Abstract
African American (AA) women have a higher incidence of triple-negative breast cancer (TNBC: negative for the expression of estrogen receptor, progesterone receptor, and HER2 gene amplification) than Caucasian (CA) women, explaining in part their higher breast cancer mortality. However, there have been inconsistent data in the literature regarding survival outcomes of TNBC in AA versus CA women. We performed a retrospective chart review on 493 patients with TNBC first seen at the Washington University Breast Oncology Clinic (WUBOC) between January 2006 and December 2010. Analysis was done on 490 women (30 % AA) for whom follow-up data was available. The median age at diagnosis was 53 (23–98) years and follow-up time was 27.2 months. There was no significant difference between AA and CA women in the age of diagnosis, median time from abnormal imaging to breast biopsy and from biopsy diagnosis to surgery, duration of follow-up, tumor stage, grade, and frequency of receiving neoadjuvant or adjuvant chemotherapy and pathologic complete response rate to neoadjuvant chemotherapy. There was no difference in disease free survival (DFS) and overall survival (OS) between AA and CA groups by either univariate or multivariate analysis that included age, race, and stage. The hazard ratio for AA women was 1.19 (CI 0.80–1.78, p = 0.39) and 0.91 (CI 0.62–1.35, p = 0.64) for OS and DFS, respectively. Among the 158 patients who developed recurrence or presented with stage IV disease (AA: n = 36, CA: n = 122), no racial differences in OS were observed. We conclude that race did not significantly affect the clinical presentation and outcome of TNBC in this single center study where patients received similar therapy and follow-up.
Keywords: Race, Triple-negative breast cancer, Outcome, Retrospective study
Introduction
African American women have a lower incidence of breast cancer, but a higher mortality rate when compared to other racial groups [1–4]. Factors potentially contributing to the racial disparity in breast cancer outcome include socioeconomic status, cultural beliefs, insurance variables that could lead to delays in diagnosis and treatment, decreased compliance with recommended screening, increased comorbidities, decreased adherence to therapy, and potential biological factors [1, 5–21]. However, the higher incidence of triple-negative breast cancer (TNBC) observed in AA women is suggested as one of the most important factors [21–24]. TNBC is a subgroup of breast cancers characterized by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 gene amplification. TNBC is often associated with a higher tumor grade and an aggressive clinical course, manifested as early relapse, a tendency for visceral metastasis and shorter survival [25–27]. Compared to Caucasian women, African American women are twice as likely to develop TNBC [21–23].
An outstanding question is whether the biology of TNBC differs between African American and Caucasian women. Only a few studies have focused on the TNBC population in particular and compared the outcomes between African American and Caucasian women. Some reported a worse survival for African American women with TNBC [21–24]. However, others concluded no survival difference between the two races [28–30]. To investigate this issue further, we reviewed the clinical presentation and outcome of patients with TNBC who sought care in the Washington University Breast Oncology Clinic in St. Louis and compared the outcomes between African American and Caucasian women.
Patients and methods
Patient population
We constructed a database of 493 patients, including only African Americans and non-Hispanic Caucasians, with a diagnosis of TNBC. These are consecutive patients who presented for their first visit to the Breast Oncology Clinic at Washington University in Saint Louis, MO between January 1st 2006 and December 16th 2010. Four hundred and ninety patients were included in the analysis, two were not included because of unknown date of diagnosis and 1 because of lack of follow-up data. For all patients, including those diagnosed elsewhere and having received medical oncology care before treatment at our institution, data collection, follow-up time, and analysis were from the time of initial diagnosis. Exclusion criteria included ER or PR or HER2 positivity, unknown date of diagnosis, an additional malignancy, male sex, race other than African American or Caucasian, Hispanic ethnicity, and unknown race or ethnicity. This study was approved by Washington University Institutional Review Board.
Tumor staging and pathology
Initial pathological stage was determined based on American Joint Committee on Cancer Criteria at the time of diagnosis. Biopsy specimens from other institutions, when available, were reviewed by pathologists at Barnes Jewish Hospital. Histological grade and ER, PR, and Her-2/neu status were recorded from pathology reports. ER and PR were considered negative if immunohistochemistry indicated an Allred score below 3 or less than 1 % tumor cells staining positive. HER2 was considered to be negative by FISH or 0 or 1+ on immunohistochemistry. Pathologic complete response (pCR) was defined as absence of residual invasive disease in the breast and axillary lymph nodes.
Clinical parameters
We recorded clinical parameters including race, ethnicity, age, time from imaging to biopsy diagnosis, tumor characteristics, time from biopsy diagnosis to surgery, surgical intervention, pathological stage, whether patients received neoadjuvant and/or adjuvant treatment, pathologic response to neoadjuvant therapy, menopausal status, follow-up information, recurrence information and date of death (obtained from the Social Security Death Index).
Outcome measures
The primary outcomes included overall survival (OS) and disease free survival (DFS). OS was defined as the time from diagnosis to death due to any cause, and survivors were censored at the date of last contact. DFS was defined as time from surgery to any invasive breast recurrence (local or distant) or death, whichever occurred first. Those patients alive and recurrence-free were censored at date of last contact. Patients who presented with stage IV disease at diagnosis or who had not undergone surgery were excluded from DFS analysis. Time from recurrence to death was defined as time from recurrence or diagnosis (if stage IV disease at initial presentation) to death due to any cause, and survivors were censored at date of last contact.
Statistical analysis
The distribution of demographic and clinical characteristics between racial groups (African American and Caucasian) was compared using Chi square test, Wilcoxon rank sum test, or two-sample t test as appropriate. Survival curves by racial groups were estimated using the Kaplan–Meier product-limit method and compared by log-rank test. Univariate Cox proportional hazard models were fit to identify factors significantly related to OS or DFS. To assess whether the racial status was an independent predictor of survival, a multivariate Cox model was constructed to adjust for other demographic and clinical characteristics that were significant in the univariate analyses. Two-way interaction terms between racial status and other factors in the multivariate Cox model were also assessed. All analyses were two-sided and significance was set at a p value of 0.05. Statistical analyses were performed using SAS (SAS Institutes, Cary, NC).
Results
Comparison of patient characteristics between races
Among the 490 patients with TNBC who initially presented to the Washington University Breast Oncology Clinic between January 2006 and December 2010, 146 patients were African American (30 %) and 344 patients were Caucasian (70 %). Table 1 shows the comparison of patient characteristics between races. Median age at diagnosis was 53 years for both races. Most patients had high grade tumors and early stage breast cancer at initial presentation. There was no statistical difference between races in age, menopausal status, tumor grade, and stage at diagnosis.
Table 1.
Comparison of characteristics between african american and Caucasian women
| Race | AA (n = 146) | CA (n = 344) | p |
|---|---|---|---|
| Menopausal status | 0.39 | ||
| Premenopausal | 49 (33.6 %) | 120 (34.9 %) | |
| Postmenopausal | 88 (60.3 %) | 191 (55.5 %) | |
| Unknown | 9 (6.10 %) | 33 (9.60 %) | |
| Age (median in years, range) | 53, 27–84 | 53, 23–98 | 0.43 |
| Median days from imaging to biopsy diagnosis (range) | 7 (0–80) | 8 (0–137) | 0.82 |
| Median days from biopsy diagnosis to surgery in those without | 26 (0–326) | 21 (0–453) | 0.01 |
| Neoadjuvant chemotherapy (range) | (n = 88) | (n = 216) | |
| Median days from biopsy diagnosis to surgery in those with | 174 (89–271) | 165 (8–574) | 0.23 |
| Neoadjuvant chemotherapy (range) | (n = 41) | (n = 98) | |
| Chemotherapy for stage I–III disease | 0.21 | ||
| Neoadjuvant and adjuvant chemotherapy | 9 (6.6 %) | 21 (6.4 %) | |
| Neoadjuvant chemotherapy alone | 35 (25.5 %) | 82 (25.0 %) | |
| Adjuvant chemotherapy alone | 67 (48.9 %) | 186 (56.7 %) | |
| No chemotherapy | 26 (19.0 %) | 39 (11.9 %) | |
| (n = 137) | (n = 328) | ||
| Response to neoadjuvant chemotherapy | 0.89 | ||
| pCR | 11/44 (25.0 %) | 22/103 (21.4 %) | |
| Pathological stage | 0.42 | ||
| pCR | 11 (7.53 %) | 23 (6.69 %) | |
| I | 39 (26.7 %) | 113 (32.8 %) | |
| IIA | 40 (27.4 %) | 86 (25.0 %) | |
| IIB | 22 (15.1 %) | 34 (9.88 %) | |
| III | 24 (16.4 %) | 67 (19.5 %) | |
| IV | 9 (6.16 %) | 16 (4.65 %) | |
| Unknown | 1 (0.68 %) | 5 (1.45 %) | |
| Histology grade | 0.98 | ||
| I/II | 20 (13.7 %) | 46 (13.4 %) | |
| III | 125 (85.6 %) | 290 (84.3 %) | |
| Unknown | 1 (0.68 %) | 8 (2.33 %) |
Sixty-five patients (26 African American and 39 Caucasian) who presented with early stage disease (stage I: n = 37, stage II: n = 18, stage III: n = 10) did not receive neoadjuvant or adjuvant chemotherapy. The most common reasons for not receiving chemotherapy included stage I disease for which chemotherapy was not recommended by the treating physician (n = 22), age/co-morbidities (n = 11), and patient refusal (n = 11). The percentage of patients who received neoadjuvant or adjuvant chemotherapy was not different between races (Table 1). In addition, there was no racial difference in the rate of pCR to neoadjuvant chemotherapy (Table 1).
To investigate potential diagnostic and treatment delays, we reviewed time from the date of abnormal imaging study to biopsy diagnosis and the date from biopsy diagnosis to surgery. The median time from the date of abnormal imaging study to biopsy was similar in both ethnic groups. The median time from biopsy diagnosis to surgery without neoadjuvant chemotherapy was longer in African American than Caucasian women (African American: 26 days vs Caucasian: 21 days, p = 0.01), although the clinical significance is uncertain. The median time from biopsy diagnosis to surgery with neoadjuvant chemotherapy was similar in both groups (Table 1).
Comparison of survival outcomes between races
The median follow-up time was 27.2 months (with an inter-quartile range (IQR) of 13.5–46.1 months). Duration of follow-up was not significantly different between races, median for African Americans was 24.4 months (IQR: 13.5–40.5 months) and for Caucasians was 28.9 months (IQR: 13.2–47.3 months). There were 120 deaths due to any cause (25.3 % in African Americans and 24.1 % in Caucasians) and 134 recurrences (24.7 % in African Americans and 35.4 % in Caucasians) during the follow-up period. There was no significant difference in DFS between groups (HR 0.78, p = 0.21). There was also no difference in OS between races (HR 1.19, p = 0.38). In addition, there was no significant difference between races in OS for those who presented with recurrence or stage IV disease, with a median time to death of 17.5 months for African Americans and 22.6 months for Caucasians. The estimated 3-year OS was 71.0 % for African Americans and 80.0 % for Caucasians, while the 3-year DFS was 66.8 % for African Americans and 62.8 % for Caucasians (Fig. 1).
Fig. 1.
Comparison of survival outcomes between African American and Caucasian Women. K-M survival curves of OS for all patients (a), DFS for patients with early stage breast cancer (b), OS for patients with recurrent or stage IV at presentation (c), by race are shown
Univariate and multivariate analysis of other variables on survival outcomes
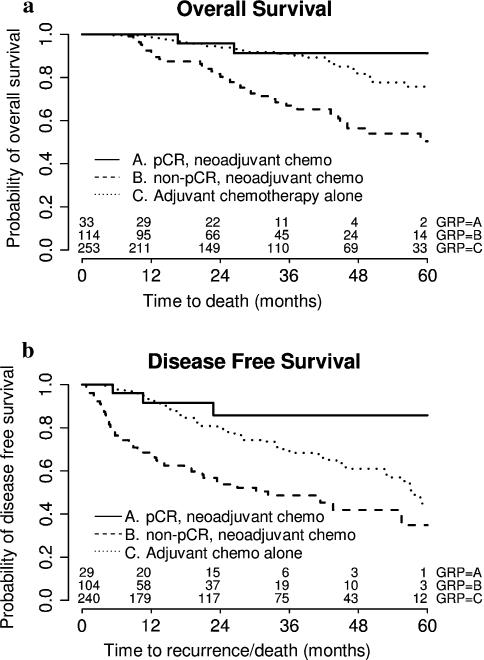
In univariate analysis, menopausal status and grade were not predicative of DFS or OS. Older age was associated with a trend toward worse OS (p = 0.07), but not DFS (p = 0.64). Pathological stage was significantly predicative of DFS and OS. Stage IIB or III disease predicted significantly worse DFS, while stage III or IV at presentation predicted significantly worse OS (Table 2). The status of response to neoadjuvant chemotherapy was also significantly related to DFS and OS. Those who did not achieve a pCR with neoadjuvant chemotherapy experienced significantly worse DFS and OS compared to those who achieved a pCR with neoadjuvant chemotherapy (Table 2; Fig. 2).
Table 2.
Univariate analysis of variables with respect to survival outcomes
| Variable | DFS |
OS |
||
|---|---|---|---|---|
| HR (95 % CI) | HR (95 % CI) | p | ||
| Race | 0.21 | 0.38 | ||
| African American | 0.78 (0.53–1.15) | 1.19 (0.81–1.76) | ||
| Caucasian | Reference group | Reference group | ||
| Age (per 1 yr increase) | 1.00 (0.99–1.01) | 0.64 | 1.01 (1.00–1.03) | 0.07 |
| Chemotherapy for stage I–III disease | <0.001 | <0.001 | ||
| pCR, neoadjuvant chemotherapy | Reference group | Reference group | ||
| Non-pCR, neoadjuvant chemotherapy | 4.01 (1.45–11.12) | 0.008 | 5.61 (1.35–23.26) | 0.02 |
| Adjuvant chemotherapy alone | 1.68 (0.61–4.63) | 0.31 | 1.87 (0.45–7.80) | 0.39 |
| Menopausal statusa | 0.32 | 0.90 | ||
| Premenopausal | 1.20 (0.84–1.70) | 1.03 (0.69–1.54) | ||
| Postmenopausal | Reference group | Reference group | ||
| Pathological stage | <0.001 | <0.001 | ||
| pCR | Reference group | Reference group | ||
| I | 1.56 (0.55–4.39) | 0.40 | 2.64 (0.63–11.2) | 0.19 |
| IIA | 1.65 (0.58–4.66) | 0.34 | 1.52 (0.35–6.69) | 0.58 |
| IIB | 3.30 (1.14–9.54) | 0.03 | 3.95 (0.91–17.2) | 0.07 |
| III | 4.89 (1.76–13.6) | 0.002 | 6.91 (1.66–28.7) | 0.01 |
| IV | Not included | 44.1 (10.3–190.0) | <0.001 | |
| Grade | 0.13 | 0.75 | ||
| 3 | 1.50 (0.89–2.55) | 1.09 (0.63–1.88) | ||
| 1 and 2 | Reference group | Reference group | ||
Not considered in the multivariate analysis due to its high correlation with age
Fig. 2.
Comparison of survival outcomes among patients with early stage disease who received neoadjuvant or adjuvant chemotherapy. K-M survival curves of OS (a) and DFS (b) comparing patients who achieved pCR to neoadjuvant chemotherapy, non-pCR to neoadjuvant chemotherapy and those who received adjuvant chemotherapy alone are shown
In multivariate analysis, pathological stage was the only significant independent predictor of DFS and OS. The survival differences between races remained insignificant for DFS (HR 0.91, p = 0.64) and OS (HR 1.19, p = 0.39) after adjusting for pathological stage (Table 3).
Table 3.
Multivariate analysis of variables with respect to survival outcomes
| Variable | DFS |
OS |
||
|---|---|---|---|---|
| HR (95 % CI) | p | HR (95 % CI) | p | |
| Race | 0.64 | 0.39 | ||
| African American | 0.91 (0.62–1.35) | 1.19 (0.80–1.78) | ||
| Caucasian | Reference group | Reference group | ||
| Age (per 1 yr increase) | Not included | 1.01 (1.00–1.03) | 0.08 | |
| Pathological stage | <0.001 | <0.001 | ||
| pCR | Reference group | Reference group | ||
| I | 1.55 (0.55–4.39) | 0.41 | 2.50 (0.59–10.64) | 0.22 |
| IIA | 1.66 (0.59–4.71) | 0.34 | 1.46 (0.33–6.45) | 0.61 |
| IIB | 3.16 (1.09–9.17) | 0.03 | 3.93 (0.91–17.09) | 0.07 |
| III | 4.88 (1.75–13.60) | 0.003 | 7.17 (1.72–29.9) | 0.01 |
| IV | Not included | 39.9 (9.04–169.0) | <0.001 | |
Discussion
The purpose of this study was to examine differences in clinical presentation, treatment, and survival between African American and Caucasian women diagnosed with TNBC who presented for medical oncology therapy in a single academic center. The survival outcomes assessed included DFS, OS, and OS after recurrence. Our study indicated no racial differences in survival outcomes between African American and Caucasian women with TNBC and suggested that TNBC in African American women is not intrinsically different from that in Caucasians.
Previous studies that focused on racial disparities in outcomes of TNBC yielded inconsistent data, some suggested a worse outcome in the African American population [21, 22, 24], others did not [28–30] (Table 4). Our data support the conclusion that TNBC does not have worse survival outcomes for African American women when compared to Caucasians. Several factors were different between some of the prior studies and ours. One, we had a self-selected patient population who sought medical oncology treatment at a single medical center. As shown in Table 1, the median time from an abnormal imaging study to diagnostic biopsy, stage at presentation, and treatment with chemotherapy were similar between races. Therefore, diagnostic delays and treatment differences reported in other studies were not apparent in our patient population. In two recent studies, by Dawood et al. and Sparano et al. [28, 30], patients received the same treatments and there was no racial difference in TNBC survival outcomes. These results, coupled with ours, suggest that when treatment is similar between races, that there is no racial difference in TNBC survival. This argues against the idea that a biological reason explains racial differences in survival seen in earlier studies and suggests that disparities in management may have affected outcomes.
Table 4.
Studies comparing survival outcomes between races for women with TNBC
| Study | Study population | Period diagnosed | Median age in years (range)a | Patient (n)a | TNBC or Basal-like (n) | Median F/u in years (range)a | Outcome |
|---|---|---|---|---|---|---|---|
| Lund et al. [21] | 3 counties in metropolitan Atlanta, GA (multi-center population-based, case control study) | May 1990–Dec 1992 | 20–54 | 476 | 135 AA: 56 CA: 79 |
11.4 | HR for OS 2.0 (95 % CI 1.0–3.7) for AA (adjusted for age, stage, grade, poverty index, treatment and treatment delay) |
| O'Brien et al. [29] | 24 counties of eastern and central North Carolina (multi-center, population-based, case control study), enriched for AA and age < 50 years | 1993–2001 | 20–74 | 1,149 | 197 AA: 117 CA: 80 |
9 (0.2–13.7) | HR for breast cancer specific survival 1.3 (95 % CI 0.8–2.3) for AA (adjusted for age, date and stage of diagnosis) |
| Sparano et al. [30] | Participants in a randomized adjuvant chemotherapy trial E1199 | Oct 1999–Jan 2002b | 51 (19–84) | 4, 817 | 886 AA:129 Non-AA: 757 |
7.9 (0–9.9)c | HR for breast cancer specific survival 0.81 (95 % CI 0.51–1.28, p = 0.37) for AA |
| Bauer et al. [22] | A population-based registry covering the state of California | Jan 1999–Dec 2003 | TNBC median 54, other breast cancer types median 60 | 51,074 | 6,370 AA: 636 CA: 3959 |
N/A | 5 yr survival: AA 14 % stage III/IV, CA 36 % stage III/IV, Hispanic 37 % stage III/IV |
| Dawood et al. [28] | Prospective cohort study of database patients at MD Anderson Cancer Center | 1996–2005 | AA: 47 (22–75) Other: 48 (25–78) | 471 | 471 AA: 100 Other: 371 |
2 (0.02–9.75) | HR for DFS 1.08 (95 % CI 0.69–1.68) for CA. HR for OS 1.08 (95 % CI 0.69–1.68, p = 0.735) for CA |
| Sachdev [24] | Retrospective cohort study of patients presenting to the University of Tennessee Cancer Institute | Sep 2003–Dec 2008 | 51 (26–82) | 124 | 124 AA: 88 CA: 36 |
1.91 (0.08–10.3) | HR for DFS 0.62, p = 0.29 for CA, HR for breast cancer specific survival 0.36, p = 0.18 for CA |
| Pacheco et al. (current study) | Retrospective cohort study of patients presenting to the Washington University Saint Louis Breast Oncology Clinic | Jan 1st 2006–Dec 16th 2010 | 53 (23–98) | 490 | 490 AA: 146 CA: 344 |
2.26 (0.01–16.2) | HR for DFS 0.91 (CI 0.62–1.35) p = 0.64 for AA, HR for OS 1.19 (CI 0.80–1.78) p = 0.39 for AA (adjusted for age and stage) |
Values represent those for all breast cancer subtypes combined
Enrolled during this time period for adjuvant therapy, not diagnosed during this time period
Median follow-up for surviving patients
Second, this study has a large sample size compared to many of the previous studies, which allowed an adequate power for the intended comparison. Many of the prior studies, with the exception of the studies by Dawood et al. [28], Sparano et al. [30], and the California Cancer Registry [22], only had approximately 100–200 TNBC patients [21, 24, 29]. Thus, some of these earlier, smaller studies, may not be as representative of the TNBC population.
Third, the vast majority of patients in our study (85.1 %) were diagnosed from 2006 onwards. This is in contrast to some of the prior studies, in which patients were diagnosed during the 1990s. Diagnostic and treatment differences in these time periods could have contributed to differences in outcomes. This is supported by a French study that showed improved disease-specific and event-free survival for breast cancer patients when comparing later to earlier treatment time periods (1990–1993, 1994–1997, and 1998–2001) [31]. In addition, some of the studies assessing patients diagnosed in the early 1990s may have selected patient populations before the full effect of mammographic screening had a chance to take place [32]. Also, environmental (e.g., diet, exercise, radiation or chemical exposure) and cultural differences (e.g., beliefs of cancer risk, beliefs surrounding the utility of mammography, perceived levels of discomfort of mammography, fear of cancer discovery, and fatalistic views of cancer diagnosis) during earlier and later diagnostic time periods could have contributed to different outcomes [33, 34].
One potential limitation of our study is the relatively short follow-up. Although comparable or longer in duration than those reported by Dawood et al. [28] and Sachdev et al. [24], the median follow-up of our study was shorter than several previous studies that included breast cancers of various receptor subtypes [21, 22, 29, 30] (Table 4). However, since TNBC has an early relapse pattern, with the highest risk of relapse within the first 2–3 years of diagnosis [26, 33], the median follow-up of 27.2 months in our study is likely to have captured most recurrence outcomes. In addition, we took a time-to-event analysis approach in the assessment of OS and DFS, which automatically accounts for the length of follow-up time. In such a time-to-event setting, the statistical power was determined primarily by the number of events, and a considerable proportion of events were detected in our study (120 deaths due to any cause and 134 recurrences).
In our study, achieving pCR to neoadjuvant chemotherapy was associated with a significantly improved DFS and OS. There was no difference in the rate of pCR between African American and Caucasian women. These results are in agreement with those reported by Dawood et al. [28] and are consistent with the well accepted observation that achieving pCR is predicative of improved survival outcomes for patients with TNBC. Another finding is that patients who did not achieve pCR experienced worse survival outcomes compared to those who received only adjuvant chemotherapy. Similar results have been observed in other population studies [34]. This is likely due to the higher tumor stage of those who went on neoadjuvant versus adjuvant chemotherapy. In our study, 39.1 % of patients who received neoadjuvant chemotherapy without a pCR had pathologic stage III disease, which is significantly higher than the 15.5 % of stage III disease in those who received only adjuvant chemotherapy (p < 0.0001).
In summary, our study suggests that African American women with TNBC have similar outcomes as Caucasian women in a single center study where access to care and treatment are similar. These results are in agreement with three other studies [28–30]. Our data suggests there is likely no biological difference between races for TNBC tumors. Future studies comparing the molecular characteristics of TNBC from different races are needed.
Acknowledgments
We would like to thank patients who participated in this study and physicians, nurses, and research coordinators at Washington University Breast Cancer Oncology Clinic for their care of these patients. The authors also wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
This study was partly presented at the 2012 Annual Meeting of the American Society of Clinical Oncology, 1–5 June, 2012, Chicago, IL, USA.
Conflict of interest All authors disclose no conflict of interest.
Contributor Information
Jose M. Pacheco, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, USA
Feng Gao, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO, USA.
Caroline Bumb, Section of Breast Oncology, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 South Euclid Avenue, P.O. Box 8056, St. Louis, MO 63110, USA.
Matthew J. Ellis, Section of Breast Oncology, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 South Euclid Avenue, P.O. Box 8056, St. Louis, MO 63110, USA Alvin J. Siteman Cancer Center, Washington University School of Medicine, St. Louis, MO, USA.
Cynthia X. Ma, Section of Breast Oncology, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 South Euclid Avenue, P.O. Box 8056, St. Louis, MO 63110, USA Alvin J. Siteman Cancer Center, Washington University School of Medicine, St. Louis, MO, USA.
References
- 1.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 2.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88(1):114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Parker SL, Davis KJ, Wingo PA, Ries LA, Heath CW., Jr Cancer statistics by race and ethnicity. CA Cancer J Clin. 1998;48(1):31–48. doi: 10.3322/canjclin.48.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 5.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. NEJM. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 6.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 7.Moskowitz MA, Ash A, Shwartz M, Marwill SL, Freund KM, McCarthy EP, Burns RB. Black women receive less mammography even with similar use of primary care. Ann Intern Med. 1996;125(3):173–182. doi: 10.7326/0003-4819-125-3-199608010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 9.Furberg H, Millikan R, Dressler L, Newman B, Geradts J. Tumor characteristics in African American and white women. Breast Cancer Res Treat. 2001;68(1):33–43. doi: 10.1023/a:1017994726207. [DOI] [PubMed] [Google Scholar]
- 10.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 11.Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR, Neugut AI. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 12.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279(22):1801–1807. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 13.McWhorter WP, Mayer WJ. Black/white differences in type of initial breast cancer treatment and implications for survival. Am J Public Health. 1987;77(12):1515–1517. doi: 10.2105/ajph.77.12.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 15.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 16.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Bindman R, Miglioretti DL, Lurie N, Abraham L, Barbash RB, Strzelczyk J, Dignan M, Barlow WE, Beasley CM, Kerlikowske K. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 18.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 19.Trock B, Rimer BK, King E, Balshem A, Cristinzio CS, Eng-strom PF. Impact of an HMO-based intervention to increase mammography utilization. Cancer Epidemiol Biomarkers Prev. 1993;2(2):151–156. [PubMed] [Google Scholar]
- 20.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 21.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, Flagg EW, O'Regan RM, Gabram SG, Eley JW. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 22.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 23.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 24.Sachdev JC, Ahmed S, Mirza MM, Farooq A, Kronish L, Jahanzeb M. Does race affect outcomes in triple negative breast cancer? Breast Cancer (Auckl) 2010;4:23–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 26.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 27.Peddi PF, Ellis MJ, Ma C. Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer. 2012;2012:217185. doi: 10.1155/2012/217185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawood S, Broglio K, Kau SW, Green MC, Giordano SH, Meric-Bernstam F, Buchholz TA, Albarracin C, Yang WT, Hennessy BT, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27(2):220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104(5):406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallol N, Desandes E, Lesur-Schwander A, Guillemin F. Disease-specific and event-free survival in breast cancer patients: a hospital-based study between 1990 and 2001. Rev Epidemiol Sante Publique. 2006;54(4):313–325. doi: 10.1016/s0398-7620(06)76727-x. [DOI] [PubMed] [Google Scholar]
- 32.Schootman M, Jeffe D, Reschke A, Aft R. The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast Cancer Res Treat. 2004;85(3):219–222. doi: 10.1023/B:BREA.0000025410.41220.67. [DOI] [PubMed] [Google Scholar]
- 33.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong Y-N, Blayney DW, Niland JC, Winer EP, Weeks JC. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher CS, Ma CX, Gillanders WE, Aft RL, Eberlein TJ, Gao F, Margenthaler JA. Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann Surg Oncol. 2011;19(1):253–258. doi: 10.1245/s10434-011-1877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]