1). Selective attention demand was controlled by manipulating pitch intervals. The low attention demand level contained sounds that were easier to discriminate (pitches at 3/8 tone intervals in twelve-tone equal temperament scale. Nontarget sound frequencies: 427.48 and 446.41 Hz). In high attention demand blocks, the sounds were harder to discriminate (1/4 tone intervals. Nontarget sound frequencies: 440 and 452.90 Hz). Alertness demand was controlled by manipulating the time interval at which stimuli were presented. For blocks with low alertness demand this interval was fixed to 720 ms. During blocks with high alertness demand, the interval was jittered (mean 720 ms, randomly chosen from 520, 620, 670, 720, 770, 820, and 920 ms). Each of the 4 block types was presented twice per run in randomized block order. Eighty sounds were presented in each block in randomized stimulus order.
Figure 1.
The experimental design and paradigm. (A) In a 2 × 2 factorial block design, the factors alertness demand and selective attention demand were each presented at 2 levels. (B) Example time courses for stimulus blocks corresponding to the 2 × 2 design in A (real experimental blocks contained 4 times as many stimuli). Each vertical line represents one sound and its height represents the pitch. The target sound (highlighted with arrows ↓) was identical throughout the experiment and had the highest pitch. In a sustained task, participants pressed a response button whenever they heard the target. Stimuli occurred at regular intervals in the low alertness conditions (left) and at jittered irregular intervals in the high alertness condition (right). The pitch of the nontarget sounds was closer to—and thus harder to discriminate from—the target sound in the high attention condition (bottom) when compared with the low attention condition (top).
A passive auditory localizer run consisted of 3 20-s blocks (15 s interblock interval) of stimulation with the target sound at 0.5 s ISIs.
Participants were instructed to keep their eyes closed throughout all functional runs to minimize potential contributions from eye movements and spatial attention shifts to brain activity.
Behavioral Analysis
Mean reaction times (RTs) and performance accuracy as measured by d′ were calculated for each of 4 conditions and entered into ANOVAs with the factors alertness demand (2 levels) and selective attention demand (2 levels).
Canonical general linear model Data Analysis
For each of 4 block types of the 2 × 2 design, separate event-related regressors were included for hits (targets detected within 800 ms of stimulus onset), correct rejections (withheld response to nontargets), false alarms (button press for nontargets), and misses (undetected targets) corresponding to the respective sound onset time. An additional regressor was added to capture variance related to the 3 reminder sounds prior to block onsets. Covariates of no interest consisted of 6 head motion parameters and signals from all CSF and all out-of-brain voxels. As performance levels were very high, many runs occurred without false alarms or without misses. Therefore, all contrasts of interest were created based on hits and correct rejections only, which captured 96% of trials (=sounds). Contrasts of interest included the main effect of the experiment (across all blocks), the effect of alertness demand (high > low), and the effect of selective attention demand (high > low).
Linear combinations of the parameter estimates reflecting the above contrasts were extracted from independently defined ICNs and averaged across all respective ICN voxels. ICNs were defined using seed-based resting-state functional connectivity. Spherical (6 mm radius) seeds from previous literature were centered over right and left dorso-lateral prefrontal cortex (DLPFC) for the FP network (Talairach coordinates [±43 22 34], (Dosenbach et al. 2007)), over right and left intra-parietal sulcus (IPS) for the DAT network ([±27–58 49], (Fox et al. 2006)), and over dorsal anterior cingulate cortex (dACC) and right anterior thalamus for the CO network ([0 15 40] (Sadaghiani et al. 2010), and [10–15 8], (Dosenbach et al. 2007)). The BOLD signal time courses from all participant-specific gray matter voxels within the 2 seeds of each network were averaged into one time course that served as a regressor in a separate general linear model for each network. Covariates of no interest consisted of 6 head motion parameters and signals from all CSF, all white matter, all gray matter and all out-of-brain voxels. Contrast images corresponding to the seed regressor were created for each participant and entered into a second-level one-sample t-test. Network regions were defined at P < 0.05 FWE-corrected and clusters >20 voxels. Voxels outside of a generic gray matter mask from WFU Pickatlas (Wake Forest University School of Medicine, http://www.fmri.wfubmc.edu/cms/software) were excluded from the ICN volumes of interest. Left and right early auditory cortices were defined using the passive auditory localizer (P < 0.05 FWE-corrected, extent >20 voxels) and were merged serving as an additional volume of interest. For visualization, ICN maps were rendered onto an inflated canonical average brain (FreeSurfer, CorTech, http://surfer.nmr.mgh.harvard.edu).
The contrast values extracted from each ICN were entered into a 3-way repeated-measures ANOVA with the factors alertness demand (2 levels), attention demand (2 levels), and network (CO, FP, DAT). Appropriate right-sided post hoc t-tests were performed.
In addition to the above-described volume of interest-based analyses and to illustrate the spatial distribution of the effects, first-level contrast images were entered into a second-level one-sample t-test. Activity is visualized at P < 0.005 uncorrected, extent >50 voxels. The alertness contrast was then masked with a generic gray matter mask from WFU Pickatlas to split a large cortico-subcortical cluster into separate regions and thus allow for appropriate cluster-level significance assessment. Additionally, hypothesis-driven small-volume-corrected analyses were performed for the ICNs. Activations significant at FWE-corrected cluster level are reported explicitly.
Functional Connectivity Analysis
For connectivity analyses, nodes of ICNs were defined from a functional atlas published by Power et al. (2011). In this atlas, peak MNI coordinates of nodes from CO, FP, DAT, and several other ICNs were defined based on meta-analysis of functional imaging data across various tasks in conjunction with resting-state functional connectivity. We created spheres of 6 mm radius around these peak coordinates provided as Supplementary Material of Power et al. for CO, FP, and DAT. Note that CO nodes are provided separately for 2 subsystems of CO, here denoted as partial CO (COP) and salience (SAL) networks. This allowed us to study their connectivity properties separately. To ensure specificity of the atlas nodes for our particular dataset, we excluded nodes that did not (partially) overlap with our seed-based ICNs. This allowed us the use of 13 (of 14) nodes for COP, 13 (of 18) nodes for SAL, 20 (of 25) nodes for FP, and 9 nodes (of 11) for DAT.
Signal time courses were extracted from nonsmoothed images for 1) resting-state data, 2) for the overall task period (concatenation of all task blocks irrespective of task condition), and 3) for each of 4 block conditions separately (high and low alertness, high and low attention). For each node, voxel-wise time courses were averaged across all subject-specific gray matter voxels.
We aimed at investigating functional connectivity within and across the ICNs after minimizing influence from co-fluctuations of task-evoked activity. To this end, we took 2 steps. First, all voxels showing a differential effect of alertness or selective attention demands in the canonical general linear model (GLM) analysis (high > low) at a lenient threshold of P < 0.005 uncorrected and extent >0 voxels were excluded before averaging node time courses. This conservative step reduced the volume of each network by ∼10% (COP: −9.5%, SAL: −12.1%, FP: −7.2%, DAT: −13.6%). Second, for each region, we used the residual time courses after regressing out the estimated task-evoked activity according to the event-related regressors specified above.
To account for the effect of head motion, 6 head motion parameters were regressed out. Furthermore, to minimize nonlinear effects of head motion that cannot be captured by the conventional 6 rigid-body head motion parameters, we added an individual co-regressor (stick function) for each motion outlier on the basis of signal variance across all voxels of each volume relative to the subsequent volume. To this end, we used the measure DVARS as introduced by (Power et al. 2012) and implemented in the fsl_motion_outliers function of the FSL toolbox. The overall number of motion outliers defined by DVARS (and hence the number of covariate stick functions) were 3.4% of volumes during task runs (11.9 ± 7.1 of 347 volumes), and 2.5% of volumes during resting state (4.6 ± 3.9 of 180 volumes). Additionally, signals from all gray matter, white matter, CSF, and out-of-brain voxels were regressed out. We repeated the main analysis across the experimental conditions also without global gray matter signal regressor (see Supplementary Material).
To assess the effect of the experimental manipulations on information exchange within and between the networks of interest, we calculated the Fisher-transformed Pearson's correlation between all nodes of all networks. Within-network correlations were then assessed by averaging correlations across all region-pairs within each network. Additionally, for between-network correlations, values were averaged for all region-pairs across pairs of networks. The main effect of task was tested in a 2-way repeated-measures ANOVA with the factors network (CO, FP, DAT) and state (rest, task). Parallel to behavioral and activation analyses, the effect of experimental conditions was tested using a 3-way repeated-measures ANOVA with the factors network (CO, FP, DAT), alertness demand (2 levels), and selective attention demand (2 levels). Appropriate post hoc t-tests were applied.
Relation of Brain Activity, Performance and Prior Experience
To further understand the cognitive functions maintained by the different networks of interest, brain activity was assessed as a function of performance accuracy across participants. For each network, the activity was determined as the parameter estimates of the canonical GLM analysis averaged across all respective voxels. Correlations were assessed between-network activity across all blocks irrespective of condition and the overall accuracy (d′) across all blocks. Furthermore, for each network the differential effect (high > low) of alertness demand and of selective attention demand on d′ and on network activity were correlated with each other.
Results
Behavioral measures were tested in two-way ANOVAs with the factors tonic alertness demand (high, low) and selective attention demand (high, low). In an analogs approach, volume-of-interest (VOI)-based network activity and network connectivity were tested in three-way ANOVAs with the factors alertness demand, selective attention demand, and network (CO, FP, DAT).
Behavioral Results
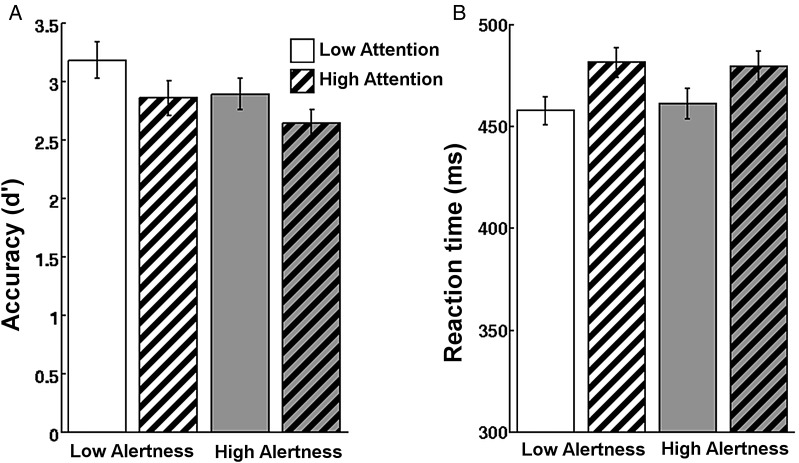
As hypothesized, both experimental factors affected behavior (Fig. 2). Importantly, this effect was dissociable between the 2 factors. Accuracy as measured by d′ decreased significantly with increased alertness demand (F1,19 = 41.3; P < 0.001) and with increased selective attention demand (F1,19 = 20.7; P < 0.001). No interaction was observed (F1,19 = 0.4) indicating independent effects. Reaction times (RTs) were slowed as a function of increased attention demand (F1,19 = 37.8; P < 0.001). No main effect of alertness demand (F1,19 = 0.1) or interaction between factors was observed in RTs (F1,19 = 0.7). This suggests that the accuracy effect (d′) of selective attention demand is likely influenced by a different, RT-sensitive, process than that of tonic alertness demand.
Figure 2.
Both experimental factors affected behavior and the effect was dissociable. (A) Accuracy as measured by d′ decreases with higher tonic alertness demand and with higher selective attention demand (P < 0.001). No interaction was observed. (B) Conversely, RT increased only as a function of selective attention demand but was unaffected by alertness. There was no interaction.
Activation Results
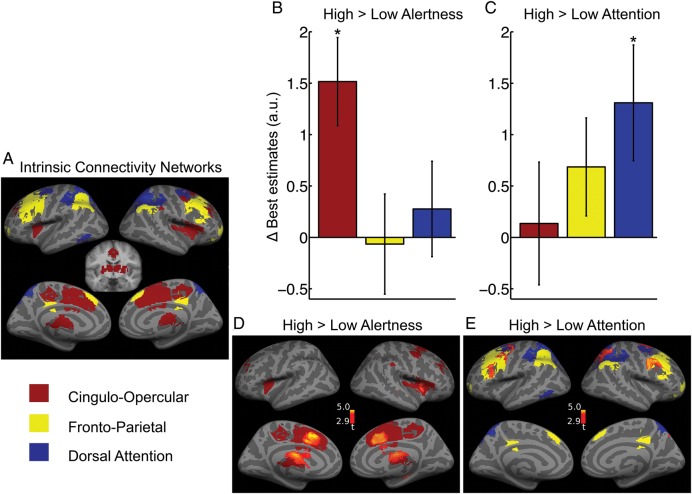
To dissociate the unique role of the 3 major task-positive networks, we applied VOI-based analyses. For activation analysis, the networks of interest were defined based on their intrinsic functional connectivity (ICNs) using seed-based functional connectivity during resting state (Fig. 3A). In accordance with previous literature (Fox et al. 2006; Dosenbach et al. 2007; Seeley et al. 2007; Sadaghiani et al. 2010), the resulting cingulo-insular (CO) network comprised bilaterally the dACC, anterior insula/frontal operculum, dorsal anterior prefrontal cortex (PFC), thalamus, and right anterior inferior parietal lobe (IPL). The DAT network comprised bilaterally the IPS, frontal eye fields (FEF), and left middle temporal complex. The FP network comprised bilaterally the DLPFC, rostro-lateral PFC, posterior IPL, para-cingulate gyrus, and midcingulate gyrus.
Figure 3.
Effects of alertness and attention demands on activation levels. (A) Task-positive ICNs defined using resting-state seed-based functional connectivity. FWE-corrected P < 0.05, extent >20 voxels. Coronal slice shows subcortical areas of CO (y = −10). (B and C) Change in estimated activity levels with experimental conditions for the average signal in each of the 3 ICNs. Only the CO ICN showed higher activity under heightened alertness demand (B). Only the DAT ICN increased activity due to increased selective attention demand (C). Error bars show ± standard error. (D and E) Voxel-wise mapping of the contrasts previously investigated in (B and C). The differential activation is overlaid on the corresponding ICNs. P < 0.005 uncorrected, extent >50 voxels.
As predicted, the main effect of the experiment across all conditions comprised extended activations across the task-positive networks most prominently in CO and FP networks, as well as activity in auditory and left sensory-motor cortices (Supplementary Fig. 1). VOI-based assessment of the main effect averaged across all respective voxels revealed significant activation in CO (t19 = 9.17, P < 0.001) and FP networks (t19 = 3.43, P = 0.001) and the auditory VOI (t19 = 3.63, P < 0.001). No significant effect was observed in the DAT network.
In a VOI-based analysis, the activity estimated for each of 4 experimental conditions was averaged across all voxels of each ICN and entered into an ANOVA with the factors network, tonic alertness demand, and selective attention demand. A significant interaction was observed between the factors network and alertness showing that the effect of alertness demands differed across networks (F1.94,36.89 = 4.22, P = 0.023). Post hoc one-sided t-tests were performed for the effect of alertness (high > low) within each network. Elevated alertness demands increased activity only in the CO network (t19 = 3.53, P = 0.001) but not in the FP (t19 = −0.13) or DAT networks (t19 = 0.60) (Fig. 3B). Alertness and selective attention demands did not interact. Likewise, there was no interaction of network with attention demands indicating that attention effects did not reach a significant difference across networks. Nevertheless, note that, in exploratory t–tests of attention effects within each network, higher selective attention demands increased activity in the DAT network (t19 = 2.33, P = 0.015) but not in the CO (t19 = 0.23) or FP (t19 = 1.43) networks (Fig. 3C). No significant contrast effects were observed for the auditory cortex (alertness contrast t19 = 0.70, attention contrast t19 = 0.48).
It has been suggested that the CO network subdivides into 2 subsystems (Power et al. 2011); a more dorsal network corresponding more closely to the CO network as previously described in Dosenbach et al. (2006, 2007) (here labeled “partial” CO network or COP), and a more ventral network more closely corresponding to the salience (SAL) network as introduced by Seeley et al. (2007). Power et al. describe the COP subsystem to be posterior and dorsal in dACC, lateral in anterior PFC, and dorsal in anterior insula relative to the SAL subsystem. We investigated whether the effect of tonic alertness differed between these 2 subdivisions of CO using the signal averaged across all spherical nodes of COP and of SAL, respectively (Power et al. 2011, see VOI definition described in connectivity section). Both subsystems were similarly affected by alertness demands (COP t = 2.45, P = 0.012, SAL t = 2.21, P = 0.02), and a pair-wise t-test found no difference between the 2 (COP vs. SAL t = 0.06, P = 0.96).
To visualize the topography and spatial specificity of the above-described effects, voxel-wise contrasts are presented (Fig. 3D,E). This supplementary analysis is presented at uncorrected level for illustration purposes. Corrected significance is explicitly stated in Supplementary Table 1. Importantly, this analysis investigates whether contrast effects occurred in regions beyond those studied and established in the VOI-based analysis. High versus low alertness demand increased activation selectively in regions of the CO network namely right anterior insula, bilateral dACC, and bilateral thalamus extending into the striatum (the latter likewise previously observed as part of CO network (Sadaghiani et al. 2010), Fig. 3D). Additionally, there was a small activation in right posterior hippocampus. High versus low selective attention demand activated right IPS and right and left FEFs of the DAT network, as well as right and left DLPFC of the FP network (Fig. 3E, Supplementary Table1). Hence, the voxel-wise differential contrast effects were largely confined to CO in the alertness contrast, and to DAT and FP networks in the selective attention contrast.
Connectivity Results
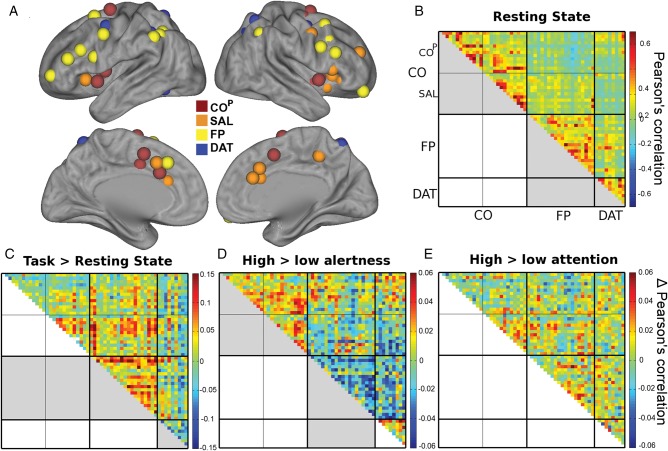
Alertness and attention demands may influence not only activity levels in the underlying attentional networks but also the communication between network regions. We therefore investigated functional connectivity within and across ICNs based on Pearson's correlation between region-wise time courses after accounting for estimated task-evoked activity. To this end, we defined the nodes of each network from a functional atlas published by Power et al. (2011). The choice of the atlas was made to allow a separate investigation of the suggested functional subdivisions of the CO network (COP and SAL). Below, CO without superscript denotes the overall system comprising both COP and SAL nodes. The COP, SAL, FP, and DAT spherical nodes centered on peak coordinates from this atlas are visualized in Figure 4A.
Figure 4.
Effects of overall task, alertness, and selective attention demands on functional connectivity. (A) Spherical VOIs from a functional atlas (Power et al. 2011). (B) Resting-state connectivity confirms strong connectivity between COP and SAL, and (to a lesser extend) between FP and DAT. (C) During task when compared with rest, an increase in connectivity is observed in the FP network as well as between FP and CO networks. DAT network becomes less integrated. (D) Matrix shows differences in pair-wise correlations for high > low alertness demand. The CO network showed a significant connectivity increase while the FP network reduced connectivity under high alertness. The previously strong correlations between FP and DAT networks significantly decreased with heightened alertness. (E) Matrix shows differences in pair-wise correlations for high > low attention. This contrast did not show any differences within or across networks. Thick black divisions indicate network boundaries. Thin black lines subdivide CO into COP and SAL. Significant contrasts in between or within-network correlations (averaged across region-pairs) are marked in gray in the lower triangle of matrices. Note that the scale in B represents correlations, while the scales in C and D) represent differences in correlations.
As a confirmatory analysis to insure that the regions of the Power et al. atlas are delineating the ICNs accurately in the current dataset, “resting-state” within-network and between-network average correlations were entered into two-sided t-tests (P-values Bonferroni corrected for 6 tests; Fig. 4B). As expected, there was very strong within-network connectivity (all t19 >10, P < 0.001 for CO, FP, and DAT). The very strong correlations between COP and SAL confirm that they form subsystems of a common network. In addition, correlations were significant between FP and DAT (t19 = 4.57, P = 0.001) in line with previously observed close ties between DAT and FP during resting state (Dosenbach et al. 2007).
To assess how connectivity changed from resting state to active task performance, a two-factorial ANOVA of network (CO, FP, DAT) and state (rest, task) was performed. Task-based correlations were derived from time courses of all task blocks concatenated irrespective of task condition and after accounting for evoked responses (Fig. 4C). We observed an interaction of network and task (F1.83,34.71 = 18.22, P < 0.001). Post hoc t-tests revealed that FP increased connectivity (t19 = 3.2, P = 0.005) while DAT reduced connectivity (t19 = −4.4, P < 0.001) during task. No change was observed in CO (t19 = −1.52) or its subsystems tested separately. Additionally, the effect of task was tested for between-network connectivity. FP and CO showed strong increase in coupling during task performance (t19 = 2.9, P = 0.028, after Bonferroni correction for the 3 between-network ANOVAs).
Finally and importantly, we investigated the differential effect of the experimental conditions on connectivity. The differences between the resulting correlation matrices for high > low alertness and high > low selective attention are presented in Figure 4D and E. Correlations were averaged across region-pairs within networks and entered into a three-factorial ANOVA with the factors network (CO comprising COP and SAL nodes, FP, DAT), alertness and selective attention demands. A significant interaction was observed between the factors network and alertness showing that the effect of tonic alertness demands on connectivity differed across networks (F1.47,27.85 = 11.59, P = 0.001). Post hoc two-sided t-tests were performed for the effect of alertness demand (high > low) within each network. The CO network showed a significant effect of alertness (t19 = 3.87, P = 0.001). When investigating the CO subsystems separately, both showed a significant positive effect of alertness demands (COP: t19 = 2.79, P = 0.012; SAL: t19 = 2.26, P = 0.036), and there was no difference between the subsystems. The FP network showed a negative effect, i.e., lower connectivity under high alertness demands (t19 = −3.65, P = 0.002). The DAT network showed a strong trend toward higher connectivity during high alertness (t19 = 2.07, P = 0.053). In the context of the above-described decrease in DAT connectivity during task when compared with resting state, this result indicates a stronger decrease in DAT connectivity during low than high alertness. The effect of the selective attention factor did not interact with the network factor (F1.78,33.4 = 0.13) nor was a main effect of attention observed. Alertness and selective attention factors were independent (see Supplementary Fig. 2). Repeating this analysis without global gray matter regression resulted in equivalent outcome (see Supplementary Materials). For completeness, connectivity “between” networks was assessed. To this end, additional two-way ANOVAs with the factors alertness and selective attention demands were calculated for each pair of networks (i.e., between CO and FP, CO and DAT, and DAT and FP). We observed that connectivity between DAT and FP networks reduces under high alertness demands (F1,19 = 7.36, P = 0.042, after Bonferroni correction for the 3 ANOVAs calculated).
The Relation of Activity and Performance
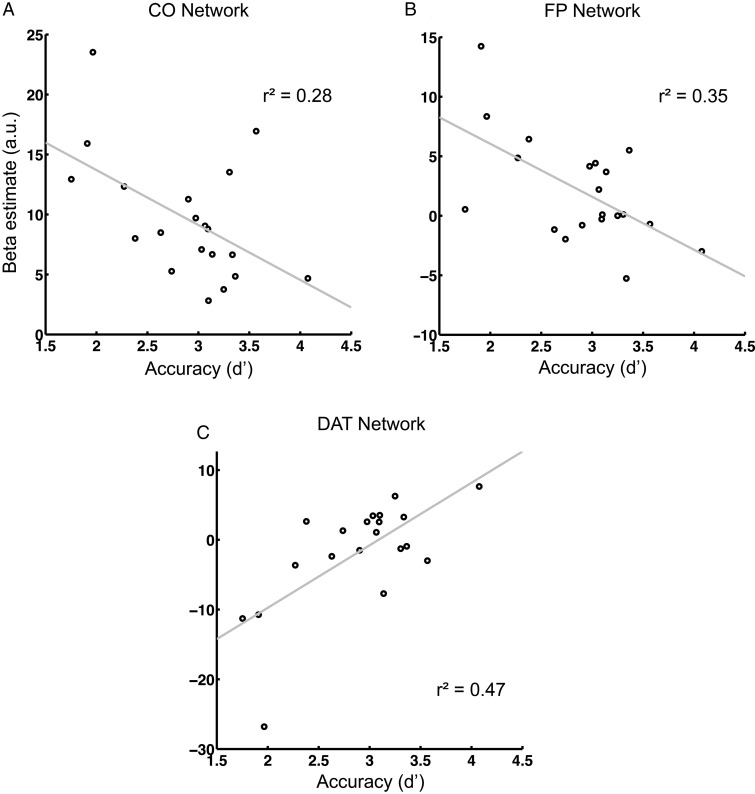
To further understand and dissociate the cognitive functions maintained by the task-positive networks, their activity was correlated to performance accuracy across participants. Subjects' overall accuracy as measured by d′ explained interindividual variability in overall network activity during the task (both measures assessed across all experimental conditions). In CO and FP networks, task-evoked activity was lower the better the subjects performed the task (P = 0.017 and P = 0.006, respectively, Fig. 5A,B). Conversely, in the DAT network, activity correlated positively with overall accuracy (P < 0.001, Fig 5C). Differences between experimental conditions (high > low alertness and high > low attention) did not correlate with differences in d′ across conditions. For completeness, we tested for a relation between overall RTs and overall activity and found no significant effect.
Figure 5.
The dependence of overall network activity on overall accuracy. The better the participant performed, the less strongly the CO and FP networks were engaged (A and B) and the more the DAT network was activated (C).
Autonomic Responses
In a different line of research, the CO network has been suggested to underlie homeostatic salience and interoceptive-autonomic processing (Seeley et al. 2007). Measures of autonomic arousal including beat-to-beat heart rate have been observed to co-vary with activity in core regions of the CO network across experimental conditions (Critchley 2005). To assess whether there was a substantial contribution of autonomic arousal to CO network effects across our conditions, we investigated respiration rate, heart rate, and heart rate variability. None of these autonomic measures differed across experimental conditions (see Supplementary Material). The lack of effects from the alertness factor (all P > 0.2) on the measured physiological parameters may indicate that any fMRI effects from the alertness factor are not substantially driven by autonomic arousal responses.
Discussion
We demonstrated that the functional role of the CO network is dissociable from other attentional control networks through independent engagement of tonic alertness in a factorial design. Both tonic alertness and selective attention demands affected detection accuracy showing that both factors increased difficulty of the task. This increase in difficulty did not interact between the 2 factors (no statistical interaction in accuracy effects), and was carried by different processes; while the accuracy decrease in response to heightened tonic alertness demands did not coincide with changes in RTs, increased selective attention demands resulted in significantly higher RTs. The latter is well described by the common notion of evidence accumulation in perceptual decisions where a less discriminable sensory input signal (pitch) results in longer diffusion time of the decision process before a decision boundary is reached (Ratcliff and McKoon 2008). This strongly contrasts with tonic alertness that is not stimulus content-specific and thus not associated with trial-by-trial perceptual decision time. Following the behavioral dissociation, the CO network increased activity when the task became more demanding in terms of intrinsically maintained tonic alertness rather than feature-selective attention. Conversely, selective attention to pitch increased activity in the DAT network with a trend in the FP network but not in the CO network.
Connectivity effects paralleled the activity effects in the CO network. After accounting for task-evoked responses, functional connectivity in ongoing activity increased selectively in the CO network when tonic alertness demands increased. This suggests that higher within-network communication is important in the maintenance of tonic alertness by the CO network. FP and DAT showed higher baseline between-network connectivity at rest in line with their associated nature in phasic aspects of control. This between-network connectivity decreased during high alertness conditions. We speculate that under ISI jittering in the high tonic alertness condition temporally coordinated phasic actions between DAT and FP may be reduced. Conversely, there was no resting baseline connectivity between FP and CO networks (over and above global correlation that were regressed out) but connectivity became significant when subjects moved from passive resting state to task performance (without further modulation across task conditions). This observation strengthens a recently emerging picture that CO and FP networks build up information exchange in the fulfillment of cognitive demands (Cohen and D'Esposito 2011; Repovš and Barch 2012).
Individual variability in performance and in network engagement helps to further delineate the possible behavioral processes subserved by the CO network. As introduced earlier, tonic alertness denotes mentally effortful, endogenously increased responsiveness (Warm 1984; Posner 2008). In the current experiment, jittered ISIs made task performance more difficult as reflected in decreasing d′, while stimulus content remained unchanged. As timing of the stimuli became unpredictable through jittering, it was presumably more difficult to process pitch in a well-timed manner. Furthermore, jittering added a task-irrelevant dimension to the stimuli that participants spontaneously reported as being distracting and that was compared with “stuttering” auditory input, which had to be actively suppressed. We thus propose that both of these aspects lead to higher tonic alertness demands in the ISI jittering condition. The negative relationship of CO network activity to overall accuracy further supports an account of this function as mentally effortful engagement. That is, the more difficult and effortful the task for a given participant as indexed by poorer overall performance accuracy, the higher the CO network activity. In summary, the function of the CO network can be described as the process of intrinsically maintaining cognitive faculties available for current processing requirements. Conversely, selective attention allows enhanced processing of specific sensory input (Driver 2001). The latter consistently manifests as an increase of local gain in the neural populations encoding the respective stimulus or feature (Chawla et al. 1999; Kastner and Ungerleider 2000). Thus, the higher the overall activity in the DAT network, the more effectively the participant engaged selective attention to pitch resulting in higher overall performance accuracy. Note that the FP network likewise showed a negative relation between overall task activity and overall accuracy similar to the CO network. This is in line with our proposal that FP network underlies another aspect of alertness—described as “phasic” alertness—that acts on an event-by-event basis (Sadaghiani et al. 2012).
These results are in good agreement with the neural mechanism that we have previously proposed for tonic alertness. This mechanism was based on the selective positive correlation of CO network activity with global upper α-band oscillation power (Sadaghiani et al. 2010). These oscillations are a signature of active inhibition of local neural activity (Klimesch et al. 2007; Mathewson et al. 2011). We thus proposed that tonic alertness involves a generalized “windshield wiper” mechanism, and that α-oscillations serve this purpose by rhythmically and synchronously clearing the flood of information globally across the cortex on a rapid time scale of ∼100 ms cycles (Sadaghiani et al. 2010). This mechanism, which we propose is deployed by the CO network, can effectively increase signal-to-noise ratio in cortical processing. In sensory settings such as the current paradigm, it results in a reduction of distraction and hence an enhancement of detection of relevant sensory information. The mechanistic interpretation of tonic alertness as suppression of irrelevant information fits well with task-irrelevant ISI jittering being perceived as distracting by the participants, and inducing increased CO network activity. Note however that while the presence of task-irrelevant input features (such as in our paradigm) can enhance the engagement and facilitate the study of this rhythmic clearing function, distracting features are not necessary prerequisites for tonic alertness. This function can engage not only during specific tasks at hand where relevance or irrelevance of inputs is known, but also when anticipating potential upcoming inputs in the absence of prior knowledge about them or their relevance. In the absence of a priori knowledge, CO network activity may increase preparedness to process and to respond by clearing currently ongoing activity in other cortical areas in a distributed and anatomically unselective manner (for more detailed discussion of this proposed mechanism see (Sadaghiani et al. 2010)).
Our description of CO network function differs from other mechanisms proposed for this network. Although closely related, the sustained alertness function has important differences to the task-set maintenance function proposed by Dosenbach et al. (2006) in that it is independent of a specific task-set or content (see Introduction). Note that in the context of our simple perceptual task of discriminating sinusoidal sounds, an increase in tonic alertness (as we have defined it) as a result of stimulus jittering is a more likely explanation than an increase of task-set complexity. Another prominent functional interpretation for the CO network that proposes a role in “salience” detection and interoceptive-autonomic processing (Seeley et al. 2007) is less related to the view that we propose. Regarding this function, an increased engagement of salience detection under ISI jittering is unlikely since in our simple task context ISI jittering probably did not increase homeostatic relevance of stimuli or task. In this context, we also cautiously note that we did not observe any changes in autonomic arousal responses such as respiration rate, heart rate, and heart rate variability across low and high tonic alertness blocks. This may indicate that CO network effects from the alertness manipulation were not substantially driven by autonomic responses (although negative findings should be interpreted with caution). It has been suggested that saliency is processed in another, more ventrally extended control system that overlaps with the CO network as defined in our study (Power et al. 2011). Current evidence of a dorsal versus ventral dissociation of the anterior insula/frontal operculum (Kurth et al. 2010; Seeley 2010; Chang et al. 2013) might support a respective subdivision of the CO network. We investigated activity and functional connectivity separately for the 2 proposed subsystems COP and SAL. Both subdivisions were significantly more active and showed significantly more connectivity under high tonic alertness with no difference between the subdivisions. Although our data support processing of tonic alertness in both proposed subsystems of the CO network, it is possible that other functions such as those related to task-set maintenance and salience are processed by overlapping structures within the CO network.
Beyond the functional interpretation, additional divergence of views regarding the CO network is apparent in its anatomical delineation in contradistinction to other task-positive networks by different laboratories. Vincent et al. (2008) describe a FP control system that combines CO and FP networks. The CO and FP networks underlie closely related functions. Their correlation structure can thus emerge as connected at a less fine level of the intrinsic connectivity hierarchy. Nevertheless, their functions are dissociable. We suggest that the core function of the CO network is maintaining tonic alertness while the FP network underlies phasic control such as adaptive adjustments in alertness and executive functions (Sadaghiani et al. 2012). The close functional relation is supported by the observation that FP network activity showed strong negative correlation to performance accuracy similar to the CO network. However, while CO network activity was sensitive to the ISI manipulation but not the pitch manipulation, the opposite behavior was observed for the FP network. This suggests a functional dissociation between CO and FP networks where the latter is acting in a more content-dependent manner. This finding is in line with lesion data that support a dissociation of FP and CO networks (Nomura et al. 2010). Along a different vein, Dosenbach et al. (2007) have defined the FP network to include areas of the DAT network. Although DAT and FP activity showed similar behavior with respect to the 2 experimental factors (albeit at varying degrees), the relation of network activity to performance was completely opposite for the 2 networks supporting a functional dissociation between them.
Taken together, the current ICN-based approach in combination with a factorial design supports a functional dissociation between the CO, FP, and DAT networks and confirms the hypothesis that intrinsically maintained tonic alertness is a core function of the CO network. The notion of tonic alertness is well suited to provide a link across various research fields. It makes a direct connection to psychological frameworks of attentional control functions and the respective behavioral research described in the Introduction section (e.g., Posner 2008, Robertson and Garavan 2004, Warm 1984). This notion furthermore bridges to electrophysiological research on tonic alertness (Makeig and Inlow 1993) and the underlying neurophysiological mechanism discussed above (Sadaghiani et al. 2010). The interpretation of CO network function within an attentional framework moreover finds support in our recent observation that core CO network regions selectively show the brain's highest density of nicotinic acetylcholine receptors, which are strongly implicated in attentional control (Picard et al. 2013). This cross-disciplinary approach promises to facilitate understanding of the foundational functions maintained by the CO network.
Supplementary Material
Supplementary can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Institute of Health (MH63901). S.S. was supported by a postdoctoral fellowship of the Deutsche Forschungsgemeinschaft, Germany.
Supplementary Material
Notes
We thank Gaël Varoquaux and Jean-Baptiste Poline for helpful discussions of connectivity analyses. Conflict of Interest: None declared.
References
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. 2013. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. 1999. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 2:671–676. [DOI] [PubMed] [Google Scholar]
- Cohen JR, D'Esposito M. 2011. The comparison of task-related networks and resting state networks during working memory. Quebec City: OHBM. [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3:201–215. [DOI] [PubMed] [Google Scholar]
- Critchley HD. 2005. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 493:154–166. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. 2006. A core system for the implementation of task sets. Neuron. 50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J. 2001. A selective review of selective attention research from the past century. Br J Psychol. 92:53–78. [PubMed] [Google Scholar]
- Driver J, Frackowiak RSJ. 2001. Neurobiological measures of human selective attention. Neuropsychologia. 39:1257–1262. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. 2000. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23:475–483. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. 2000. Visual attention: insights from brain imaging. Nat Rev Neurosci. 1:91–100. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. 2000. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 23:315–341. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. 2007. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 53:63–88. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. 2010. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackworth N. 1948. The breakdown of vigilance durning prolonged visual search. Q J Exp Psychol. 1:6–21. [Google Scholar]
- Maeda F, Kanai R, Shimojo S. 2004. Changing pitch induced visual motion illusion. Curr Biol. 14:R990–R991. [DOI] [PubMed] [Google Scholar]
- Makeig S, Inlow M. 1993. Lapses in alertness: coherence of fluctuations in performance and EEG spectrum. Electroencephalogr Clin Neurophysiol. 86:23–35. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Beck DM, Fabiani M, Ro T, Gratton G. 2011. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Percept Sci. 2:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D'Esposito M. 2010. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci USA. 107:12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R. 1998. The attentive brain. Cambridge (MA): MIT Press. [Google Scholar]
- Picard F, Sadaghiani S, Leroy C, Courvoisier DS, Maroy R, Bottlaender M. 2013. High density of nicotinic receptors in the cingulo-insular network. Neuroimage. 79:42–51. [DOI] [PubMed] [Google Scholar]
- Posner MI. 2008. Measuring alertness. Ann N Y Acad Sci. 1129:193–199. [DOI] [PubMed] [Google Scholar]
- Posner MI, Boies SJ. 1971. Components of attention. Psychol Rev. 78:391–408. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. 2008. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 20:873–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovš G, Barch DM. 2012. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, Garavan H. 2004. Vigilant attention. In: The cognitive neurosciences. 3rd ed Cambridge (MA): MIT Press; p. 563–578. [Google Scholar]
- Sadaghiani S, Kleinschmidt A. 2013. Functional interactions between intrinsic brain activity and behavior. NeuroImage. 80:379–386. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. 2009. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 29:13410–13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Maier JX, Noppeney U. 2009. Natural, metaphoric, and linguistic auditory direction signals have distinct influences on visual motion processing. J Neurosci. 29:6490–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, D'Esposito M, Kleinschmidt A. 2012. Alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J Neurosci. 32:14305–14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, Kleinschmidt A. 2010. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 30:10243–10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. 2010. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct. 214:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, Longoni F, Fimm B, Dietrich T, Weis S, Kemna S, Herzog H, Willmes K. 2004. Network for auditory intrinsic alertness: a PET study. Neuropsychologia. 42:563–568. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm JS. 1984. Sustained attention in human performance. New York: Wiley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.