Abstract
Background
CD4 counts guide antiretroviral therapy (ART) initiation and prophylaxis for opportunistic infections. It is unclear whether normal CD4 counts translate to normalized immune responses among ART-treated adults. We compared antigen-specific CD4 T-cell immune responses among ART-treated adults with CD4 ≥ 500 cells/μl, optimal immune responders (O-IR), and their age-matched healthy HIV-negative counterparts.
Methods
In a sample-based case–control study, cryopreserved peripheral blood mononuclear cells from 15 O-IR after 7 years of ART and 15 healthy controls, were analyzed for CD4+ T-cell proliferation using CFSE dye and cytokine production.
Results
CD4 T-cell proliferation, upon stimulation with PPD and pneumococcal polysaccharide antigen, was lower among O-IR relative to HIV-negative controls; p = 0.016 and p = 0.016 respectively. CD4 T-cell production of IL-2 was lower among O-IR relative to HIV-negative control p = 0.002. CD4 T-cell proliferation upon stimulation with SEB and CMV antigens was similar among O-IR and HIV-negative controls p = 0.971 and p = 0.480, respectively, and so was IL-4 and IFN γ production; p = 0.528 and p = 0.892, respectively.
Conclusion
Seven years of suppressive ART caused partial CD4 T-cell function recovery in an African HIV treatment cohort, despite restoration of CD4 T-cell counts to levels ≥ 500 cells/μl. The role innate immunity in the recovery of immune function during long-term ART should be investigated to guide decisions on continued prophylaxis against opportunistic infections.
Keywords: CD4 T-cell proliferation, Cytokine production, ART, Immune recovery, Uganda, Sub-Saharan Africa
1. Background
CD4 T-cells are important cells of the immune system that trigger other cells of the immune system through cytokine production in the hosts’ fight against infections [1]. In the absence of good CD4 T-cell numbers and a robust CD4 T-cell immune response during HIV infection, pathogen clearance is made difficult and hosts remain susceptible to many opportunistic infections. The hallmark of HIV infection is a gradual drop in CD4+ T-cell counts, an acquired immune deficiency syndrome (AIDS), leading to increased risk of AIDS-associated illnesses and opportunistic infections [2]. Not only does HIV-1 infection decrease the CD4+ T-cell count, but it also distorts the repertoire of CD4+ T-cell immune responses causing considerable variation in antigen specific immune responses [3,4]. Antiretroviral therapy (ART) causes a rapid reduction in HIV-RNA viral load and restoration of CD4+ T-cell numbers for most patients [5,6]. In addition, ART causes a reduction in the amounts of circulating inflammatory cytokines and chemokines hitherto elevated during infection [7]. Consequently, ART prevents the onset of symptoms and progression to AIDS, translating into improved health and prolonged survival of people infected with HIV. Full restoration of circulating CD4 T-cell numbers and function occurs in a minority of patients, despite several years of sustained viral suppression; phenotypic abnormalities and functional defects in lymphocyte subsets often persist [4,8,9]. Whereas some patients restore circulating CD4 T-cell numbers to levels above 500 cells/μl, similar to HIV-negative adults [10,11], it remains unclear whether CD4 T-cell responses recover to levels similar to HIV-negative individuals. Consequently, in sub-Saharan Africa, ART-treated HIV-infected adults remain on cotrimoxazole prophylaxis for opportunistic infections despite normal absolute CD4 counts.
Whereas CD4 count is a primary measure of immune status during HIV-infection, and guides decisions to initiate ART and prophylaxis for opportunistic infections [12], normal absolute CD4 counts may not translate to normal immune responses to invading pathogens among people living with HIV/AIDS [7,13]. This study determined CD4 T-cell proliferation and cytokine production among ART-treated adults with CD4 counts ≥ 500 cells/μl after seven years of suppressive ART (O-IR). CD4 T-cell immune responses to common antigens such as Staphylococcus aureus endotoxin (SEB), pneumococcal polysaccharide, purified protein derivative (PPD) and cytomegalovirus (CMV) were compared among ART-treated O-IR and age-matched healthy HIV-negative controls. Results from this study inform the development of clinical studies to determine clinical benefits of long-term ART including the feasibility of discontinuation of cotrimoxazole prophylaxis and immune responses to specific vaccines among HIV-infected adults receiving life-long ART.
2. Methods
2.1. Study design and participants
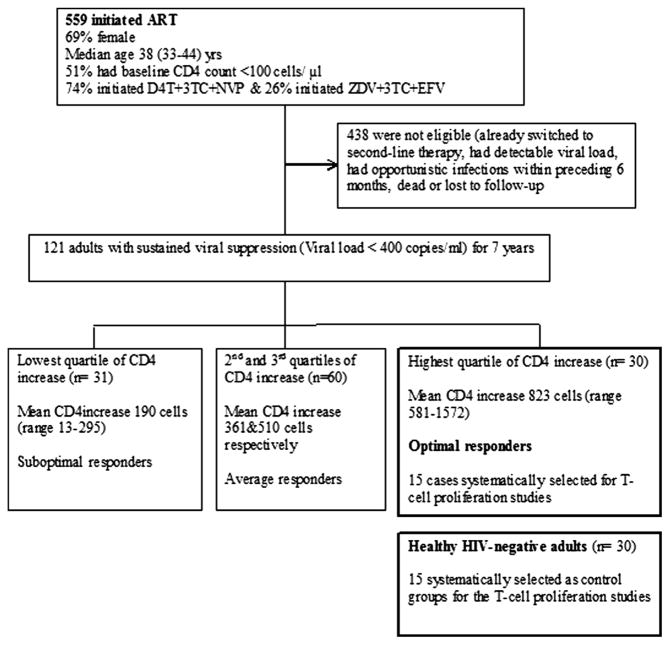
This study was a sample-based case–control study of ART-treated O-IR; individuals in the highest quartile of CD4 count recovery after seven years of suppressive first-line ART within the Infectious Diseases Institute (IDI) HIV treatment research cohort (cases) with mean CD4 increase of 823 (range 581–1572) cells/μl and age-matched healthy HIV-negative controls within the same community (Fig. 1). Between April 2004 and April 2005, a total of 559 consecutive ART-naïve HIV-infected patients were initiated on HAART and enrolled into the IDI prospective observational research cohort, previously described by Kamya et al. [14]. Patients were initiated on first-line ART at CD4 counts ≤ 200 cells/μl according to Ugandan guidelines for ART initiation at the time. Drugs were provided through the Global Fund (a generic combined formulation of stavudine [d4T], lamivudine [3TC], and nevirapine [NVP]) and the US President’s Emergency Plan for AIDS Relief (a combined formulation of zidovudine [ZDV] and 3TC plus efavirenz [EFZ] or NVP). Patients with toxicity to ZDV were changed to tenofovir [TDF]. All patients received cotrimoxazole (or dapsone) prophylaxis according to the national policy to provide cotrimoxazole to all people living with HIV. Adherence to ART was encouraged by at least 3 individual group counseling sessions. Patients were reviewed monthly by the study physicians that evaluated among others, adherence to medication, toxicities and acute infections. HIV RNA viral loads, complete blood counts and CD4 lymphocyte counts were measured 6 monthly. CD4 increases (difference pre-ART CD4 count and CD4 count at seven years) were grouped into four quartiles and individuals within the highest quartile were considered as optimal immune responders (O-IR) as shown in Fig. 1. Healthy HIV-negative controls, defined as age-matched (±5 years the age of the cases), were consecutively selected from the HIV-negative patient register of the Mulago hospital routine HIV testing program. All participants provided written informed consent for use of their stored biological samples for further immunological studies. This study was approved by the School of Biomedical Sciences Institutional review board with final approval by the Uganda National Council for Science and Technology. The assays were conducted at the translational laboratory at Makerere University College of Health Sciences. Flow cytometry assays were conducted using an eight-color FACS Canto II (BD Biosciences) flow cytometer.
Fig. 1.
Cohort profile of the Infectious Diseases Institute HIV treatment research cohort after seven years of therapy.
2.2. Carboxyfluoresceinsuccinimidyl ester (CFSE) staining and stimulation for CD4 T-cell proliferation assays
This study utilized cryopreserved peripheral blood mono-nuclear cells (PBMC) that were previously collected using the Ficoll-Paque method and stored in liquid nitrogen (−196 °C). PBMC were thawed, and washed and rested in 5 ml s of R10 medium (RPMI media containing 10%, v/v fetal calf serum) for 4 h, after which they were washed and viability determined using trypan blue dye. Minimum viability was 80%. Proliferation assays were used to assess antigen-specific immune responses among O-IR and the HIV-negative individuals. CD4+ T-cell proliferation was measured using carboxyfluoresceinsuccinimidyl ester (CFSE) on day 5 of culture after stimulation with peptide antigens including purified protein derivative (PPD), Statens Serum Institute, Cytomegalovirus (CMV) antigen from the Native Antigen company Oxford shire, pneumococcal polysaccharide antigen (Staten Serum Institute, 76852) and staphylococcal enterotoxin B (SEB), Sigma–Aldrich, St. Louis, MO, USA. The final concentration of the antigens used was 10 μg/ml for PPD, 10 μg/ml for pneumococcal polysaccharide, 5 μg/ml of SEB antigen, 2 μg/ml of CMV antigen and 5 μg/ml of PHA for every 1 million PBMCs per well. PHA was used as a positive control and the unstimulated wells as negative controls. Brefeldin A (3 μg/ml) was added on culture day 4 to block the secretory pathways of cells and trap secreted cytokines intracellular. On culture day 5, the cells were harvested for immunophenotyping.
2.3. Immunophenotyping
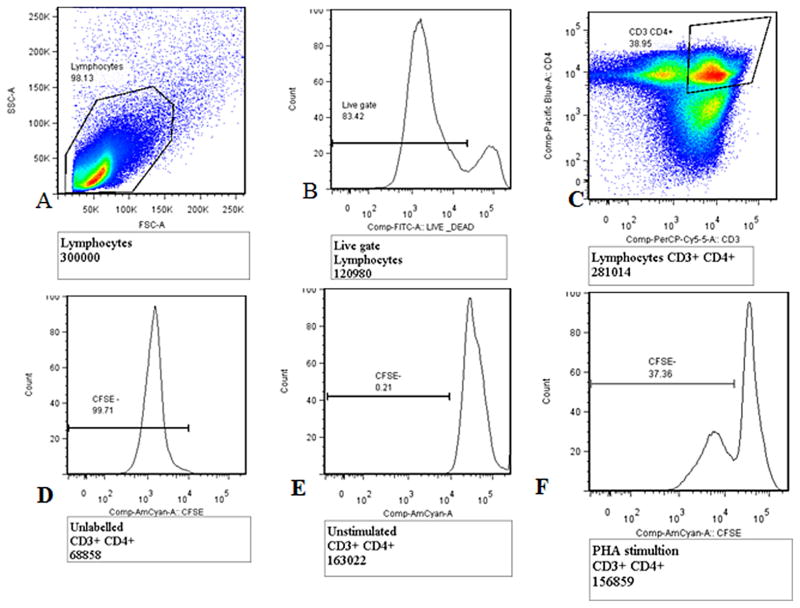
The cells were re-suspended in FACs buffer (5% FBS, 0.01% sodium azide and 1X PBS) and were ready for staining. Cell surface staining was done using live/dead marker-FITC, anti-CD3 PerCP cy 5.5 and anti-CD4 Pacific blue (BD Biosciences San Jose, CA). Cells were incubated at 4 °C in the dark for 30 min and then washed with FACs buffer. Stained cells were acquired on a FACS Canto II flow cytometer (BD Biosciences, San Jose, CA). At least 300,000 events in the lymphocyte gate were acquired. The flow cytometry data analysis was performed using FlowJo software (TreeStar, Version X). Only data with a minimum of 300,000 acquired events of lymphocytes was analyzed. CD4 T-cell proliferation was analyzed using the gating strategy in Fig. 2.
Fig. 2.
Gating strategy for CD4 T-cell proliferation using carboxy fluorescein succinimidyl ester (CFSE) dye. (A) Total lymphocyte gate, (B) live/dead lymphocytes, (C) the CD3+ CD4+ T-cell gate, (D) unlabeled CD3+ CD4+ T-cells, (E) unstimulated CD3+ CD4+ T-cells (CFSE negative control) and (F) CD4 T-cell proliferation (CD3+ CD4+ CFSE−). T-cells, with PHA stimulation as a positive control.
2.4. Measurement of cytokine production
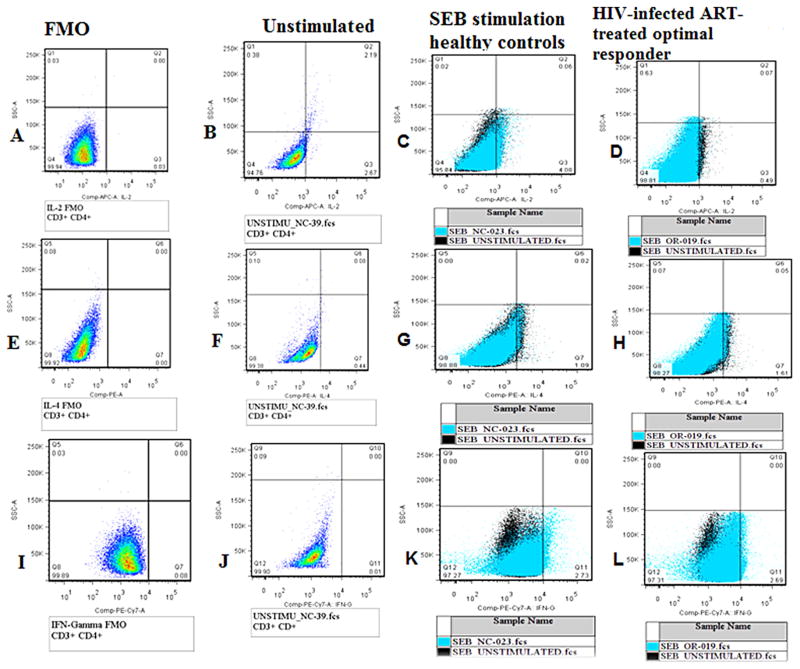
Intracellular staining of cells was done to measure interleukin-2 (IL-2 APC), interferon-gamma (IFN-γ PE.cy 7) and interleukin-4 (IL-4 PE) – BD Biosciences San Jose, CA. Flow cytometry techniques were used to measure the quantities of secreted cytokines in each individual cell. Cells were fixed and permeabilised to allow specific anti-cytokine fluorescence antibody conjugates to enter into the cell. Cells were washed by adding 500 μl of FACs buffer and centrifuged at 1800 rpm for 3 min. Fluorochrome-conjugated antibodies were added and incubated for 30 min at 4 °C in the dark. Cells were washed with FACs buffer and acquired using a Fac-sCanto II flowcytometer. The gating was standardized and set using fluorescence minus one control for IL-2 APC, IFN-γ PE.cy 7 and IL-4 PE (Fig. 3).
Fig. 3.
Gating strategy cytokine production. (A–D) IL2 production (A – FMO, B – unstimulated, C – healthy control and D – ART-treated optimal immune responder(O-IR)), (E–H) IL4 production (E – FMO, F – unstimulated, G – healthy control and H – ART-treated optimal immune responder), (I–L) interferon gamma production (I – FMO, J – unstimulated, K – healthy control and L – ART-treated optimal immune responder). Analysis of cytokine production IL-2, IL-4 and IFN-gamma was determined after subtraction of the background (unstimulated sample – black color) from the stimulated sample (blue color) as shown in C, D, G, H, K, and L.
2.5. Statistical analysis
The flow cytometry data analysis was performed using FlowJo software (TreeStar, Version X) and transferred to STATA version 12.0 and Graph Pad Prism 6.0 for analysis. Demographic and clinical characteristics of participants were presented using frequency tables. Medians for specific immune responses (CD4 T-cell proliferation and cytokine production) were measured and compared between optimal immune responders and HIV-negative individuals using Mann–Whitney U-test for non-parametric tests. Level of significance was measured by p values ≤ 0.05
2.6. Analysis for CD4 T-cell proliferation
CD4+ T-cell proliferation was measured as the proportion of CFSE negative cells (CD3+ CD4+ CFSE−) on day 5 for stimulation with antigens SEB, PPD, CMV and pneumococcal polysaccharide, antigens as shown by the gating strategy in Fig. 2. Lymphocytes were gated off the forward scatter side scatter plot, CD3+ CD4+ T-cells were gated off the lymphocyte gate and CFSE negative cells (due to dye dilution) were used to measure proliferation. Unlabeled samples were used to set cut-offs for CFSE-negative (CFSE−) T-cells and unstimulated CFSE-labeled samples were used to set cut-offs for CFSE-positive (CFSE+) T-cells (Fig. 2).
2.7. Analysis of cytokine production
From the lymphocyte and CD3+ CD4+ gates shown in Fig. 2 above, IL-2, IL-4 and IFN-γ gamma production by CD4 T-cells was measured by the proportions of CD3+ CD4+ IL-2+, CD3+ CD4+ IL-4+ and CD3+ CD4+ IFN-γ+ cells upon stimulation with SEB antigen. Cytokine production cut-offs were determined using fluorescence minus one controls for IL-2 APC, IFN-γ PE.cy 7 and IL-4 PE. Individual background cytokine production was controlled for by using the unstimulated well as a negative control. Cytokine production upon stimulation by SEB, was determined by subtraction of background cytokine production (unstimulated well) from the stimulated well for each of the samples (Fig. 3).
3. Results
3.1. Participants characteristics
Optimal immune responders (with current CD4 counts ≥ 500 cells/μl) had initiated ART at a median CD4 count of 97 [interquartile range (IQR) 11–158], median age was 40 (IQR 38–46) years and 26(87%) were female. Sixty-three percent (19/30) of healthy HIV-negative controls were female and their median age was 36 (IQR 33–42) years (Table 1).
Table 1.
Characteristics of optimal immune responders with sustained viral load after seven years of antiretroviral therapy (ART) and healthy HIV negative adults.
| Patient characteristics | ART-treated optimal respondersa | Healthy HIV-negative controls |
|---|---|---|
| Age in years (median) | 40 (38, 46) | 36 (33, 42) |
| Female gender n (%) | 26 (87) | 19 (63) |
| Baseline CD4 count cells/μl; median (IQR) | 97 (11, 158) | N/A |
| Current CD4 cells/μl median (IQR) | 607 (759, 996) | N/A |
| BMI; median (IQR) | 23 (20,25) | 26 (22, 30) |
| Family history of | 6.7 | 7.9 |
| hypertension (%) | ||
| Family history of diabetes | 3.3 | 3.2 |
| Febrile illness | 0 | 0 |
| Current regimen | ||
| ZDV-3TC-NVP (%) | 56.7 | N/A |
| ZDV-3TC-EFV (%) | 30.0 | N/A |
| TDF-3TC-EFV (%) | 3.3 | N/A |
All HAART-treated adults had sustained viral suppression for seven years. IQR, interquartile range, BMI, body mass index, ZDV, zidovudine, 3TC, lamivudine, EFV, efavirenz, TDF, tenofovir.
3.2. CD4 T-cell proliferation among ART-treated optimal immune responders (O-IR)
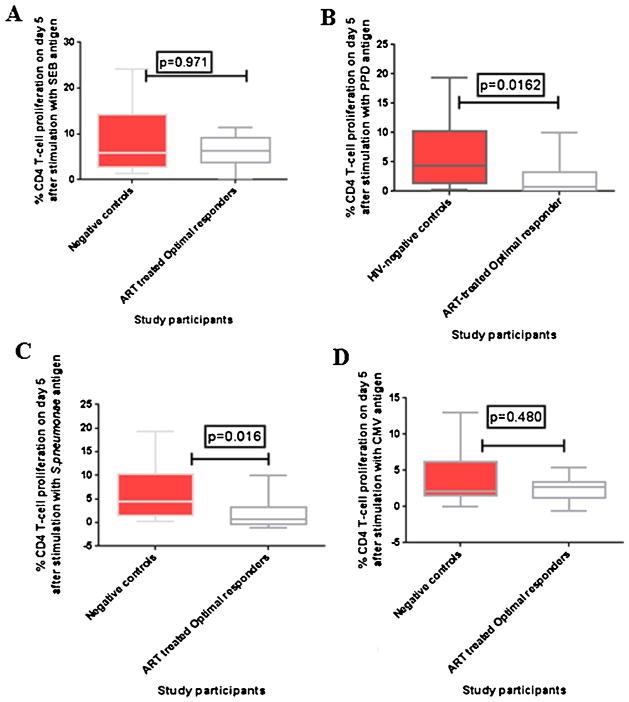
Upon stimulation with PPD and pneumococcal polysaccha-ride antigens, CD4+ T-cell proliferation was significantly lower among HIV-infected ART-treated optimal immune responders than healthy HIV-negative controls; p = 0.0162 and p = 0.0164 respectively. CD4+ T-cell proliferation upon stimulation with SEB antigen, was slightly lower in the HIV-infected ART-treated O-IR than healthy HIV-negative controls although the difference was not statistically significant; p = 0.971. Similarly, CD4+ T-cell proliferation upon stimulation with CMV was lower among ART-treated O-IR than healthy HIV-negative controls, although the difference was not statistically significant, p = 0.480 (Fig. 4 and Table 2). There were variations in the median % proliferation levels of the two groups with O-IR ranging from (2.7 to 11.4) for SEB, (0.6 to 11.6) for PPD, (0 to 10.0) for pneumococcal polysaccharide antigen and (0 to 5.4) for CMV, and HIV-negative controls ranging from (1.4 to 24.2) for SEB, (0.3 to 19.4) for PPD, (0.26 to 19.4) for pneumococcal polysaccharide antigen and (13.0) for CMV (Table 2).
Fig. 4.
CD4 T-cell proliferation among ART-treated optimal responders and age-matched healthy HIV-negative adults, on day 5 of stimulation. (A–D) Percentages of proliferated CD4 T-cells on day 5 after stimulation with SEB, PPD, pneumococcal polysaccharide and CMV antigens respectively. The boxes and whiskers represent medians, 5th and 95th percentiles. The Mann–Whitney U-test for non-parametric tests was used to compare ART-treated optimal responders and healthy controls, with statistical significance at p values ≤ 0.05.
Table 2.
CD4 T-cell proliferation and cytokine production among ART-treated optimal responders and healthy HIV-negative adults after stimulation with SEB, PPD, CMV antigens and pneumococcal polysaccharide type 2.
| Parameter | ART-treated optimal responders | Healthy-HIV-negative people | p-Value |
|---|---|---|---|
| CD4 T-cell proliferation | |||
| SEB antigen, median % | 6.32 | 5.84 | 0.971 |
| PPD antigen, median % | 0.74 | 4.430 | 0.016 |
| Pneumococcal polysaccharide antigen | 0.74 | 4.43 | 0.016 |
| CMV antigen, median % | 2.650 | 2.050 | 0.480 |
| Cytokine production after stimulation with SEB antigen | |||
| IL-2, median % | 0 | 0.74 | 0.0002 |
| IL-4, median % | 2.50 | 2.57 | 0.528 |
| IFN-gamma, median % | 2.15 | 3.72 | 0.891 |
ART, antiretroviral therapy, SEB, Staphylococcal enterotoxin B, PPD, purified protein derivative, CMV, cytomegalovirus, IL-2, interleukin-2, IL-4, Interleukin-4, IFN-γ, interferon gamma.
3.3. CD4 T-cell cytokine production among O-IR
We measured CD4 T-cell cytokine production among ART-treated O-IR and healthy HIV-negative controls. In general, cytokine production was lower among ART-treated O-IR than healthy HIV-negative controls upon stimulation with SEB antigen. CD4+ IL-2 cytokine production was significantly lower among ART-treated O-IR than healthy HIV-negative controls, p = 0.002. However, IL-4 and IFN gamma production were comparable among ART-treated O-IR and healthy HIV-negative controls, p = 0.528 and p = 0. 892, respectively (Fig. 5). There were variations in cytokine production within each of the two groups with medians of O-IR ranging from (0.0 to 1.2) for IL-2, (0.0 to 11.1) for IL-4 and (0.53 to 14.0) for IFN-γ and ranging from (0.0 to 3.0) for IL-2,(0.0 to 11.2) for IL-4 and (0.6 to 24.2) for IFN-γ among healthy controls (Table 2).
Fig. 5.
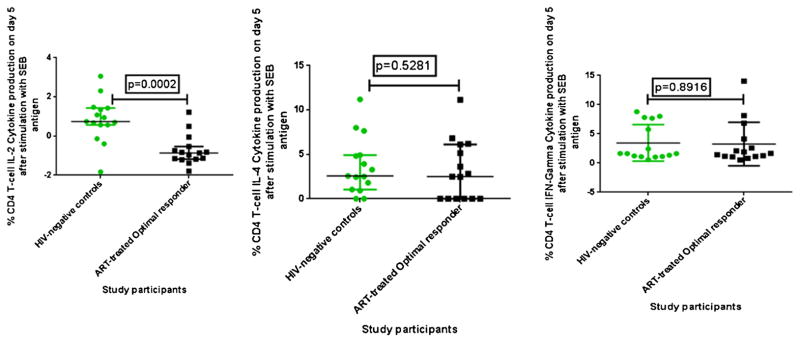
Cytokine production after day 5 of stimulation with SEB among ART-treated optimal immune responders and age-matched healthy HIV-negative individuals. (A–C) Percentages of CD4 T-cells producing IL-2, IL-4 and interferon (IFN)-gamma cytokines on day 5 respectively.
4. Discussion
4.1. Low CD4 T-cell proliferation after 7 years of therapy
We found that ART-treated optimal immune responders (O-IR), with CD4 counts ≥ 500 cells/μl (from a nadir below 200 cells/μl), had lower CD4+ T-cell immune responses to pneumococcal polysaccharide antigen and PPD relative to their healthy HIV-negative counterparts. Our findings are consistent with previous reports that despite sustained HIV-RNA viral suppression and restoration of circulating CD4+ T-cell numbers to levels above 450 cells/μl, specific immune responses among ART-treated adults remain dependent upon the pre-ART CD4 counts [15]. Functional defects in pneumococcal-specific T-cell memory and regulation were previously reported during the course of HIV disease [16]. Our study participants initiated ART at advanced stages of HIV disease which makes complete restoration of their immune responses very difficult. ART initiation during advanced HIV disease has been associated with incomplete restoration of other immune responses, due to several factors including persistent immune activation, microbial translocation, co-infections and immunosenescence [17]. For example, plasmacytoid dendritic cells and natural killer (NK) cells were not completely reconstituted after one year of effective antiretroviral therapy [17]. We previously demonstrated incomplete restoration of the NK cell repertoire after four years of suppressive ART within our HIV treatment research cohort [9]. Although we did not evaluate innate immune function recovery in this study, it is possible that poor recovery of innate immune function components such as dendritic cells and macrophages (required for antigen presentation) could affect the adaptive T-cell responses. We therefore recommend comprehensive assessment of innate functional recovery among ART-treated adults to ensure appropriate host immune responses to invading pathogens. Our study findings imply that despite sustained viral suppression and restoration of CD4 counts to normal levels [18], ART-treated individual may remain more susceptible to common infections relative to their HIV-negative counterparts.
In addition, we found that CD4 IL-2 cytokine production was lower among ART-treated O-IR than healthy HIV negative controls. This finding is consistent with the low CD4+ T cell proliferation, observed using the CFSE dye experiments, given that IL-2 is necessary for CD4+ T-cell proliferation. Our findings are consistent with previous reports that IL-2 production was lower among ART-treated individuals than healthy controls after three years of therapy in a European cohort [19]. Our cohort initiated ART at CD4 < 200 cells/μl, an advanced stage of HIV disease that is associated with loss of the fibroblastic reticular cell network due to excess collagen deposition, subsequently affecting CD4 T-cell function including IL-2 cytokine production [20]. In a study done by Spitsin et al. IL-2 production by CD4 T-cells did not improve after 2 months on ART in the USA, where patients had elevated levels of programmed death 1 (PD-1) and ex vivo blockade of PD-1 augmented T-cell cytokine production [21]. Therefore, down regulation of PD-1 expression may be important in enhancing immune recovery in HIV infection [21]. Our results and previous reports suggest that normalization of CD4 T-cell immune responses may not be achieved by ART alone, especially when individuals initiate ART at advanced stages of HIV disease. There is need for interventions to enhance functional immune recovery during ART, particularly in resource-limited settings where late initiation of ART still prevails due to logistical challenges to handle overwhelming numbers of people living with HIV/AIDS [19].
Evidence suggests that irreversible loss of T-cell immune repertoire during chronic HIV disease, could be limited with ART initiation at earlier stages of disease before severe immune dysfunction [15]. Therefore, initiation of ART during early HIV disease before severe immune dysfunction is a potential strategy to optimize immune recovery during ART. For example, in a European cohort where patients were initiated on ART during the four months window after HIV-infection, a quick restoration of the CD4 T-cell count occurred and was associated with recovery of the CD4 T-cell immune responses [15]. Similarly, Laurence Weiss et al. showed that CD4 IL-2 production improved after 12 months of suppressive ART among adults that initiated therapy at earlier stages of HIV disease with CD4 counts above 200 cells/μl [22]. Therefore early ART initiation at CD4 counts below 500 cells/μl, as recommended by the 2013 WHO guidelines [12], should be scaled up in resource-limited settings in order to optimize individual benefits of antiretroviral therapy.
4.2. Partial restoration of immune responses
We found that CD4+ T-cell immune responses to SEB and CMV were comparable among ART-treated O-IR and healthy HIV-negative adults. In addition, IL-4 and interferon gamma cytokine production were similar among ART-treated O-IR and healthy HIV-negative adults. Although cytokine production varies with specific antigens, our study assessed cytokine production upon stimulation with SEB, a super-antigen which stimulates immune responses in majority of individuals. The observed recovery of some immune response parameters could be attributed to gradual or variable recovery of immune responses toward different opportunistic pathogens [23]. For example, HIV-1-specific lymphoproliferative responses were restored among ART-treated adults with viral suppression relative to individuals with active viral replication in a Caucasian population [24]. Our study demonstrates partial restoration of immune function as evidenced by CD4 T-cell immune responses to PPD and pneumococcal polyssacharide antigen that were not restored to levels comparable to HIV-negative individuals. Similarly, Rios LS et al. showed that ART-treated children had restored immune responses to CMV after 2 months of therapy although immune responses to tetanus toxoid remained suboptimal [25]. A study done by Laurence Weiss et al. had partially similar findings with CD4 T-cell IFN-γ production similar among ART-treated HIV-infected patients and HIV-negative adults after stimulation with phorbol myristate acetate (PMA) and ionomycin although CD4 IL-4 production was not restored [22].
A major strength was using two complimentary methods for determining antigen-specific CD4 T-cell immune responses to common antigens in this setting [16,26,27]. Many studies have looked at proliferation and discontinuation of prophylaxis without analysis of cytokine production profiles [28,29]. In addition, our assessment had representation of both TH1 (IL-2 and interferon gamma) and TH2 (IL-4) cytokine profiles. We also had a unique cohort that had suppressive ART for seven years to provide analysis of immune responses after long-term therapy in an African cohort. Most previous studies examined immune responses after six and twelve months [18,20]. Our study provides valuable data on ‘normalization’ of immune responses to levels similar to HIV-negative individuals with CD4 counts ≥ 500 cells/μl, as reported in a Ugandan population [18]. ‘Normalization’ of CD4 counts as well as immune responses should therefore become a goal of long-term ART to optimize the quality of life of individuals on life-long treatment. This study adds to the emerging literature on the ability of pneumococcal polysaccharide antigens to stimulate antibody-independent CD4+ T-cell responses [30–32]. It is important to explore and document the potential zwitterionic properties of SSI pneumococcal polysaccharide antigen and its role in protection against invasive pneumococcal disease among people living with HIV-AIDS. Immune responses to fungal infections were not studied, however previous studies showed good immune responses to Candida albicans among ART-treated patients [28]. In view of the important role that cytokine production plays during HIV co-infections, we recommend larger studies to understand cytokine production in response to other common antigens that ART-treated adults might be exposed to during long-term ART. Our results are complimentary to relevant clinical studies to understand the clinical consequences of discontinuation of cotrimoxazole prophylaxis among individuals that attain relatively normal CD4 counts during ART.
4.3. Clinical implications
Given that seven years of suppressive ART did not restore immune responses to levels comparable to healthy HIV negative individuals, ART-treated O-IR may not respond adequately to common infections such as Streptococcus pnuemonae, Mycobacterium tuberculosis or specific vaccines. Additional protection may be required in form of prophylaxis to common infections among ART-treated adults irrespective of high CD4 T-cell counts. It is likely that prolonged ART (beyond seven years) could further restore the immune responses to levels comparable to healthy HIV-negative individuals. We may also have to consider other clinical interventions to enhance recovery of immune responses; for example, reduction of cell death mechanisms using adjuvant blockade of PD-1 that has been promising in in vitro experiments [21]. Furthermore, there is need to understand functional recovery of the innate immune system including monocytes and natural killer cells that could contribute to functional recovery of the adaptive T-cell responses. In addition, there is need to understand immune recovery among HIV-infected adults that initiate ART at the CD4 count cut-off of 500 cells/μl as recommended in the 2013 WHO guidelines for HAART initiation [12]. It is also important to note that many African countries still face logistical and systemic challenges to achieve universal access to HAART, and patients still initiate HAART at advanced stages of HIV disease [33]. Hence strategies to optimize immune recovery remain relevant, particularly to individuals that initiate ART with severe immune suppression due to advanced HIV disease.
4.4. Conclusions
Seven years of suppressive ART caused partial recovery of CD4 T-cell proliferation and cytokine production in an adult African HIV treatment cohort, despite restoration of CD4 T-cell counts to levels ≥ 500 cells/μl. The role innate immunity in the recovery of immune function during long-term ART should be investigated. Long-term prophylaxis for opportunistic infections should be considered in cases of partial immune recovery.
Acknowledgments
The authors thank the Infectious Diseases Institute (IDI) research cohort and staff for accepting to participate in this study. We acknowledge the translational laboratory, at Makerere University College of health Sciences where the laboratory assays were conducted. This work was made possible by Grand Challenges Canada, RS145 (held by Damalie Nakanjako), a Wellcome Trust Uganda post-doctoral fellowship in Infection and Immunity (held by Damalie Nakanjako), funded by a Wellcome Trust Strategic Award, grant number 084344, as well as the Medical Education for Equitable Services to All Ugandans a Medical Education Partnership Initiative grant number 5R24TW008886 from the Office of Global AIDS Coordinator and the U. S. Department of Health and Human Services, Health Resources and Services Administration and National Institutes of Health that supported Rose Nabatanzi.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
RN, DN, MJ, JO, AK and HMK made substantial contribution to the conception, design and interpretation of the data. NR, LB, AK, MJ and DN made substantial contribution to the data collection, flow cytometry assays and data analysis. DN, ADK, MRK contributed substantially to the clinical cohort from which the samples were collected. NR and DN drafted the manuscript. All authors reviewed the manuscript and approved the final version for publication.
Contributor Information
Rose Nabatanzi, Email: rosemagala@yahoo.com.
Lois Bayigga, Email: baylois@gmail.com.
Isaac Ssinabulya, Email: ssinabulyaisaac@gmail.com.
Agnes Kiragga, Email: akiragga@idi.co.ug.
Andrew Kambugu, Email: akambugu@idi.co.ug.
Joseph Olobo, Email: jolobo@yahoo.co.uk.
Moses Joloba, Email: moses.joloba@case.edu.
Moses R. Kamya, Email: mkamya@infocom.co.ug.
Harriet Mayanja-Kizza, Email: hmk@chs.mak.ac.ug.
References
- 1.Owen J, Punt J, Stranford S. Kuby Immunology. 2013 [Google Scholar]
- 2.Jansen CA, De Cuyper IM, Steingrover R, Jurriaans S, Sankatsing SU, Prins JM, et al. Analysis of the effect of highly active antiretroviral therapy during acute HIV-1 infection on HIV-specific CD4 T cell functions. AIDS. 2005;19:1145–54. doi: 10.1097/01.aids.0000176214.17990.94. [DOI] [PubMed] [Google Scholar]
- 3.Keane NM, Price P, Lee S, Almeida CA, Stone SF, James I, et al. Restoration of CD4 T-cell responses to cytomegalovirus is short-lived in severely immunodeficient HIV-infected patients responding to highly active antiretroviral therapy. HIV Med. 2004;5:407–14. doi: 10.1111/j.1468-1293.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Valdez H, Connick E, Smith KY, Lederman MM, Bosch RJ, Kim RS, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 5.Erb P, Battegay M, Zimmerli W, Rickenbach M, Egger M. Effect of antiretroviral therapy on viral load, CD4 cell count, and progression to acquired immunodeficiency syndrome in a community human immunodeficiency virus-infected cohort. Swiss HIV Cohort Study Arch Intern Med. 2000;160:1134–40. doi: 10.1001/archinte.160.8.1134. [DOI] [PubMed] [Google Scholar]
- 6.Nakanjako D, Kiragga A, Ibrahim F, Castelnuovo B, Kamya MR, Easterbrook PJ. Sub-optimal CD4 reconstitution despite viral suppression in an urban cohort on antiretroviral therapy (ART) in sub-Saharan Africa: frequency and clinical significance. AIDS Res Ther. 2008;5:23. doi: 10.1186/1742-6405-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS. 2011;25:1823–32. doi: 10.1097/QAD.0b013e3283489d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis. 2011;11:43. doi: 10.1186/1471-2334-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayigga L, Nabatanzi R, Sekiziyivu PN, Mayanja-Kizza H, Kamya MR, Kambugu A, et al. High CD56++CD16− natural killer (NK) cells among suboptimal immune responders after four years of suppressive antiretroviral therapy in an African adult HIV treatment cohort. BMC Immunol. 2014;15:2. doi: 10.1186/1471-2172-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–13. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Geneva: 2013. [Google Scholar]
- 13.Lederman MM. Immune restoration and CD4+ T-cell function with antiretro-viral therapies. AIDS. 2001;15(Suppl 2):S11–5. doi: 10.1097/00002030-200102002-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kamya MR, Mayanja-Kizza H, Kambugu A, Bakeera-Kitaka S, Semitala F, Mwebaze-Songa P, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 15.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glennie SJ, Sepako E, Mzinza D, Harawa V, Miles DJ, Jambo KC, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS ONE. 2011;6:e25610. doi: 10.1371/journal.pone.0025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117:5582–90. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 18.Lugada ES, Mermin J, Kaharuza F, Ulvestad E, Were W, Langeland N, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11:29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;81:13904–15. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitsin S, Tustin NB, Riedel E, Tustin R, Murray JB, Peck LM, et al. Programmed death 1 receptor changes ex vivo in HIV-infected adults following initiation of highly active antiretroviral therapy. Clin Vaccine Immunol. 2012;19:752–6. doi: 10.1128/CVI.00093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss L, Ancuta P, Girard PM, Bouhlal H, Roux A, Cavaillon NH, et al. Restoration of normal interleukin-2 production by CD4+ T cells of human immunodeficiency virus-infected patients after 9 months of highly active antiretroviral therapy. J Infect Dis. 1999;180:1057–63. doi: 10.1086/315025. [DOI] [PubMed] [Google Scholar]
- 23.Miller V, Mocroft A, Reiss P, Katlama C, Papadopoulos AI, Katzenstein T, et al. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA study. Ann Intern Med. 1999;130:570–7. doi: 10.7326/0003-4819-130-7-199904060-00005. [DOI] [PubMed] [Google Scholar]
- 24.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J Virol. 2002;76:5925–36. doi: 10.1128/JVI.76.12.5925-5936.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rios LS, Vallochi AL, Muccioli C, Campos-Machado MA, Belfort R, Rizzo LV. Cytokine profile in response to cytomegalovirus associated with immune recovery syndrome after highly active antiretroviral therapy. Can J Ophthalmol. 2005;40:711–20. doi: 10.1016/S0008-4182(05)80087-0. [DOI] [PubMed] [Google Scholar]
- 26.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–13. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 27.Guwatudde D, Nakakeeto M, Jones-Lopez EC, Maganda A, Chiunda A, Mugerwa RD, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–98. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blankson JN, Gallant JE, Siliciano RF. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J Infect Dis. 2001;183:657–61. doi: 10.1086/318545. [DOI] [PubMed] [Google Scholar]
- 29.Ledergerber B, Mocroft A, Reiss P, Furrer H, Kirk O, Bickel M, et al. Discontinuation of secondary prophylaxis against Pneumocystis carinii pneumonia in patients with HIV infection who have a response to antiretroviral therapy. N Engl J Med. 2001;344:168–74. doi: 10.1056/NEJM200101183440302. [DOI] [PubMed] [Google Scholar]
- 30.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch MCD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]