Abstract
BACKGROUND
Δ9-Tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH), and cannabinol (CBN) were measured in breath following controlled cannabis smoking to characterize the time course and window of detection of breath cannabinoids.
METHODS
Exhaled breath was collected from chronic (≥4 times per week) and occasional (<twice per week) smokers before and after smoking a 6.8% THC cigarette. Sample analysis included methanol extraction from breath pads, solid-phase extraction, and liquid chromatography–tandem mass spectrometry quantification.
RESULTS
THC was the major cannabinoid in breath; no sample contained THCCOOH and only 1 contained CBN. Among chronic smokers (n = 13), all breath samples were positive for THC at 0.89 h, 76.9% at 1.38 h, and 53.8% at 2.38 h, and only 1 sample was positive at 4.2 h after smoking. Among occasional smokers (n = 11), 90.9% of breath samples were THC-positive at 0.95 h and 63.6% at 1.49 h. One occasional smoker had no detectable THC. Analyte recovery from breath pads by methanolic extraction was 84.2%–97.4%. Limits of quantification were 50 pg/pad for THC and CBN and 100 pg/pad for THCCOOH. Solid-phase extraction efficiency was 46.6%–52.1% (THC) and 76.3%–83.8% (THCCOOH, CBN). Matrix effects were −34.6% to 12.3%. Cannabinoids fortified onto breath pads were stable (≤18.2% concentration change) for 8 h at room temperature and −20°C storage for 6 months.
CONCLUSIONS
Breath may offer an alternative matrix for testing for recent driving under the influence of cannabis, but is limited to a short detection window (0.5–2 h).
Cannabis, the most widely used illicit drug worldwide (1), is the most frequently detected illicit substance among roadside surveyed drivers (2). Cannabis intoxication leads to cognitive and psychomotor impairment resulting in altered perception, reaction time, short-term memory, attention, and motor skills (3–7). Identification of recent cannabis smoking and intoxication or impairment is critical to drug testing in the workplace, drug treatment facilities, and in driving under the influence of drugs (DUID)3 programs.
Δ9-Tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is metabolized to 11-hydroxy-THC and 11-nor-9-carboxy-THC (THCCOOH) (8–9). THC and phase I metabolites also undergo glucuronidation to facilitate excretion. Base hydrolysis efficiently cleaves ester-linked THCCOOH-glucuronide (10–12), while enzymatic hydrolysis is required for THC- and 11-hydroxy-THC-glucuronide cleavage (12–13). Cannabinol (CBN), a natural cannabinoid plant constituent and THC degradation product, is 10% as potent as THC (14–15) and has been detected in cannabis smokers’ oral fluid (16) and in nonsmoking individuals passively exposed to cannabis smoke (17).
Previous studies demonstrated presence of several drugs, including THC, in exhaled breath (18–22). Three previous investigations documented THC in self-reported cannabis smokers’ exhaled breath (20–22). Participants smoked 2 cigarettes containing 150 μg THC/kg body weight over 30 min, with breath collected 10 min to 22 h postsmoking (22). Due to collection device limitations and poor recovery, THC was present in breath in 10 (71.4%) of study participants only 10 min postsmoking; at 20 min, THC was below the limit of detection (LOD) in all samples. More recently, THC exceeded 770 pg/polymeric breath pad in 10 individuals 1–2 h after self-reported smoking with a 10-min breath collection (20).
We investigated exhaled breath cannabinoid concentrations following controlled cannabinoid smoking. There is strong interest in finding alternatives to urine and blood for identifying recent drug use (23–24). To date, oral fluid is the most studied and promising alternative matrix for DUID programs (16, 25–27). However, THC is detected in oral fluid for 48 h in chronic smokers during sustained abstinence (26). Exhaled breath may offer an alternative to oral fluid testing as cannabinoid detection in this matrix may better coincide with impairment.
In the present study, we used SensAbues breath collection devices and a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method to quantify breath cannabinoids in chronic and occasional cannabis smokers following controlled smoked THC administration.
Materials and Methods
PARTICIPANTS
Cannabis smokers ages 18–45 years with normal cardiac function were recruited. Chronic cannabis smokers reported mean cannabis smoking ≥4 times/week in the past 3 months and had cannabinoid-positive urine at recruitment. Occasional cannabis smokers reported mean cannabis smoking <2 times/week in the past 3 months. Group classification was initially based on urine screening results and self-reported cannabis use. Analysis of oral fluid, whole blood, plasma, and urine samples collected from these participants within 24 h effectively validated classification in all but 2 cases. Two occasional smokers were reclassified as chronic smokers based on oral fluid THCCOOH (16), urine THCCOOH-glucuronide (28), and whole blood/plasma THCCOOH and THCCOOH-glucuronide concentrations (29). The study was institutional review board approved and written informed consent was obtained from all participants. Participants resided on the closed research unit 16–20 h before and 30 h after dosing.
CANNABIS ADMINISTRATION AND BREATH COLLECTION
Cannabis cigarettes containing 6.8% THC (54 mg) were obtained through the NIDA Chemistry and Physiological Systems Research Branch. Participants smoked a single cigarette ad libitum over 10 min. Breath samples were collected with SensAbues devices for 3 min at admission and −1, 0.5, 1, 2, 3, 4, 5, 6, 8, 10.5, 13.5, and 21 h postsmoking. SensAbues devices contain a mouthpiece and polymeric filter pad enclosed in a plastic collection chamber (21). Participants were asked to breathe normally, inhaling through their nose and exhaling through the SensAbues mouthpiece during sampling. After 3 min, the mouthpiece was discarded; the pad-containing collection chamber was sealed at both ends and stored at −20 °C within 2 h. A new device was used for each collection. Devices protect against oral fluid contamination during sampling with barrier ledges inside the mouthpiece (21). Food and beverage intake was restricted 10 min before each collection. Breath collection devices were not stored in the smoking room; 0.5 h after smoking, clean collection devices were brought to the participant in the smoking room, which contained a special exhaust system to remove cannabis smoke. The 1–22-h collections occurred in a different room from the smoking room.
CALIBRATOR SOLUTION PREPARATION
THCCOOH, THC, and CBN standards were purchased from Cerilliant Corporation. Eight calibrators were prepared by fortifying clean pads with 50 μL methanolic stock solutions (1–200 ng/mL THCCOOH, THC, and CBN) yielding 50, 100, 250, 500, 1000, 2500, 5000, and 10 000 pg/pad calibrator concentrations. QC solutions were prepared from different standard ampoules, with THCCOOH, THC, and CBN pad fortification producing 150, 750, and 7500 pg/pad QC samples. An internal standard solution containing 10 ng/mL of d9-THCCOOH, d3-THC, and d3-CBN was prepared in methanol with standards obtained from Cerilliant Corporation and Lipomed (d3-CBN). Each calibrator, QC, and authentic sample was fortified with 50 μL internal standard mix. A hydrolysis control sample prepared with each extraction monitored THCCOOH-glucuronide hydrolysis. THCCOOH-glucuronide was prepared from a purchased Cerilliant Corporation standard; fortification of 50 μL of a 100-ng/mL solution resulted in 1500 pg/pad THCCOOH-glucuronide. All solutions were stored at −20 °C.
SAMPLE PREPARATION
Sample preparation modeled a previously described method (20) with modifications. Breath pads were removed from collection devices with sterile tweezers, transferred to individual 20-mL amber-glass screw-top vials, and fortified with internal standard. Tweezers were cleaned thoroughly with methanol between pads. Calibrators and QCs were prepared similarly with fortified blank pads transferred to 20-mL vials. After addition of 6 mL methanol, vials were shaken for 5 min on a horizontal shaker at 260 oscillations/min. Vials were allowed to sit for 5 min. The breath pads absorbed approximately 3 mL methanol; the remaining 3 mL was removed to a 16- × 100-mm screw-top glass tube. An additional 3 mL methanol was added to the pads, vials were shaken for 5 min, and 3 mL methanol was removed. This procedure was repeated for a total of 3 times. The methanol was dried under nitrogen to 0.5 mL, 100μL of 10 mol/L sodium hydroxide was added, and samples were incubated for 20 min at 60 °C. Following hydrolysis, samples were neutralized with 200 μL of 5 mol/L acetic acid and 6 mL of 2 mol/L ammonium acetate (pH 6.5). Samples were applied to SSTHC solid-phase extraction (SPE) columns (60 mg/10 mL; UCT) preconditioned with 1 mL methanol and water. Columns were washed with 1 mL water:acetonitrile:ammonium hydroxide (84:15:1, v/v/v) and dried under a vacuum for 15 min. Analytes were eluted in 10-mL glass tubes with 3 mL hexane:ethyl acetate:acetic acid (49:49:2, v/v/v). Eluents were dried under nitrogen at 40 °C and reconstituted in mobile phase (40:60 solvent A:solvent B, see below).
CANNABINOID QUANTIFICATION
THCCOOH, THC, and CBN were quantified by LC-MS/MS with an AB Sciex 5500 Qtrap mass spectrometer with a TurboV electrospray ionization (ESI) source (AB Sciex). The mass spectrometer was interfaced to a Shimadzu UFLCXR system with 2 LC-20ADXR pumps, a SIL-20ACXR autosampler, and a CTO-20 AC column oven (Shimadzu Corporation). LC conditions on a Phenomenex Kinetex C18, 100- × 2.1-mm, 2.6-μm column used gradient elution with 15 mmol/L ammonium acetate (adjusted to pH 3.1 with formic acid) (solvent A) and acetonitrile (solvent B). With constant 0.5 mL/min flow, 60% B was increased to 95% B over 3.5 min, held at 95% B for 1.5 min, and reequilibrated to 60% B for 1.5 min; total run time was 6.5 min. The column oven and autosampler temperatures were 40 and 4 °C, respectively. Injection volume was 15 μL.
Data were acquired with multiple reaction monitoring (MRM) of 2 transitions for each analyte and internal standard. ESI operated in negative mode for the first 2.25 min quantifying THCCOOH (342.9–299.0, 342.9–245.2) and d9-THCCOOH (352.0–308.2, 352.0–254.2). During the remaining 4.25 min, ESI operated in positive mode quantifying THC (315.1–193.0, 315.1–123.0), d3-THC (318.1–196.0, 318.1–123.0), CBN (311.1–223.2, 311.1–194.9), and d3-CBN (314.1–223.2, 314.1–195.0). Compound-specific optimized MS/MS parameters were achieved via 10 μL/min infusion of 50 ng/mL reference solutions. Optimized source parameters were gas 1, 0.31 MPa; gas 2, 0.48 MPa; curtain gas, 0.24 MPa; source temperature, 650 °C; and ion source voltage ±4500 V. Linear regression with 1/x2 weighting was employed for all analytes. Analyst 1.5.1 was used for data collection and processing. Repeated-measures Friedman tests were used to compare concentration changes across collection times and nonparametric t-tests to assess differences between groups for collection times and concentrations.
METHOD VALIDATION
Specificity was assessed by relative retention time, precursor mass, and fragment ions. Sensitivity was defined by LOD and limits of quantification (LOQ). Decreasing concentrations of drug-fortified meconium were analyzed to empirically determine LOD and LOQ. LOD was defined as the concentration with signal/noise ratio ≥3, symmetrical peak shape, transition peak area ratios ±20% mean calibrator ratios, and retention time ±0.1 min mean calibrator retention time. LOQ was defined by LOD criteria in addition to signal/noise ratio ≥10 and quantification within ±15% of target (n = 5). Linearity (R2) was evaluated with least squares regression lines with ≥7 nonzero calibrators on 5 days. Assay imprecision and accuracy were determined from 4 replicates at 3 QC concentrations analyzed in 5 batches with separate calibration curves.
Extraction efficiency and matrix effect were determined according to Matuszewski et al. (30) with 3 replicates of QC samples fortified onto blank breath pads before SPE, into eluents after SPE, and into neat samples prepared in initial mobile phase at equivalent concentrations. Analyte recovery from breath pads and loss during sample drying before SPE also were investigated with 3 QC replicates fortified into blank pad–collected methanol in a 16- × 100-mm glass tube before sample evaporation and into methanolic samples after hydrolysis.
Carryover was determined by injecting a sample containing internal standard after a sample containing 2 times the upper limit of quantification (n = 3). Base hydrolysis efficiency was determined by comparing mean molar THCCOOH-glucuronide concentrations to mean molar free THCCOOH concentrations (n = 3). Endogenous interferences were investigated in 3 breath samples collected with SensAbues devices from 10 individuals. One set of samples (n = 10) was confirmed negative and extracted as authentic samples with only internal standard added. Other sets were fortified at low (n = 10), medium (n = 5), and high (n = 5) QC concentrations. These experiments were designed to detect if endogenous interferences in breath bias cannabinoid breath quantification. Exogenous interferences (Table 1) were tested by fortifying blank pads with low QC, internal standard solution, and the potential interference at 10 000 pg/pad. No interference was determined if the QC quantified ±20% of target and had stable retention time and correct transition ratios.
Table 1.
Potential exogenous interferences tested by fortification of QC (150 pg/pad) samples at 10 000 pg/pad.
| 11-Hydroxy-Δ9-tetrahydrocannabinol | Cocaine | Norbuprenorphine |
| 2-Ethyl-5-methyl-3,3-diphenylpyrroline | Codeine | Norcocaethylene |
| 3,4-Methylenedioxyamphetamine | Cotinine | Norcocaine |
| 3,4-Methylenedioxyethyl-amphetamine | Dextromethorphan | Norcodeine |
| 3,4-Methylenedioxymethamphetamine | Diazepam | Norcotinine |
| 3-Methoxymethamphetamine | Diphenhydramine | Nordiazepam |
| 4-Bromo-2,5-dimethoxyphenethylamine | Ecgonine | Norfluoxetine |
| 4-Hydroxy-3-methoxyamphetamine | Ecgonine ethyl ester | Normorphine |
| 6-Acetylcodeine | Ecgonine methyl ester | Nornicotine |
| 6-Acetylmorphine | (±)-Ephedrine | Noroxycodone |
| 7-Aminoclonazepam | Flunitrazepam | Noroxymorphone |
| 7-Aminoflunitrazepam | Fluoxetine | Oxazepam |
| 7-Aminonitrazepam | Flurazepam | Oxycodone |
| Acetaminophen | Hydrocodone | Oxymorphone |
| Acetylsalicylic acid | Hydromorphone | Paraxetine |
| Alprazolam | Ibuprofen | Pentazocine |
| Amphetamine | Imipramine | Phencyclidine |
| Anhydroecgonine methyl ester | Ketamine | Phentermine |
| Benzodioxolylbutanamine | Lorazepam | P-hydroxyamphetamine |
| Benzoylecgonine | Methadone | P-hydroxybenzoylecgonine |
| Bromazepam | Methamphetamine | P-hydroxycocaine |
| Brompheniramine | M-hydroxybenzoylecgonine | P-hydroxymethamphetamine |
| Buprenorphine | M-hydroxycocaine | P-methoxyamphetamine |
| Caffeine | Morphine | P-methoxymethamphetamine |
| Cannabidiol | Morphine-3-glucuronide | Propoxyphene |
| Cannabigerol | Morphine-6-glucuronide | (±)-Pseudoephedrine |
| Cathinone | N-ethylamphetamine | Temazepam |
| Chlorpheniramine | Nicotine | Trans-3′-hydroxycotinine |
| Clomipramine | Nitrazepam | |
| Clonazepam | Norbenzoylecgonine | |
| Clonidine | 3,4-Methylenedioxy-N-methyl-butanamine | |
| Cocaethylene | 2-Ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine |
Cannabinoid stability was tested with 3 QC replicates under the following conditions: 1 and 8 h room temperature; 72 h 4 °C; 1 week and 1, 3, and 6 months −20 °C; and 3 freeze/thaw cycles (23 h at −20 °C followed by 1 h at room temperature). Stability samples were prepared by fortifying blank pads and placing them in individual 20-mL screw-top glass vials for the storage duration. After storage, pads were fortified with internal standard and extracted with freshly prepared calibrators. Stability sample concentrations were compared to target QC concentrations. Autosampler stability was assessed by reinjecting QC samples after 72 h and comparing calculated concentrations to original values with the initial calibration curve.
Results
METHOD PERFORMANCE
LOQs were 50 pg/pad for THC and CBN, and 100 pg/pad for THCCOOH. LODs were all 50 pg/pad. Lower LODs for THC and CBN were not achieved, determined on the basis of qualifying MRM signal/noise. Linearity extended to 10 000 pg/pad for all analytes; R2 values (n = 5) were ≥0.996. Mean accuracies of 4 QC replicates on 5 days (n = 20) at 3 QC concentrations (150, 750, and 7500 pg/pad) were 101.9%–111.0% for THCCOOH, 98.9%–99.9% for THC, and 96.6%–97.9% for CBN, respectively. Between-run imprecision (n = 20) ranged from 2.8% to 8.6% for THCCOOH, 4.6% to 5.2% for THC, and 3.7% to 3.9% for CBN across the 3 QC concentrations. Maximum within-run imprecision (n = 4) across 5 days for the 3 QC concentrations was 3.4% to 6.8% for THCCOOH, 6.1% to 9.1% for THC, and 5.3% to 6.5% for CBN.
Overall extraction efficiencies ranged from 33.8% to 73.5% across the linear range for all analytes (Table 2). Matrix effects were −34.6% to 12.3% (Table 2). Matched deuterated internal standards had similar extraction efficiencies (34.8%–75.3%) and matrix effects (−37.5% to 18.6%). Additional recovery experiments indicated high recovery (84.2%–97.4%) of cannabinoids from breath pads during methanolic incubation and agitation (Table 2). Recovery during SPE was greater for THCCOOH (76.3%) and CBN (82.3%–83.8%) than for THC (46.6%–52.1%) (Table 2).
Table 2.
Breath pad recovery, overall extraction efficiency, and matrix effect for cannabinoids in breath collected with the SensAbues device.
| Low QC (150 pg/pad)
|
High QC (7500 pg/pad)
|
|||||
|---|---|---|---|---|---|---|
| THCCOOH | THC | CBN | THCCOOH | THC | CBN | |
| Direct recovery from pada | 85.0% | 85.5% | 84.2% | 97.4% | 83.0% | 91.0% |
|
| ||||||
| Evaporation loss prior to SPEb | 10.4% | 24.1% | 5.0% | 15.1% | 10.7% | 2.0% |
|
| ||||||
| SPE recoveryc | 76.3% | 52.1% | 83.8% | 76.3% | 46.6% | 82.3% |
|
| ||||||
| Overall extraction efficiencyd | 58.1% | 33.8% | 67.1% | 63.0% | 34.6% | 73.5% |
|
| ||||||
| Matrix effecte | 12.3% | −8.3% | −32.9% | 9.6% | −16.1% | −34.6% |
Mean analyte peak areas of fortified breath pad samples divided by mean analyte peak areas of extracted blank pads fortified after methanolic collection in the 16- × 100-mm screw-top tubes.
Mean analyte peak areas of extracted blank pads fortified after methanolic collection divided by mean analyte peak areas of extracted blank pads fortified after hydrolysis and acid neutralization just prior to SPE.
Mean analyte peak areas of extracted blank pads fortified after acid neutralization prior to SPE divided by mean analyte peak areas of blank samples fortified after SPE.
Mean analyte peak areas of samples fortified onto breath pads divided by mean analyte peak areas of blank samples fortified after SPE.
Negative values represent matrix suppression; positive values indicate matrix enhancement.
No carryover was seen after injecting 20 000 pg/pad THCCOOH, THC, and CBN. During validation, THCCOOH-glucuronide hydrolysis efficiency was 90.8% (n = 3); consistent hydrolysis was demonstrated with a hydrolysis control in each run. No endogenous interferences (n = 30) or exogenous compounds (Table 1) altered QC quantification.
Cannabinoids were stable (≤18.2% concentration change) under all tested conditions (Table 3). When stored at room temperature for 1 and 8 h, fortified breath pads demonstrated a ≤10.9% concentration change. After 6 months at −20 °C, all cannabinoids showed losses of 10.8%–18.2% of targeted QC concentrations. Cannabinoids also were stable (<7.0% concentration change) after 72 h on the 4 °C autosampler.
Table 3.
Stability of cannabinoids at low (150 pg/pad), medium (750 pg/pad), and high (7500 pg/pad) concentrations.a
| Stability condition | THCCOOH | THC | CBN |
|---|---|---|---|
| 1 h Room temperature low | −1.8% | −10.9% | −9.8% |
| 1 h Room temperature medium | 6.7% | −5.9% | −5.4% |
| 1 h Room temperature high | 10.9% | −5.2% | −6.5% |
| 8 h Room temperature low | 8.9% | 3.8% | 5.3% |
| 8 h Room temperature medium | −7.4% | −2.1% | −3.4% |
| 8 h Room temperature high | −4.8% | −2.0% | −3.9% |
| 72 h 4 °C low | 14.0% | 2.4% | −4.2% |
| 72 h 4 °C medium | 4.2% | −1.4% | −3.4% |
| 72 h 4 °C high | 7.6% | 0.4% | 1.1% |
| 3 Freeze/thaw cycles low | 10.2% | −1.6% | −4.7% |
| 3 Freeze/thaw cycles medium | 5.9% | −2.6% | −3.7% |
| 3 Freeze/thaw cycles high | 7.4% | −0.7% | −2.5% |
| 1 week −20 °C low | 10.4% | −3.6% | −4.9% |
| 1 week −20 °C high | 0.8% | −7.8% | −5.6% |
| 1 month −20 °C low | −14.9% | −1.1% | −5.8% |
| 1 month −20 °C high | 13.7% | −5.8% | −3.6% |
| 3 month −20 °C low | −3.1% | 1.6% | −4.7% |
| 3 month −20 °C high | 1.0% | −0.8% | −1.6% |
| 6 month −20 °C low | −17.3% | −18.2% | −18.0% |
| 6 month −20 °C high | −10.8% | −17.3% | −13.3% |
| 72 h 4 °C autosampler low | −7.0% | 0.0% | −1.7% |
| 72 h 4 °C autosampler medium | −0.5% | 0.3% | 3.5% |
| 72 h 4 °C autosampler high | −2.2% | −2.2% | −1.2% |
Mean percentage difference (n = 3); positive values indicate percentage increase from original QC concentrations.
BREATH CANNABINOIDS
Thirteen chronic and 11 occasional cannabis smokers (ages 19–41 years) completed the study (Table 4). Each participant provided 13 breath samples except participant Y, who was unable to provide samples beyond 4 h after smoking owing to drug response.
Table 4.
Demographics and self-reported cannabis smoking histories for 24 cannabis smokers.
| Participant | Racea | Sex | Age, years | BMI, kg/m2 | Days smoked in past 14 | Mean use, joints | Age first smoked, years | Lifetime years smoked |
|---|---|---|---|---|---|---|---|---|
| Chronic smokers | ||||||||
| A | AA | M | 29.6 | 27.6 | 11 | 4/day | 12 | 17.6 |
| B | AA | M | 19.4 | 22.6 | 13 | 5/day | 15 | 4.4 |
| D | W | M | 25.5 | 23.0 | 14 | 20/day | 13 | 12.5 |
| E | AA | F | 19.9 | 32.4 | 14 | 3.5/day | 11 | 8.9 |
| F | AA | M | 24.2 | 27.4 | 12 | 1.5/day | 13 | 11.2 |
| G | W | F | 22.9 | 24.8 | 14 | 6/day | 16 | 6.9 |
| H | AA | M | 37.3 | 23.0 | 14 | 3/day | 25 | 12.3 |
| I | AA | F | 27.6 | 35.4 | 14 | 4/day | 18 | 9.6 |
| J | AA | F | 26.9 | 20.4 | 14 | 21/day | 14 | 12.9 |
| K | AA | M | 23.4 | 24.3 | 14 | 6/day | 19 | 4.4 |
| L | AA | M | 28.7 | 28.1 | 14 | 6/day | 14 | 14.7 |
| M | AA | M | 28.0 | 19.4 | 2b | 2/monthb | 14 | 14.0 |
| N | AA | M | 23.8 | 30.7 | 1b | 4/monthb | 14 | 9.8 |
| Median | 25.5 | 24.8 | 14.0 | 4.5/day | 14.0 | 11.2 | ||
| Mean | 25.9 | 26.1 | 13.5 | 6.9/day | 15.2 | 10.7 | ||
| SD | 4.7 | 4.7 | 1.0 | 6.5/day | 3.7 | 3.9 | ||
| Occasional smokers | ||||||||
| O | W | M | 25.6 | 29.4 | 0 | 2/month | 16 | 9.6 |
| P | W | M | 25.4 | 23.7 | 0 | 2/month | 13 | 12.4 |
| Q | W | M | 23.7 | 24.1 | 2 | 7/month | 16 | 7.7 |
| R | AA | M | 38.2 | 21.0 | 2 | 2/month | 19 | 19.2 |
| S | M | M | 41.3 | 22.0 | 5 | 10/month | 16 | 25.3 |
| T | U | F | 34.9 | 31.7 | 1 | 2/month | 13 | 21.9 |
| U | AA | F | 36.5 | 47.8 | 2 | 4/month | 18 | 18.5 |
| V | M | M | 22.5 | 25.2 | 0 | 6/month | 13 | 9.5 |
| W | W | F | 34.2 | 26.6 | 1 | 0.25/month | 14 | 20.2 |
| X | AA | M | 31.7 | 21.8 | 0 | 8/month | 16 | 15.7 |
| Y | AA | M | 31.9 | 22.6 | 0 | 2/month | 15 | 16.9 |
| Median | 31.9 | 24.1 | 1 | 2/month | 16 | 16.9 | ||
| Mean | 31.4 | 26.9 | 1.2 | 4.1/month | 15.4 | 16.1 | ||
| SD | 6.3 | 7.7 | 1.5 | 3.1/month | 2.0 | 5.7 | ||
AA, African American; W, white; M, mixed; U, unknown.
Self-reported data not consistent with biological sample concentrations. Data excluded from mean and median.
Breath collection did not always occur at the intended time because the study design was complex and also involved collection of other matrices. Following cannabis smoking, dry mouth frequently occurs, making oral fluid collection difficult. Because oral fluid collections occurred before breath collections in this study design, breath collections were delayed early in the timeline (0.5–3 h), with prolonged oral fluid collection times. Mean (range) times for breath collection shortly after smoking among chronic smokers were 0.89 h (0.78–1.01 h), 1.38 h (1.29–1.54 h), 2.38 h (2.25–2.53 h), and 3.23 h (3.11–3.42 h). Among occasional smokers, actual breath collection times shortly after smoking were 0.95 h (0.74–1.10 h), 1.49 h (1.21–2.07 h), 2.51 h (2.17–2.81 h), and 3.35 (3.12–3.64 h). Collection times among occasional users were delayed longer than among chronic users 1–3 h after collection (P = 0.0315–0.0422); all other collection times were not significantly different between groups.
THC was the major cannabinoid detected in breath samples; no sample tested positive for THCCOOH and only 1 sample contained CBN. Among chronic smokers, breath samples were THC positive at 0.89 h (100%), 1.38 h (76.9%), and 2.38 h (53.8%) after smoking, with 2 participants’ breath positive at admission and 1 breath sample positive after 4.2 h (but not at 3.1 h) (Table 5). Among occasional smokers, breath samples were positive for THC only after 0.95 h (90.9%) and 1.49 h (63.6%). One occasional smoker (W) had no detectable THC in any breath sample. In all positive samples from chronic smokers, the median (range) of THC breath concentrations was 94.8 pg/pad (50.5–409 pg/pad) and among occasional smokers, 61.0 pg/pad (50.2–118 pg/pad), with an extreme outlier of 1170 pg THC (Table 5). Reasons for this high THC concentration could include device malfunction resulting in oral fluid or smoke contamination.
Table 5.
Breath THC concentrations (pg/pad) from chronic and occasional cannabis smokers after smoking a single 6.8% THC cigarette within 10 min.
| Time after smokinga,b | Chronic cannabinoid smokers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | D | E | F | G | H | I | J | K | L | M | N | |
| Admission | —c | — | — | — | — | — | — | 90.1 | — | 102.0 | — | — | — |
| −1.00 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 0.50 | 50.7 | 292.0 | 409.0 | 147.0 | 177.0 | 84.5 | 161.0 | 182.0 | 65.8 | 171.0 | 117.0 | 51.4 | 55.5 |
| 1.00 | 109.0 | 209.0 | 159.0 | 71.4 | 89.6 | 51.4 | 159.0 | 159.0 | — | 90.9 | 85.3 | — | — |
| 2.00 | — | 67.6 | 86.3 | 50.5 | — | — | 97.6 | 74.3 | — | 94.8 | 54.0 | — | — |
| 3.00 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 4.00 | — | — | — | — | — | — | — | — | — | 98.0 | — | — | — |
| Time after smokingb,d | Occasional cannabinoid smokers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | P | Q | R | S | T | U | V | W | X | Y | |
| 0.50 | 118.0 | 1170.0e | 60.4 | 56.4 | 87.4 | 50.2 | 101.0 | 54.9 | — | 106 | 61.5 |
| 1.00 | 53.0 | 74.3 | 50.8 | 83.7 | 53.0 | — | 92.3 | — | — | — | 50.4 |
Actual mean (range) collection times. Admission: −18.35 h (−19.37 to −17.33 h); −1 h: −0.82 h (−0.97 to −0.59 h); 0.5 h: 0.89 h (0.78–1.01 h); 1 h: 1.38 h (1.29–1.54 h); 2 h: 2.38 h (2.25–2.53 h), 3 h: 3.23 h (3.11–3.42 h), 4 h: 4.27 h (4.11–4.45 h).
All samples collected from chronic cannabis smokers 5–21 h postsmoking were <LOQ. All occasional smoker breath samples were <LOQ at admission and at −1 and 2–21 h postsmoking.
—, <LOQ.
Actual mean (range) collection times, 0.5 h: 0.95 h (0.74–1.10 h); 1 h: 1.49 h (1.21–2.07 h).
CBN 150 pg/pad.
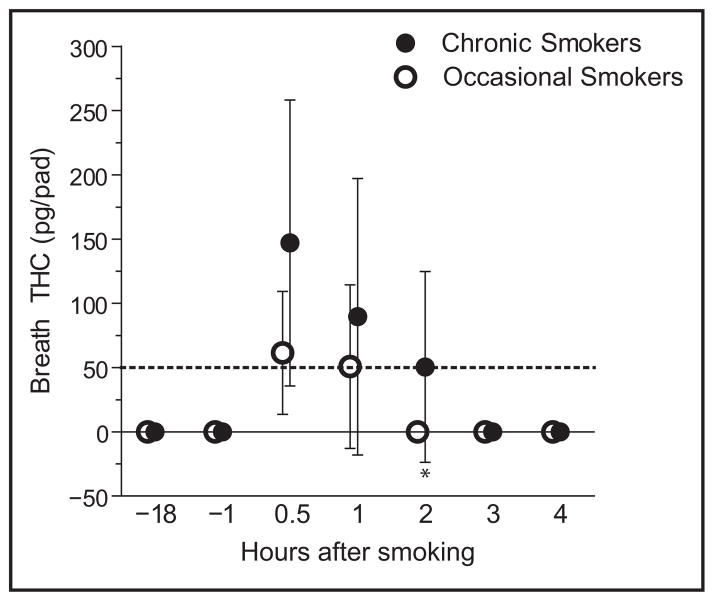
THC breath concentrations significantly decreased with time after smoking in both groups (P <0.0001) (Fig. 1). Among chronic smokers with positive breath samples at 0.5 and 1 h postsmoking, THC breath concentrations decreased for 9 (90%) of participants, with decreases ranging from 1.2% to 61.1% (Table 5). Further decreases were seen at 2 h for most chronic smokers; THC breath concentration decreases from 1–2 h postsmoking ranged from 29.3% to 67.7% for 6 participants. Positive admission breath samples for participants I and K showed similar THC concentrations as those for their 1–2-h postsmoking collections. Among occasional smokers with positive breath samples at 0.5 and 1 h postsmoking, the majority showed THC breath concentration decreases (8.6%–93.9%). Median breath THC concentrations were significantly different between groups only at 2 h postsmoking (P = 0.0156, Fig. 1).
Fig. 1. Median (interquartile range) THC breath concentrations from chronic and occasional cannabinoid smokers after smoking a single 6.8% THC cigarette for 10 min.
Dashed line indicates limit of quantitation. *P = 0.0156, group comparison.
All participants produced THC-negative breath samples 1 h before smoking. In all but 1 participant (K), once breath samples were negative (<LOQ) for THC, they remained negative for the duration of the monitoring period (21 h postsmoking). Participant K produced THC-positive breath samples from 0.5 to 2 h and at 4 h after smoking, although his 3-h breath collection had no detectable THC. This participant’s 2-and 4-h breath samples had similar concentrations (Table 5).
Discussion
Cannabis is the most commonly used illicit drug and is frequently identified in drugged driving cases. Detection of recent cannabis smoking is important for documenting accompanying impairment. Here we describe cannabinoid concentrations in exhaled breath following controlled smoked cannabis administration; these data characterize breath cannabinoids, the duration of detection, and peak concentrations. Breath collection is noninvasive and easily observed, and samples can be collected roadside. Breath alcohol tests are widely employed by law enforcement to provide evidence of recent alcohol consumption during roadside stops. Exhaled breath analysis also is evolving as a new frontier in lung and cardiovascular disease testing (31–32).
THC, THCCOOH, and CBN quantification in breath were achieved with low LOQs (50 pg/pad THC and CBN and 100 pg/pad THCCOOH). THC’s LOQ was the same as for the previously published method (20). Although THCCOOH’s LOQ was higher than that in the previously published method (50 pg/pad), THCCOOH was not detected in either study. No previous quantification method for breath cannabinoids included CBN. 11-hydroxy-THC is found in low concentrations in blood (29) and is rarely found in oral fluid following smoking (16); therefore, this analyte was not included in this assay.
Overall extraction efficiencies of THCCOOH (58.1%–63.0%) and CBN (67.1%–73.5%) were greater than that for THC(33.8%–34.6%). By fortifying QC samples throughout our procedure, we determined that cannabinoid breath pad recovery was high (>83%) and the largest THC loss (47.9%–53.4%) occurred during SPE, yielding low overall extraction efficiency (Table 2). However, compromises were necessary to recover THCCOOH, a compound with different physicochemical properties. Similar sweat-patch THC recovery (44%–46%) was reported by Saito et al. (33). During method development, breath pad cannabinoids were extracted with different solvents and non-SPE procedures, leading to large matrix effects (−98%). With the validated SPE method, the matrix effect was reasonable for all cannabinoids (<±35%). Inclusion of matched deuterated internal standards compensated for these effects.
Breath pad cannabinoids were stable under all test conditions. THC breath pad removal after the test period was similar to that of freshly prepared calibrators and QC samples; all fortified stability QC samples yielded quantitative values ±18.2% of expected values (Table 3). After 6 months, frozen, fortified cannabinoid concentrations showed consistent decreases of −10.8% to −18.2% of expected concentrations; stability beyond 6 months is unknown.
The cannabinoid detection window in breath is short, ranging from 0.5 to 2 h, and in 1 case 0.5 to 4 h, after smoking a single cannabis cigarette in chronic and occasional cannabis smokers. Only THC was identified, except for CBN in 1 sample 0.5 h after smoking. With the short detection window for THC, breath collection may offer an alternative matrix to oral fluid for DUID and “for cause” workplace drug testing when testing occurs <2 h after smoking. Further work is needed to validate the predictive value of cannabinoid breath testing. THC in oral fluid can be detected for 48 h in chronic smokers during sustained abstinence (26); therefore, exhaled breath may offer a cannabinoid detection alternative and better coincide with impairment 1–2 h after smoking (3).
Occasional cannabis smokers may have a shorter window of detection for breath cannabinoids compared to chronic users, as no occasional smoker produced a positive sample beyond 1 h after smoking and 1 occasional smoker produced no positive samples (Table 5). However, in some cases windows of detection for these 2 groups were similar, with 3 chronic users positive only at 0.5 h and another 3 only until 1 h after smoking. A similar window of detection (1–2 h) was reported previously for breath THC based on self-reported cannabis smoking (20). The short window of detection may be due to THC’s rapid elimination from blood following smoking (29). While this short window of breath THC detection coincides with impairment, a major limitation is missed detection of occasional smokers shortly after smoking. Our study design involved collection of additional matrices before breath that delayed collection times shortly after smoking. This delay may have limited our detection capabilities, because most 0.5-h samples were collected closer to 1 h after smoking.
Future research must determine if THC’s window of detection in breath is extended in chronic cannabis smokers during sustained abstinence, since residual excretion is seen in blood (34), plasma (35), oral fluid (26), and sweat (36); however, low THC breath concentrations in chronic cannabis smokers in the present study suggest prolonged excretion would not be expected. The effect of passive cannabis exposure on breath THC concentrations also remains unknown. Recent studies demonstrate detectable THC in oral fluid following passive cannabis exposure (17). Further studies are needed to determine if passive cannabis exposure results in cannabinoid breath concentrations.
These first breath cannabinoid data following controlled cannabis administration suggest that the cannabinoid detection window in breath is short, ranging from 0.5 to 2 h, and reflects parent THC only. Breath cannabinoids have a short detection window, coinciding with possible impairment 1–2 h after smoking, making this alternative matrix applicable for DUID and “for cause” workplace cannabinoid drug testing; however, the driving impairment window extends beyond the detectability of breath cannabinoids.
Acknowledgments
This work was presented at the American Academy of Forensic Science, Washington, DC, February 2013.
The authors acknowledge the contributions of the clinical staff of the Intramural Research Program, National Institute on Drug Abuse, and the Behavioral Pharmacology Research Unit, Johns Hopkins Bayview Medical Center, as well as the University of Maryland, Baltimore, a member of the Graduate Partnership Program, NIH.
Footnotes
Nonstandard abbreviations: DUID, driving under the influence of drugs; THC, Δ9-tetrahydrocannabinol; THCCOOH, 11-nor-9-carboxy-THC; CBN, cannabinol; LOD, limit of detection; LC-MS/MS, liquid chromatography-tandem mass spectrometry; SPE, solid phase extraction; ESI, electrospray ionization; MRM, multiple reaction monitoring; LOQ, limit of quantitation.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: S.K. Himes, National Institute on Drug Abuse; M.A. Huestis, National Institute on Drug Abuse.
Consultant or Advisory Role: None declared.
Stock Ownership: O. Beck, part ownership in SensAbues producing the sampling device.
Honoraria: None declared.
Research Funding: Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health.
Expert Testimony: None declared.
Patents: O. Beck, does not have patent number.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.United Nations Office on Drugs and Crimes. World Drug Report. United Nations Publications; 2009. [Accessed December 2012]. Sales No. E. 11.XI. 10. http://www.unodc.org/documents/data-and-analysis/WDR2011/WDR2011-web.pdf. [Google Scholar]
- 2.Lacey JH, Kelley-Baker T, Furr-Holden D, Voas RB, Romano E, Ramirez A, et al. [Accessed December 2012];2007 National roadside survey of alcohol and drug use by drivers: drug results. 2009 http://www.nhtsa.gov/DOT/NHTSA/Traffic%20Injury%20Control/Articles/Associated%20Files/811249.pdf.
- 3.Hartman RL, Huestis MA. Cannabis Effects on Driving Skills. Clin Chem. 2013;59:478–92. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. Acute marijuana effects on human risk taking. Neuropsychopharmacology. 2005;30:800–9. doi: 10.1038/sj.npp.1300620. [DOI] [PubMed] [Google Scholar]
- 5.Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109–19. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- 7.Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol. 2005:445–77. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–9. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga T, Iwawaki Y, Watanabe K, Yamamoto I, Kageyama T, Yoshimura H. Metabolism of delta 9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995;56:2089–95. doi: 10.1016/0024-3205(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 10.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. Detection of conjugated 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. J Anal Toxicol. 2007;31:187–94. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 11.Coulter C, Garnier M, Moore C. Analysis of tetrahydrocannabinol and its metabolite, 11-nor-delta(9)-tetrahydrocannabinol-9-carboxylic acid, in oral fluid using liquid chromatography with tandem mass spectrometry. J Anal Toxicol. 2012;36:413–7. doi: 10.1093/jat/bks039. [DOI] [PubMed] [Google Scholar]
- 12.Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, McWilliams ME, et al. Cannabinoids in humans. II. The influence of three methods of hydrolysis on the concentration of THC and two metabolites in urine. J Anal Toxicol. 1995;19:292–8. doi: 10.1093/jat/19.5.292. [DOI] [PubMed] [Google Scholar]
- 13.Gray TR, Barnes AJ, Huestis MA. Effect of hydrolysis on identifying prenatal cannabis exposure. Anal Bioanal Chem. 2010;397:2335–47. doi: 10.1007/s00216-010-3772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner CE, ElSohly MA, Boeren EG. Constituents of cannabis sativa L. XVII. a review of the natural constituents. J Nat Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 15.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb Exp Pharmacol. 2005:657–90. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem. 2012;58:748–56. doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, et al. Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–30. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Beck O, Leine K, Palmskog G, Franck J. Amphetamines detected in exhaled breath from drug addicts: a new possible method for drugs-of-abuse testing. J Anal Toxicol. 2010;34:233–7. doi: 10.1093/jat/34.5.233. [DOI] [PubMed] [Google Scholar]
- 19.Beck O, Sandqvist S, Bottcher M, Eriksen P, Franck J, Palmskog G. Study on the sampling of methadone from exhaled breath. J Anal Toxicol. 2011;35:257–63. doi: 10.1093/anatox/35.5.257. [DOI] [PubMed] [Google Scholar]
- 20.Beck O, Sandqvist S, Dubbelboer I, Franck J. Detection of delta9-tetrahydrocannabinol in exhaled breath collected from cannabis users. J Anal Toxicol. 2011;35:541–4. doi: 10.1093/anatox/35.8.541. [DOI] [PubMed] [Google Scholar]
- 21.Beck O, Stephanson N, Sandqvist S, Franck J. Detection of drugs of abuse in exhaled breath from users following recovery from intoxication. J Anal Toxicol. 2012;36:638–46. doi: 10.1093/jat/bks079. [DOI] [PubMed] [Google Scholar]
- 22.Manolis A, McBurney LJ, Bobbie BA. The detection of delta 9-tetrahydrocannabinol in the breath of human subjects. Clin Biochem. 1983;16:229–33. doi: 10.1016/s0009-9120(83)90070-x. [DOI] [PubMed] [Google Scholar]
- 23.Gallardo E, Queiroz JA. The role of alternative specimens in toxicological analysis. Biomed Chromatogr. 2008;22:795–821. doi: 10.1002/bmc.1009. [DOI] [PubMed] [Google Scholar]
- 24.Frederick DL. Toxicology testing in alternative specimen matrices. Clin Lab Med. 2012;32:467–92. doi: 10.1016/j.cll.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Desrosiers NA, Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. On-site test for cannabinoids in oral fluid. Clin Chem. 2012;58:1418–25. doi: 10.1373/clinchem.2012.189001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–36. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 27.Wille SM, del Ramirez-Fernandez MM, Samyn N, De Boeck G. Conventional and alternative matrices for driving under the influence of cannabis: recent progress and remaining challenges. Bio-analysis. 2010;2:791–806. doi: 10.4155/bio.10.29. [DOI] [PubMed] [Google Scholar]
- 28.Skopp G, Pötsch L, Ganβmann B, Mauden M, Richter B, Aderjan R, Mattern R. Freie und glucuronidierte Cannabinoide im Urin—Untersuchungen zur Einschätzung des Konsumverhaltens. Reschtsmedzin. 1999;10:21–8. [Google Scholar]
- 29.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–14. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 31.Cikach FS, Jr, Dweik RA. Cardiovascular biomarkers in exhaled breath. Prog Cardiovasc Dis. 2012;55:34–43. doi: 10.1016/j.pcad.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Kant KD, van der Sande LJ, Jobsis Q, van Schayck OC, Dompeling E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: a systematic review. Respir Res. 2012;13:117. doi: 10.1186/1465-9921-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito T, Wtsadik A, Scheidweiler KB, Fortner N, Takeichi S, Huestis MA. Validated gas chromatographic-negative ion chemical ionization mass spectrometric method for delta(9)-tetrahydrocannabinol in sweat patches. Clin Chem. 2004;50:2083–90. doi: 10.1373/clinchem.2004.034868. [DOI] [PubMed] [Google Scholar]
- 34.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, et al. Do delta 9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–8. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, Huestis MA. Implications of plasma delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33:469–77. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huestis MA, Scheidweiler KB, Saito T, Fortner N, Abraham T, Gustafson RA, Smith ML. Excretion of delta9-tetrahydrocannabinol in sweat. Forensic Sci Int. 2008;174:173–7. doi: 10.1016/j.forsciint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]