Abstract
Background
Percutaneous access for mitral interventions is currently limited to transapical and transseptal routes, both of which have shortcomings. We hypothesized that the left atrium could be accessed directly through the posterior chest wall under imaging guidance.
Methods and Results
We tested percutaneous transthoracic left atrial access in 12 animals (10 pigs and 2 sheep) under real-time MRI or X-ray fluoroscopy plus C-arm CT guidance. The pleural space was insufflated with CO2 to displace the lung, an 18Fr sheath was delivered to the left atrium, and the left atrium port was closed using an off-the-shelf nitinol cardiac occluder. Animals were survived for a minimum of 7days. The left atrium was accessed and the port was closed successfully in 12/12 animals. There was no procedural mortality and only one hemodynamically insignificant pericardial effusion was observed at follow-up. We also successfully performed the procedure on three human cadavers. A simulated trajectory to the left atrium was present in all of 10 human cardiac CT angiograms analyzed.
Conclusions
Percutaneous transthoracic left atrium access is feasible without instrumenting the left ventricular myocardium. In our experience, MRI offers superb visualization of anatomic structures with the ability to monitor and address complications in real-time, although X-ray guidance appears feasible. Clinical translation appears realistic based on human cardiac CT analysis and cadaver testing. This technique could provide a direct non-surgical access route for future transcatheter mitral implantation.
Keywords: structural heart disease, magnetic resonance imaging, cardiac valvular surgery, mitral valve, transapical, interventional MRI, transcatheter mitral valve replacement, percutaneous mitral valve repair
Transcatheter mitral valve-in-valve or valve-in-ring implantation is feasible using prostheses designed for the aortic valve1, 2. Implantation in the native mitral annulus presents distinct challenges: available aortic prostheses are too small, valve fixation is difficult because the annulus is elastic, and the sub-valvular apparatus, which plays an important role in left ventricular function, should not be disrupted. At least four dedicated devices have undergone early human testing3–5. These are bulky and require large caliber access ports (up to 32Fr), mostly transapical.
Whether transapical access is associated with higher mortality than transfemoral remains unclear6–8. The higher mortality reported in some studies may reflect inclusion of higher risk patients or operator experience. Nonetheless, magnetic resonance imaging (MRI) and echocardiography detect apical wall motion abnormalities after transapical access, particularly in patients with increased left ventricle (LV) diameter, which can lead to long-term reduction in global LV function9–11. In the PARTNER trial quality-of-life assessment, transcatheter aortic valve replacement via transapical approach demonstrated no benefit compared with conventional surgery12. Morbidity and mortality are likely even higher in patients with mitral valve disease because of preexisting LV dysfunction. Truly percutaneous transapical access using nitinol devices for closure is possible13, but complications do occur including pneumothorax, cardiac tamponade, LV pseudoaneurysm and hemothorax related to coronary or intercostal vessel laceration or bleeding from the LV puncture site14.
Alternative approaches have been explored for mitral valve interventions: direct trans-atrial via mini-thoracotomy15, transjugular transseptal16, 17, and transfemoral transseptal18. However, a mini-thoracotomy still confers surgical morbidity. Transseptal delivery of large mitral implants has been demonstrated, but achieving coaxiality with the mitral valve can remain challenging. A ‘straight shot’ to the mitral valve that permits large sheath access but does not violate the LV myocardium would be desirable, and could reduce the engineering constraints of miniaturization, reduce procedural complexity and improve patient outcomes.
Percutaneous left atrial (LA) access was first performed in the 1950s using long needles through the posterior chest wall to sample pressure 19, 20. At first glance, delivering large sheaths via this approach appears challenging because of interposed lung, but there is extensive surgical evidence that temporarily collapsing a lung to perform an intra-thoracic intervention is safe21. In fact, diagnostic thoracoscopy with iatrogenic lung deflation is commonly performed in awake patients and confers extremely low morbidity and mortality22. Percutaneous transthoracic cardiac catheterization has also been performed in children with no alternative access, through the anterior chest into the pulmonary venous atrium and through the lower back into the inferior vena cava23, 24.
We hypothesized that with imaging guidance and percutaneous techniques, it is possible to access the LA directly through the posterior chest wall by first displacing a lung with gas, then delivering a large sheath, and finally closing the LA port using off-the-shelf nitinol cardiac occluder devices. Compared with percutaneous transapical LV closure, we believe that closing a port in the lower pressure LA may be preferable. Because of anatomic differences between large mammals and human, we tested this hypothesis in two different large animal models (porcine and ovine) and then explored feasibility of clinical translation with human cardiac computed tomography (CT) analysis and human cadaver testing. We also explored different image guidance modalities, MRI and X-ray fluoroscopy, to simplify translation into patients.
Methods
Animal experiments
The institutional animal care and use committee approved all procedures, which were performed according to contemporary NIH guidelines. Animals were anesthetized with ketamine (25mg/kg), midazolam (15mg/kg) and glycopyrrolate (0.01mg/kg), and maintained with on sevoflurane (1–4%) with and mechanical ventilation. Femoral arterial and venous access was obtained with ultrasound guidance with animals supine.
The technique was developed in non-survival experiments on 10 naïve Yorkshire swine, not further described here. Subsequently, survival experiments were performed in 10 naïve Yorkshire swine with median bodyweight 51kg (47–54kg) and 2 naïve Dorset sheep (28kg, 36kg), all of which were survived for at least 7days before euthanasia and necropsy.
Imaging
Experiments were performed first using MRI at 1.5T (Aera, Siemens) and later using biplane X-ray fluoroscopy enhanced by C-arm CT (Artis Zee and DynaCT, Siemens). Experiments are summarized in Figure 1. For MRI, trajectories were planned on isotropic 3D images, and the procedure was performed using custom MRI-antenna needles25 and real-time MRI at frame rates up to 15fps. MRI parameters and devices are provided in the data supplement.
Figure 1.
Experiment design
Pericardial auto-transfusion catheter placement
An 8.3Fr multi-sidehole subxiphoid pericardial drain was placed after trans-atrial microcatheter CO2 insufflation as described26, and connected to the femoral vein to facilitate immediate auto-transfusion for blood salvage. 500–1000IU of unfractionated heparin was infused into the pericardial space to prevent in situ thrombus.
Pleural CO2 insufflation to displace lung
A needle was advanced through the left lateral chest wall during small puffs of iodinated or gadolinium contrast (for X-ray or MRI visualization, respectively) until the pleural space was entered (Figure 2). This was exchanged for an 8.3Fr multi-sidehole pleural drain positioned in a dependent position to aspirate fluid but not CO2. As with the pericardium, 500–1000IU of unfractionated heparin was infused into the pleural space to prevent in situ thrombus formation and facilitate auto-transfusion. The pleural space was insufflated with CO2 titrated to displace lung sufficiently to allow a clear trajectory to the LA. After insufflation, the ventilation rate was increased and tidal volumes reduced to keep airway pressure low.
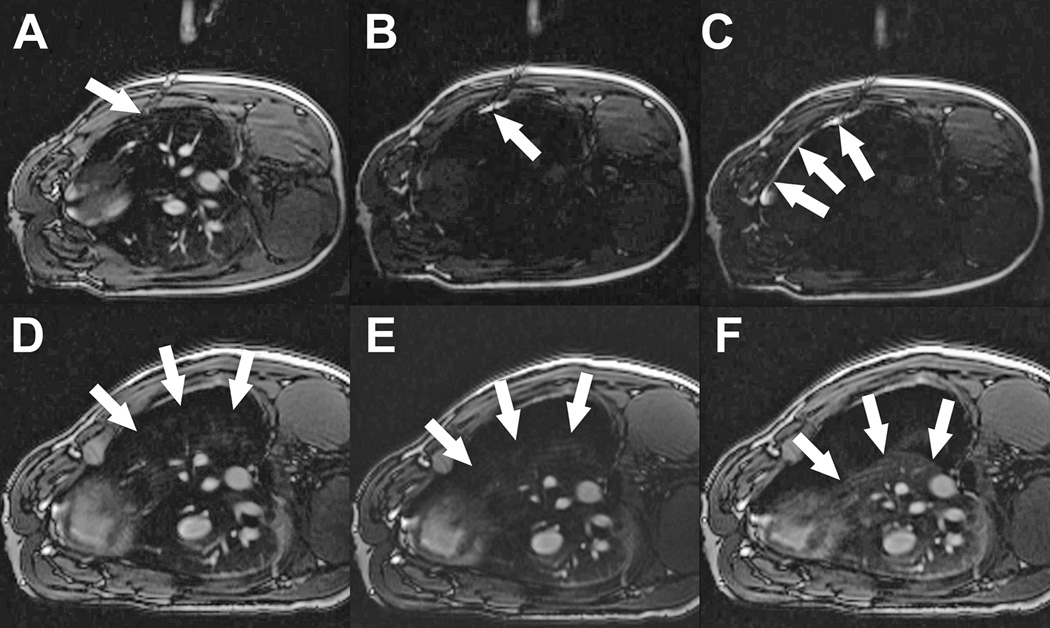
Figure 2. MRI guided pleural insufflation to displace lung.
(A) Needle (arrow) is advanced through the chest wall towards the pleura. (B) ‘Saturation mode’ MRI darkens tissue to enhance visibility of gadolinium contrast. Small aliquots of contrast injected through the needle (arrow) show that the tip is still within the chest wall. (C) After advancing the needle further, contrast flows freely around the lung in the pleural space (arrows). Saturation mode MRI is then toggled off. (D, E, F) After insertion of a drain, the pleural space is insufflated with CO2 despite positive pressure ventilation to deflate the lung. Arrows indicate lung margin during progressive pleural insufflation.
Transthoracic left atrial trajectory planning and access
3D MRI or X-ray (C-arm CT and biplane fluoroscopy contrast angiograms) of the LA was acquired at baseline and after pleural insufflation. For X-ray guidance we used a superimposed C-arm CT of LA and LV angiograms (syngo InSpace EP, Siemens). Swine and sheep were positioned respectively on the right side or prone, to optimize working angles. A 20cm needle was used to puncture through the posterior or lateral chest wall, pass through the empty pleural space, and enter the LA posteriorly (Figure 3, Figure 4). Needle tip position was confirmed by atrial pressure waveform and contrast injection. A stiff nitinol guidewire (Nitrex, Covidien) was introduced through the mitral valve to the LV apex, over which a 18Fr sheath (outer diameter 7mm, Large Check-Flo, Cook) and a custom-shortened dilator was advanced into the LA without pre-dilatation. Sheath position and relation to the mitral valve were confirmed using MRI or biplane contrast angiography.
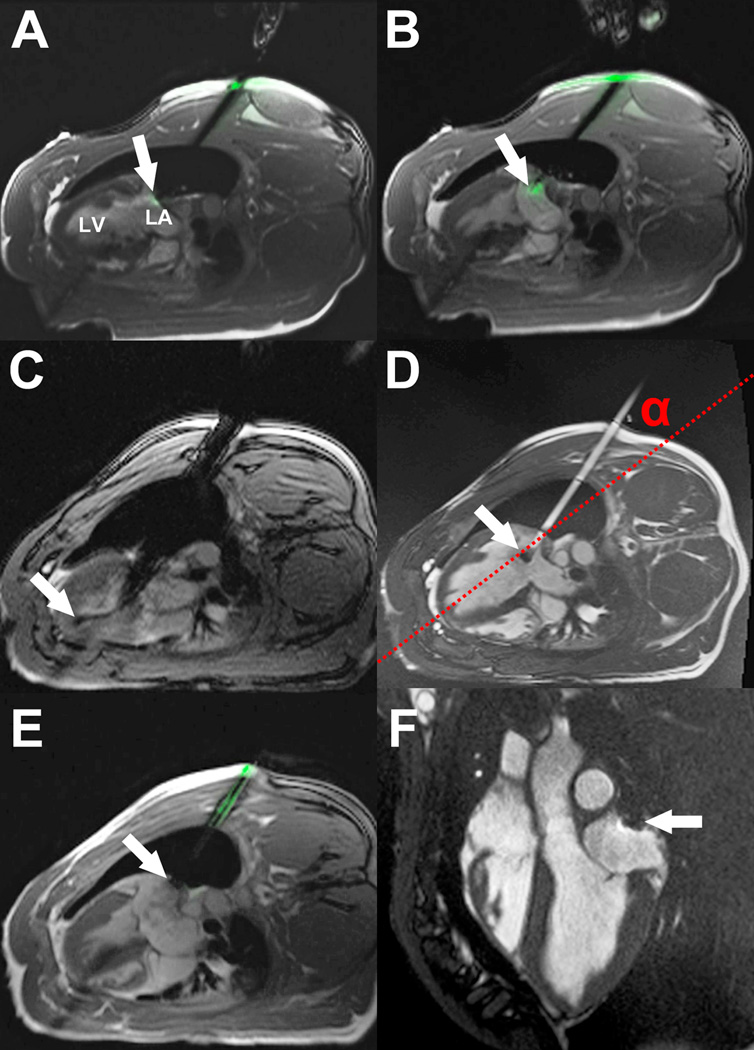
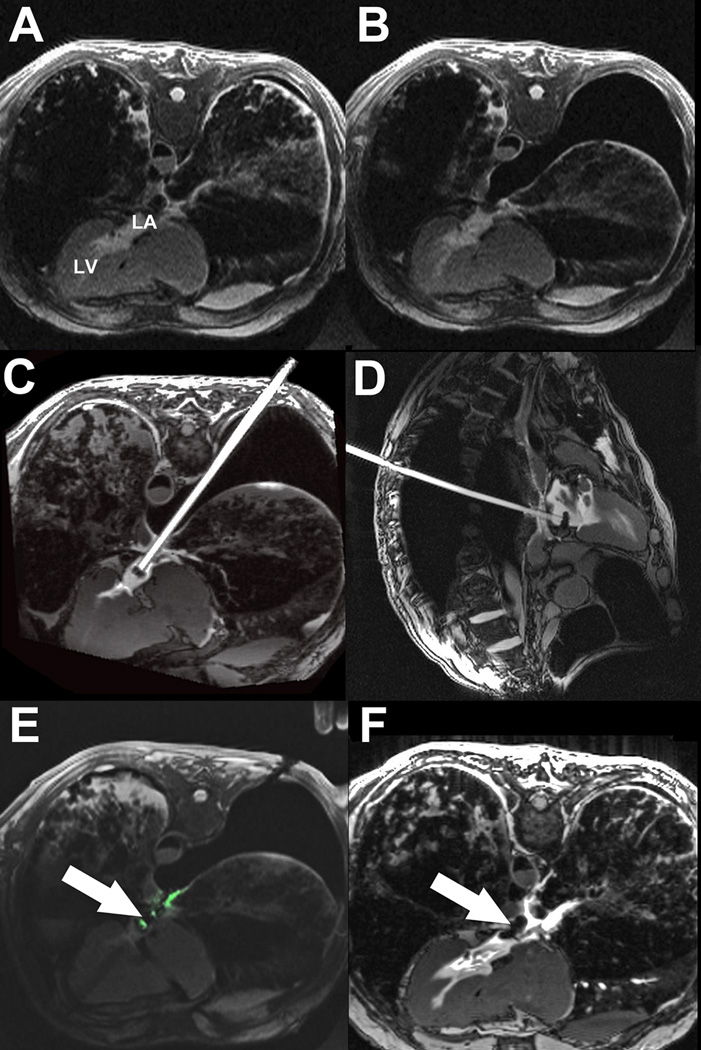
Figure 3. Real-time MRI guided left atrial access and closure in swine.
(A) An active visualization MRI needle incorporating MRI electronics for enhanced visibility, is introduced through the chest wall into the empty pleural space. The tip is positioned against the posterior LA wall (arrow). (B) LA puncture: the active needle tip is clearly seen within the LA (arrow). (C) Delivery of a large 18Fr sheath into the LA. The guidewire is visible in the apex of the LV (arrow). (D) Filled with gadolinium contrast, the sheath trajectory to the LA is apparent. There is a passive marker at the tip of the sheath (arrow). The angle α represents the offset between the sheath trajectory and the long axis of the LV (dotted red line). (E) A nitinol cardiac occluder device is loaded onto an active delivery cable, which incorporates MRI electronics for visualization. The distal disk is deployed within the LA and pulled back against the posterior wall (arrow). The proximal disk is deployed outside the heart. (F) 7 days later, the nitinol cardiac occluder device remains in the intended position, with no pericardial effusion.
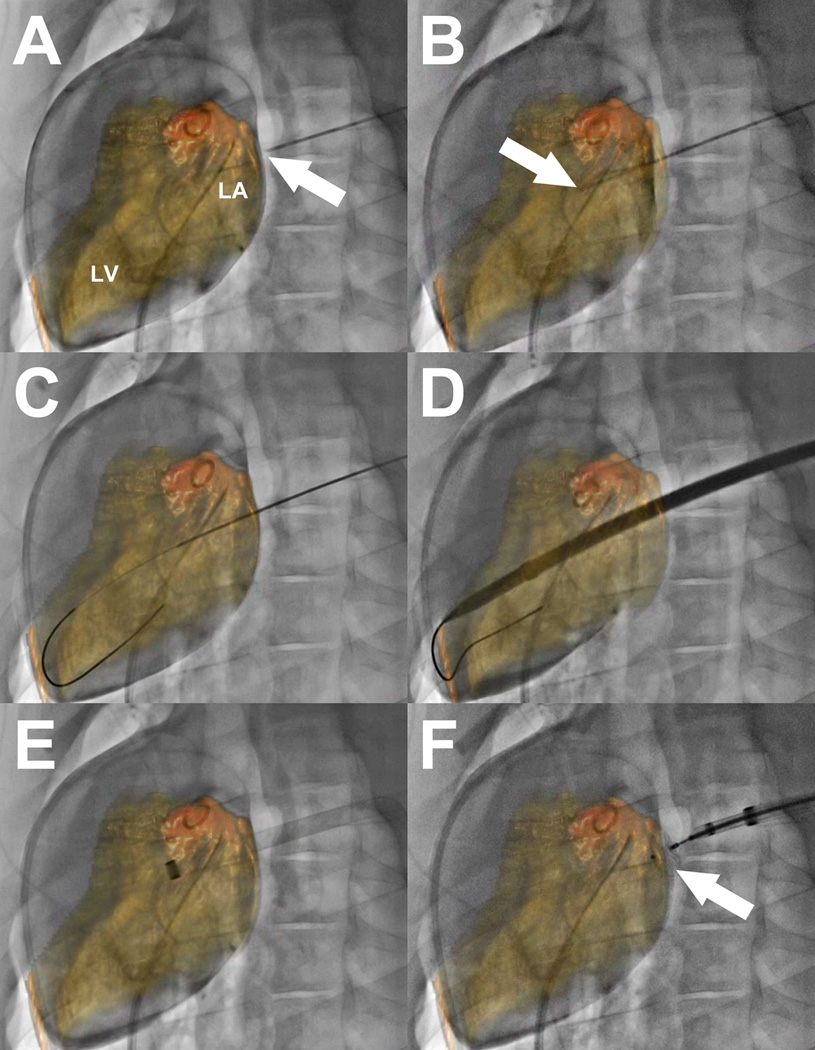
Figure 4. X-ray fluoroscopy and C-arm CT guided left atrial access and closure in swine.
(A) A pigtail catheter is positioned in the LA via transseptal puncture. Contrast-enhanced cone-beam angiography is performed and LA and LV are segmented and overlaid on the fluoroscopic images. The needle tip (arrow) for LA puncture is positioned against the posterior wall of the LA. (B) The needle (arrow) is advanced into the LA. (C) A stiff wire is introduced through the mitral valve into the LV apex. (D) A large sheath is advanced over the wire into the heart. (E) After withdrawing the sheath dilator, the sheath tip is confirmed within the LA. (F) The LA port is closed with a nitinol cardiac occluder device (arrow).
Left atrial closure
The LA port was closed with an off-the-shelf 5 or 6mm nitinol atrial septal occluder (St Jude Medical, MN; Lepu Medical, Beijing, China). The distal disc was deployed within the LA and the sheath and delivery catheter were withdrawn in tandem until the disc was in apposition with the LA wall. The proximal disc was deployed outside the heart within the pleural space and the device was released.
Human cadaver study and human CT analysis
To explore feasibility for humans, we tested percutaneous transthoracic LA access and closure in three human cadavers (one under MR and two under X-ray guidance). After femoral access, the cadavers were rolled prone and all procedural steps were identical to those previously described for animal procedures, using a larger sheath (Ascendra, Edwards Lifesciences, 26Fr/30Fr inner/outer diameter).
We also examined 10 random cardiac CT angiograms having full chest wall coverage among patients with mitral valve disease, dilated LA, or previous sternotomy in the NHLBI anonymized and de-linked database. This does not constitute human subjects research under US 45CFR§46.102(f). Each CT was examined for potential trajectories to the LA.
Statistical analysis
Hemodynamic data are reported in the Table as median with first and third quartiles (Q1–Q3). Each hemodynamic parameter was analyzed in a linear mixed model with ARMA(1,1) correlation structure assumed within each animal. The model fit mean levels of each parameter at each of the four post-baseline follow-up measurements with baseline as the reference. Since there were four follow-up measures for each of three hemodynamic parameters we used a Bonferroni correction factor of 12 to account for multiple comparisons. A multiplicity-corrected p value ≤0.05 was considered statistically significant.
Results
Pericardial access for auto-transfusion catheter placement
Trans-atrial pericardial access with a 2.8Fr microcatheter, insufflation of the pericardial space with CO2, placement of a sub-xiphoid 8.3Fr drain and withdrawal of the trans-atrial microcatheter was uncomplicated in all 12 animals.
Pleural insufflation
Pleural access and left lung deflation with CO2 was uncomplicated in all 12 animals. 600–900mL of CO2 was required to displace the lung sufficiently to establish an unobstructed trajectory to the LA in both species. Pleural pressures remained low (8–10mmHg) and, despite lung displacement, ventilation was well tolerated with no increase in expired CO2 or evidence of hemodynamic compromise (Table). Reductions in mean arterial pressure and heart rate were observed after pleural insufflation, which were likely caused by an increase in the inhaled anesthetic agent concentration required to allow for the reduction in ventilator tidal volumes. However, these changes were not statistically significant.
Table. Hemodynamics.
Mean arterial pressure, heart rate and expired CO2 in swine (n=10) undergoing direct percutaneous LA access. The two sheep were smaller animals with lower baseline blood pressure so were excluded from the analysis of hemodynamics. No differences retained statistical significance after correction for multiplicity.
| n=10 swine | MAP (mmHg) |
HR (bpm) |
Expired CO2 (mmHg) |
|---|---|---|---|
| Baseline | 61(53–74) | 96(77–102) | 29(27–32) |
| After lung deflation | 57(46–66) | 88(72–98) | 28(26–29) |
| After insertion of left atrial sheath | 52(45–64) | 87(69–97) | 29(27–31) |
| After closure | 50(47–59) | 81(67–91) | 31(29–33) |
| Follow up | 56(48–72) | 102(84–113) | 30(30–33) |
Data are presented as median (Q1–Q3). MAP: mean arterial blood pressure; HR: heart rate.
Trajectory planning
In swine, pleural insufflation caused the heart to rotate, displacing the long axis of the LV by 7° (6–14°) compared with baseline. A direct trajectory to the LA, avoiding important anatomic structures was achievable in all animals. Distance from skin to LA was 10cm (9–11cm) in swine and 11cm in both sheep.
Transthoracic left atrial access and closure
The LA was successfully accessed in 10/10 swine, including 8 under MR guidance (Figure 3, Video 1) and 2 under X-ray guidance (Figure 4, Video 2). All succeeded in a single needle pass except in one, wherein a custom needle that had become ‘dull’ required a second pass. The stiff nitinol guidewire was positioned in the LV apex, over which the 18Fr sheath was advanced without pre-dilation. Sheath advancement displaced the LA wall by 25mm (20–30mm) (under X-ray guidance), but the wall recoiled as soon as the sheath tip entered the LA. No pericardial or pleural effusions developed during LA puncture or while the large sheath remained in place. 3D and cine MRI confirmed that the sheath trajectory was offset relative to the long axis of the LV by an angle α of up to 30° (Figure 3). The LA port was successfully closed with a single nitinol cardiac occluder device in all animals. During sheath withdrawal and LA port closure, accumulated blood was auto-transfused intermittently using the pre-positioned pericardial and pleural drains. Median pericardial and pleural auto-transfusion volumes were 55mL (40–73mL) and 10mL (10–75mL) respectively. Animals were stable hemodynamically throughout and no arrhythmias were observed (Table).
The LA was successfully accessed with a single pass and closed under MRI guidance in 2/2 sheep. One animal had minimal pericardial effusion (50mL). The second required higher than expected intra-procedural auto-transfusion (300mL) without any hemodynamic compromise. Pleural effusion was negligible in both animals (10ml and 20mL).
There was no procedural mortality and all animals recovered uneventfully. At the end of the procedure, the pericardial and pleural spaces were aspirated to dryness, any remaining pleural CO2 was aspirated and the drains were withdrawn.
Follow up
No complications were observed during the follow up period of 7.5days (7–8.5days). Follow up MRI confirmed stable position of the LA occluder device in all animals. 12/12 animals had no pleural effusion. 11/12 animals had no detectable or trace pericardial effusion 0mL(0–6mL). One pig, in which the LA was punctured twice, had a large but hemodynamically insignificant pericardial effusion (660mL) at 7days follow up. No other complications were observed in any animal and all skin incisions healed well. On necropsy, the nitinol cardiac occluder device was well seated and fibrosed to the LA wall in all animals (data not shown).
Human CT analysis
Cardiac CT angiograms from 10 patients (age 68years (59–73years), 8/10 male) with full chest wall coverage were examined. A direct trajectory to the LA through the right posterior chest wall was achievable in all (Figure 5, Video 3). The right lung would need to be displaced by pleural insufflation, but the trajectory avoided important structures including descending aorta and esophagus. The mean angle α between the achievable trajectory and the true long axis of the LV was 5° (4–7°). Mean intercostal distance at the point of entry through the chest wall was 12mm (11–15mm).
Figure 5. Human cardiac CT angiography analysis.
Axial (A) and sagittal (B) CT cross sections showing simulated trajectory to the left atrium (red line). The angle α represents the offset between the simulated trajectory and the true long axis of the LV. (C) 3D reconstruction showing skin entry point (yellow arrow). The LA is highlighted in green and the esophagus in purple.
We also tested whether there was a trajectory to the mitral valve from a right mini thoracotomy incision, entering the right atrium, and passing through the interatrial septum. In all 10 patients this was achievable but the offset angle between the trajectory and the true long axis of the LV was 56° (51–58°).
Human cadaver study
In the prone position, right pleural insufflation and deflation of the right lung was straightforward whether under MR or X-ray guidance. This exposed a direct trajectory to the posterior wall of the LA avoiding other important structures, such as aorta and esophagus in all three cadavers. The LA was successfully entered and a large 26Fr sheath was delivered. Pleural insufflation did not cause the heart to shift orientation within the thorax. As a result, the sheath trajectory remained well aligned with the long axis of the LV. The LA port was closed using a nitinol cardiac occluder device and the right lung was re-inflated by aspirating the pleural CO2 (Figure 6, Video 4).
Figure 6. Realtime MRI guided left atrial access and closure in a human cadaver.
(A) Axial MRI before lung deflation. (B) MRI in the same plane after right lung deflation. An unobstructed trajectory to the LA is apparent. (C) Axial MRI of large sheath in the LA filled with gadolinium contrast. (D) Sagittal MRI of sheath in the LA. (E) Deployment of a nitinol cardiac occluder device (arrow). The delivery cable incorporates MRI electronics to enhance device visualization. (F) After lung re-inflation, the device can be clearly seen across the posterior wall of the LA (arrow).
Discussion
We describe a novel percutaneous technique to access the left heart to deliver interventional devices. The technique combines several innovations: (1) Fully percutaneous transthoracic LA access with a needle followed by a large sheath; (2) Pericardial auto-transfusion catheter placement; (3) Pleural insufflation and lung displacement to create an unobstructed trajectory to the LA; and (4) Closure of the LA port using off-the-shelf nitinol cardiac occluder devices. We demonstrated pre-clinical feasibility in two large animal models (porcine and ovine) and feasibility of clinical translation through performing the procedure in human cadavers and simulating on human cardiac CT images. Real-time MRI provided optimum usability, for example continuous depiction of the deflated lung, but X-ray fluoroscopy assisted by C-arm CT provided adequate confirmation of lung displacement during sheath traversal of the pleural space for LA entry.
Pleural insufflation to create unobstructed trajectory to LA
Thoracoscopy to inspect the pleura, perform biopsy or deliver therapy is an established technique usually performed under moderate sedation27. More advanced procedures, for example lobectomy or management of spontaneous pneumothorax, are typically performed under general anesthesia with dual-lumen endotracheal tube to facilitate single lung ventilation, and have excellent peri-operative outcomes21, 28, 29. However there is evidence that such interventions can be well tolerated in conscious non-intubated patients30 even for long procedures and in older sicker patients31. Based on this extensive clinical experience, there is reason to expect that single lung deflation for the purpose of transthoracic LA access may be tolerated in patients.
Choice of imaging modality to guide LA access
The superior soft tissue visualization and multiplanar viewing capabilities of MRI is appealing to guide minimally invasive interventions, particularly interventions that violate vascular boundaries, in which every anatomic structure traversed must be visualized. MRI provides this capability. In this study, we demonstrated that LA access and closure could be safely performed using real-time MRI guidance. MRI also permitted easy access to the pleural space and titration of pleural insufflation to displace lung. Crucially, MRI afforded continuous monitoring for development of pericardial and pleural effusions during LA puncture and closure. Any accumulation of blood in the pericardial or pleural space was immediately identified and auto-transfused.
Nonetheless, we explored whether the procedure could be performed using X-ray fluoroscopy. A transseptal pigtail catheter was positioned in the LA. Biplane contrast angiography and intra-pericardial iodinated contrast delineated LA anatomy adequately. This enabled a trajectory to the LA to be planned in orthogonal X-ray projections. It was difficult to definitely establish adequate lung displacement using fluoroscopy only, but C-arm CT provided clear visualization of lung tissue and assurance of satisfactory lung displacement. In humans, where the preferred LA puncture site should be posterior and mid-way between left and right pulmonary vein ostia, dual transseptal guidewires in left and right pulmonary veins could offer a precise fluoroscopic target for LA puncture. Echocardiography could provide a substitute for real-time MRI to monitor for pericardial blood accumulation and guide auto-transfusion.
We also explored using color overlays of the LA and LV, segmented from contrast-enhanced C-arm CT using software designed to guide electrophysiological procedures. The fusion images enhanced our ability to predict trajectories and determine optimal LA puncture location (Figure 4). Additionally, the overlays aided in selecting optimal X-ray projection angles and may have reduced overall iodinated contrast usage.
Closure of LA port
There is clinical precedent for the use of off-label nitinol cardiac occluder devices to seal large holes in vascular structures, including the LV apex or abdominal aorta13, 32. Even in the high pressure LV, hemostasis was rapidly achieved with reversal of anticoagulation. Hemostasis should be easier to achieve in the LA because the pressure is much lower than in the LV or systemic arteries, even in patients with mitral valve disease. In some animals, blood did accumulate in the pericardial or pleural space, but was easily managed by aspiration and auto-transfusion, akin to standard surgical blood salvage technique. After aspirating to dryness, all drains were removed post procedure for animal comfort – although in humans they would be left in overnight. Only one large but hemodynamically insignificant pericardial effusion was observed at follow up, in the one animal in which two LA punctures were made because a custom needle had become ‘dull’. The animal recovered uneventfully and the effusion would not have accumulated had the pericardial drain remained in place immediately post-procedure.
Feasibility of clinical translation
We tested LA access and closure in two large animal models in order to determine whether the approach was feasible in different anatomies. The trajectories for unobstructed access to the LA were different in the two species but easily achievable by deflating the left lung. Importantly, all animals were hemodynamically stable with a large sheath in the LA. This approach could circumvent many of the risks associated with trans-apical access in patients with advanced mitral valve disease, in particular acute hemodynamic instability and late LV pseudoaneurysm, ventricular arrhythmias and decrement in LV function.
Human cardiac CT angiogram analysis confirmed that a theoretical trajectory exists in humans. In swine, pleural insufflation rotated the heart in the thorax, which resulted in an offset between the long axis of the heart and the achieved trajectory (Figure 3). Because the orientation of the heart in the human mediastinum is different, the trajectory is different from that in swine or sheep. In humans, the right lung would be deflated to puncture the LA posteriorly between the pulmonary veins (Figure 5). In this configuration, right lung deflation would deflect the right pulmonary veins away from the needle trajectory, exposing the posterior wall of the LA. This trajectory avoids other important structures such as the aorta and esophagus. The mean intercostal distance at the point of entry through the chest wall was sufficient to accommodate a sheath 32Fr or larger.
To demonstrate that this trajectory was feasible, we accessed the LA in human cadavers and delivered a large sheath under either MRI or X-ray guidance (Figure 6). Pleural insufflation did not shift the orientation of the heart within the thorax in cadavers. We then closed the LA port with a nitinol cardiac occluder device and re-expanded the right lung.
Limitations
There are anatomical differences between humans and swine. For example, the descending aorta and esophagus vary in position within the thorax. Procedural tomography (such as C-arm CT or real-time MRI) is therefore recommended for trajectory planning. Pulmonary disease or chronic heart failure could increase the risk of single lung ventilation. Clotting disorders could increase bleeding. Off-label nitinol cardiac occluder devices may not assure hemostasis. Prior cardiothoracic surgery may cause pleural adhesions, which could impede lung displacement. Because our technique involves two points of fixation (chest wall and left atrial wall), sheath maneuverability is constrained. However, because excellent alignment with the LV long axis can be achieved by careful trajectory planning, we do not anticipate that excessive sheath manipulation would be required to achieve coaxial alignment to perform a mitral valve intervention.
Technical considerations for clinical translation
To perform this procedure in patients under X-ray fluoroscopy guidance, we envision the following provisions. (1) The procedure would be performed under general anesthesia with a dual lumen endotracheal tube and/or bronchial blockers to facilitate intentional right lung collapse and single lung ventilation. (2) The patient would be positioned initially supine for standard percutaneous access and then rolled prone for transthoracic atrial access. (3) A temperature or transesophageal echocardiography probe would be used to identify the esophagus. (4) A pericardial drain may not be required in patients with prior sternotomy and pericardial adhesions. (5) Respiration would be suspended during LA puncture. (6) The guidewire would be advanced through the mitral valve into the LV over a balloon catheter to prevent chordal entrapment. (7) Anticoagulation would be reversed with protamine immediately prior to cardiac occluder device deployment. (8) A coaxial buddy wire would be used during LA port closure to allow LA access to be reestablished rapidly in case of inadvertent pull through of the nitinol cardiac occluder device. (9) Pleural and/or pericardial drains would be left in place for a short period after the procedure to drain any effusions.
Conclusion
Fully percutaneous transthoracic LA access is feasible to deliver large interventional devices to the mitral valve without violating the LV myocardium. The procedure can be guided by a variety of imaging modalities, although in our experience MRI guidance offers the greatest visualization of anatomic structures with the ability to monitor and address complications in real-time, for example pericardial effusion. Clinical translation appears feasible based on human cardiac CT analysis and human cadaver testing. This technique could provide a direct and coaxial access route for transcatheter mitral valve interventions in the future.
Supplementary Material
Acknowledgments
We thank Katherine Lucas, Shawn Kozlov and Joni Taylor for their help with animals, and Gaetano Paone and Adam Greenbaum of Henry Ford Hospital for their thoughtful comments.
Sources of Funding
This work was supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (Z01-HL005062 and Z01-HL006040).
Footnotes
Disclosures
None.
References
- 1.Seiffert M, Conradi L, Baldus S, Schirmer J, Knap M, Blankenberg S, Reichenspurner H, Treede H. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv. 2012;5:341–349. doi: 10.1016/j.jcin.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Descoutures F, Himbert D, Maisano F, Casselman F, de Weger A, Bodea O, Van der Kley F, Colombo A, Giannini C, Rein KA, De Bruyne B, Petronio AS, Dahle G, Alfieri O, Vahanian A. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg. 2013;44:e8–e15. doi: 10.1093/ejcts/ezt155. [DOI] [PubMed] [Google Scholar]
- 3.De Backer O, Piazza N, Banai S, Lutter G, Maisano F, Herrmann HC, Franzen OW, Sondergaard L. Percutaneous transcatheter mitral valve replacement: An overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv. 2014;7:400–409. doi: 10.1161/CIRCINTERVENTIONS.114.001607. [DOI] [PubMed] [Google Scholar]
- 4.Cheung A, Stub D, Moss R, Boone RH, Leipsic J, Verheye S, Banai S, Webb J. Transcatheter mitral valve implantation with tiara bioprosthesis. EuroIntervention. 2014;(10 Suppl U):U115–U119. doi: 10.4244/EIJV10SUA17. [DOI] [PubMed] [Google Scholar]
- 5.Bapat V, Buellesfeld L, Peterson MD, Hancock J, Reineke D, Buller C, Carrel T, Praz F, Rajani R, Fam N, Kim H, Redwood S, Young C, Munns C, Windecker S, Thomas M. Transcatheter mitral valve implantation (tmvi) using the edwards fortis device. EuroIntervention. 2014;(10 Suppl U):U120–U128. doi: 10.4244/EIJV10SUA18. [DOI] [PubMed] [Google Scholar]
- 6.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O'Brien S, Holmes D. Outcomes following transcatheter aortic valve replacement in the united states. JAMA. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 7.Schymik G, Wurth A, Bramlage P, Herbinger T, Heimeshoff M, Pilz L, Schymik JS, Wondraschek R, Suselbeck T, Gerhardus J, Luik A, Gonska BD, Posival H, Schmitt C, Schrofel H. Long-term results of transapical versus transfemoral tavi in a real world population of 1000 patients with severe symptomatic aortic stenosis. Circ Cardiovasc Interv. 2015;8:e000761. doi: 10.1161/CIRCINTERVENTIONS.113.000761. [DOI] [PubMed] [Google Scholar]
- 8.Greason KL, Suri RM, Nkomo VT, Rihal CS, Holmes DR, Mathew V. Beyond the learning curve: Transapical versus transfemoral transcatheter aortic valve replacement in the treatment of severe aortic valve stenosis. J Card Surg. 2014;29:303–307. doi: 10.1111/jocs.12323. [DOI] [PubMed] [Google Scholar]
- 9.Meyer CG, Frick M, Lotfi S, Altiok E, Koos R, Kirschfink A, Lehrke M, Autschbach R, Hoffmann R. Regional left ventricular function after transapical vs. Transfemoral transcatheter aortic valve implantation analysed by cardiac magnetic resonance feature tracking. Eur Heart J Cardiovasc Imaging. 2014;15:1168–1176. doi: 10.1093/ehjci/jeu103. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg LM, Tamas E, Vanky F, Engvall J, Nylander E. Differences in recovery of left and right ventricular function following aortic valve interventions: A longitudinal echocardiographic study in patients undergoing surgical, transapical or transfemoral aortic valve implantation. Catheter Cardiovasc Interv. 2013;82:1004–1014. doi: 10.1002/ccd.24812. [DOI] [PubMed] [Google Scholar]
- 11.Barbash IM, Dvir D, Ben-Dor I, Corso PJ, Goldstein SA, Wang Z, Bond E, Okubagzi PG, Satler LF, Pichard AD, Waksman R. Impact of transapical aortic valve replacement on apical wall motion. J Am Soc Echocardiogr. 2013;26:255–260. doi: 10.1016/j.echo.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: Results from the partner (placement of aortic transcatheter valve) trial (cohort a) J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 13.Jelnin V, Dudiy Y, Einhorn BN, Kronzon I, Cohen HA, Ruiz CE. Clinical experience with percutaneous left ventricular transapical access for interventions in structural heart defects a safe access and secure exit. JACC Cardiovasc Interv. 2011;4:868–874. doi: 10.1016/j.jcin.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Dudiy Y, Kliger C, Jelnin V, Elisabeth A, Kronzon I, Ruiz CE. Percutaneous transapical access: Current status. EuroIntervention. 2014;(10 Suppl U):U84–U89. doi: 10.4244/EIJV10SUA12. [DOI] [PubMed] [Google Scholar]
- 15.Bruschi G, Barosi A, Colombo P, Botta L, Oreglia J, De Marco F, Paino R, Klugmann S, Martinelli L. Direct transatrial transcatheter sapien valve implantation through right minithoracotomy in a degenerated mitral bioprosthetic valve. Ann Thorac Surg. 2012;93:1708–1710. doi: 10.1016/j.athoracsur.2011.08.084. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer U, Frerker C, Busse C, Kuck KH. Transjugular and transseptal treatment of a degenerated mitral valve prosthesis with a balloon-expandable biological valve. Heart Lung Circ. 2012;21:836–840. doi: 10.1016/j.hlc.2012.03.125. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko T, Swain JD, Loberman D, Welt FG, Davidson MJ, Eisenhauer AC. Transjugular approach in valve-in-valve transcatheter mitral valve replacement: Direct route to the valve. Ann Thorac Surg. 2014;97:e161–e163. doi: 10.1016/j.athoracsur.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer U, Frerker C, Schewel D, Thielsen T, Meincke F, Kreidel F, Kuck KH. Transfemoral and transseptal valve-in-valve implantation into a failing mitral xenograft with a balloon-expandable biological valve. Ann Thorac Surg. 2012;94:2115–2118. doi: 10.1016/j.athoracsur.2012.04.123. [DOI] [PubMed] [Google Scholar]
- 19.Fisher DL. The use of pressure recordings obtained at transthoracic left heart catheterization in the diagnosis of valvular heart disease. J Thorac Surg. 1955;30:379–392. [PubMed] [Google Scholar]
- 20.Wood EH, Sutterer W, Swan HJ, Helmholz HF., Jr. The technic and special instrumentation problems associated with catheterization of the left side of the heart. Proc Staff Meet Mayo Clin. 1956;31:108–115. [PubMed] [Google Scholar]
- 21.Naunheim KS, Mack MJ, Hazelrigg SR, Ferguson MK, Ferson PF, Boley TM, Landreneau RJ. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg. 1995;109:1198–1203. doi: 10.1016/S0022-5223(95)70203-2. discussion 1203-1194. [DOI] [PubMed] [Google Scholar]
- 22.Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJ, Downer NJ, Gleeson FV, Howes TQ, Treasure T, Singh S, Phillips GD. Local anaesthetic thoracoscopy: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii54–ii60. doi: 10.1136/thx.2010.137018. [DOI] [PubMed] [Google Scholar]
- 23.Maher KO, Murdison KA, Norwood WI, Jr, Murphy JD. Transthoracic access for cardiac catheterization. Catheter Cardiovasc Interv. 2004;63:72–77. doi: 10.1002/ccd.20080. [DOI] [PubMed] [Google Scholar]
- 24.Cheatham JP, McCowan TC, Fletcher SE. Percutaneous translumbar cardiac catheterization and central venous line insertion: An alternative approach in children with congenital heart disease. Catheter Cardiovasc Interv. 1999;46:187–192. doi: 10.1002/(SICI)1522-726X(199902)46:2<187::AID-CCD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Saikus CE, Ratnayaka K, Barbash IM, Colyer JH, Kocaturk O, Faranesh AZ, Lederman RJ. Mri-guided vascular access with an active visualization needle. J Magn Reson Imaging. 2011;34:1159–1166. doi: 10.1002/jmri.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers T, Ratnayaka K, Schenke WH, Faranesh AZ, Mazal JR, O'Neill WW, Greenbaum AB, Lederman RJ. Intentional right atrial exit for microcatheter infusion of pericardial carbon dioxide or iodinated contrast to facilitate sub-xiphoid access. Catheter Cardiovasc Interv. 2014 doi: 10.1002/ccd.25698. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 28.Sedrakyan A, van der Meulen J, Lewsey J, Treasure T. Video assisted thoracic surgery for treatment of pneumothorax and lung resections: Systematic review of randomised clinical trials. BMJ. 2004;329:1008. doi: 10.1136/bmj.38243.440486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 30.Wu CY, Chen JS, Lin YS, Tsai TM, Hung MH, Chan KC, Cheng YJ. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg. 2013;95:405–411. doi: 10.1016/j.athoracsur.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 31.Chen KC, Cheng YJ, Hung MH, Tseng YD, Chen JS. Nonintubated thoracoscopic lung resection: A 3-year experience with 285 cases in a single institution. J Thorac Dis. 2012;4:347–351. doi: 10.3978/j.issn.2072-1439.2012.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenbaum AB, O'Neill WW, Paone G, Guerrero ME, Wyman JF, Cooper RL, Lederman RJ. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: Initial human experience. J Am Coll Cardiol. 2014;63:2795–2804. doi: 10.1016/j.jacc.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.