Summary
Infection with the opportunistic enteric pathogen Clostridium difficile is an increasingly common clinical complication that follows antibiotic treatment-induced gut microbiota perturbation. Innate lymphoid cells (ILCs) are early responders to enteric pathogens; however, their role during C. difficile infection is undefined. To identify immune pathways that mediate recovery from C. difficile infection, we challenged C57BL/6, Rag1−/−, which lack T and B cells, and Rag2−/− Il2rg−/− (Ragγc−/−) mice, which additionally lack ILCs, with C. difficile. In contrast to Rag1−/− mice, ILC-deficient Ragγc−/− mice rapidly succumbed to infection. Rag1−/−, but not Ragγc−/− mice, upregulate expression of ILC1 or ILC3 associated proteins following C. difficile infection. Protection against infection was restored by transferring ILCs into Ragγc−/− mice. While ILC3s made a minor contribution to resistance, loss of IFN-γ or T-bet-expressing ILC1s in Rag1−/− mice increased susceptibility to C. difficile. These data demonstrate a critical role for ILC1s in defense against C. difficile.
Introduction
Clostridium difficile is the leading cause of hospital-acquired gastrointestinal infections with C. difficile related deaths having increased by 400% over the past decade. (Hunt and Ballard, 2013; Lucado et al., 2012). C. difficile establishes infection following disruption of the indigenous microbiota, usually the result of preceding antibiotic treatment (Rupnik et al., 2009; Viswanathan et al., 2010). Antibiotic treatments for C. difficile infection (CDI) are often ineffective, (Huang et al., 2009; Johnson, 2009; Pepin et al., 2005) and complications associated with recurrent CDI in hospitalized patients interfere with medical therapies until infection is brought under control (Chopra et al., 2010; Dubberke et al., 2010).
While progress has been made in identifying specific commensal bacterial species that can prevent C. difficile replication (Buffie et al., 2015; Lawley et al., 2012; Seekatz et al., 2014; Tvede et al., 2015), the role of the host's natural defenses in limiting disease severity is not well understood. In humans, circulating antibodies specific for C. difficile toxin-A correlate with less severe infections and lower relapse rates, suggesting that humoral immunity contributes to defense in chronic and recurrent infections (Kyne et al., 2000, 2001). A recently developed mouse model has provided insights into innate immune defenses against CDI (Chen et al., 2008). Inoculation of antibiotic pre-treated mice with C. difficile spores results in acute colitis, with growth of vegetative, oxygen intolerant, and toxin-secreting C. difficile bacteria (Chen et al., 2008). The tcdA and tcdB genes of C. difficile that encode toxin A and toxin B respectively, glycosylate and inactivate Rho GTPases of colonic epithelial cells, leading to loss of intestinal barrier integrity and infiltration of inflammatory cells into the colonic lamina propria (Just et al., 1995; Lyras et al., 2009; Voth and Ballard, 2005). In mice, the acute phase of CDI progresses for 2 to 5 days following spore inoculation and is followed by recovery, with weight gain and resolution of diarrhea despite continued growth of C. difficile bacteria and toxin A and B production (Buffie et al., 2012; Lawley et al., 2009). The host mechanisms underlying recovery from acute CDI remain incompletely defined, however innate immune signaling pathways have been implicated in early host defense. Loss of specific innate immune defense components, such as MyD88, ASC, NOD1 or infiltrating neutrophils increases mortality in mice during acute CDI (Hasegawa et al., 2012; Hasegawa et al., 2011; Jarchum et al., 2012; Lawley et al., 2009).
Innate lymphoid cells (ILCs) reside in the intestine and contribute to restoration of intestinal integrity following infection (Artis and Spits, 2015). Type-1 ILCs (ILC1s) produce IFN-γ and TNF-α and limit Toxoplasma gondii replication in the intestine (Bernink et al., 2013; Fuchs et al., 2013; Klose et al., 2014). ILC2s provide resistance against helminth infections via IL-5 and IL-13 secretion while (ILC3s) contribute to defense against invasive bacteria such as Citrobacter rodentium via production of IL-22 (Artis and Spits, 2015). Further, Nfil3−/− mice, which have a developmental defect in ILC maturation resulting in absence of all ILC subsets, are more susceptible to CDI (Geiger et al 2014). However, the contributions of different ILC subsets to resolution of the acute phase of CDI remain undefined.
In this report we examine the role of ILCs in the innate immune response to CDI. We find that recovery from the acute phase of CDI does not depend on the adaptive immune system but that the loss of ILCs markedly increases acute phase mortality. Adoptive transfer of CD90+ CD127+ ILCs into highly susceptible, ILC-deficient mice enabled mice to recover from CDI. While selective loss of ILC3s or IL-22 modestly reduced resistance to acute CDI, loss of T-bet expressing ILC1s or the ILC1-derived effector cytokine IFN-γ markedly increased morbidity and mortality. Our results reveal a cooperative role for two ILC subsets in host defense against acute CDI, with ILC3s making a minor contribution to the more significant impact of ILC1s in the recovery phase.
Results
Absence of innate lymphoid cells increases susceptibility to acute C. difficile infection
ILCs reside within the intestinal wall, including the epithelial layer and the underlying lamina propria (Lp), and contribute to the maintenance of tissue integrity following infection-mediated damage (Artis and Spits, 2015; Diefenbach, 2013). The role ILCs in the early host response to CDI, however, is undefined. To address this question we used a murine model of CDI. C57BL/6, Rag1−/−, and Rag2−/−, common gamma chain double knockout (Ragγc−/−) mice were cohoused for three weeks to equilibrate the intestinal microbiota (Ubeda et al., 2012). Mice were then treated with neomycin (0.25g/L) and vancomycin (0.25g/L) in the drinking water for three days followed by a single intraperitoneal (i.p.) injection of clindamycin (Buffie et al., 2012; Lewis et al., 2015; Rupnik et al., 2009; Wren et al., 2005) (Figure S1A). Antibiotic-treated mice were orally challenged with a low dose of C. difficile spores (200 spores), an inoculum that likely approximates both the route and dose acquired by humans in a healthcare setting. We have used the VPI 10463 C. difficile strain, which produces high amounts of toxin A and B and causes intestinal epithelial damage leading to the recruitment of infiltrating inflammatory immune cells into the intestine (Chen et al., 2008).
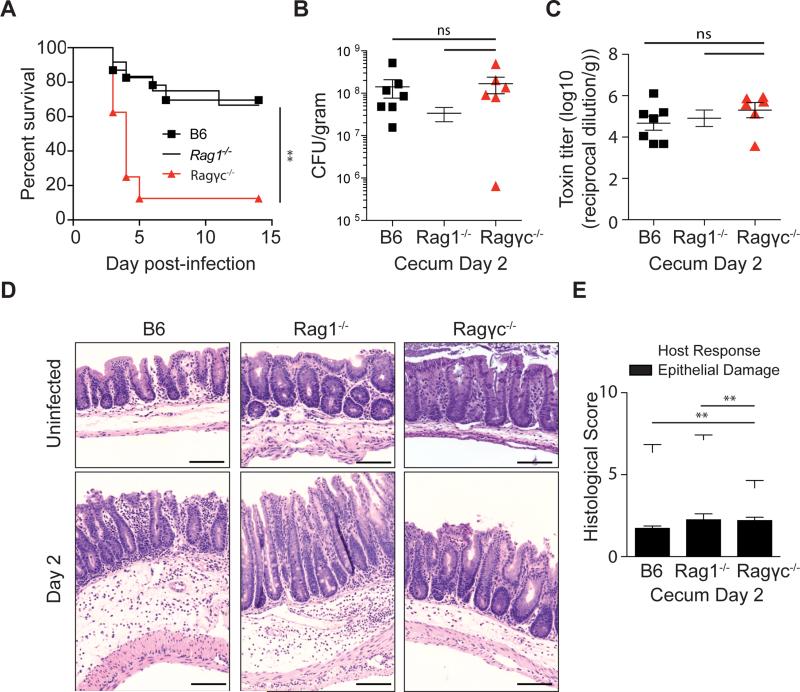
C57BL/6 and Rag1−/− mice had similar survival (Figure 1B), weight loss (Figure S1B), C. difficile burden (Figure S1C), C. difficile toxin levels (Figure S1D), and tissue damage in the large intestine (Figure S1D,E) following infection, indicating that the innate immune response is capable of mediating host recovery from acute CDI. In contrast, Ragγc−/− mice were susceptible to acute CDI, did not progress to the recovery phase and succumbed within five days of spore inoculation (Figure 1B). Increased mortality in Ragγc−/− mice was not the result of an elevated intestinal C. difficile burden (Figure 1C) or increased toxin levels (Figure 1D) following infection. Deep-sequencing of 16S rRNA isolated from the fecal pellets of cohoused Ragγc−/− and C57BL/6 mice demonstrated intestinal microbiota equilibration prior to infection (Figure S2A-C), suggesting that an aberrant intestinal microbiota in Ragγc−/− mice did not prevent recovery from acute CDI. Histological examination of the cecum two days following infection revealed that C57BL/6, Rag1−/−, and Ragγc−/− mice had similar damage to the epithelial layer but that Ragγc−/− mice had decreased edema and fewer infiltrating immune cells (Figure 1F,G). At day two post infection, all three groups had detectable levels of albumin in the cecal content (Figure S2D), indicating intestinal epithelial damage, but only Ragγc−/− mice had incidences of LPS in the serum (Figure S2E), Consistent with a compromised intestinal barrier, blooms of bacteria were detected by fluorescence in situ hybridization within the large intestinal crypts and the submucosa of Ragγc−/− mice at day two post-infection (Figure S2F). These data suggest that common-γ chain-dependent ILCs are essential for recovery from acute CDI.
Figure 1. Absence of innate lymphoid cells leads to increased susceptibility to C. difficile.
(A) C57BL/6, Rag1−/−, Ragγc−/− mice were inoculated with 200 spores of C. difficile (VPI 10463 strain) and were assessed for survival following infection. Survival curve is a combination of 5 independent experiments (C57BL/6 n=23, Rag1−/− n=12, Ragγc−/− n=8). At day 2 post-infection (B) C. difficile burden and (C) toxin levels were measured from the cecal content. (D) Representative H&E stained cecal sections from antibiotic-treated, uninfected and day 2 infected C57BL/6, Rag1−/−, Ragγc−/− mice. Scale bar = 100μm. (E) Pathology score of histological tissue sections based on cellular infiltration/edema (host response), and epithelial layer degeneration (n=9-11). *p<0.05, **p<0.01. ns = not significant. Data shown are mean ± SEM. See also Figure S1 and S2.
Rag1−/−, but not Ragγc−/− mice upregulate expression of ILC1 or ILC3 associated proteins following C. difficile infection
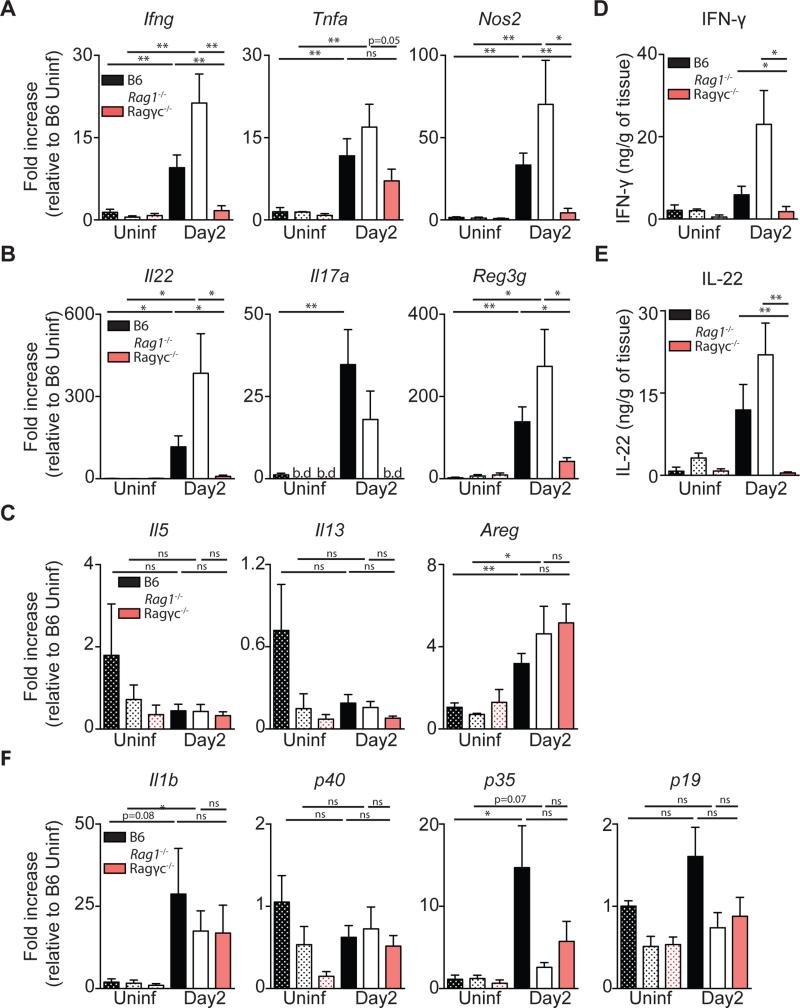
Reduced recovery of Ragγc−/− mice during CDI suggests that ILCs enable the host to re-establish a homeostatic state despite continued infection with toxin-producing bacteria. To begin to dissect the relative contributions of ILC1s, ILC2s and ILC3s following CDI, C57BL/6, Rag1−/− and Ragγc−/− mice were assessed for expression of ILC1, 2, and 3 associated genes in the colon. Two days following spore inoculation, C57BL/6 and Rag1−/− mice had increased expression of Ifng (Figure 2A), Il17a, and Il22 (Figure 2B) mRNA compared to uninfected C57BL/6 or Rag1−/− mice, respectively, while infected Ragγc−/− mice failed to upregulate transcription of these genes. Further, Nos2 (Figure 2A) and Reg3g (Figure 2B), IFN-γ and IL-22 inducible genes respectively, were significantly increased in C57BL/6 and Rag1−/− mice compared to Ragγc−/− mice following infection, suggesting an impairment in the ILC1 and ILC3 response axis. ILC2 associated effector molecules, Il5, Il13, and Areg were not differentially expressed between day two infected C57BL/6, Rag1−/− and Ragγc−/− mice (Figure 2C), suggesting that ILC2s are not major contributors to recovery from acute CDI. Supernatants from cecal tissue explants confirmed significantly decreased production of IFN-γ (Figure 2D) and IL-22 (Figure 2E) in day two infected Ragγc−/− mice compared to day two infected C57BL/6 and Rag1−/− mice on the protein level. ILC1s can be stimulated by IL-12 (composed of the p35 and p40 subunits) to produce IFN-γ while ILC3s are activated by IL-23 (composed of the p19 and p40) and/or IL-1β to produce IL-22 (Artis and Spits, 2015). Induction of Il1b and p35 mRNA and constitutive expression of p40 and p19 mRNA in the colon was comparable in day two infected C57BL/6, Rag1−/− and Ragγc−/− mice, (Figure 2F), suggesting that the upstream machinery for ILC activation remained intact in Ragγc−/− mice following infection. These data demonstrate that induction of IFN-γ and IL-22-mediated effector mechanisms following CDI is absent in susceptible, ILC-deficient Ragγc−/− mice.
Figure 2. C57BL/6 and Rag1−/−, but not Ragγc−/− mice upregulate expression of ILC1 or ILC3 associated proteins following C. difficile infection.
Fold Induction of (A) ILC1, (B) ILC3, (C) ILC2 associated effector molecules in the colon at day 2 post-infection relative to uninfected C57BL/6 mice and normalized to Hprt. (D) IFN-γ and (E) IL-22 protein in the supernatant of cecal tissue explants. (F) Fold induction of genes upstream of ILC1 and ILC3 activation (n=7-13) *p<0.05, **p<0.01. Data shown are mean ± SEM. b.d. = below detection.
Innate lymphoid cells are the predominant source of IL-22 and IFN-γ following C. difficile infection
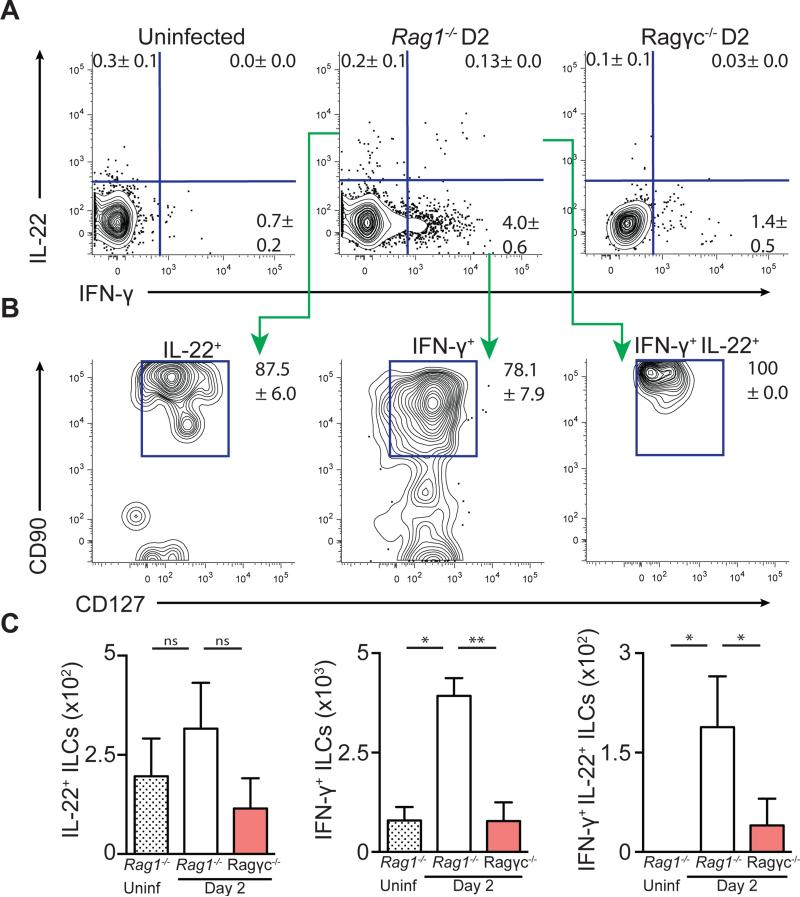
To identify the early cellular source of IFN-γ and IL-22 following CDI, Rag1−/− and Ragγc−/− mice were sacrificed at day two post infection and cells isolated from the large intestine were assessed for cytokine production by flow cytometric analysis. In the absence of any ex vivo stimulation, IL-22+, IL-22+/IFN-γ+, and IFN-γ+ cells were recovered from day two infected Rag1−/− mice (Figure 3A). These IFN-γ and IL-22 expressing cells predominantly expressed CD90 and CD127 (Figure 3B), canonical surface markers for ILCs (Sonnenberg et al., 2013), and were significantly increased compared to infected Ragγc−/− mice and uninfected Rag1−/− mice (Figure 3C). A similar induction of IFN-γ+ and IL-22+, CD90+, CD127+ ILCs were observed in the mesenteric lymph nodes (mLN) of day two infected Rag1−/− mice compared to uninfected control mice (Figure S3A-C). To assess whether ILCs are the predominant early source of IFN-γ and IL-22 in fully immunocompetent hosts, CD4+ T cells and ILCs from the mLN of uninfected or day two infected C57BL/6 mice were assessed for cytokine production (Figure S3D). While there was no induction of cytokine-producing CD4+ T cells at day two post infection (Figure S3E), there was a significant increase in IL-22+ and IFN-γ+ ILCs in infected C57BL/6 mice compared to uninfected controls (Figure S3F). Further analysis of these cytokine-producing ILCs revealed that the majority of IL-22-producing cells were CD4+ LTi-like cells while the majority of IFN-γ-producing cells were NK1.1+, NKp46+ ILC1s (Figure S3G). Combined, these data demonstrate that IL-22- and IFN-γ-producing ILCs are induced in the large intestine and associated lymphoid tissue in both C57BL/6 and Rag1−/− mice following CDI. Further, these CD90+ CD127+ ILCs are the major early source of IL-22 and IFN-γ following CDI and are absent in Ragγc−/− mice.
Figure 3. IL-22 and IFN-γ expressing ILCs are induced in the large intestine following C. difficile infection.
Cells isolated from the large intestine intraepithelial compartment of uninfected Rag1−/−, day 2 infected Rag1−/−, or day 2 infected Ragγc−/− mice were incubated in media in the presence of brefeldin A (BFA) and assessed for IL-22 and IFN-γ production. (A) Frequency of IL-22+, IFN-γ+, and IL-22+/ IFN-γ+ cells. FACS plots gated on live, CD45+, Gr-1neg cells. (B) Expression of CD90 and CD127 on cytokine positive cells from day 2 infected Rag1−/− mice. (C) Number of IL-22+, IFN-γ+, and IL-22+/IFN-γ+ ILCs. Data representative of 3 independent experiments (n=4-5.) *p<0.05, **p<0.01. Data shown are mean ± SEM. See also Figure S3.
Adoptive transfer of ILCs into Ragγc−/− mice restores host defense against C. difficile
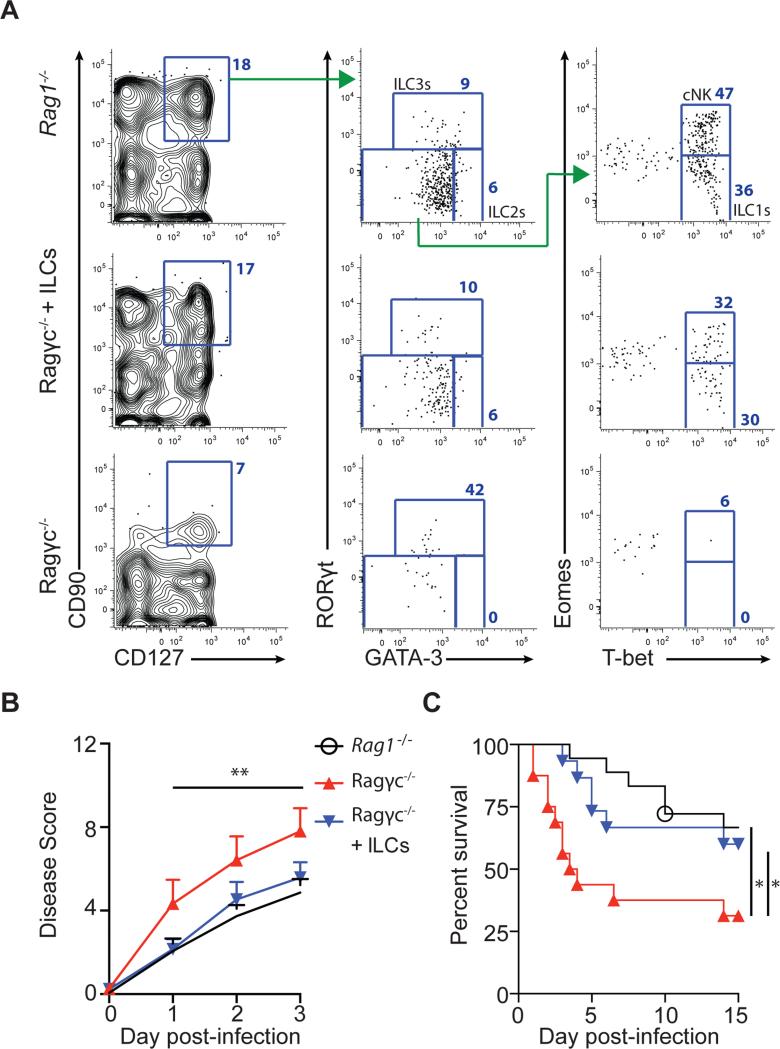
Ragγc−/− mice lack IL-22 and IFN-γ producing ILCs following inoculation with C. difficile and are unable to recover from acute infection. To assess if this cell population is sufficient to reestablish resistance in Ragγc−/− mice against acute CDI, CD90+, CD127+ ILCs were sorted from the spleen, lymph nodes, and intestines of Rag1−/− mice and adoptively transferred into recipient Ragγc−/− mice. The sort-purified population of CD90+ CD127+ ILCs consisted of T-bet expressing ILC1s, GATA-3 expressing ILC2s, and RORγt expressing ILC3s (Figure S4A) and, as previously reported by (Klose et al., 2013), three weeks following adoptive transfer into recipient Ragγc−/− mice, donor CD90+, CD127+ ILCs were present in the large intestine (Figure 4A), spleen (Figure S4B), small intestine (Figure S4C). Transcriptional profiling of donor ILCs in Ragγc−/− ILC recipients confirmed the presence of all three ILC subsets as well as Eomes+, T-bet+ ‘classical’ NK cells (Gordon et al., 2012) (Figure 4A, Figure S4). Ragγc−/− ILC recipient mice received a second transfusion of CD90+ CD127+ ILCs and were challenged with C. difficile spores along with Rag1−/− and Ragγc−/− mice. As shown previously (Figure 1), Ragγc−/− mice had reduced recovery from acute CDI compared to Rag1−/− mice (Figure 4B), as determined by a morbidity score consisting of weight loss, body temperature, diarrhea severity and lethargy (Warren et al., 2012), and rapidly succumbed to infection (Figure 4C). In contrast, Ragγc−/− ILC recipient mice had reduced morbidity and mortality following infection compared to Ragγc−/− mice not receiving ILCs (Figure 4B,C), demonstrating the capacity of ILCs to promote recovery from acute CDI.
Figure 4. Adoptive transfer of ILCs into Ragγc−/− mice restores host defense following C. difficile infection.
(A) Reconstitution of ILC compartment in the large intestine Lp of recipient Ragγc−/− mice 3 weeks after ILC transfer. Rag1−/−, Ragγc−/−, and Ragγc−/− +ILCs mice were infected with C. difficile and were assessed for (B) disease severity and (C) survival following infection. Combination of 5 independent experiments (n=15-18). *p<0.05, **p<0.01. Data shown are mean ± SEM. See also Figure S4.
Selective loss of ILC3s or ILC3 effector molecules reveals their limited role in recovery following acute C. difficile infection
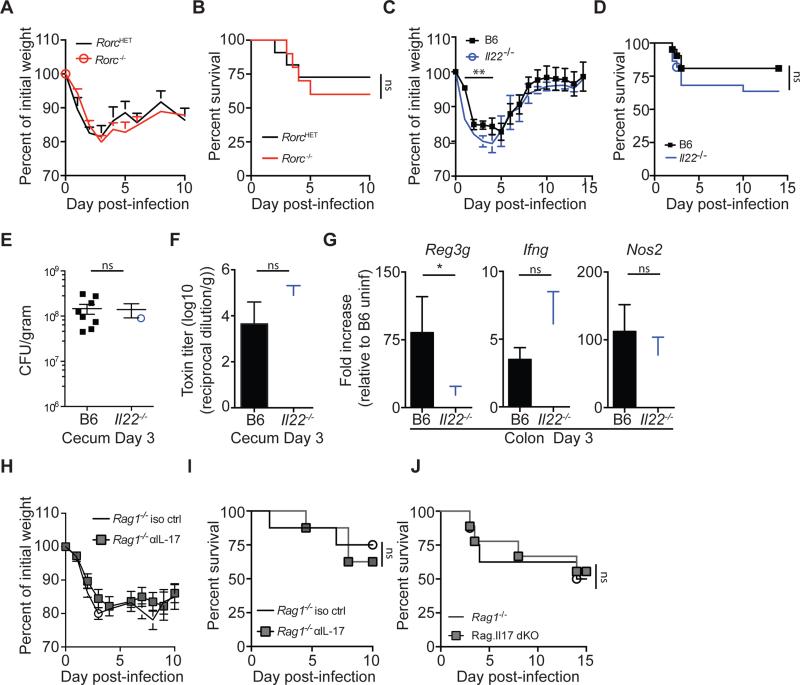
Adoptive transfer of CD90+ CD127+ ILCs into Ragγc−/− mice improved survival following C. difficile challenge, demonstrating the contribution of these cells to the re-establishment of gut homeostasis following acute phase infection. However, the relative contribution of distinct ILC subsets to host defense against acute C. difficile remained unresolved. RORγt-expressing ILC3s provide protection against the attaching and effacing bacterial pathogen Citrobacter rodentium via IL-22 production (Satoh-Takayama et al., 2008; Sonnenberg et al., 2011). IL-22 is also produced by ILCs following CDI (Figure 2,3), and thus could contribute to recovery from acute CDI. To address the relative contribution of ILC3s in host defense against CDI, Rorc knockout (KO) mice, and heterozygous littermate control mice were challenged with C. difficile. Surprisingly, no statistically significant difference in weight loss (Figure 5A) or survival (Figure 5B) was detected between the Rorc KO and heterozygous littermate control mice. Rorc KO mice lack all lymphoid structures and have an altered intestinal T cell repertoire (Eberl and Littman, 2004; Eberl et al., 2004), complicating interpretation of experimental results with these mice. To address this issue, we used Il22−/− mice and assessed the host response following acute CDI. Il22−/− mice had significant, albeit transiently increased weight loss between days 2-5 of infection compared to control C57BL/6 mice (Figure 5C), but no statistically significant difference in rate of survival (Figure 5D). At day three post infection, Il22−/− and C57BL/6 mice had equivalent C. difficile burdens (Figure 5E) and toxin levels (Figure 5F) in the cecum, indicating similar establishment of CDI. Further, while expression of IL-22 dependent Reg3g was significantly decreased in Il22−/− mice compared to C57BL/6 mice, there was no difference in the induction of Ifng or IFN-γ inducible Nos2 at day three post infection (Figure 5G). These data suggest that in the absence of ILC3-mediated IL-22 production, the ILC1 response remains intact.
Figure 5. Selective loss of ILC3s or ILC3 effector molecules reveals their limited role in recovery following acute C. difficile infection.
(A) Weight loss and (B) survival of RorcHET or Rorc KO mice following CDI. Combination of 3 independent experiments (n=10-11). (C-G) C57BL/6 and Il22−/− mice were infected with C. difficile spores and assessed for (C) weight loss and (D) survival. Weight loss curve is representative of 4 independent experiments. Survival curve is a combination of 4 experiments (n=21-22). (E) C. difficile burden and (F) toxin levels in the cecal content at day 3 post-infection. (G) Fold induction of Reg3g, Ifng, and Nos2 in the colon of day 3 infected C57BL/6 and Il22−/− mice relative to uninfected C57BL/6 mice and normalized to Hprt (n=7-8). (H) Weight loss and (I) survival of Rag1−/− or Rag1−/− mice treated with anti-IL-17a neutralizing antibody (n=8). (J) Survival of Rag1−/− or Rag.Il17 dKO− mice (n=7-9). *p<0.05, **p<0.01. Data shown are mean ± SEM. See also Figure S5.
Our observations demonstrating a modest impact of IL-22 deficiency on recovery from acute CDI contrast with those recently reported by (Hasegawa et al., 2014). To reconcile our apparently disparate observations, we pretreated Il22−/− and C57BL/6 mice with a cocktail of six broad spectrum antibiotics and then orally challenged mice with a vegetative culture of C. difficile (instead of spores), as described in (Hasegawa et al., 2012; Hasegawa et al., 2014) (Figure S5A). This inoculum resulted in 5×107 to 1×108 CFUs of the vegetative form as well as ~600 spores per mouse (Figure S5B). Under these conditions, infected Il22−/− mice had higher disease severity (Figure S5C) and significantly increased mortality compared to infected C57BL/6 mice (Figure S5D). These results indicate that the relative contributions of innate immune cells to infection by the same pathogen can differ, depending on the context in which the host is exposed to the pathogen. Our results, we believe, uncover innate immune mechanisms that are involved in early defense against CDI acquired by ingestion of spores, the most likely mechanism of transmission in a hospital setting, while the results of Hasegawa et al. may reveal mechanisms of immune defense during more severe and perhaps later stages of C. difficile induced sepsis.
It is possible that ILC3s contribute to resolution of acute CDI in an IL-22 independent manner. IL-17a is another effector cytokine produced by ILC3s that is upregulated following acute CDI (Figure 2B, (Sadighi Akha et al., 2013)) and has been implicated in host defense against bacterial and fungal pathogens (Gladiator et al., 2013; Ye et al., 2001). To assess the potential role for IL-17a in host defense against C. difficile, we administered anti-IL17a neutralizing or isotype control antibodies to Rag1−/− mice followed by oral challenge with C. difficile spores. Rag1−/− mice receiving anti-IL17a blockade did not have enhanced weight loss (Figure 5H) or mortality (Figure 5I) following infection compared to isotype treated Rag1−/− mice. Anti-IL-17a treatment did increase susceptibility to pulmonary Klebsiella pneumoniae infection, a pathogen where IL-17 is known to be critical for host defense (Ye et al., 2001), demonstrating the efficacy of the IL-17 blockade therapy (Figure S5E-G). To further assess the contribution of ILC-derived IL-17a, Rag1−/− mice were crossed with Il17a−/− mice to generate Rag1−/− Il17a−/− mice (Rag.Il17 dKO). Rag.Il17 dKO mice infected with C. difficile spores did not exhibit increased mortality following infection compared to Rag1−/− mice (Figure 5J). Combined, these data suggest that ILC3-derived IL-17a does not significantly contribute to host defense against acute CDI. Our finding that the loss of ILC3 associated effector molecules only modestly impacts recovery from acute CDI suggests that other ILC subsets are important contributors to host defense against CDI.
T-bet expressing ILC1s are critical for host defense against C. difficile
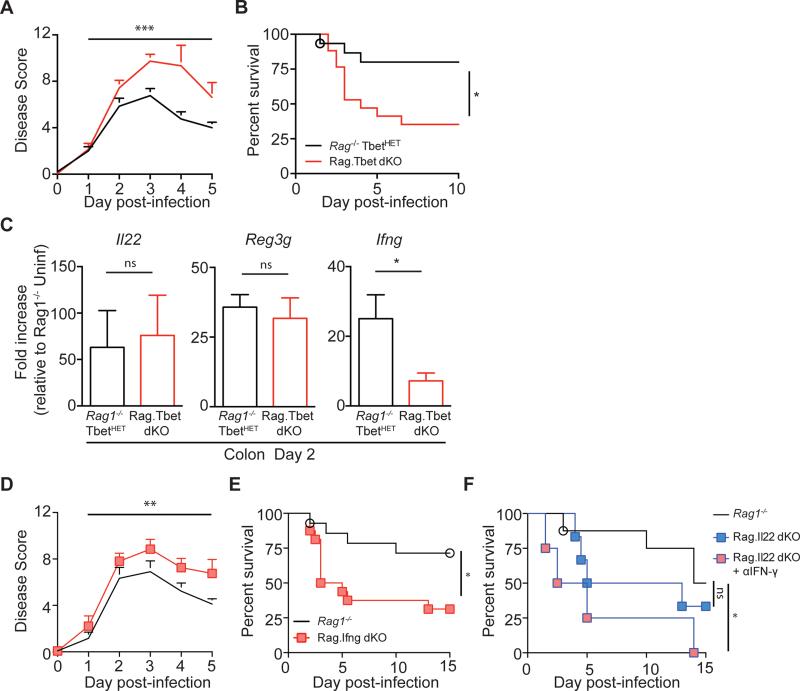
Ablation of ILC3-mediated effector molecules did not recapitulate the phenotype observed in Ragγc−/− mice following CDI, and the ILC1 response remained intact in C. difficile infected Il22−/− mice, suggesting that an ILC1-dependent mechanism could be important in mediating protection. ILC1 maturation is dependent on expression of the transcription factor T-bet (Klose et al., 2014). C57BL/6 and Rag1−/− mice do not differ in their ability to recover from acute CDI (Figure S1, (Hasegawa et al., 2014)), therefore to assess ILC1 contributions to defense against acute CDI, Rag1−/− mice were crossed with Tbx21−/− mice to generate Rag1−/− Tbx21−/− mice (Rag.Tbet dKO). Rag.Tbet dKO mice may develop spontaneous colitis depending on the composition of the host microbiota (Garrett et al., 2007; Powell et al., 2012), however, the Rag.Tbet dKO mice generated for these studies did not exhibit any overt signs of colitis. Eight-week old naïve, Rag1−/− and Rag.Tbet dKO mice had similar numbers of ILC2s (Figure S6A), ILC3s (Figure S6A), and Eomes+, NK1.1+ ‘classical’ NK cells (Figure S7B), while having significantly fewer Eomesneg, NK1.1+ ‘ILC1s’ (Figure S6B), which predominantly express T-bet (Figure S6C), in the large intestine lamina propria (Figure S6D), mLN (Figure S6E), spleen (Figure S6F), and liver (Figure S6G). Following CDI, disease severity (Figure 6A) and mortality (Figure 6B) were markedly increased in Rag.Tbet dKO mice compared to littermate Rag1−/−, TbetHET mice. The greater than 50% mortality rate observed in Rag.Tbet dKO mice was similar to that observed in Ragγc−/− mice (Figure 1B), indicating that T-bet expressing ILC1s make a major contribution to recovery from acute CDI. While induction of Il22 and IL-22 dependent Reg3g genes was comparable between Rag.Tbet dKO and Rag1−/−, TbetHET mice at day two post CDI, Ifng expression was significantly decreased in Rag.Tbet dKO mice, indicative of a defective ILC1 response in these mice (Figure 6C). To assess whether ILC1s act through IFN-γ to protect the host against C. difficile, Rag1−/− Ifng−/− double knockout mice (Rag.Ifng dKO) were generated and challenged with C. difficile spores along with cohoused Rag1−/− control mice. Similar to Rag.Tbet dKO mice, Rag.Ifng dKO mice had significantly higher disease scores than infected Rag1−/− mice (Figure 6D) and rapidly succumbed to infection (Figure 6E). Our results demonstrate the critical role of T-bet+, IFN-γ expressing ILC1s in recovery from acute CDI. Lastly, we crossed Rag1−/− mice with Il22−/− mice to generate Rag1−/− Il22−/− mice (Rag.Il22 dKO) and used these mice in combination with IFN-γ blockade to assess the additive impact of these ILC-derived cytokines. Similar to Il22−/− mice (Figure 5C,D), Rag.Il22 dKO mice exhibited an intermediate phenotype following CDI with a trend toward increased susceptibility following CDI (Figure 6F). However, Rag.Il22 dKO mice that were also treated with IFN-γ blockade rapidly succumbed to infection (Figure 6F) demonstrating the cooperative role of ILC1s and ILC3s in host defense against acute CDI.
Figure 6. Ablation of ILC1s or ILC1-associated IFN-γ leads to increased susceptibility to C. difficile infection.
(A) Disease severity and (B) survival of Rag1−/− TbetHET or Rag.Tbet dKO mice following CDI. Combination of 3 independent experiments (Rag1−/− TbetHET n=14, Rag.Tbet dKO n=16). (C) Fold induction of Il22, Reg3g, and Ifng in the colon of day 2 infected Rag1−/− TbetHET or Rag.Tbet dKO mice relative to uninfected Rag1−/− TbetHET mice and normalized to Hprt (n=7). (D) Disease severity and (E) survival of Rag1−/− or Rag.Ifng dKO mice following CDI. Combination of 4 independent experiments (n=15-17). (F) Survival of Rag1−/−, Rag.Il22 dKO or Rag.Il22 dKO mice treated with αIFN-γ neutralizing antibody (n=4-8). *p<0.05, **p<0.01, ***p<0.001. Data shown are mean ± SEM. See also Figure S6.
Discussion
CDI and associated destruction of the colonic epithelial barrier result from the failure of microbiota-mediated colonization resistance. Upon in vivo colonization, germination, vegetative growth and toxin production by C. difficile, innate immune defenses are engaged that are essential for host survival. Previous studies have demonstrated the importance of MyD88- and NOD-mediated signaling and neutrophil recruitment in limiting damage to the large intestine and for survival following CDI (Hasegawa et al., 2011; Jarchum et al., 2012; Lawley et al., 2009). In this report, we demonstrate that innate immune defenses mediated by ILCs are activated and contribute to recovery from acute CDI. Adoptive transfer of ILCs into highly susceptible Ragγc−/− mice markedly reduces mortality resulting from acute CDI. These observations and the finding that Nfil3−/− mice are more susceptible to C. difficile and C. rodentium infection despite intact T and B cell function, highlight the importance of ILCs during CDI. Type 3 ILCs and the production of IL-22 have been implicated in defense against colonic infection with C. rodentium (Satoh-Takayama et al., 2008; Sonnenberg et al., 2011; Zheng et al., 2008). In contrast, our studies demonstrate that recovery from acute CDI was largely mediated by ILC1s and IFN-γ, with a contribution from ILC3s and IL-22. The distinct contributions of ILC1s and ILC3 subsets to these two colonic bacterial pathogens likely result from distinct pathogenic mechanisms, with C. difficile mediating epithelial cell damage by exotoxin secretion while C. rodentium directly contacts epithelial cell membranes (Mundy et al., 2005; Viswanathan et al., 2010).
The outcome of CDI is influenced by the virulence of C. difficile strains, the composition of the host's intestinal microbial communities and the host's immune response. Infection of mice with highly virulent strains of C. difficile in a setting of severe microbiota dysbiosis, following administration of multiple broad-spectrum antibiotics, can overwhelm innate immune defenses and lead to mortality (Buonomo et al., 2013; Chen et al., 2008). Infection of mice with less virulent C. difficile isolates or following less severe alteration of the microbiota leads to acute infection, with diarrhea and weight loss, followed by recovery that is mediated by the innate immune system (Buffie et al., 2012; Jarchum et al., 2012). In this setting, innate immune responses enable mice to regain weight, resolve diarrhea and survive long-term, despite continuing to harbor toxin-producing C. difficile bacilli in their large intestine. Inflammation is part of the host's innate immune response to infection. However, if left unchecked, the proinflammatory immune response can cause tissue destruction that is detrimental (Chovatiya and Medzhitov, 2014). In the context of CDI it is unclear whether the strong inflammatory response observed in some patients drives disease severity or is a result of pathogen-induced damage to the intestinal epithelium. Recovery from acute C. difficile colitis likely requires engagement of mechanisms that restrict systemic dissemination of intestinal bacteria and their byproducts, potentially by activating their uptake and destruction by phagocytic cells in the lamina propria and by re-establishing the intestinal epithelial barrier. ILC1s and IFN-γ-mediated defenses may contribute to enhanced clearance of bacteria that enter the lamina propria, thereby limiting their dissemination to the liver and the bloodstream. Bacteria that do disseminate can activate ILC3s and are cleared via IL-22 activation of the complement pathway (Hasegawa et al., 2014). This inflammatory response, however, must be tightly regulated to maintain protective features of the inflammatory immune response while limiting tissue pathology.
While our study implicates innate IFN-γ production in recovery from acute CDI, it is possible that ILC1s are not the sole source of this cytokine. For example, classical Eomes-expressing NK cells produce IFN-γ and, in some settings, neutrophils, and inflammatory monocytes produce IFN-γ (Gordon et al., 2012; Kraaij et al., 2014; Spees et al., 2014). Further, T-bet-expressing ILC1s may express or induce additional effector mechanisms that act in conjunction with IFN-γ to elicit host defenses against C. difficile. ILC1s express CD160, (Fuchs et al., 2013), a ligand that is essential for IFN-γ secretion by NK cells (Tu et al., 2015) and can coproduce TNF-α along with IFN-γ (Klose et al., 2014). Antibody blockade of either CD160 or TNF-α during CDI results in decreased induction of anti-microbial defense genes, suggesting that these may be additional ILC1-mediated effector mechanisms that contribute to recovery from acute CDI (McDermott et al., 2014; Sadighi Akha et al., 2014).
A recent report demonstrated a role for IL-22 in defense against CDI via activation of the C3 complement pathway. In the absence of IL-22, strains of Enterobacteriaceae translocated from the intestinal lumen into peripheral organs following C. difficile induced damage to the intestinal epithelial barrier (Hasegawa et al., 2014). In contrast, another study demonstrated that abrogation of the IL-22 signaling pathway via deletion of IL-23 improved survival of C. difficile infected mice (Buonomo et al., 2013). These observations suggest a context dependent role for ILC3s and IL-22 in limiting systemic infection by oxygen tolerant pathobionts that, if present within the gut lumen, can translocate into the bloodstream following toxin-induced damage to the epithelial barrier by C. difficile. Our results, in combination with these studies, implicate a cooperative role between ILC1s and ILC3s. ILC1s which are prominent in intestinal intraepithelial space (Bernink et al., 2013; Fuchs et al., 2013), may limit C. difficile damage by activating local defenses at the site of infection, while ILC3s activate innate immune responses in peripheral organs such as the liver and lung to clear any translocated pathogenic bacteria. Indeed, systemic administration of bacterial flagellin can elicit IL-22 production by ILC3s (Kinnebrew et al., 2012) and can protect against acute C. difficile mortality (Jarchum et al., 2011). Thus, similar to the role of CD4+ T helper type 1 cells of the adaptive immune system, ILC1s produce effector cytokines that activate the anti-bacterial defense programs of bystander immune cells at the site of infection. Without the requirement of antigen specificity and subsequent clonal expansion, ILC1s can rapidly respond to infection and provide initial host defenses against invasive bacteria. This report identifies the critical role of ILC1s in host defense against C. difficile and suggests the potential for a cooperative role of ILC1s and ILC3s in limiting pathology following pathogen induced damage to the epithelial barrier.
Experimental Procedures
Mice
C57BL/6, Rag1−/−, Rorcgfp/gfp (Rorc KO), Tbx21−/−, Ifng−/− mice were purchased from the Jackson Laboratory. Ragγc−/− mice were purchased from Taconic Farms. Il22−/− mice were provided by R. Flavell (Yale University, New Haven, CT). All knockout mouse strains were derived on a C57BL/6 background. Rag.Tbet dKO, Rag.Ifng dKO, Rag.Il17 dKO, and Rag.Il22 dKO mice were generated by breeding Rag1−/− mice with Tbx21−/−, Ifng−/− Il17a−/−, or Il22−/− mice. All mice were bred and maintained under specific pathogen-free conditions at the Memorial Sloan Kettering Research Animal Resource Center. Sex and age-matched controls were used in all experiments according to institutional guidelines for animal care. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Memorial Sloan Kettering Cancer Center.
Antibiotic pretreatment, CDI and mouse monitoring
Mice were cohoused for three weeks prior to antibiotic treatment then supplemented with neomycin (Sigma) (0.25 g/L) and vancomycin (Novaplus) (0.25 g/L) in the drinking water for 3 days. One day following cessation of antibiotic water, mice received 200 μg of clindamycin (Sigma) by i.p. injection. Twenty-four hours later, mice received 200 C. difficile spores (VPI10463 strain ATCC# 43255) via oral gavage. For IL-17a or IFN-γ blockade experiments, mice received 400 μg of anti-mIL17a antibody (clone 17F3 BioXcell), anti-mIFN-γ antibody (clone XMG1.2 BioXcell) or mouse IgG1 isotype control antibody (clone MOPC-21 BioXcell) i.p. every other day starting at day −1 of infection. After infection, mice were monitored and scored for disease severity by four parameters: weight loss (>95% of initial weight = 0, 95-90% initial weight = 1, 90-80% initial weight = 2, <80% = 3), surface body temperature (>32°C = 0, 32-30°C = 1, 30-28°C = 2, <28°C = 3), diarrhea severity (fo rmed pellets = 0, loose pellets = 0, liquidly discharge = 2, no pellets/caked to fur = 3), morbidity (score of 1 for each symptoms with max score of 3; ruffled fur, hunched back, lethargy, ocular discharge).
Cell isolation and ex vivo cytokine detection
Single cell suspensions from the mLN, IEL, or Lp were cultured in a 96-well plate in complete media and BFA alone (Golgiplug, eBioscience) for 3 hours at 37°C. Following incubation, cells were stained for surface molecules, fixed, and intracellularly stained for IL-22 (clone 1H8PWSR, eBioscience) and IFN-γ (clone XMG1.2 BD Biosciences). For detailed description of procedure see Supplemental Experimental Procedures.
ILC adoptive transfer
Donor Rag1−/− mice received 3 i.p. injections of 2.5 μg rhIL-7 (eBioscience)/ 15 μg anti-IL-7 complex (clone M25 BioXcell) every other day to expand the ILC population (Boyman et al., 2008). Twelve hours following last injection, the spleen, lymph nodes, small and large intestine were processed into single cell suspensions as described above. Samples were stained with fluorescently conjugated antibodies and DAPI then sorted on a BD Aria on the basis of DAPIneg, CD45+, Gr-1neg, F4/80neg, CD90+, CD127+ with >92% purity obtained. Approximately 2×105 sort-purified ILCs were transferred by retro-orbital injection into Ragγc−/− mice. Three weeks following transfer, recipient mice, along with Rag1−/− and Ragγc−/− control mice, received 200 μg of clindamycin by i.p. injection on two consecutive days then were challenged with 200 C. difficile spores (VPI10463) (Buffie et al., 2012). Recipient Ragγc−/− mice received an additional ILC transfer twenty-four hours following infection and were monitored for morbidity and mortality.
Tissue RNA isolation, cDNA preparation and RT-PCR
RNA was isolated from colon tissue using mechanical homogenization and TRIzol isolation (Invitrogen) according to the manufacturer's instructions. cDNA was generated using QuantiTect reverse transcriptase (Qiagen). RT-PCR was performed on cDNA using Taqman primers and probes in combination with Taqman PCR Master Mix (ABI) and reactions were run on a RT-PCR system (Step-one Plus; Applied Biosystems). Gene expression is displayed as fold increase over uninfected C57BL/6 mice and normalized to Hprt.
Histology sectional and pathology scoring
Colon tissues were fixed with 4% paraformaldehyde, embedded in paraffin and 5 μm sections were cut and stained with hematoxylin and eosin. H&E-stained colon tissue sections were blindly scored as described in (Jarchum et al., 2012) based on epithelial degeneration/cell death, edema and cellular infiltration with each parameter scored from 0-3.
Statistical analysis
Results represent means ± SEM. Statistical significance was determined by the unpaired t-test, Mann-Whitney test for n ≤ 5, two-way ANOVA test for timecourse experiments, and logrank test for survival curve. Statistical analyses were performed using Prism GraphPad software v6.0. (* p < 0.05; ** p < 0.01; *** p < 0.001).
Supplementary Material
Highlights.
Recovery from acute C. difficile infection is independent of adaptive immunity.
Lack of Innate Lymphoid Cells leads to mortality following C. difficile infection.
Transfer of ILCs into susceptible hosts restores protection against C. difficile.
Type-1 ILCs mediate IFN-γ-dependent protection against C. difficile.
Acknowledgements
We would like to acknowledge members of the Pamer laboratory for helpful discussions and critical reading of the manuscript. We thank the Memorial Sloan Kettering Flow Cytometry and Cell Sorting Core the Molecular Cytology Facility at Memorial Sloan Kettering Cancer Center (Core grant P30 CA008748) for technical advice and expertise. The Lucille Castori Center for Microbes, Inflammation and Cancer for assistance with high throughput sequencing and analysis. This research is supported by the US National Institutes of Health (RO1 AI042135 and AI095706 to E.G.P), the Irvington Institute Postdoctoral Fellowship of the Cancer Research Institute (M.C.A.), a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH (B.B.L) (award number T32GM007739, award to the Weill Cornell/Rockefeller/Sloan Kettering Tri Institutional MD-PhD Program), and the Gilliam pre-doctoral fellowship from the Howard Hughes Medical Institute (S.G.C.).
Abbreviations used
- ILC
Innate Lymphoid Cells
- Ragγc−/−
Rag2, common gamma chain double knockout mice
- mLN
mesenteric lymph nodes
- KO
knockout
- PBS
phosphate-buffed saline
- IEL
intraepithelial lymphocyte compartment
- Lp
lamina propria
- i.p.
intraperitoneal
- CDI
C. difficile infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.C.A. and E.G.P. designed the experiments and wrote the manuscript. M.C.A. performed experiments and most analyses. L.L. and B.B.L. performed 16S amplicon quantification, MiSeq sequencing and analyzed sequencing data. S.C. performed FISH staining. H.X. performed K. pneumoniae infection. B.B.L., B.S., R.A.C. assisted in bacterial culturing, and qRT-PCR reactions. I.L., and R.C. maintained and screened mouse strains.
References
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. Journal of immunology. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infection and immunity. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo EL, Madan R, Pramoonjago P, Li L, Okusa MD, Petri WA., Jr. Role of interleukin 23 signaling in Clostridium difficile colitis. The Journal of infectious diseases. 2013;208:917–920. doi: 10.1093/infdis/jit277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chopra T, Alangaden GJ, Chandrasekar P. Clostridium difficile infection in cancer patients and hematopoietic stem cell transplant recipients. Expert Rev Anti Infect Ther. 2010;8:1113–1119. doi: 10.1586/eri.10.95. [DOI] [PubMed] [Google Scholar]
- Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A. Innate lymphoid cells in the defense against infections. Eur J Microbiol Immunol (Bp) 2013;3:143–151. doi: 10.1556/EuJMI.3.2013.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubberke ER, Reske KA, Srivastava A, Sadhu J, Gatti R, Young RM, Rakes LC, Dieckgraefe B, DiPersio J, Fraser VJ. Clostridium difficile-associated disease in allogeneic hematopoietic stem-cell transplant recipients: risk associations, protective associations, and outcomes. Clin Transplant. 2010;24:192–198. doi: 10.1111/j.1399-0012.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nature immunology. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12 and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. Journal of immunology. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Kamada N, Jiao Y, Liu MZ, Nunez G, Inohara N. Protective role of commensals against Clostridium difficile infection via an IL-1beta-mediated positive-feedback loop. Journal of immunology. 2012;189:3085–3091. doi: 10.4049/jimmunol.1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Yada S, Liu MZ, Kamada N, Munoz-Planillo R, Do N, Nunez G, Inohara N. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 2014;41:620–632. doi: 10.1016/j.immuni.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Nunez G, Inohara N. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. Journal of immunology. 2011;186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- Huang H, Weintraub A, Fang H, Nord CE. Antimicrobial resistance in Clostridium difficile. Int J Antimicrob Agents. 2009;34:516–522. doi: 10.1016/j.ijantimicag.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Hunt JJ, Ballard JD. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol Mol Biol Rev. 2013;77:567–581. doi: 10.1128/MMBR.00017-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infection and immunity. 2011;79:1498–1503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infection and immunity. 2012;80:2989–2996. doi: 10.1128/IAI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. The Journal of infection. 2009;58:403–410. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, Goppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- Kraaij MD, Vereyken EJ, Leenen PJ, van den Bosch TP, Rezaee F, Betjes MG, Baan CC, Rowshani AT. Human monocytes produce interferon-gamma upon stimulation with LPS. Cytokine. 2014;67:7–12. doi: 10.1016/j.cyto.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. The New England journal of medicine. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, Mastroeni P, Scott P, Raisen C, Mottram L, et al. Antibiotic treatment of clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infection and immunity. 2009;77:3661–3669. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS pathogens. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BB, Buffie CG, Carter R, Leiner I, Toussaint NC, Miller L, Gobourne A, Ling L, Pamer E. Loss of microbiota-mediated colonization resistance to Clostridium difficile infection is greater following oral vancomycin as compared with metronidazole. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucado J, Gould C, Elixhauser A. Clostridium Difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs (Rockville (MD)) 2012 [Google Scholar]
- Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AJ, Higdon KE, Muraglia R, Erb-Downward JR, Falkowski NR, McDonald RA, Young VB, Huffnagle GB. The Role of Gr-1 Cells and TNFalpha Signaling During Clostridium difficile Colitis in Mice. Immunology. 2014 doi: 10.1111/imm.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cellular microbiology. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674–684. doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature reviews. Microbiology. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- Sadighi Akha AA, McDermott AJ, Theriot CM, Carlson PE, Jr., Frank CR, McDonald RA, Falkowski NR, Bergin IL, Young VB, Huffnagle GB. IL22 and CD160 play additive roles in the host mucosal response to Clostridium difficile infection in mice. Immunology. 2014 doi: 10.1111/imm.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha AA, Theriot CM, Erb-Downward JR, McDermott AJ, Falkowski NR, Tyra HM, Rutkowski DT, Young VB, Huffnagle GB. Acute infection of mice with Clostridium difficile leads to eIF2alpha phosphorylation and pro-survival signalling as part of the mucosal inflammatory response. Immunology. 2013 doi: 10.1111/imm.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, Young VB. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5:e00893–00814. doi: 10.1128/mBio.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Mjosberg J, Spits H, Artis D. SnapShot: innate lymphoid cells. Immunity. 2013;39:622–622. doi: 10.1016/j.immuni.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees AM, Kingsbury DD, Wangdi T, Xavier MN, Tsolis RM, Baumler AJ. Neutrophils are a source of gamma interferon during acute Salmonella enterica serovar Typhimurium colitis. Infection and immunity. 2014;82:1692–1697. doi: 10.1128/IAI.01508-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu TC, Brown NK, Kim TJ, Wroblewska J, Yang X, Guo X, Lee SH, Kumar V, Lee KM, Fu YX. CD160 is essential for NK-mediated IFN-gamma production. The Journal of experimental medicine. 2015;212:415–429. doi: 10.1084/jem.20131601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvede M, Tinggaard M, Helms M. Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea: results from a case series of 55 patients in Denmark 2000-2012. Clin Microbiol Infect. 2015;21:48–53. doi: 10.1016/j.cmi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. The Journal of experimental medicine. 2012;209:1445–1456. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan VK, Mallozzi MJ, Vedantam G. Clostridium difficile infection: An overview of the disease and its pathogenesis, epidemiology and interventions. Gut microbes. 2010;1:234–242. doi: 10.4161/gmic.1.4.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, et al. Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Antimicrob Agents Chemother. 2012;56:4103–4111. doi: 10.1128/AAC.00360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren SM, Ahmed N, Jamal A, Safadi BY. Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg. 2005;140:752–756. doi: 10.1001/archsurg.140.8.752. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. The Journal of experimental medicine. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.