Abstract
Rationale
Abnormal phenotypic switch of vascular smooth muscle cell (VSMC) is a hallmark of vascular disorders such as atherosclerosis and restenosis after angioplasty. MicroRNAs (miRNAs) have emerged as important regulators for VSMC function, and we recently identified miR-663 as critical for controlling human aortic smooth muscle cell proliferation.
Objective
To investigate whether miR-663 plays a role in human VSMC phenotypic switch and the development of neointima formation.
Methods and Results
By using quantitative reverse-transcription polymerase chain reaction, we found that miR-663 was significantly downregulated in human aortic VSMCs on platelet-derived growth factor treatment, whereas expression was markedly increased during VSMC differentiation. Furthermore, we demonstrated that overexpression of miR-663 increased expression of VSMC differentiation marker genes, such as smooth muscle 22α, smooth muscle α-actin, calponin, and smooth muscle myosin heavy chain, and potently inhibited platelet-derived growth factor-induced VSMC proliferation and migration. We identified the transcription factor JunB and myosin light chain 9 as downstream targets of miR-663 in human VSMCs, because overexpression of miR-663 markedly inhibited expression of JunB and its downstream molecules, such as myosin light chain 9 and matrix metalloproteinase 9. Finally, we showed that adeno-miR-663 markedly suppressed the neointimal lesion formation by ≈50% in mice after vascular injury induced by carotid artery ligation, specifically via decreased JunB expression.
Conclusions
These results indicate that miR-663 is a novel modulator of human VSMC phenotypic switch by targeting JunB/myosin light chain 9 expression. These findings suggest that targeting miR-663 or its specific downstream targets in human VSMCs may represent an attractive approach for the treatment of proliferative vascular diseases.
Keywords: migration, miR-663, proliferation, vascular remodeling, vascular smooth muscle cells
Vascular smooth muscle cells (VSMCs) within the middle layer of the vessel wall perform both contractile and synthetic functions in response to various cellular stimuli.1,2 In a cellular process known as phenotype switching, VSMCs alternate between a contractile (also termed differentiated) state and a synthetic phenotype or dedifferentiated state. Dedifferentiated VSMCs have increased rates of proliferation, migration, and synthesis of extracellular matrix components, as well as reduced expression of differentiation markers (ie, smooth muscle α-actin [SMA], smooth muscle 22α [SM22α], smooth muscle myosin heavy chain).1,3 Pathological phenotype switching plays a major role in the development of a number of cardiovascular diseases, such as atherosclerosis, postangioplasty restenosis, and hypertension.4 VSMC phenotype can be dramatically modulated by numerous environmental cues, including growth factor/cytokines, cell–cell contact, cell adhesions, extracellular matrix interactions, mechanical force, and injury stimuli1,4 In particular, platelet-derived growth factor (PDGF) promotes the synthetic VSMC phenotype and increases smooth muscle cell (SMC) proliferation and subsequent migration into the neointima layer after artery injury.5 However, the molecular mechanisms underlying the SMC phenotypic switch still remains unclear.
MicroRNAs (miRNAs) are a class of conserved, small (approximately 22 nucleotides), single-stranded noncoding RNAs that control diverse biological functions by induction of target mRNA degradation or blockage of translation.6,7 Increasing evidence demonstrates that miRNAs regulate a variety of major cellular functions, such as proliferation,8 migration,9 and apoptosis10; thus, they have been implicated in the development of cardiovascular diseases. Several miRNAs, such as miR-221, miR-222, miR-21, miR-146a, miR-133, miR-143, and miR-145, have been shown to play pivotal roles in the modulation of VSMC function and phenotype switching in animal models.11-15 For example, miR-143 and miR-145 are highly expressed in rodent VSMCs; in response to PDGF-BB stimulation, expression is downregulated.12 Furthermore, knockout studies using mice with miR-143/145 deletion have shown that loss of miR-143 and miR-145 significantly compromises the VSMC contractile phenotype.12,15 Importantly, overexpression of miR-145 has been shown to inhibit SMC proliferation and neointima formation after balloon injury by directly repressing the transcription factor KLF5, suggesting that modulation of miRNA expression may have therapeutic potential.15 Other aspects of PDGF-mediated VSMC phenotype switch is mediated by induction of miR-221.16,17 In response to PDGF treatment, the level of mature miR-221 was increased, which in turn leads to increased VSMC proliferation and migration as well as reduced expression of contractile genes, through targeting the cyclin-dependent kinase inhibitors p27Kip1 and p57Kip2.17 miR-133 has been shown to reduce VSMC proliferation and migration through suppressing the transcription factor Sp-1 expression in rat SMCs. All together, these studies provide significant novel insights into the critical importance and functional complexities of miRNAs in the regulation of VSMC phenotype as well as the development of vascular diseases.
miRNAs are expressed in a tissue-specific manner; thus, VSMCs from different species or tissues may display substantial differences in terms of the expression pattern and functional significance. For example, miR-1 was expressed more in skeletal and cardiac muscle tissue, whereas its expression was almost absent in vascular smooth muscle tissue.18 Although significant progress has been made in the understanding of VSMC biology regulated by miRNAs in animal models of vascular injury, little is known about the miRNAs that are expressed specifically in human VSMCs. Therefore, clarification of their expression and function would be fundamentally important for understanding the molecular mechanisms involved in human VSMC phenotypic modulation and identification of novel therapeutic targets. In this study, we identified miR-663, whose expression was sharply downregulated in human proliferative VSMCs, as a novel modulator for VMSC phenotypic regulation and neointimal formation in vivo.
Methods
Cell Culture
Human aortic SMCs (Lonza, Walkersville, MD) were cultured in growth media SmGM-2 (Lonza) in 5% fetal bovine serum (FBS), and HEK293 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% FBS.
Oligonucleotide Transfection
For miR-663 overexpression, miR-663 mimic (Qiagen) was added to the complexes at the final concentrations of 10, 20, and 40 nmol/L. For miR-663 knockdown, the miR-663 inhibitor (Qiagen) was added to the complexes at a final concentration of 50 nmol/L. Knockdown of JunB expression was performed using JunB siRNA (Sigma). Cells were transfected using HiPerFect Transfection Reagent (Qiagen) according to the manufacturer’s protocol.
Polymerase Chain Reaction Analysis and RNA Analysis by Quantitative Reverse-Transcription Polymerase Chain Reaction
Total RNAs were extracted from human aortic VSMCs and mouse carotid arteries by using either miRNeasy Mini Kit (Qiagen) or RNeasy kit (Qiagen). Polymerase chain reaction (PCR) analysis of miR-663 was performed using the primers (forward: GAG AGG ATC CTG AGT TTG TGG CTG TG; reverse: GAG TAA GCT TGC AGA CAG GCA AGG G). Primer sequences used for the detection of human matrix metalloproteinase 2 (MMP-2), MMP-9, JunB, calponin, SM22α, SMA, myocardin, and MYH11 are listed in Online Table II. The expression of miR-663 relative to U6 and the expression levels of MMP-2, MMP-9, JunB, calponin, SM22α, SMA, myocardin, and MYH11 relative to 18S were determined using the 2−ΔΔCt method.
Western Blot
Western blot analysis was performed essentially as previously described.19 Briefly, cell lyses were resolved by SDS-PAGE and transferred to nitrocellulose membrane. Blots were blocked with 5% nonfat milk in phosphate-buffered saline (PBS) with 0.1% Tween20 and developed with diluted antibodies to JunB (1:1000 dilution; Cell Signaling), SM22α (1:300 dilution; Santa Cruz Biotech), SMA (1:800 dilution; Sigma), myosin regulatory light chain 9 (Myl9; 1:1000 dilution; Cell Signaling), α-tubulin (1:1000 dilution; Cell Signaling), and GAPDH (1:1000 dilution; Santa Cruz), followed by incubating with either IRDye 700 or 800 secondary antibodies and visualized using Odyssey Infrared Imaging System software (Li-Cor, Lincoln, NE).
Luciferase Reporter Assay
The fragment of the 3′-UTR of JunB or Myl9 mRNA containing the putative miR-663–binding sequence was cloned into a RenSP luciferase reporter and then cotransfected with vehicle (vehicle control), miR-663 mimic, or a nontargeting mimic control into HEK293 cells according to the instructions provided by Switchgear Genomics. Forty-eight hours after transfection, LightSwitch Luciferase Assay Reagent was added to measure luciferase activity.
Mutagenesis
Two potential miR-663 target sites located at JunB and Myl9 3′-UTRs were mutated using the QuickChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA).
Generation of Recombinant Adenovirus
The adenovirus expressing miR-663 (Ad-miR-663) and control viruses expressing green fluorescent protein (Ad-GFP) were made using RAPAd Universal Adenoviral Expression System (Cell Biolabs) according to the manufacturer’s instructions.
SMC Scratch Wound Assay
SMCs were starved in starvation medium (0.5% FBS) for 48 hours. A linear wound was then gently introduced in the center of the cell monolayer using 200 μL tip and then subjected to stimulation with or without human PDGF-BB (Peprotech) at a final concentration of 20 ng/mL and monitored for an additional 24 hours. Images were captured using an Olympus IX 71 microscope equipped with a diagnostic instrument RT SPOT Digital Camera (Canon).
VSMC Proliferation Assay In Vitro
VSMC proliferation in vitro was determined by either MTT assay using Vybrant MTT Cell Proliferation Assay Kit (Invitrogen) or BrdU incorporation assay (Roche Applied Science) according to the protocols provided by manufacturers.
Adventitial Gene Transfer and Carotid Artery Ligation Injury
Briefly, male C57BL/6N mice (20–25 g) were anesthetized with an intraperitoneal injection of avertin (400 mg/kg). The left common carotid artery was ligated with a 6-0 silk suture so that the common carotid artery blood flow was completely disrupted. The common carotid artery was dissected free of the surrounding connective tissue and Ad-miR-663 (1010 pfu/mL) or Ad-LacZ (1010 pfu/mL) was suspended together in 50 μL pluronic F127 gel (BASF Corp; 25% wt/vol) and applied around the carotid artery. Carotid arteries were harvested 2 weeks after ligation. Animals were anesthetized and perfused with 0.9% NaCl, fixed with 4% paraformaldehyde, and embedded in paraffinum. Tissue was sectioned at 6 μm and stained with hematoxylin and eosin and was examined by a light microscope (Nikon); the neointimal area was measured by the computer program ImageJ2x. This study was reviewed and approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. The use of human tissues was reviewed and approved by the Thomas Jefferson University, and all work with human samples conform the principles outlined in the Declaration of Helsinki.
Immunofluorescence
Briefly, the sections were incubated with anti-PCNA antibodies (1:50 dilution; Cell Signaling), JunB antibody (1:50 dilution; Cell Signaling), and SMA antibody (1:500 dilution; Sigma), respectively, followed by fluorescein-conjugated secondary antibodies (1:300 dilution; Invitrogen). Cell nuclei were stained with DAPI.
In Situ Hybridization of miR-663 in Human Aortic SMC
Human aortic SMCs were fixed in 4% paraformaldehyde, digested with proteinase K (10 μg/mL) for 1 minute, and then postfixed with 4% paraformaldehyde. Slides were prehybridized in hybridization buffer (50% formamide, 5× SSC, 0.1% Tween20, 50 μg/mL heparin, and 500 μg/mL yeast tRNA) at 55°C for 2 hours and hybridized with hsa-miR-663 probes (LNA mercury probe; Exiqon) at 55°C overnight. Subsequently, antidigoxigenin-POD antibody (Roche) was added and the signals were detected using the tyramide signal amplification system (PerkinElmer).
Statistical Analysis
Data were expressed as mean±SD and analyzed for statistical significance by the unpaired Student t test or ANOVA using SPSS software (version 18.0). P<0.05 was considered statistically significant in all experiments.
Results
Downregulation of miR-663 in Human Proliferative Aortic SMCs
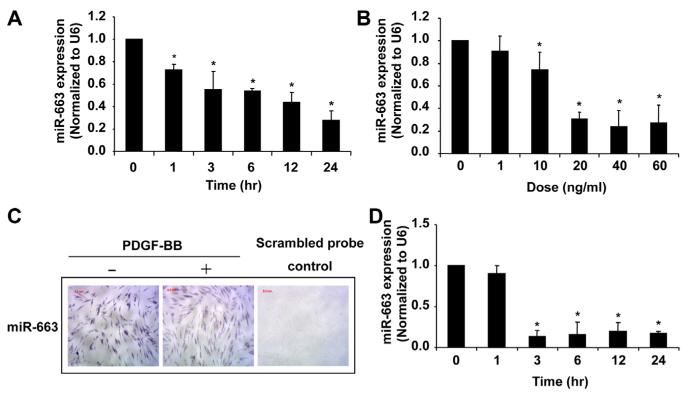
To identify the miRNAs specifically implicated in the regulation of human VSMC function, we performed miRNA microarray in human aortic SMCs after PDGF-BB stimulation for 3, 6, and 24 hours. Results demonstrated that miR-663 was the most downregulated miRNA at all time points (Online Table I). To corroborate these findings, expression of miR-663 was further evaluated in PDGF-induced proliferative human aortic VSMCs by quantitative reverse-transcription PCR (qRT-PCR). As shown in Figure 1A and 1B, treatment of human SMCs with PDGF-BB significantly downregulated the expression of miR-663 in a time-dependent and dose-dependent manner. Notably, this inhibitory effect was confirmed by in situ hybridization for miR-663 in PDGF-treated SMCs and quiescent SMCs (Figure 1C). To test the specificity of this response, human aortic SMCs were treated with 5% FBS, another potent stimulant for VSMC proliferation. Similarly, as shown in Figure 1D, miR-663 was rapidly downregulated, with a maximal downregulation of miR-663 expression by ≈90% at 3 hours after FBS stimulation.
Figure 1. MicroRNA (miR)-663 is significantly downregulated in proliferating vascular smooth muscle cells (VSMCs).
A, Platelet-derived growth factor-BB (PDGF-BB; 20 ng/mL) caused a time-dependent decrease in miR-663 expression in human aortic VSMCs, as determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (n=4). *P<0.05 compared with 0 h group. B, PDGF-BB caused a dose-dependent decrease in miR-663 expression in human aortic VSMCs at 24 h after PDGF treatment, as determined by qRT-PCR (n=4). *P<0.05 compared with that in VSMCs without PDGF-BB. C, In situ hybridization (violet) shows miR-663 was significantly decreased in PDGF-BB–stimulated (20 ng/mL) VSMCs. D, Serum (5%) caused a time-dependent decrease in miR-663 expression in human aortic VSMCs, as demonstrated by qRT-PCR (n=4). *P<0.05 compared with that without 5% fetal bovine serum treatment.
To investigate whether miR-663 expression also changes in other types of VSMCs, we examined the expression of miR-663 in human pulmonary artery SMCs (PASMCs). Although basal expression of miR-663 in human PASMCs is ≈3-fold higher than that in human aortic SMCs, we found that miR-663 expression is similarly downregulated in proliferating PASMCs (Online Figure I). Interestingly, the downregulation was also seen in the lung vessels of patients with pulmonary artery hypertension (Online Figure I), implicating the functional significance of miR-663 in human proliferative vascular diseases.
miR-663 Is a Novel Phenotypic Marker for VSMCs In Vitro
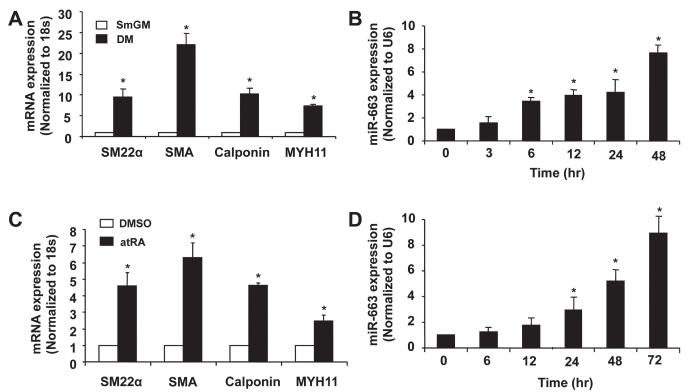
To investigate whether miR-663 modulates VSMC phenotype, we examined expression in differentiated SMCs induced by either SMC differentiation medium or all transretinoic acid (atRA).20,21 Culturing of SMCs in SMC differentiation medium for 48 hours induced differentiation, as indicated by increased expression of VSMC differentiation markers, such as SM22α, SMA, calponin, and MYH11 (Figure 2A). Accordingly, in a time-dependent manner, the expression of miR-663, as determined by qRT-PCR, was markedly increased after exposure to differentiation medium (Figure 2B). Similarly, atRA markedly induced expression of VSMC differentiation marker genes (Figure 2C), which was accompanied by ≈8-fold increased expression of miR-663 (Figure 2D), after 72 hours of atRA treatment. Together, these findings suggest that miR-663 is important for VSMC phenotypic switch.
Figure 2. MicroRNA (miR)-663 is upregulated in differentiated human aortic smooth muscle cells (SMCs).
A, Vascular SMCs (VSMCs) cultured in SMC differentiation medium (DM) for 48 h caused a significantly increased expression of VSMC differentiation marker genes, such as smooth muscle α-actin, smooth muscle 22α, calponin, and smooth muscle myosin heavy chain, as determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (n=5).*P<0.05 compared with SMC growth medium (smGM). B, Human aortic SMCs were cultured in SMC differentiation medium to induce SMC differentiation and the expression of miR-663 was measured by qRT-PCR at different time points (n=5). *P<0.05 compared with 0 h group. C, Human aortic SMCs were treated with all transretinoic acid (atRA; 1 mmol/L) or dimethyl sulfoxide (DMSO) and the expression of SMC differentiation marker genes was determined by qRT-PCR after 72 h of atRA treatment (n=5). *P<0.05 compared with control D, miR-663 was significantly upregulated in atRA-treated VSMCs, as determined by qRT-PCR miRNA assay (n=5). *P<0.05 compared with 0 h.
miR-663 Regulates the Expression of Smooth Muscle Marker Genes In Vitro
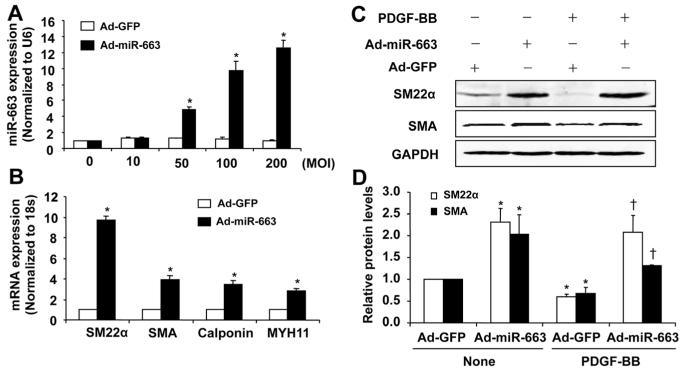
To investigate the role of miR-663 in SMC phenotypic switch, we performed gain-of-function studies by using adenovirus-mediated delivery of miR-663 into VSMCs. As shown in Figure 3A, transduction of SMC with Ad-miR-663 resulted in increased expression of miR-663 in a dose-dependent manner. This increase was associated with markedly increased expression of VSMC differentiation marker genes, such as SM22α, SMA, calponin, and MYH11, as determined by qRT-PCR (Figure 3B). Next, we tested whether miR-663 prevents PDGF-BB–induced downregulation of smooth muscle marker genes. As shown in Figure 3C and 3D, overexpression of miR-663 significantly increased the protein levels of SM22α and SMA, as determined by Western blot analysis, under both quiescent and PDGF-stimulated conditions. These results support the notion that miR-663 is a novel modulator for VSMC phenotypic switch.
Figure 3. MicroRNA (miR)-663 regulates the expression of vascular smooth muscle cell (VSMC) contractile genes.
A, Adenovirus-mediated overexpression of miR-663 (Ad-miR-663) with a multiplicity of infection (MOI) of 100 increases miR-663 levels in a dose-dependent manner (n=4). *P<0.05 vs control viruses expressing green fluorescent protein (Ad-GFP). B, Seventy-two hours after transduction of cell with Ad-miR-663 (MOI=100), the expression of VSMC differentiation marker genes in human aortic SMCs was determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (n=5). *P<0.05 compared with Ad-GFP group. C, Ad-miR-663 (MOI=100) increases the expression of SMC differentiation marker genes in human aortic SMCs in the presence and absence of platelet-derived growth factor (PDGF; 20 ng/mL) treatment, as determined by Western blot analysis. D, Densitometric analysis of SMC differentiation marker genes expression as determined by Western blot (n=5). *P<0.05 vs Ad-GFP without PDGF treatment; †P<0.05 vs Ad-GFP with PDGF (20 ng/mL) treatment.
miR-663 Is a Novel Regulator of VSMC Proliferation and Migration
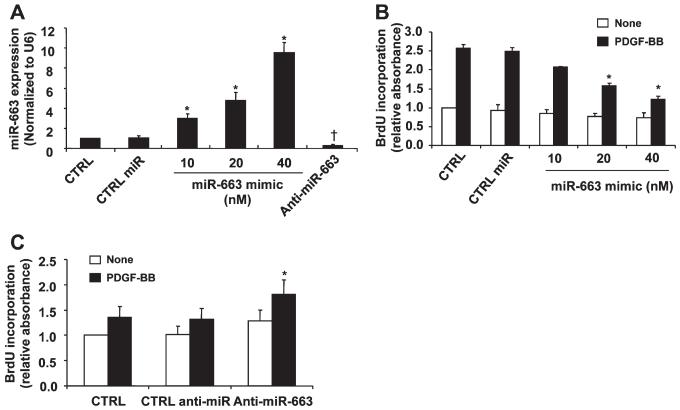
VSMC differentiation is accompanied by decreased proliferation and migration of VSMCs. To determine the potential roles of miR-663 in VSMC proliferation and migration, human aortic SMCs were transfected with miR-663 mimic, anti-miR-663, or negative controls. As shown in Figure 4A, transfection of miR-663 mimics caused a dose-dependent increase of intracellular miR-663 expression, whereas miR-663 inhibitor markedly reduced endogenous levels of miR-663 in VSMCs. Accordingly, transfection of miR-663 mimics significantly inhibited PDGF-BB–induced SMC proliferation, as determined by BrdU incorporation assay (Figure 4B). Furthermore, downregulation of miR-663 expression by anti-miR-663 enhanced SMC proliferation in response to low-dose PDGF-BB (2 ng/mL; Figure 4C). These findings indicate that miR-663 effectively inhibits human SMC proliferation.
Figure 4. Role of microRNAs (miR)-663 in vascular smooth muscle cell (VSMC) proliferation.
A, Human aortic VSMCs were transfected with control miR mimics, miR-663 mimics, or miR-663 inhibitors, respectively, and then subjected to quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis of miR-663 level (n=4). *P<0.05 vs CTRL miR; †P<0.05 vs control (CTRL) miR. B, miR-663 mimic inhibits platelet-derived growth factor-BB (PDGF-BB)-induced VSMC proliferation in a dose-dependent manner as measured by BrdU incorporation (n=4). *P<0.05 vs CTRL miR with PDGF treatment. C, Forty-eight hours after transfection with either anti-miR-663 (50 nmol/L) or CTRL anti-miR (50 nmol/L), SMCs were stimulated with or without PDGF-BB (2 ng/mL) for 24 h. Anti-miR-663 (50 nmol/L) significantly increased human aortic VSMCs proliferation stimulated by PDGF-BB (2 ng/mL) as determined by BrdU incorporation (n=5). *P<0.05 compared with CTRL miR treated with PDGF-BB (2 ng/mL) for 24 h.
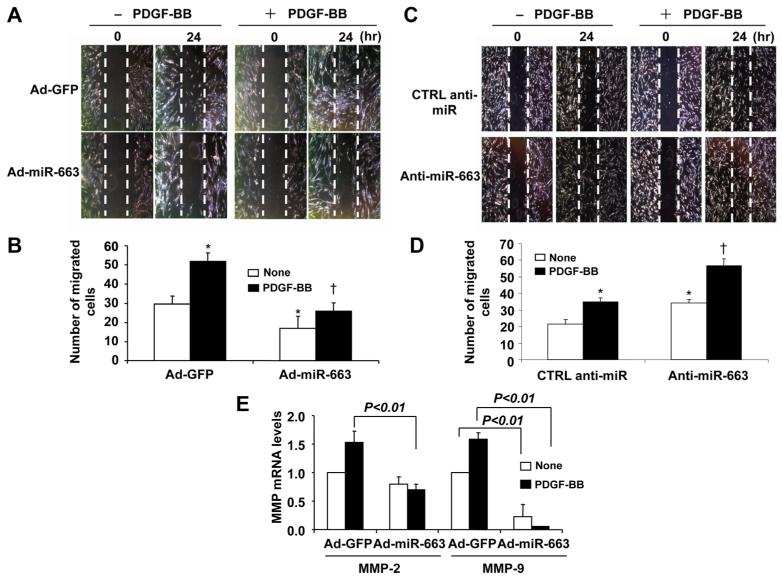
To evaluate the role of miR-663 in SMC migration, we performed the scratch-wound healing assay. As shown in Figure 5A and 5B, adenovirus-mediated overexpression of miR-663 inhibited SMC migration under both quiescent and PDGF-stimulated conditions. In addition, we showed that transfection of SMCs with anti-miR-663 further augmented both basal and PDGF-induced SMC migration (Figure 5C and 5D). Furthermore, MMP-2 and MMP-9, which are implicated in SMC migration,22 were significantly inhibited by overexpression of miR-663 (Figure 5E). Together, our results indicate that miR-663 is an inhibitor of SMC migration.
Figure 5. Role of microRNA (miR)-663 in vascular smooth muscle cell (VSMC) migration.
A, Forty-eight hours after transduction of adenovirus-mediated overexpression of miR-663 (Ad-miR-663; MOI=100), VSMCs were starved and cell migration was measured after platelet-derived growth factor (PDGF; 20 ng/mL) stimulation for 24 h by scratch-wound assay. Migrated cells were quantitated and the results are shown in (B). The data are means±SD of the number of migrated cells from 3 independent experiments. *P<0.05 vs control viruses expressing green fluorescent protein (Ad-GFP) without PDGF treatment; †P<0.05 vs Ad-GFP with PDGF treatment. C, Forty-eight hours after transfected with anti-miR-663 (50 nmol/L) or anti-miR control (CTRL) (50 nmol/L), smooth muscle cells (SMCs) were subjected to injury by scraping and then were stimulated with or without PDGF-BB (20 ng/mL) for 24 h. Migrated cells were quantitated using Image J software program and the results are shown in (D). The data are means±SD of the number of migrated cells from 3 independent experiments. *P<0.05 vs CTRL anti-miR without PDGF treatment; †P<0.05 vs CTRL anti-miR with PDGF treatment. E, Ad-miR-663 decreases matrix metalloproteinase 2 (MMP-2) and MMP-9 mRNA levels in human aortic SMCs as measured by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (n=5).
Identification of JunB and Myl9 as Target Genes of miR-663 in VSMCs
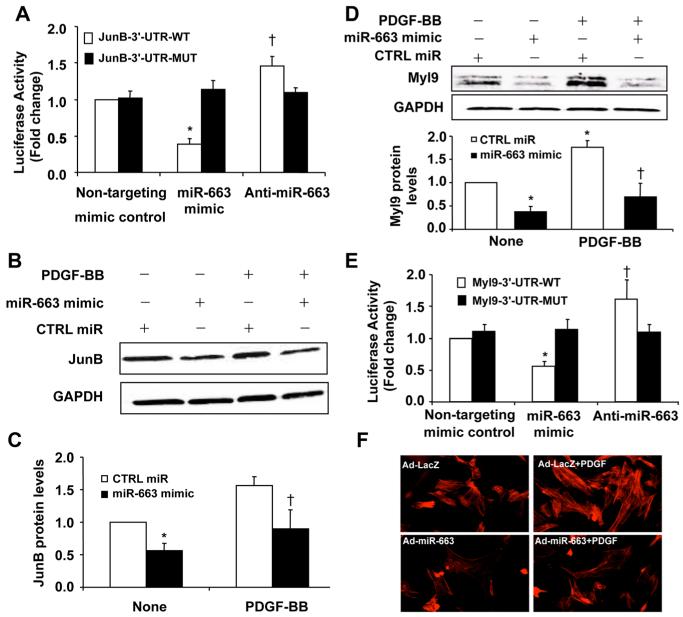
In an attempt to identify targets of miR-663, we used TargetScan and identified JunB as a potential target gene of miR-663 (Online Figure IIA). As shown in Figure 6A, overexpression of miR-663 mimic, but not the control mimics, substantially repressed the activity of luciferase fused with WT-3′-UTR of JunB but had no effect on the luciferase activity, when the miR-663–binding sites in the 3′-UTR of JunB were mutated (Figure 6A), suggesting that miR-663 can directly bind to the 3′-UTR of JunB and regulate its expression.
Figure 6. Identification of JunB and myosin light chain 9 (Myl9) as target genes of microRNA (miR)-663 in vascular smooth muscle cells (VSMCs).
A, miR-663 mimic (50 nmol/L) or nontargeting control (50 nmol/L) was cotransfected with the luciferase reporter carrying WT-JunB 3′-UTR or mutated JunB 3′-UTR. Forty-eight hours after transfection, renilla luciferase activities were measured (n=5). *P<0.05 vs JunB-3′-UTR-WT transfected with mimic control; †P<0.05 vs JunB-3′-UTR-WT transfected with mimic control. B, Effect of miR-663 on JunB expression in platelet-derived growth factor-BB (PDGF-BB; 20 ng/mL)-stimulated VSMCs. C, Densitometric analysis of JunB protein levels as measured by Western blot (n=5). *P<0.05 vs control (CTRL) miR without PDGF treatment; †P<0.05 vs CTRL miR with PDGF treatment. D, Effect of miR-663 on Myl9 expression in PDGF-BB (20 ng/mL)-stimulated VSMCs. Densitometric analysis of Myl9 protein levels as measured by Western blot (n=5). *P<0.05 vs CTRL miR without PDGF treatment; †P<0.05 vs CTRL miR with PDGF treatment. E, miR-663 mimic (50 nmol/L) or nontargeting control (50 nmol/L) was cotransfected with the luciferase reporter carrying WT-Myl9 3′-UTR or mutated Myl9 3′-UTR. Forty-eight hours after transfection, renilla luciferase activities were measured (n=5). *P<0.05 vs Myl9-3′-UTR-WT transfected with mimic control; †P<0.05 vs Myl9-3′-UTR-WT transfected with mimic control. F, VSMCs were transduced with either Ad-LacZ or Ad-miR-663. Forty-eight hours after transduction, SMCs were starved for 48 h and then stimulated with 25 ng/mL PDGF-BB for 20 min. Stress fiber formation was examined by staining cells with Alexa Fluor 546 phalloidin.
To further verify that JunB is a functional target gene of miR-663 in VSMCs, we transfected VSMCs with either miR-663 mimic or control mimics, and the levels of JunB expression were determined by Western blot. As shown in Figure 6B and 6C, overexpression of miR-663 significantly inhibited the expression of JunB in VSMCs, under both basal and PDGF-stimulated conditions. Because Myl9 has been identified as a critical downstream target of JunB and implicated in the regulation of SMC migration,23 we attempted to investigate whether miR-663 affects the expression of Myl9. Treatment of SMCs with PDGF markedly increased the expression of Myl9 in VSMCs (Figure 6D). Consistent with the effect of miR-663 on JunB expression, overexpression of miR-663 markedly inhibited the expression of Myl9 under both basal and PDGF-stimulated conditions (Figure 6D).
Targetscan reveals not only JunB as a miR-663 target but also Myl9 with an 8-mer conserved site (Online Figure III). Overexpression of miR-663 mimic markedly inhibited the activity of luciferase fused with 3′-UTR of Myl9 (Figure 6E), indicating that miR-663 may directly affect Myl9 expression. Accordingly, PDGF-induced stress fiber formation was significantly inhibited by miR-663 (Figure 6F). Collectively, these results suggest that miR-663 directly targets both JunB and Myl9 to potently inhibit the JunB/Myl9 pathway in VSMCs.
Role of JunB in VSMC Proliferation and Migration
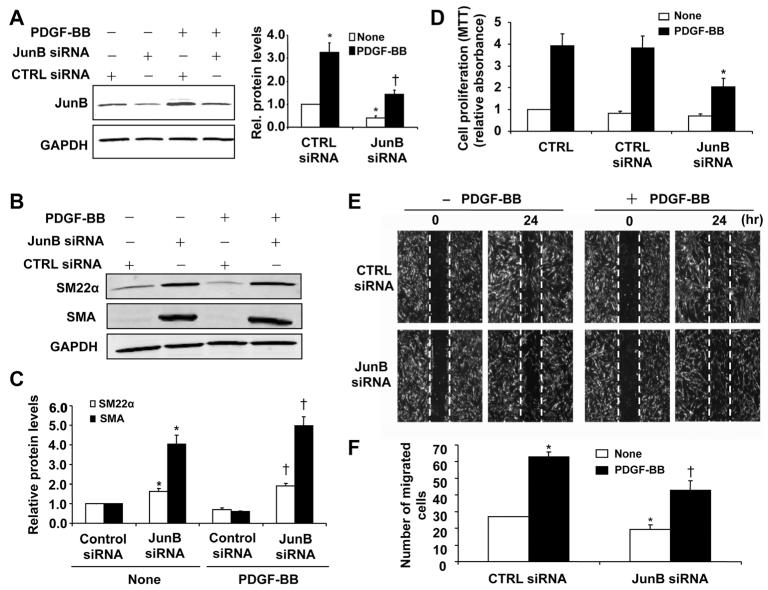
Activation of transcriptional factor AP-1 is implicated in VSMC proliferation and neointimal formation in vivo,24,25 and JunB is the major component of AP-1 complex formed in VSMCs in response to serum and PDGF-BB stimulation.25 To further substantiate the functional significance of JunB in SMC function, we performed loss-of-function study by using JunB siRNA. As shown in Figure 7A, transfection of VSMCs with JunB siRNA significantly inhibited both basal and PDGF-BB–induced JunB expression. Accordingly, JunB knockdown markedly increased the expression of SMC contractile genes such as SM22α and SMA, as determined by Western blot analysis (Figure 7B and 7C). In addition, the PDGF-BB–induced SMC proliferation was significantly inhibited by knockdown of JunB expression (Figure 7D). Likewise, both basal and PDGF-BB–stimulated SMC migration was also markedly attenuated in cells transfected with JunB siRNA (Figure 7E and 7F). To further define the importance of JunB in miR-663–mediated VSMC function, we examined the effect of anti-miR-663 on JunB and Myl9 expression. Transfection of VSMCs with anti-miR-663 significantly increased expression of both JunB and Myl9, whereas knockdown of JunB by specific siRNA in VSMCs markedly attenuated the effect of anti-miR-663 on VSMC proliferation, migration, phenotypic switch, and expression of Myl9 (Online Figure IV). Together, these results suggest that JunB is a critical regulator for SMC differentiation, proliferation, and migration, and is involved in miR-663–mediated effect in VSMC function
Figure 7. JunB is critically implicated in vascular smooth muscle cell (VSMC) proliferation and migration.
A, Silencing of JunB by specific small interfering RNA (JunB siRNA; 100 nmol/L) reduced JunB expression as determined by Western blot. *P<0.05 vs control (CTRL) siRNA without platelet-derived growth factor (PDGF) treatment; †P<0.05 vs CTRL siRNA with PDGF (20 ng/mL) treatment. B, Silencing JunB increases the expression of SMC marker genes smooth muscle 22α (SM22α) and smooth muscle α-actin (SMA), as determined by Western blot analysis. C, Densitometric analysis of SM22α and SMA protein levels as measured by Western blot (n=5). *P<0.05 vs CTRL siRNA without PDGF treatment; †P<0.05 vs CTRL siRNA with PDGF (20 ng/mL) treatment. D, JunB knockdown attenuated PDGF-BB (20 ng/mL)-induced proliferation in human aortic SMCs as determined by MTT assay (n=4). *P<0.05 vs control siRNA with PDGF-BB treatment. E, VSMCs were transfected with JunB siRNA (100 nmol/L). Forty-eight hours after transfection, VSMCs were starved and cell migration was measured after PDGF-BB (20 ng/mL) stimulation for 24 h by scratch-wound assay. F, Quantitation of migrated cells. The data are means±SD of the number of migrated cells from 3 independent experiments. *P<0.05 vs CTRL siRNA without PDGF treatment; †P<0.05 vs CTRL siRNA with PDGF treatment.
miR-663 Inhibits Neointimal Formation in Mice After Carotid Artery Ligation
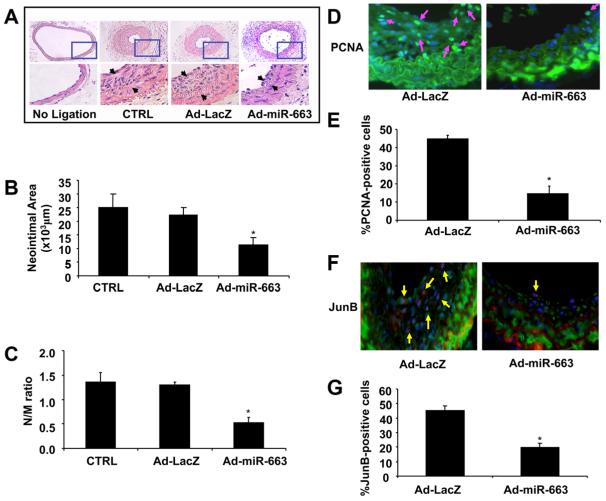
Although miR-663 is expressed only in human and nonhuman primates,26,27 the binding site of miR-663 in JunB 3′-UTR is conserved among different species, such as human, rat, and mouse (Online Figure IIA). Thus, to investigate whether miR-663 inhibits SMC proliferation and migration in VSMCs from other species, we performed studies using mouse SMCs. Similar to findings in human cells, overexpression of miR-663 inhibited the expression of JunB in mouse SMCs (Online Figure IIB). To determine whether miR-663 is involved in vascular lesion formation in vivo, a mouse carotid artery ligation model was used and the neointimal formation was examined at 14 days after vascular injury. The carotid arteries of mice were transduced with either Ad-miR-663 (1010 pfu/mL), Ad-LacZ, or pluronic F127 gel. Fourteen days after transduction, the expression of miR-663 in mouse carotid arteries was markedly increased compared with Ad-LacZ-treated group, as determined by qRT-PCR (Online Figure VA), and the transduction efficiency was ≈43% (Online Figure VB). No neointimal formation was observed in the unligated left common carotid arteries. In contrast, flow cessation led to a substantial increase in neointimal formation in carotid arteries transduced with either gel alone or the gel with Ad-LacZ (Figure 8A). Transduction of carotid arteries with Ad-miR-663, however, substantially inhibited the carotid artery ligation–induced neointimal formation by nearly 60%, which is associated with decreases in both neointimal area (Figure 8B) and the intima-to-media ratio (Figure 8C) in Ad-miR-663–treated arteries. Furthermore, immunofluorescent staining of PCNA, JunB, and SMA was performed in ligated carotid artery. As shown in Figure 8D and 8E, the ratio of PCNA-positive nuclei to total cells was significantly decreased in Ad-miR-663–transduced arteries as compared with Ad-LacZ–treated arteries. Moreover, the number of JunB-positive cells was also substantially reduced in Ad-miR-663–treated arteries (Figure 8F and 8G). Taken together, these results suggest that miR-663 inhibits the vascular injury–induced neointimal formation in vivo by suppressing JunB-mediated SMC proliferation.
Figure 8. MicroRNA (miR)-663 attenuates neointimal formation in a mouse model of carotid artery ligation.
A, Adenovirus-mediated overexpression of miR-663 (Ad-miR-663) significantly reduced neointimal formation in vivo. Representative hematoxylin and eosin (H&E)-stained carotid artery slices from mouse treated with Ad-LacZ or Ad-miR-663 at 14 days after carotid artery ligation. B and C, The effect of miR-663 on vascular neointimal lesion formation in mouse carotid arteries at 14 days after ligation injury as quantitated by neointimal area and neointima/media (N/M) ratio. *P<0.05 vs Ad-LacZ or control (CTRL) D, Representative immunofluorescent staining of PCNA in mouse carotid arteries at 14 days after ligation injury. Green is the PCNA that represents proliferating cells; blue is the cell nuclear staining by DAPI. E, Quantification of PCNA-positive cells showed that fewer cells were proliferating in the injured arteries treated with Ad-miR-663 compared with Ad-LacZ-treated vessels. *P<0.05 vs Ad-LacZ. F, Representative immunofluorescent staining of JunB in mouse carotid arteries at 14 days after ligation injury. Green is smooth muscle α-actin; purple is JunB staining; and blue is nuclear staining by DAPI. G, Quantification of JunB-positive cells showed that JunB was downregulated in Ad-miR-663-treated vessels compared with Ad-LacZ-treated vessels. *P<0.05 vs Ad-LacZ.
Discussion
Despite significant progress in our understanding of VSMC biology, the molecular mechanism underlying the human VSMC phenotypic switch still remains elusive. In the present study, we identified miR-663 as a novel modulator implicated in human SMC differentiation, proliferation, and migration. We showed that the expression of miR-663 is markedly downregulated in human proliferative VSMCs and is upregulated in differentiated VSMCs. Both loss-of-function and gain-of-function studies suggest that miR-663 plays a key role in VSMCs phenotypic switch in vitro and in vivo. Furthermore, we demonstrated that miR-663 inhibits VSMC proliferation and migration in vitro and neointimal formation in vivo, most likely through targeting the transcription factor JunB and its downstream targets Myl9 and MMPs.
The functional significance of miR-663 in human diseases has only recently been explored. Accumulating evidence suggests that miR-663 is critically involved in cell growth and differentiation. atRA, as used in the present study to induce SMC differentiation, has been shown to induce the differentiation of acute myeloid leukemia cell line HL-60 by upregulating the miR-663 expression.28 In human gastric cancer cells, the expression of miR-663 has been shown to be downregulated, and ectopic overexpression of miR-663 represses gastric cancer cell proliferation.29 Moreover, in vascular endothelial cells, miR-663 expression is increased by oscillatory shear stress and plays a key role in inflammatory response of endothelial cells.26 Resveratrol (trans-3,4′,5-trihydroxystilbene), a natural antioxidant with cardiovascular and cancer-preventive properties, has been shown to increase miR-663 in colorectal cancer cells and to decrease AP-1 activity in human THP-1 monocytic cells and blood monocytes.27 Because resveratrol has important biological effects in cardiovascular system,30 it would be interesting to investigate whether miR-663 is involved in resveratrol-induced antiproliferative effects in VSMCs. Nevertheless, at this time, little is known about the role of miR-663 in VSMC biology.
Mechanistically, we identified transcription factor JunB and Myl9 as critical targets of miR-663 in VSMCs. JunB is a major component of the AP-1 complex in VSMCs, and its expression can be significantly induced by multiple stimuli, such as PDGF, FBS, thrombin, and vascular injury.25,31 The members of AP-1 family transcription factors, such as Jun, Fos, and ATF proteins, have been implicated in VSMC migration and proliferation in vitro and in vivo. For instance, inhibition of AP-1 by decoy oligodeoxynucleotides has been shown to inhibit VSMC proliferation in vitro and neointimal formation in vivo,24 further implicating the critical role of AP-1 in SMC function. In addition, AP-1 has been shown to regulate SMC migration through regulating expression of MMP-9.32 Importantly, JunB has been recently identified as a key regulator involved in actomyosin-based contractility and motility in VSMCs by targeting Myl9.23 Myl9 (MLC2), a component of the actomyosin contractile apparatus, has been implicated in actomyosin-based cell contractility and motility.33 In VSMCs, JunB has been shown to specifically bind to the CRE sites of the Myl9 promoter, thus regulating the expression of Myl9. In JunB-deficient SMCs, the expression of Myl9 was markedly decreased, which led to the impaired stress fiber formation and motility of VSMCs.23 In accordance with this observation, we found that overexpression of miR-663 markedly suppressed the PDGF-induced expression of both JunB and Myl9 in VSMCs, hence contributing to its inhibitory effects on VSMC migration. Although miR-663 is expressed exclusively in human and nonhuman primates, the binding site of miR-663 in JunB 3′-UTR is conserved among different species, such as human, rat, and mouse levels, which prompted us to investigate the functional significance of miR-663 in vivo by using mouse carotid artery ligation model. Strikingly, overexpression of miR-663 markedly inhibited the neointimal formation, which is accompanied by decreased expression of JunB and SMC proliferation marker, PCNA. It is worth noting that deficiency of JunB in mesenchyme did not significantly affect vascular remodeling in mouse DOCA-salt model.23 Although these findings appear to contradict results in our study,23 it is important to mention that our study focused only on VSMCs, suggesting that JunB may have unique functional roles in different mesenchymal cell types (eg, VSMCs versus vascular endothelial cells). In addition, comparison between studies is complicated by the fact that different vascular injury models were used. Nevertheless, our results are the first to identify miR-663 as a critical modulator in regulating VSMC proliferation and migration, at least in part, through targeting the JunB/Myl9 pathway.
The modulation of VSMC phenotype is of great importance for the resolution of vascular injury.4 Recent studies have implicated miRNAs, such as miR-143/145, miR-146a, miR-31, miR-221, and miR-133a, as critical targets of multiple growth factor signaling pathways that regulate VSMC phenotype.3,34 The molecular mechanisms underlying the regulation of SMC differentiation by these miRNAs are divergent but are mainly involved in the direct or indirect regulation of the expression of transcriptional factors, such as serum response factor, myocardin and myocardin-related transcription factors (MRTFs), and Kruppel-like zinc finger family.34,35 In the present study, we examined the effect of miR-663 on these transcriptional factors and found that modulation of miR-663 expression did not significantly affect expression of FOSB, myocardin, MRTF-A, CEBPB, and KLF4, which are the major regulators for expression of SMC marker genes (Online Figure VI), suggesting that JunB regulates expression of SMC marker genes, possibly through a mechanism yet to be determined.
In summary, for the first time to our knowledge, we identified miR-663 as a critical regulator in human VSMC differentiation, proliferation, and migration by targeting, at least partly, the JunB/Myl9 pathway. Moreover, the expression of miR-663 was substantially downregulated in pulmonary artery SMCs in patients with pulmonary artery hypertension, indicating a pathological role of miR-663 in proliferative vascular diseases. Interestingly, JunB deficiency has been recently shown to prevent mice from experiencing development of hypertension and its associated adaptive responses, such as cardiac hypertrophy and retina artery constriction, without a significant effect on the blood pressure at baseline.23 Thus, modulation of JunB/Myl9 expression by miR-663 may have potential as a therapeutic target for treatment of cardiovascular diseases such as atherosclerosis and restenosis.
Supplementary Material
Novelty and Significance.
What Is Known?
In response to vascular injury, vascular smooth muscle cells (VSMCs) undergo phenotypic switch from the contractile state to the synthetic state.
Platelet-derived growth factor (PDGF), a key player in occlusive vascular diseases, promotes the synthetic VSMC phenotype and increases VSMC proliferation and migration.
MicroRNAs (miRNAs) are noncoding small RNAs that play important roles in regulating VSMC function.
What New Information Does This Article Contribute?
Treatment of human VSMCs with PDGF-BB is associated with a reduced level of miR-663.
Overexpression of miR-663 is associated with increased expression level of the molecular markers of VSMC differentiation and reduced expression level of the transcription factor JunB and myosin light chain 9.
Targeted overexpression of miR-663 suppresses VSMC proliferation and migration in vitro and reduces vascular injury–induced neointimal formation in vivo.
Understanding molecular mechanisms underlying VSMC phenotypic switch might provide novel therapeutic targets for the treatment of occlusive vascular diseases, such as atherosclerosis and restenosis. We show that miR-663 is highly expressed in human VSMCs and its expression is inversely associated with the proliferation and migration of VSMCs. By targeting a key transcriptional factor, JunB, miR-663 influences VSMC phenotype and neointimal formation. These findings suggest that miR-663 may play an important role in vascular remodeling and could be targeted to develop novel therapeutic strategies.
Acknowledgments
We thank Dr Ross Summer for critical reading of the manuscript and fruitful discussion.
Sources of Funding
This work was supported by the National Institutes of Health (HL103869) and grants from the Chinese Natural Science Foundation (381170114 and 81370418 to J.S., 30971231 to Y.Q., and 81100197 to N.Z.).
Nonstandard Abbreviations and Acronyms
- Ad
adenovirus
- atRA
all transretinoic acid
- miRNA
microRNA
- MMP
matrix metalloproteinase
- PDGF
platelet-derived growth factor
- SMA
smooth muscle α-actin
- SM22α
smooth muscle 22α
- VSMC
vascular smooth muscle cell
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.113.301306/-/DC1.
Disclosures
None.
References
- 1.Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–2377. doi: 10.1161/ATVBAHA.111.226670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 4.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 5.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C. MicroRNomics: a newly emerging approach for disease biology. Physiol Genomics. 2008;33:139–147. doi: 10.1152/physiolgenomics.00034.2008. [DOI] [PubMed] [Google Scholar]
- 10.Xu P, Guo M, Hay BA. MicroRNAs and the regulation of cell death. Trends Genet. 2004;20:617–624. doi: 10.1016/j.tig.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YLX, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. Microrna-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, Liu Y, Cheang TY, Huang XL, Wang SM. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 19.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Han M, Zhao XM, Wen JK. Kruppel-like factor 4 is required for the expression of vascular smooth muscle cell differentiation marker genes induced by all-trans retinoic acid. J Biochem. 2008;144:313–321. doi: 10.1093/jb/mvn068. [DOI] [PubMed] [Google Scholar]
- 21.Chanchevalap S, Nandan MO, Merlin D, Yang VW. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Kruppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 23.Licht AH, Nübel T, Feldner A, Jurisch-Yaksi N, Marcello M, Demicheva E, Hu JH, Hartenstein B, Augustin HG, Hecker M, Angel P, Korff T, Schorpp-Kistner M. Junb regulates arterial contraction capacity, cellular contractility, and motility via its target Myl9 in mice. J Clin Invest. 2010;120:2307–2318. doi: 10.1172/JCI41749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn JD, Morishita R, Kaneda Y, Lee SJ, Kwon KY, Choi SY, Lee KU, Park JY, Moon IJ, Park JG, Yoshizumi M, Ouchi Y, Lee IK. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ Res. 2002;90:1325–1332. doi: 10.1161/01.res.0000023200.19316.d5. [DOI] [PubMed] [Google Scholar]
- 25.Rao GN, Katki KA, Madamanchi NR, Wu Y, Birrer MJ. JunB forms the majority of the AP-1 complex and is a target for redox regulation by receptor tyrosine kinase and G protein-coupled receptor agonists in smooth muscle cells. J Biol Chem. 1999;274:6003–6010. doi: 10.1074/jbc.274.9.6003. [DOI] [PubMed] [Google Scholar]
- 26.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–H1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tili E, Michaille JJ, Adair B, Alder H, Limagne E, Taccioli C, Ferracin M, Delmas D, Latruffe N, Croce CM. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis. 2010;31:1561–1566. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian P, Li ZW, Fang TY, Jian W, Zhuan Z, Mei LX, Yan WS, Jian N. Retinoic acid induces HL-60 cell differentiation via the upregulation of miR-663. J Hematol Oncol. 2011;4:20. doi: 10.1186/1756-8722-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, Hu H, Zhou Z, Sun L, Peng L, Yu L, Sun L, Liu J, Yang Z, Ran Y. Tumor-suppressive mir-663 gene induces mitotic catastrophe growth arrest in human gastric cancer cells. Oncol Rep. 2010;24:105–112. doi: 10.3892/or_00000834. [DOI] [PubMed] [Google Scholar]
- 30.Schreiner CE, Kumerz M, Gesslbauer J, Schachner D, Joa H, Erker T, Atanasov AG, Heiss EH, Dirsch VM. Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc Res. 2011;90:140–147. doi: 10.1093/cvr/cvq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miano JM, Vlasic N, Tota RR, Stemerman MB. Localization of Fos and Jun proteins in rat aortic smooth muscle cells after vascular injury. Am J Pathol. 1993;142:715–724. [PMC free article] [PubMed] [Google Scholar]
- 32.Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, Greene WC, Valente AJ. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem. 2006;281:15099–15109. doi: 10.1074/jbc.M600200200. [DOI] [PubMed] [Google Scholar]
- 33.Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–4733. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- 34.McDonald RA, Hata A, MacLean MR, Morrell NW, Baker AH. MicroRNA and vascular remodelling in acute vascular injury and pulmonary vascular remodelling. Cardiovasc Res. 2012;93:594–604. doi: 10.1093/cvr/cvr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazari-Jahantigh M, Wei Y, Schober A. The role of microRNAs in arterial remodelling. Thromb Haemost. 2012;107:611–618. doi: 10.1160/TH11-12-0826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.