Sir,
Burkholderia pseudomallei is the cause of melioidosis, a serious infection associated with a mortality rate of 14–43% [1]. Recommended antimicrobial therapy is ≥10 days of parenteral ceftazidime or a carbapenem, followed by oral trimethoprim/sulfamethoxazole (SXT; co-trimoxazole) to complete up to 20 weeks of therapy [2]. A previous evaluation of 1976 clinical B. pseudomallei isolated from patients in northeast Thailand between 1992 and 2003 reported that SXT resistance was detected in 13% of isolates [3]. Subsequent studies have reported much lower rates of SXT resistance for isolates from Laos (0.8%), Australia (0.4%) and Cambodia (0%) [4]. Here we report the results of a re-evaluation of SXT resistance in Thailand. Second-line oral treatment in patients infected with SXT-resistant B. pseudomallei or in whom SXT is contraindicated is amoxicillin/clavulanic acid (AMC) [1], thus we also evaluated the susceptibility of SXT-resistant isolates to AMC and doxycycline (DOX), which is used less frequently as an alternative to SXT.
Susceptibility to SXT was determined by Etest (bioMérieux, Marcy-l’Étoile, France) [3], with reading of the minimum inhibitory concentration (MIC) at the 80% inhibition point. Interpretative standards for the Etest were based on Clinical and Laboratory Standards Institute (CLSI) guidelines for broth microdilution, which classifies SXT MICs of ≤2/38 mg/L as susceptible and ≥4/76 mg/L as resistant [5]. Escherichia coli ATCC 25922 was used as the control. For SXT-resistant isolates, the Etest was used to define susceptibility to trimethoprim (TMP) alone and sulfamethoxazole (SMX) alone. Susceptibility testing to AMC and DOX was also performed using the Etest, in which the MIC was read at the point of no visible growth. Escherichia coli ATCC 35218 was used as a control for AMC, and E. coli ATCC 25922 was used as a control for TMP, SMX and DOX.
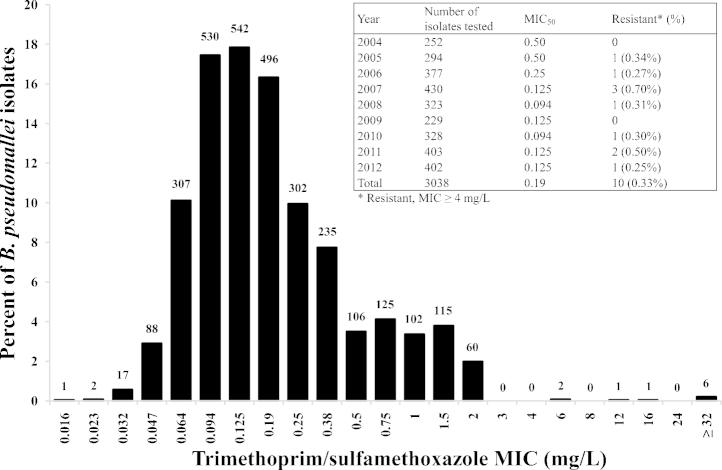
Two isolate collections were evaluated. The first was drawn from a retrospective study of 3270 patients with culture-proven melioidosis at Sappasithiprasong Hospital in northeast Thailand between 2004 and 2012. A single isolate was used per patient (the first positive culture). All isolates had been stored at −80 °C and subculture recovered isolates from 3038 patients. The Etest SXT MIC ranged from 0.016 mg/L to ≥32 mg/L [MIC50 (concentration that inhibits 50% of bacterial isolates) = 0.19 mg/L and MIC90 (concentration that inhibits 90% of bacterial isolates) = 0.75 mg/L; interquartile range 0.094–0.25 mg/L] (Fig. 1). Ten isolates (0.33%) were resistant to SXT, with an annual resistance rate ranging from 0% to 0.7%. As this is considerably lower than that reported by us previously for B. pseudomallei isolated from patients presenting to the same hospital between 1992 and 2003 [3], we re-evaluated this original collection. Previously, 258/1976 isolates were assigned as SXT-resistant based on the Etest [3], of which 255 could be recovered from frozen stocks. Etest could only confirm SXT resistance in 13 (5.1%) of these 255 isolates (Supplementary Table S1). The 23 SXT-resistant isolates from both collections were resistant to TMP and SMX when tested as separate agents (Supplementary Table S1). All SXT-resistant isolates were susceptible to AMC, but only 21 isolates (91%) were susceptible to DOX.
Fig. 1.
Trimethoprim/sulfamethoxazole minimum inhibitory concentrations (MICs) for Burkholderia pseudomallei from 3038 patients with primary melioidosis presenting to a hospital in northeast Thailand between 2004 and 2012. MICs were determined using the Etest. The numbers above each column represent the number of isolates with the corresponding MIC. The table shows the total and annual number of B. pseudomallei isolates tested, the MIC50 (concentration that inhibits 50% of bacterial isolates) and the percentage resistant.
The CLSI recommends the broth microdilution method as the standard method for MIC testing of B. pseudomallei [5]. This is impractical for such a large study collection and was therefore used to verify Etest results for a subset of isolates. These were all 13 isolates from the 1992–2003 collection that were classified as resistant in both studies as well as 15 randomly selected isolates from the 1992–2003 collection with discrepant results between the two studies. Escherichia coli ATCC 25922 was used as the control. This demonstrated complete concordance of results between broth dilution and Etest performed in this study, confirming that the previous study had overestimated resistance. The most likely explanation for the erroneous results in the original study is error in reading the 80% inhibition point. This is inherently subjective and a minor difference in the interpretation of MIC results that are close to the breakpoint can lead to false classification as resistance. The majority (68%) of MICs for isolates that were erroneously defined as resistant in the previous study were 3 mg/L or 4 mg/L, which is consistent with a minor upshift in the MIC value but a large error in susceptibility classification [3]. Inhibition zones frequently have diffuse edges, and reading against a black background aided technical observation in this study.
Our finding that 99.7% of clinical B. pseudomallei isolates were susceptible to SXT is comparable with rates reported from Laos (99.2%), Australia (99.6%) and Cambodia (100%) [4], which indicate that primary SXT resistance in B. pseudomallei is uncommon. Our study also confirmed that SXT-resistant B. pseudomallei were susceptible to AMC, the current second-line drug of choice.
Acknowledgments
The authors thank the staff at Sappasithiprasong Hospital (Thailand), Sarunporn Tandhavanant and the Mahidol–Oxford Tropical Medicine Research Unit (MORU) for their assistance. The authors also thank David Dance for comments.
Funding: This work was supported by the Wellcome Trust [grant 087769/Z/08/Z to NC] and by an ICTM grant of the Faculty of Tropical Medicine, Mahidol University (Bangkok, Thailand).
Competing interests: None declared.
Ethical approval: Not required.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijantimicag.2015.01.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Minimum inhibitory concentrations (MICs) to a range of antimicrobial drugs for 23 SXT-resistant Burkholderia pseudomallei.
References
- 1.Wiersinga W.J., Currie B.J., Peacock S.J. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 2.Chetchotisakd P., Chierakul W., Chaowagul W., Anunnatsiri S., Phimda K., Mootsikapun P. Trimethoprim–sulfamethoxazole versus trimethoprim–sulfamethoxazole plus doxycycline as oral eradicative treatment for melioidosis (MERTH): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet. 2014;383:807–814. doi: 10.1016/S0140-6736(13)61951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wuthiekanun V., Cheng A.C., Chierakul W., Amornchai P., Limmathurotsakul D., Chaowagul W. Trimethoprim/sulfamethoxazole resistance in clinical isolates of Burkholderia pseudomallei. J Antimicrob Chemother. 2005;55:1029–1031. doi: 10.1093/jac/dki151. [DOI] [PubMed] [Google Scholar]
- 4.Dance D.A., Davong V., Soeng S., Phetsouvanh R., Newton P.N., Turner P. Trimethoprim/sulfamethoxazole resistance in Burkholderia pseudomallei. Int J Antimicrob Agents. 2014;44:368–369. doi: 10.1016/j.ijantimicag.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute . CLSI; Wayne, PA: 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline—second edition. Document M45-A2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Minimum inhibitory concentrations (MICs) to a range of antimicrobial drugs for 23 SXT-resistant Burkholderia pseudomallei.