Abstract
Medication nonadherence is a vexing problem in health care necessitating patients and health professionals’ efforts to prevent, minimize, or reverse it. Research participants’ inconsistent medication taking obscures treatment efficacy and adds costs to biomedical research. Electronic monitoring devices (EMDs), like the Medication Event Monitoring System (MEMS), have grown in sophistication, providing precise, timely insights into individuals’ medication-taking patterns across clinical populations. This article reports on the desirability and feasibility study of using a wireless EMD in clinical research to promote adherence to clinical regimens and research protocols. Nonadherence in transplant patients has been linked to late acute rejection and graft loss. High levels of adherence (97.7 %) were documented for six renal transplant recipients for a mean of 6 months (M = 196.1 ± 71.2 days) who indicated acceptance of the technology. MEMS data confirmed the feasibility of using wireless EMDs to monitor medication use. Monitoring provides greater assurance that research studies reflect the biological impact of medications and provide a basis for targeting adherence enhancement efforts within research investigations.
Keywords: Adherence, Clinical trial, Electronic monitoring, Medication, Renal transplant
Introduction
Clinical trials evaluating pharmaceuticals depend heavily on research participants following the study dose protocol. Research is predicated on the assumption that participants actually take their medication in accordance with the protocol of the research arm to which they are assigned. In health care, the relationship between adherence and health outcomes may be conceptualized, albeit perhaps simplistically, as “linear and predictable with good adherence resulting in the desired health outcome” [1]. The assumption, however, that people adhere as meticulously to medication regimens as their health care providers and clinical investigators would wish, i.e., that they behave consistently over time, flies in the face of clinical and research realities [2, 3]. Hence, Urquhart [4] conceptualizes adherence as “the extent to which the patient’s actual history of drug administration corresponds to the prescribed regimen.” Adherence is critical to achieving the promise of innovative treatments as they are adapted to clinical practice.
Adherence in renal transplants
Medication nonadherence (NA) is a worldwide problem across clinical conditions [5] and is the focus of this report. DiMatteo’s [6] review calculated mean adherence of 75.2 % (range = 4.6–100 %) across 569 studies. The critical importance of adherence to immunosuppressant regimens in solid organ transplants makes it essential that organ recipients follow their regimens. Despite the role of adherence in optimizing clinical outcomes, Dew et al.’s [7] meta-analysis of post-transplant adherence in 32 studies noted mean NA for kidney recipients of 35.6 %, exceeding NA for heart (14.5 %) and liver (6.7 %) recipients. Also, despite medications’ centrality to optimizing outcomes, NA in kidney recipients was higher than NA for nine other health behaviors (e.g., diet, exercise, clinic appointment attendance). The significance of adherence for renal transplant recipients is clear: NA has been estimated to account for 16 % of kidney graft loss and 20 % of late acute rejection episodes [8, 9]. Even modest NA, i.e., missing as few as 5 % of immunosuppression doses, has been associated with up to 50 % of death-censored graft loss [10].
The prevalence of problems and severity of health consequences and costs associated with NA in renal transplantation render it essential to maximize adherence for this medically complex population. Kidney graft loss can result in multiple adverse consequences including need for hospitalization, dialysis, retransplant (thereby increasing the pool of 109,521 patients currently on the national waiting list for kidneys [11]), as well as the risk of premature mortality. In addition to such clinical outcomes, kidney graft loss due to NA is estimated to cost the US health care system about $100 million annually [12–14].
Adherence in research
In research, too, adherence is a complex phenomenon that is of critical importance to the success of an investigation. Thus far, the effects of heterogeneous adherence to the research protocol in biomedical research and on the medical literature are not fully understood [15]. Convergent studies of diverse health conditions confirm that NA continues to be a major challenge in research as well as health care [16].
Research participants may adhere better to regimens than patients do in clinical practice [17]. An analysis of 51 studies that reported drug adherence estimated mean adherence of 85.7 % (range 48–100 % [18]). However, other researchers have estimated that as many as 25–50 % of research participants may not adhere fully with some elements of trial protocols [19]. Although adherence in clinical trials is fundamental to achieving scientific objectives [20], most studies, even in high impact journals, do not document adherence rates [18]. Inadequate adherence within a study undermines its validity, confounds statistical analyses (e.g., increases probability of type II error), may preclude drawing scientific conclusions, and adds costs [2].
Research participants’ insufficient adherence can increase the sample size necessary to achieve statistical power for evaluating research hypotheses [21]. It can lengthen studies and necessitate adding trial centers. It also consumes additional staff time and resources to address the NA [22, 23]. Such concerns lead some researchers to advocate for developing uniform standards for reporting on adherence in clinical trials akin to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting on data and recruitment [18, 24]. Monitoring medication taking is a common strategy used to assess and promote regimen adherence in clinical research [3].
In biomedical research, the “80 % rule” has been a conventional threshold for regimen adherence, including medication taking [2]. Studies that fail to reach that threshold may be deemed inadequate by funding agencies as well as by reviewers and editors for publication in scientific journals. Scientific journals are reluctant to publish articles with low adherence [25]. Consequently, it is important for drug trials and other research to integrate both measurement of adherence and processes to facilitate adherence.
Assessing adherence
Because people generally take medications unsupervised, health professionals have limited understanding of their patients’ actual medication taking patterns. In clinical contexts, health professionals may query patients to detect NA. Such imprecise assessment is vulnerable to desirability bias, leading to adherence estimates that exceed measurement by electronic monitoring devices (EMDs [26]). Patients’ acknowledgement of NA may be an honest reporting of their experience. However, it also could reflect limitations of patients’ awareness or memory of their NA, or it may be a defensive or misleading socially desirable report [27]. Other approaches are also possible, such as blood or urine assays which are available for some medications to indicate levels of the medications or metabolites, or asking patients to keep written or graphed records that add structure to self-reports. Objective adherence measurement approaches include pill counts, in which staff counts patients’ remaining pills at clinic visits. The count back method is calculated by subtracting how many pills remained from how many should have remained from the number dispensed during the monitored time period. Pill counts have been the most commonly used method of measuring adherence in clinical trials [18]. Pharmacy records also indicate when patients seek refills, which can be compared to when they should have obtained refills if they have been taking doses as prescribed. Although none of these metrics related to patient medication use can be presumed to provide definitive accounting of actual medication ingestion (i.e., they all have limitations and are based on certain assumptions), they generally provide valuable insights into how consistently patients take their medications compared to their prescription. Relative advantages and limitations of monitoring approaches have been discussed elsewhere [24, 28] and warrant the consideration of clinical researchers.
Quantification of medication use has also been available in recent decades through EMDs that track patients’ use of medications using microchip technologies. EMDs are engineered to track when patients open medication vials or pillboxes. Data can be downloaded and reviewed to provide a window on a person’s medication taking. Electronic monitoring is generally regarded as one of, and arguably the best method, for measuring medication adherence (MA [26, 29]). EMD cap openings are generally presumed to be reasonable, albeit imperfect, proxies for medication taking that, shy of direct observation, serve as a “gold standard” for verifying when patients take medications [29–31]. It is noted, however, that despite the utility of this approach, there are limits (i.e., patients may not necessarily ingest the medication when they opened the vial), and patients can subvert the technology if so motivated [32]. Strategies have been suggested to overcome some of these limits [33].
Earlier reviews summarized some of the commercially available EMDs [26, 34]. Ingerski et al.’s [34] analysis was based largely on a PubMed search identifying nearly a thousand publications mentioning electronic monitoring of MA [34]. The Medication Event Monitoring System (MEMS®), the predominant electronic monitor of MA [34], has been used to evaluate MA for a broad range of conditions such as epilepsy, hypertension, HIV/AIDs, transplants, and psychiatric disorders. MEMS® has been employed extensively, including in reports in 785 peer-reviewed articles and review articles [35], as well as in books, doctoral dissertations, published symposia, and lectures, etc. [36]. Interventions involving MEMS® in clinical settings, while costly, provide one of the clearest pictures of patients’ medication use. The costs of providing monitored clinical care perhaps are best viewed relative to the overall costs for managing conditions, including NA and its consequences. For example, the estimated costs of heart failure in 2010 in the USA were $39.2 billion with NA considered to be contributing substantially to those costs [37]. To the extent that EMDs can help to reduce NA [37], the costs of using EMDs could prevent and offset other health care costs.
Recently, a second generation of medication-tracking devices (see Table 1) has been introduced, harnessing the Internet to provide closer to real-time feedback [38]. The full impact of this next generation of devices is not yet known. However, the ability to give people and their health providers contemporaneous data about their MA might have greater potential to affect behavior and facilitate interventions to improve behavior than less immediate or less precise feedback. Carver and Scheier’s [39] control systems theory and Leventhal et al.’s [40] self-regulation models underscore the importance of timely feedback in altering behavior to conform better to goals. This wireless approach also has the potential to increase patients’ sense of self-efficacy and self-determination [41, 42]. It is consistent with the theory of planned behavior [43] for changing health behaviors as well as the current interest and proliferation of approaches to self-monitoring [44].
Table 1.
Examples of Internet-based electronic medication monitors
| Product | Manufacturer |
|---|---|
| GlowCaps | Vitality, Inc. |
| http://www.glowcaps.com/ | |
| Medication Event Monitoring Systems- (MEMS®) | MWV Healthcare |
| http://www.mwvaardex.com/Products/DataCollection/MEMSCap | |
| Maya | Medminder |
| www.medminder.com | |
| Med-eMonitor | InforMedix |
| http://www.informedix.com | |
| SimpleMed | Vaica Medical |
| http://www.vaica.com/products | |
| eCAP | Mediary |
| http://informationmediary.com/ecap/ | |
| Wisepill | Wisepill Technologies |
| http://reports.mediscern.com/wisepill/ |
Objective of feasibility study
This preliminary report describes the feasibility and acceptability of using a new generation of wireless EMD in renal transplant patients who were part of a larger study of MA. These participants had already demonstrated good adherence with older, nonwireless MEMS technology. We did not know how receptive they would be to the newer technology, which is more interactive, requires placement of the vial on the reader base to upload data, and whether they would tolerate communications from a nurse coordinator that address MA records. Consequently, the present study was undertaken to ascertain participants’ willingness to use the wireless technology. Participants’ enrollment in the pilot trial using the newer wireless MEMS allowed exploration of their experience with the upgraded technology.
Methods
Six renal transplant recipients were invited, and all volunteered to use the wireless MEMS after participating in the early phase of an IRB-approved study of MA (clinicaltrials.gov #NCT00148174 [45]). The feasibility study was approved by the IRB because no additional risks were identified, expanding the earlier study to allow participants to use the wireless MEMS. In the Nevins et al. [45] study, participants were monitored with nonwireless MEMS without receiving MA feedback. They were selected for the feasibility study based on having completed the prior study and on their willingness to try the new technology. They had not participated in any research interventions to improve MA. All participants demonstrated MA ≥90 % using the standard, nonwireless MEMS cap with their immunosuppressant medication vial. Sample size was limited due to funding and time constraints. Since we were principally interested in whether the new technology was acceptable to participants, we solicited individuals who we anticipated would actually use it and would be able to provide comparative feedback based on actual experience. The study was undertaken to explore the feasibility of designing a larger clinical intervention trial using the wireless technology with a sample characterized by more heterogeneous adherence.
The sample included two women and four men at the University of Minnesota Medical Center whose mean age at transplant was 45.9 years. Their monitored p.o. immunosuppressant regimen was b.i.d. (i.e., 12 h apart, e.g., 8 A.M. and 8 P.M.). Four were taking mycophenolate mofetil (MMF; CellCept); two took enteric-coated mycophenolic acid (Myfortic). MA was tracked between 12/20/2012 and 8/2/2013 with the medAmigo wireless MEMS (see Fig. 1). The MEMS and wireless MEMS caps contain a plunger mechanism that moves each time the cap is removed from the pill vial. Integrated microcircuits record the time and date of vial openings. The functionality of the device is not affected by the physical characteristics (e.g., appearance or size) of the pill.
Fig. 1.
MEMS cap with vial on reader
The wireless MEMS can store 3800 events and has an estimated battery life >24 months. It transfers data by telemetry to a reader that downloads, encrypts, and transmits it via wireless circuits (Global System for Mobile Communications [GSM]) to a central database. Individuals’ data can be reviewed by staff administrators (e.g., research nurse) via a secure website. Reports were generated and shared with participants providing them with feedback on the consistency of their medication taking. The wireless cap features a liquid-crystal display (LCD) indicating the number of cap openings since midnight and the hours elapsed since the last opening. This feature provides a visual reminder as to when to take the next dose or reflects whether a scheduled dose might have been missed. Wireless MEMS caps fit standard medication vials (e.g., Pro Plus Vial 30, 60, or 90 dram) in a range of sizes. Participants were instructed not to open the cap except when taking a medication and to refill vials with their medications, when necessary, only when they had already opened the vials to take a prescribed dose (i.e., so as not to overestimate doses taken). Further information is available at www.medAmigo.com.
Prior to participating in the pilot, participants completed a separate MA study using nonwireless MEMS caps beginning the day following hospital discharge after renal transplant. Nonwireless MEMS were monitored about 6 months (M = 185.3 ± 49.9 days). In that study, these participants returned their nonwireless MEMS caps as requested 3-month post-transplantation for review and did not receive data reports. In the feasibility study, data collection began a few days after returning the nonwireless MEMS cap (mean 191 ± 49.4 days following transplant; range 147–285) and used the wireless MEMS for several months (M = 196.1 ± 71.2 days). They received reports about their medication taking (e.g., Figs. 2 and 3) by email approximately every 2 weeks based on the wireless cap data. They had regular phone or email contacts (≈2 weeks) with the study nurse to review their pattern of medication taking.
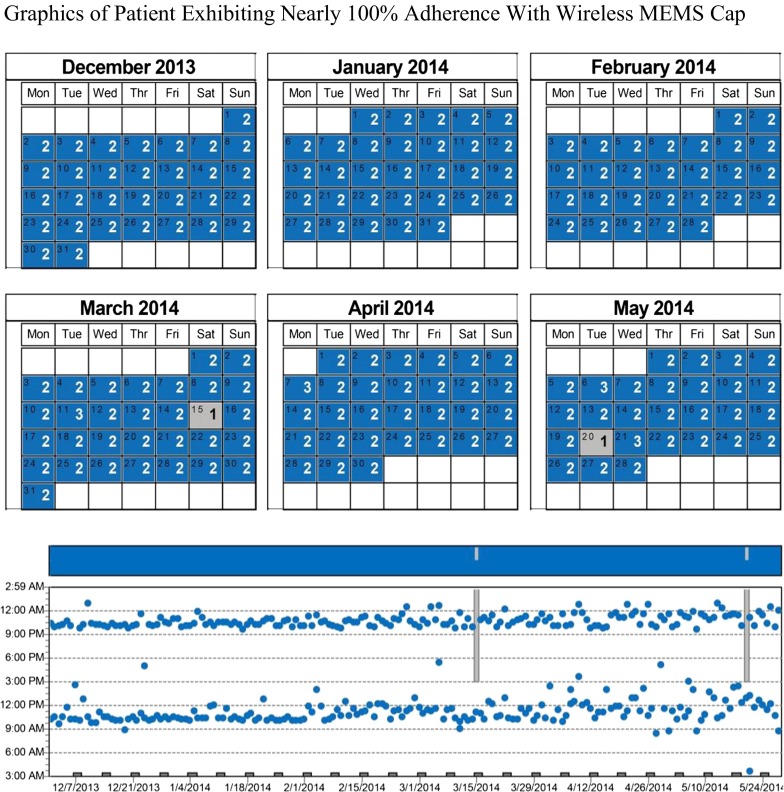
Fig. 2.
Graphics of patient exhibiting nearly 100 % adherence with wireless MEMS cap. White boxes indicate either dates not monitored at beginning or end of trial or days of week that were incorporated in an adjacent month’s data. Gray boxes indicate days on which patient did not demonstrate cap openings expected for that day (i.e., missing at least one dose). The graph displays the times of day the vial was opened and the bars represent missed doses within time frame
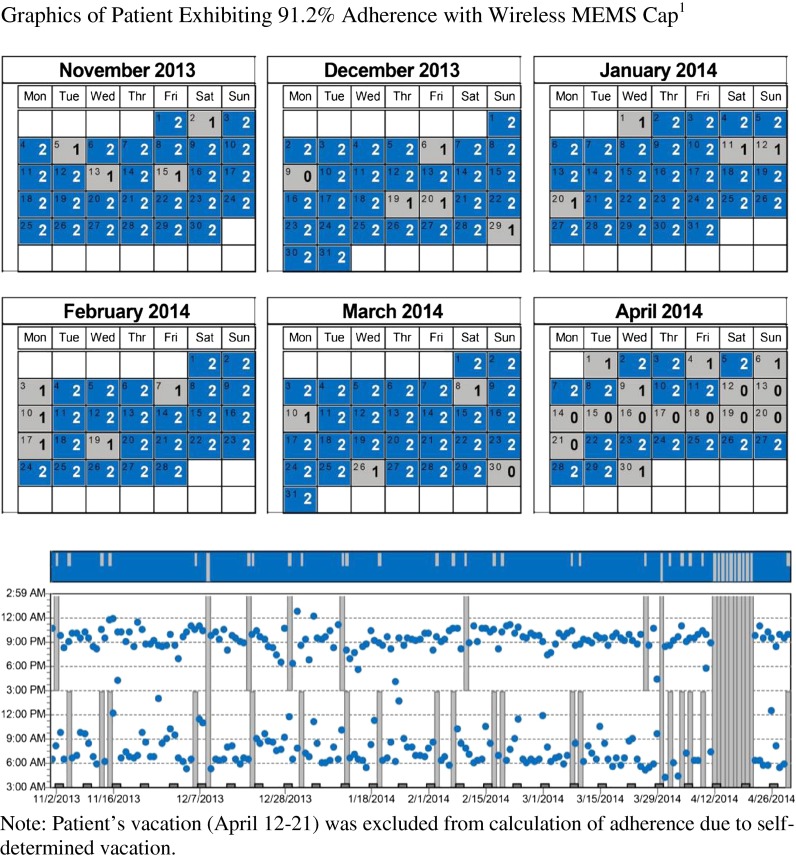
Fig. 3.
Graphics of patient exhibiting 91.2 % adherence with wireless MEMS cap1
At the conclusion, participants completed a 13-item multiple-choice questionnaire developed by the authors about their experience with the wireless technology. Descriptive statistics were calculated using Microsoft Excel. Additional qualitative data were reviewed based on email communications between participants and the research nurse and notes taken by the research nurse at the time of phone contacts. In addition to tracking wireless MEMS data, we inquired about experiences with the technology and reviewed email exchanges and nurse coordinator notes summarizing their discussions.
Results
Table 2 presents adherence and interdose interval (IDI) data for feasibility study participants. IDI refers to the time between medications taken as quantified by cap openings. Both the mean and the standard deviation of IDI are indices of consistency of medication taking. Participants’ original (i.e., nonwireless) and wireless MEMS data were fairly comparable. Mean early adherence for the first 3-month post-transplant with the nonwireless MEMS was 98 % (±2.2 %), with mean IDI in medication taking of 11.8 h (±0.5). The mean nonwireless IDI s.d. was 2.8 h (±1.4). Maximum intervals during the monitored period are also presented. For the wireless MEMS, the mean adherence calculated by the MEMS software on a daily basis was 97.7 % (±3.2 %), with a mean IDI of 12.2 h (±0.5) and IDI s.d. of 2.25 h (±1.3).
Table 2.
Renal recipients’ medication adherence and interdose intervals: sequential nonwireless and wireless MEMS caps summaries by participant
| Participant/cap | Length in days | Age at transplant | Adherence % Nonwireless/wireless | Interdose interval in hours Men Nonwireless/wireless | Interdose interval in hoursS.D. Nonwireless/wireless | Interdose interva maximum hours Nonwireless/ /wireless |
|---|---|---|---|---|---|---|
| 1 Nonwireless | 147 | 31 | 97 | 10.43 | 3.83 | 47.85 |
| 1 Wireless | 280 | 99 | 12.05 | 1.54 | 24.17 | |
| 2 Nonwireless | 185 | 35 | 100 | 11.95 | 1.34 | 23.92 |
| 2 Wireless | 197 | 99.3 | 11.91 | 2.35 | 26.02 | |
| 3 Nonwireless | 178 | 42 | 97.2 | 12.18 | 2.52 | 25.35 |
| 3 Wireless | 182 | 99.7 | 12.02 | 1.27 | 23.97 | |
| 4 Nonwireless | 181 | 68 | 100 | 11.82 | 1.68 | 23.58 |
| 4 Wireless | 141 | 99 | 12.11 | 1.53 | 24.28 | |
| 5 Nonwireless | 280 | 55 | 99.4 | 12.08 | 2.12 | 24.88 |
| 5 Wireless | 102 | 98 | 12.14 | 2.02 | 24.35 | |
| 6 Nonwireless | 141 | 43 | 94.0 | 12.61 | 5.11 | 48.63 |
| 6 Wireless | 275 | 91.2 | 13.15 | 4.79 | 35.93 |
Figures 2 and 3 present qualitative and quantitative data from two participants. Participant #1 (in Table 2) generated nearly perfectly consistent MA (Fig. 2). The calendar and time plots revealed just two missed doses over the monitored 280-day period, an adherence level >99 %.
Figure 3 presents calendar and time plots of participant #6 (in Table 2) whose data were variable over a 486-day monitoring period in which 8.8 % of doses were not recorded as having been taken on time. This participant acknowledged using an idiosyncratic system of keeping morning medication out of the MEMS-monitored vial “twisting” the cap at home, but actually taking the medication upon arrival at work. This approach undermined the ability of the system to track actual medication use. It generated data similar to patterns of lower MA. The case illustrates the importance of adhering to EMD protocols as directed to generate reliable, interpretable data. The modification complicated the protocol, confounding understanding of MA, and precluding definite verification of MA.
Participants uniformly found the wireless system preferable to the nonwireless system. All six found the LCD display to be helpful in tracking their most recent doses. For example, one described waking up during the night, worried that she had missed her last dose. She viewed the cap display to confirm that she indeed had taken it. Participants appreciated receiving their personal summary data every few weeks, especially toward the beginning of this pilot. Five participants were “very” and one was “somewhat likely” to recommend the wireless MEMS cap to others.
All participants indicated that it was feasible to ask research participants to use wireless caps for at least 1 year, with most reporting feasibility for longer periods. Three thought that they would be likely to use the wireless MEMS if it were available beyond the study. At their request, they continued using it beyond the first anniversary of their transplant because they found it helpful in maintaining their adherence.
The research nurse’s discussions with participants provided qualitative feedback about their medication taking behaviors that allowed for joint problem solving. By reviewing MEMS feedback together, participants and the nurse could have authentic, focused discussions about missed doses and individuals’ unique medication taking patterns. For example, participants who missed doses when their schedule changed (e.g., on the weekends), or at certain times of the day or week, could develop a plan for addressing this together. Participants often initiated discussions about missed or inconsistent timing of doses after reviewing their record. These discussions impressed the nurse as more collaborative, less defensive, and more productive than conversations of MA with other participants based on the nonwireless MEMS, which lacked immediate feedback.
Participants uniformly indicated that the wireless cap was easy to use. However, two acknowledged some inconvenience due to the MEMS reader requirements for space and to be plugged in. Brief problem solving helped integrate it into users’ routine. The pilot also revealed that caps needed to be open at least 3 s to register events, leading to updated instructions.
Discussion
Technological advances providing enhanced information about medication taking present increasing opportunities to maximize research participants’ and patients’ MA. The more effective medications and the more serious the condition for which they are taken, the more important it is to optimize MA. Wireless EMDs offer unprecedented potential to provide timely feedback and to generate data-driven, problem-solving discussions between research participants and research staff specifically, and between patients and caregivers in general.
Our research group has monitored renal transplant patients’ medication taking for over a decade [9, 45] using MEMS® and is currently conducting studies on the early prediction of NA and on interventions to enhance MA. Wireless technology provides a logistical breakthrough in presenting near real-time MA data and feedback, closing the gap in earlier iterations of EMD technology that required the physical return of caps to be downloaded at clinical visits, or the mailing of caps that had built in delays. In this series of studies, renal transplant recipients were willing to initiate monitoring within a few days of transplant and collectively were monitored for a total of 2289 patient monitored days: 1112 nonwireless MEMS monitored days followed by 1177 wireless MEMS monitored days in the feasibility study.
The cases delineated in Figs. 2 and 3 illustrate the calendar and intuitive graphics that can be used to discuss adherence with EMD users. This feedback loop allows researchers and clinicians to problem solve with patients about missed or late doses so as to maximize the consistency of MA. This is particularly important in transplant recipients to ensure that they are dosed steadily with sufficient immunosuppressant medication to optimally regulate their immune function and prevent rejection of transplanted organs.
These renal transplant recipients accepted the wireless MEM, found it user-friendly, tolerated daily use for months, and were open to longer monitoring. Our earlier research participants have been willing to use the nonwireless MEMS caps for multiple years. The preference expressed for the wireless MEMS caps suggests that many would be anticipated to be willing to monitor their medication usage for longer periods than were monitored in this feasibility study. Furthermore, the cap provides valuable real-time feedback to patients. In our studies with nonwireless caps, participants have been monitored for as long as 5 years [9]; so, it is reasonable to expect that research participants similarly would be willing to be monitored for extended periods with wireless EMDs. There is no known upper limit to monitoring length. The parameters of patients’ willingness to be monitored could be explored in future studies. Extended monitoring is particularly important for longer trials, such as with chronic illnesses and slowly progressing conditions.
The limited sample size and sampling are recognized limitations to this study and precluded meaningful comparisons in adherence between the wireless and nonwireless devices or exploration of effects due to time, learning, or sequence. Individuals who were willing to participate in this pilot were already known to be tolerant of the medication monitoring with the nonwireless methodology. We sought to explore whether such individuals would also tolerate the nonwireless technology. We do not know how well these results would generalize to larger samples or to naïve cap users or less adherent individuals. However, our previous research with NA patients would suggest that wireless EMDs are likely to be tolerated by most patients. We anticipate this to be a ripe area for future research with this evolving technology. Whereas medication monitoring provides robust data about use of EMDs, it does not guarantee that a person has actually taken the medication. The limitations and weaknesses of electronic monitoring have been described elsewhere [46]. The cost of the technology and the logistics of using it and integrating it within clinical protocols may be limiting factors to its adoption for research and in clinical contexts [34].
Although the added complexity inherent in monitoring adherence increases the costs of conducting research, the benefits of being able to certify some threshold of adherence and to target interventions (now possible in close to real time) to enhance MA of participants whose adherence may be problematic, adds both to the integrity of the research enterprise and to the probability that studies will succeed in answering the scientific questions they investigate. In behavioral research, treatment fidelity [47] or implementation fidelity [48] are critical concerns. Such concerns parallel the fidelity to protocol in biomedical research [49] that can potentially be enhanced by using EMDs to monitor MA.
Costs of tracking MA in research may be in part offset by being able to shorten trials and decrease sample size if research participants sustain higher levels of MA [2]. Given the fundamentally behavioral basis of taking medications, psychologists are particularly prepared to collaborate in developing and implementing MA-enhancing programs in research based on their focus on behavior, and expertise in behavioral measurement (which medication monitoring is) and in promoting behavior change.
Timely and consistent MA is a critical aspect of biomedical research. Interest in the use of increasingly sophisticated technology for monitoring MA is not limited to clinical trials. In health care clinical outcomes are increasingly tracked by health care institutions, governments, and payers, and efforts are underway for utilizing outcomes as the basis of payment as part of pay-for-performance [50]. Consistent with the triple aim [51] and health reform [52], as payment hinges increasingly on health outcomes, rather than the volume of service rendered, this technology can be employed with efforts to optimize MA so as to maximize the potential of achieving clinical outcomes, especially for patients exhibiting or at risk for NA.
Moreover, EMDs can be seen in a larger context, namely, the current zeitgeist of interest in quantifying various parameters of human performance, as in The Quantified Self [53], a collaboration of users and developers of self-tracking tools that is dedicated to increasing self-knowledge through metrics. Just as people are electronically tracking other parts of their personal regimens (e.g., minutes of exercise, steps, caloric intake) and health outcomes or progress toward health goals (e.g., weight, blood pressure, pain) through various devices (e.g., fitbit, pedometer), apps, and websites, they now have useful, accurate, timely tools for quantifying their medication usage, providing data for better understanding their behavior so as to be more capable of changing it. EMDs provide feedback consistent with control systems theory [39] and have the potential to enhance health behavior in accord with other health behavior change models such as the theory of planned behavior [37].
Wireless EMDs are valuable new collaborative tools that equip psychologists and other health professionals to provide more direct assistance to patients seeking to optimize MA in research and in clinical contexts. As medications become more powerful and expensive, and as the costs of biomedical research and clinical care mount, it is increasingly important to understand how patients actually take their medications, and to strive to ensure they are taken properly. Effective translation of clinical research results to improve clinical care demands accurate measures of MA in both research and clinical contexts. Wireless EMD technology offers feasible options for researchers and practitioners to accomplish these worthy objectives while providing patients who are striving to manage diverse conditions with the desired, timely feedback that can help them achieve critical health goals. The full impact of this new wireless technology on MA and the management of diverse health conditions will hopefully be explored through future sufficiently powered randomized controlled clinical trials and other translational research incorporating wireless EMDs.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (DK-13083). The authors thank all our subjects for their willingness to participate and also gratefully acknowledge the encouragement and support of Bernard Vrijens, Ph.D., Chief Science Officer at MWV Healthcare who provided the wireless caps and readers, as well as educational materials for their use.
Conflict of interest
All authors declare that they have no conflicts of interest.
Adherence to ethical principles
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Footnotes
Implications
Practice: New wireless medication monitoring technology can be employed to help patients track their own medication adherence and to allow researchers and health professionals to collaborate with them to optimize medication taking.
Policy: The inclusion of more precise and timely medication adherence information in clinical research has the potential to improve research and to provide greater assurance about the accuracy of the findings of clinical investigations.
Research: The use of wireless electronic medication monitoring devices is feasible to audit and may help improve medication adherence within clinical and translational research.
Contributor Information
William N. Robiner, Phone: (612) 624-1479, Email: robin005@umn.edu
Nancy Flaherty, Phone: (612) 626-5080, Email: flahe001@umn.edu.
Thyra A. Fossum, Phone: (612) 624-0513, Email: tafossum@umn.edu
Thomas E. Nevins, Phone: (612) 624-3995, Email: nevin001@umn.edu
References
- 1.Rand CS, Sevick MA. Ethics in adherence promotion and monitoring. Control Clin Trials. 2000;21:S241S–247S. doi: 10.1016/S0197-2456(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 2.Ellis S, Shumaker S, Sieber W, et al. Adherence to pharmacological interventions: current trends and future directions. Control Clin Trials. 2000;21:S218–225. doi: 10.1016/S0197-2456(00)00082-9. [DOI] [PubMed] [Google Scholar]
- 3.Robiner WN. Enhancing adherence in clinical research. Contemp Clin Trials. 2005;26:59–77. doi: 10.1016/j.cct.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Urquhart J. Role of patient compliance in clinical pharmacokinetics: Review of recent research. Clin Pharmacokinet. 1994;27:202–15. doi: 10.2165/00003088-199427030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sabate E, editor. Adherence to long-term therapies: Evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 6.DiMatteo MR. Variations in Patients' Adherence to Medical Recommendations: A Quantitative Review of 50 Years of Research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 7.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:58–73. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 8.Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int. 2005;18:1121–1133. doi: 10.1111/j.1432-2277.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 9.Nevins TE, Kruse L, Skeans MA, et al. The natural history of azathioprine compliance after renal transplantation. Kidney Int. 2001;60:1565–1570. doi: 10.1046/j.1523-1755.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 10.Dobbels, F., & De Geest, S. (2011). How to identify nonadherence to immunosuppressive medication in organ transplant recipients? Current insights and future challenges. Compliance in solid organ transplantation. In J. M. Grinyo, (Ed.), International Transplantation Updates 2011, 1-19.
- 11.Organ Procurement & Transplantation Network (2015, January 17) Retrieved January 17, 2015 from: http://optn.transplant.hrsa.gov/converge/latestData/rptData.asp.
- 12.Constantiner M, Cukor D. Barriers to immunosuppressive medication adherence in high-risk adult renal transplant recipients. Dial Transplant. 2011;40(2):60–66. doi: 10.1002/dat.20536. [DOI] [Google Scholar]
- 13.Hansen R, Seifeldin R, Noe L. Medication adherence in chronic disease: issues in posttransplant immunosuppression. Transplant Proc. 2007;39:1287–1300. doi: 10.1016/j.transproceed.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 14.Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9:2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 15.Azvolinsky A. Mystery around drug adherence still plagues medical literature. Nat Med. 2014;20:455. doi: 10.1038/nm0514-455. [DOI] [PubMed] [Google Scholar]
- 16.Blaschke TF, Osterberg L, Vrijens B, et al. Adherence to medications: Insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 17.Hughes DA, Bagust A, Haycox A, et al. The impact of non-compliance on the cost-effectiveness of pharmaceuticals: A review of the literature. Health Econ. 2001;10:601–15. doi: 10.1002/hec.609. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Peluso MJ, Gross CP, et al. Adherence reporting in randomized controlled trials. Clin Trials. 2014;11:195–204. doi: 10.1177/1740774513512565. [DOI] [PubMed] [Google Scholar]
- 19.Lasagna L, Hutt PB. Health care, research, and regulatory impact of noncompliance. In: Cramer JA, Spilker B, editors. Patient compliance in medical practice and clinical trials. New York: Raven Press; 1991. pp. 393–403. [Google Scholar]
- 20.Cramer JA, Spilker B, editors. Patient compliance in medical practice and clinical trials. New York: Raven Press; 1991. [Google Scholar]
- 21.Davis CE. Prerandomization compliance screening: a statistician’s view. In: Shumaker SA, Schron EB, Ockene JK, McBee WL, editors. The handbook of health behavior change. 2. New York: Springer; 1998. pp. 485–90. [Google Scholar]
- 22.Probstfield JL, Russell ML, Henske JC, et al. A successful program for returning dropouts to a clinical trial. Am J Med. 1986;80:777–84. doi: 10.1016/0002-9343(86)90615-7. [DOI] [PubMed] [Google Scholar]
- 23.Spilker B, Cramer JA. Patient recruitment in clinical trials. New York: Raven Press; 1992. [Google Scholar]
- 24.Williams AB, Amico KR, Bova C, et al. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2013;14:284–297. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sackett, D. L., Straus, S. E., Richardson, W. S., Rosenberg, W., & Haynes, R. B. (2000). Evidence-based medicine: how to practice and teach EBM. Edinburgh: Churchill Livingstone.
- 26.Riekert KA, Rand CS. Electronic monitoring of medication adherence: When is high-tech best? J Clin Psychol Med Settings. 2002;9:25–34. doi: 10.1023/A:1014131928789. [DOI] [Google Scholar]
- 27.Green CA. What can patient health education coordinators learn from ten years of compliance research? Patient Educ Couns. 1987;10:167–174. doi: 10.1016/0738-3991(87)90096-6. [DOI] [PubMed] [Google Scholar]
- 28.Matsui D. Medication adherence issues in patients: Focus on cost. Clin Audit. 2013;5:33–42. doi: 10.2147/CA.S30125. [DOI] [Google Scholar]
- 29.Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs. 1995;49:597–605. doi: 10.2165/00003495-199549030-00001. [DOI] [PubMed] [Google Scholar]
- 30.Zeller A, Ramseier E, Teagtmeyer A, et al. Patients' self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens Res. 2008;31:2037–2043. doi: 10.1291/hypres.31.2037. [DOI] [PubMed] [Google Scholar]
- 31.van den Boogaard J, Lyimo RA, Boeree MJ, et al. Electronic monitoring of treatment adherence and validation of alternative adherence measures in tuberculosis patients: a pilot study. Bull World Health Organ. 2011;89:632–639. doi: 10.2471/BLT.11.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denhaerynck K, Schäfer-Keller P, Young J, et al. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook P, Schmiege S, McClean M, et al. Practical and analytic issues in the electronic assessment of adherence. West J Nurs Res. 2012;34:598–620. doi: 10.1177/0193945911427153. [DOI] [PubMed] [Google Scholar]
- 34.Ingerski LM, Hente EA, Modi AC, et al. Electronic Measurement of medication adherence in pediatric chronic illness: A review of measures. J Pediatr. 2011;159:528–534. doi: 10.1016/j.jpeds.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MWV (2015). Adherence peer reviewed publications. Retrieved January 17, 2015 from: http://www.iadherence.org/www/publication.adx.
- 36.AARDEX (2004). Bibliography of scientific papers using the medication event monitoring system (MEMS®). Retrieved June 28, 2014 from http://helpdesk.aardexgroup.com/downloads/EM%20bibliography%2028feb04.pdf.
- 37.Wu J-R, Corley DJ, Lennie TA, et al. Effect of a medication-taking behavior feedback, theory-based intervention on outcomes in patients with heart failure. J Card Fail. 2012;18:1–9. doi: 10.1016/j.cardfail.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haberer JE, Robbins GK, Ybarra M, et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav. 2012;16:375–382. doi: 10.1007/s10461-011-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carver CS, Scheier MF. Control processes and self-organization as complementary principles underlying behavior. Personal Soc Psychol Rev. 2002;6:304–315. doi: 10.1207/S15327957PSPR0604_05. [DOI] [Google Scholar]
- 40.Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: A perceptual-cognitive approach. Psychol Health. 1998;13:717–733. doi: 10.1080/08870449808407425. [DOI] [Google Scholar]
- 41.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55:68–78. doi: 10.1037/0003-066X.55.1.68. [DOI] [PubMed] [Google Scholar]
- 42.Ryan, R. M., Patrick, H., Deci, E. L., & Williams, G. C. Facilitating health behaviour change and its maintenance: Interventions based on Self-Determination Theory. Eur Health Psychol. 2008; 10, 2-5. 26.
- 43.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 44.Goetz, T. (201). The decision tree: How to make better choices and take control of your health. NY: Rodale Press.
- 45.Nevins TE, Robiner WN, Thomas W. Predictive patterns of early medication adherence in renal transplantation. Transplantation. 2014;98:878–84. doi: 10.1097/TP.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitolins MZ, Rand CS, Rapp SR, et al. Measuring adherence to behavioral and medical interventions. Control Clin Trials. 2000;21:188S–194S. doi: 10.1016/S0197-2456(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 47.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 48.Carroll C, Patterson M, Wood S, et al. Implement Sci. 2007;2:40–48. doi: 10.1186/1748-5908-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarin DA, Tse T. Trust but verify: Trial registration and determining fidelity to the protocol. Ann Intern Med. 2013;159:65–67. doi: 10.7326/0003-4819-159-1-201307020-00011. [DOI] [PubMed] [Google Scholar]
- 50.Werner R, Kolstad JT, Stuart EA, et al. The effect of pay-for-performance in hospitals: Lessons for quality improvement. Health Aff. 2011;30:690–698. doi: 10.1377/hlthaff.2010.1277. [DOI] [PubMed] [Google Scholar]
- 51.Berwick DM, Nolan TW, Whittington J. The triple aim: Care, health, and cost. Health Aff. 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 52.Patient Protection and Affordable Care Act, Pub. L. No: 111–148, 124 Stat. 119. (2010). Retrieved from http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
- 53.The Quantified Self (2014) Retrieved October 12, 2014 from: http://quantifiedself.com/about/.