Abstract
Recent studies in mammals have demonstrated a central role for the circadian clock in maintaining metabolic homeostasis. In spite of these advances, however, little is known about how these complex pathways are coordinated. Here, we show that fundamental aspects of the circadian control of metabolism are conserved in the fruit fly Drosophila. We assay feeding behavior and basic metabolite levels in individual flies and show that, like mammals, Drosophila display a rapid increase in circulating sugar following a meal, which is subsequently stored in the form of glycogen. These daily rhythms in carbohydrate levels are disrupted in clock mutants, demonstrating a critical role for the circadian clock in the postprandial response to feeding. We also show that basic metabolite levels are coordinated in a clock-dependent manner and that clock function is required to maintain lipid homeostasis. By examining feeding behavior, we show that flies feed primarily during the first 4 hours of the day and that light suppresses a late day feeding bout through the cryptochrome photoreceptor. These studies demonstrate that central aspects of feeding and metabolism are dependent on the circadian clock in Drosophila. Our work also uncovers novel roles for light and cryptochrome on both feeding behavior and metabolism.
Keywords: circadian clock, cryptochrome, light signaling, metabolism, feeding behavior
The circadian clock consists of a molecular oscillator that orchestrates daily rhythms in gene expression, hormone secretion, metabolism, and behavior (Doherty and Kay, 2010; Hardin, 2005; Green et al., 2008). The evolutionary conservation of the clock, from cyanobacteria to plants and mammals, suggests that this temporal coordination is advantageous and increases fitness (Rosbash, 2009). Consistent with this, clock disruption leads to decreased life span, changes feeding activity, and causes metabolic disorders in mammals, including obesity, hepatic steatosis, hyperglycemia, and reduced levels of circulating insulin (Turek et al., 2005; Maury et al., 2010). This role for the circadian clock in metabolism appears to have a direct effect on human health as evidenced by the increased incidence of obesity and diabetes in shift workers (Knutsson, 2003). Conversely, obesity in humans and mice influences physiological and behavioral rhythms that are controlled by the circadian clock. This interplay between circadian clocks and metabolism is regulated by both the central clock, which resides in the brain and is entrained by light, and by clocks in peripheral tissues. Remarkably, in spite of the critical role of light signaling and the circadian clock in metabolic health, few studies have addressed this topic using simple genetic model systems. In particular, only 2 articles have examined the regulation of metabolism by the circadian clock in the fruit fly Drosophila, the organism in which the clock was discovered and initially characterized (Xu et al., 2008; Diangelo et al., 2011). These studies analyzed the circadian control of feeding behavior in adult flies as well as metabolic functions in the central clock neurons and a peripheral tissue, the fat body, showing that clock disruption in this tissue results in a change in feeding rhythm, food consumption, starvation sensitivity, and reduced levels of glycogen (Xu et al., 2008). Here, we develop assays that allow us to measure feeding behavior and metabolic parameters in individual flies across a circadian time course. These studies reveal an unexpected level of control for feeding. We show that, in addition to an early morning feeding bout, adult Drosophila have a late day feeding bout that is suppressed by light via the cryptochrome photoreceptor. We also show that, like mammals, flies display a postprandial metabolic response to feeding as well as the proper storage and coordination of key metabolites. Both of these responses are dependent on clock function, demonstrating that a critical role for the circadian clock in maintaining metabolic homeostasis has been conserved through evolution. These studies reveal new roles for light and cryptochrome in feeding behavior and metabolism and establish a foundation for using Drosophila as a simple genetic system to characterize the central role of the circadian clock in metabolic health.
MATERIALS AND METHODS
Locomotor Behavior Analysis
All studies were performed using Berlin-K wild-type flies and tim01 or cry01 mutants that had been crossed for at least 9 generations into the Berlin-K background. Stocks were maintained at 25 °C. Locomotor activity of adult males was assayed using the DAM-2 system (TriKinetics, Waltham, MA). Flies were entrained to 12 hours of light followed by 12 hours of darkness (12:12 LD) for 4 to 5 days, at approximately 80% relative humidity, and then transferred to constant darkness (DD) for some studies, as described. Analyses were performed using DAM software (TriKinetics) and Microsoft Excel (Redmond, WA).
Feeding Behavior Analysis
Newly emerged adult males were fed yeast paste made with 1% dextrose solution and entrained to 12:12 LD for 5 to 6 days prior to testing. In some cases, entrained flies were transferred to DD for multiple days, as described. At specified times, flies were transferred to vials containing yeast paste supplemented with 108 counts/min/mL (final volume) α-32P dCTP (PerkinElmer, Waltham, MA) and permitted to feed for 2 hours. Individual flies were immediately frozen in 0.65-mL tubes, and consumed radiation was measured by Cerenkov counting in a scintillation counter (Beckman Coulter, Brea, CA).
Metabolite Measurements
Newly emerged adult males were fed yeast paste made with 1% dextrose solution and entrained to 12:12 LD cycles for 5 to 6 days prior to testing. All DD measurements were made from flies during the second day of DD after LD entrainment. At specified times, flies were collected and frozen immediately before homogenizing individually in 100 μL of ice-cold 0.1% Tween-20. Homogenates were heat inactivated for 10 minutes at 70 °C prior to transfer to 96-well plates. Fractions of homogenates were used to assay total trehalose, glycogen, triacylglyceride, and protein levels, as described (Ruaud et al., 2010).
Statistical Analysis
All data sets were combined and subjected to the Anderson-Darling test for normal distribution. Where indicated, data failing the Anderson-Darling test were compared using the Mann-Whitney U test to determine significance. All other data sets were compared using Student’s t test. Correlation coefficients for pairwise comparisons were generated using the Spearman ρ test for comparison of nonnormally distributed data sets. These values were also calculated using linear regression analysis, revealing nearly identical significance. Comparison between correlation coefficients was performed using the Fisher z transformation. This test estimates the likelihood that variance between 2 variables from 2 independent populations would occur by chance based on the sample size and correlation coefficient.
RESULTS
The Circadian Clock and Cryptochrome Regulate Feeding Behavior
Wild-type Drosophila entrained to 12 hours of light followed by 12 hours of darkness (12:12 LD), and then shifted to constant darkness (DD), display characteristic early morning and late evening activity bouts in LD and recurring bouts of activity in DD, with a free-running rhythm of approximately 23 hours (Fig. 1A, top). As expected, this behavior is dependent on a functional clock, with flies that carry the tim01 timeless-null allele in the same genetic background as our wild-type strain displaying arrhythmic locomotor behavior in DD (Sehgal et al., 1994) (Fig. 1A, bottom). In order to monitor feeding behavior under these conditions, we developed an assay to quantify food uptake in large numbers of individual flies using a radioactive tracer and took measurements at 2-hour intervals. Using this approach, we confirmed that wild-type animals feed within the first few hours of daylight in LD (Xu et al., 2008). Our approach, however, also uncovered a late evening feeding bout in DD that appears to increase with time, revealing a previously unknown, light-dependent ultradian rhythm (Fig. 1B, top). An intact molecular clock is necessary for this rhythm because tim01 mutant flies do not feed with discernable regularity in DD, although their feeding behavior mimics that of wild-type flies in LD (Fig. 1B, bottom). There are, however, 2 significant increases in feeding behavior in tim01 mutant flies on the first day in DD, at CT 0 to 2, and at CT 16 to 18 (Fig. 1B, bottom). A similar result was observed by Xu et al. (2008) in animals that express a dominant-negative form of the clock gene. This clock-independent function may be due to a homeostatic response to starvation. Entraining wild-type flies to 12:12 LD and then restricting food after ZT 0 reveals that fasted flies will not feed in the middle of the day (ZT 4–6) but eat more than fed controls from ZT 2 to 4 and from ZT 6 to 12 (Suppl. Fig. S1). This demonstrates that flies will respond to food deprivation only during the early morning or late evening and suggests that feeding behavior is tightly regulated by circadian and homeostatic mechanisms, dependent upon light exposure and food availability. Taken together, our observations suggest that light signaling independent of the circadian clock normally suppresses evening feeding.
Figure 1.
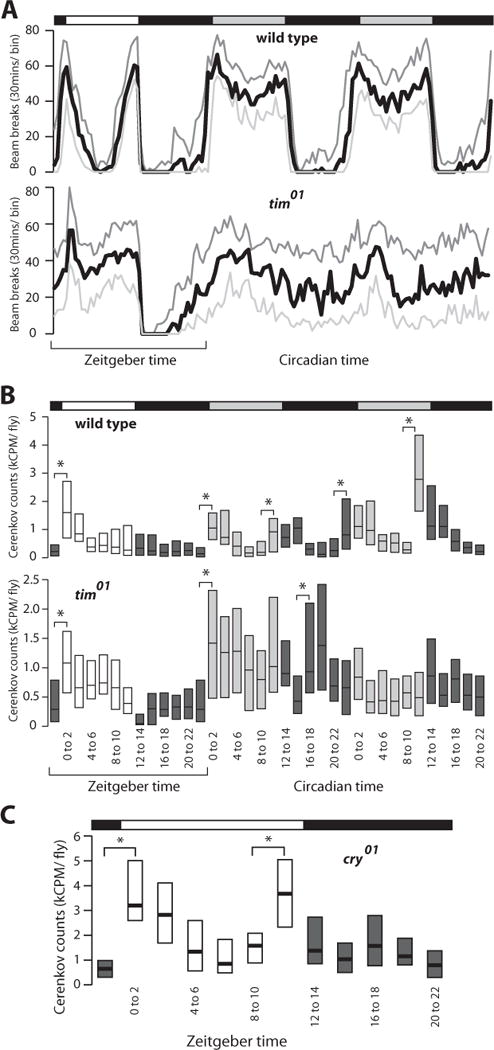
An ultradian feeding rhythm is regulated by cryptochrome and light. Bars on top of each panel indicate light:dark (LD) (white/black) or dark:dark (DD) (gray/black) cycles, while zeitgeber time or circadian time in hours is shown at the bottom. (A) Locomotor activity of wild-type flies and tim01 arrhythmic mutants under 12:12 LD and DD conditions (n = 32 for each genotype). The number of beam breaks was summed into 30-minute bins and is presented as first quartile (light gray), median (black), and third quartile (dark gray) values. (B) Feeding behavior measured as radioactive counts consumed during 2-hour intervals for wild-type flies and tim01 mutants under 12:12 LD and DD conditions (n > 1700 for each genotype). Boxes depict the interquartile range for each time point, with the median indicated by a horizontal bar and the lighting condition represented by shading. (C) Feeding behavior of cry01 mutants under 12:12 LD conditions (n > 540). All data are compiled from 4 biologically independent experiments (*p < 0.01, Student’s t test).
The cryptochrome (CRY) photoreceptor is necessary for rapid clock entrainment and light-induced phase changes in locomotor activity and adult eclosion (Stanewsky et al., 1998). CRY is thought to affect these behaviors by directly resetting the molecular clock through light-mediated degradation of TIM (Busza et al., 2004). We hypothesized that CRY might be the light-signaling factor responsible for suppression of the evening feeding bout that emerges in DD. To test this, we measured LD feeding behavior in animals that carry the cry01-null allele. Interestingly, these mutants not only display the early morning feeding bout that is seen in wild-type animals in LD, but they also display robust late evening feeding under these conditions, similar to wild-type flies in DD (Fig. 1C). This reveals that light activation of CRY inhibits feeding. In addition, the absence of a late day feeding bout in tim01 mutant animals in LD suggests that this unexpected role for CRY is independent of light-induced TIM degradation (Fig. 1B, bottom).
Major Forms of Energy Fluctuate in a Clock-Dependent Manner
Mammalian feeding behavior leads to predictable biochemical and physiological events that result in the proper uptake, metabolism, and storage of nutrients. A hallmark of this response is the rapid and significant increase in plasma glucose immediately following a meal. Although Drosophila have many of the analogous metabolic pathways and tissues necessary for this response (Baker and Thummel, 2007; Schlegel and Stainier, 2007), postprandial changes in metabolites have not been reported previously in this organism. The discrete, robust feeding bout seen in wild-type flies at 0 to 2 hours ZT provided us with an opportunity to determine if this response is conserved through evolution. Accordingly, animals were entrained to 12:12 LD for several days, after which single flies were collected at 2-hour time points, homogenized, and assayed for the predominant circulating form of sugar in flies, trehalose. Trehalose levels are lowest at ZT 2 and increase 80% approximately 4 hours after feeding (Fig. 2A, top). In addition, glycogen levels in these same animals increase >2-fold from ZT 0 to ZT 12, indicating that much of the consumed sugar is subsequently captured as stored energy (Fig. 2A, bottom). This glycogen is depleted during the remainder of the day, suggesting that it is used to support energetic needs during this time. Analysis of the data by ANOVA statistics followed by a post hoc least significant difference test confirms that glycogen levels are significantly higher early in the day and lower in late day (Suppl. Fig. S2A). The oscillation in glycogen concentration is dampened but persists in DD, suggesting that this is a clock-dependent process (Fig. 2B and Suppl. Fig. S2B). This is supported by the absence of a clear rhythm in tim01 flies under either LD or DD conditions (Fig. 2C and 2D). Although glycogen measurements fluctuate significantly in tim01 mutant flies, the entire data set fits best to a linear regression line, indicating there is no statistically significant peak and trough. In addition, no rhythmic changes are seen in protein levels in wild-type or tim01 flies or in triacylglycerol concentrations, similar to a previous report (Diangelo et al., 2011) (Fig. 3A–D). Significant changes were not seen in total levels of glycogen or trehalose in tim01 or cry01 mutant animals (Suppl. Fig. S3).
Figure 2.
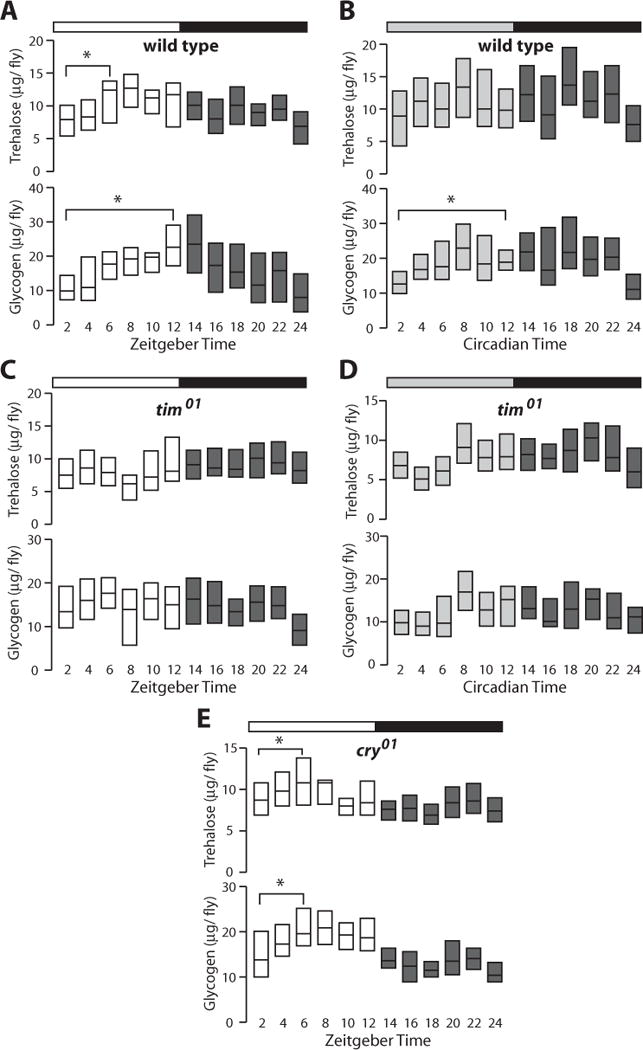
Carbohydrate metabolism is regulated by the circadian clock and feeding. Bars on top of each panel indicate light:dark (LD) (white/black) or dark:dark (DD) (gray/black) cycles, while zeitgeber time or circadian time in hours is shown at the bottom. (A) Amount of trehalose (upper) and glycogen (lower) in 12:12 LD entrained individual wild-type flies (n > 540). (B) Trehalose and glycogen measurements from wild-type flies (n > 540) taken during the second day of DD following entrainment at 12:12 LD. (C) Trehalose and glycogen measurements from 12:12 LD entrained tim01 arrhythmic mutants (n > 540). (D) Trehalose and glycogen measurements from tim01 mutants (n > 540) during the second day of DD following entrainment at 12:12 LD. (E) Trehalose and glycogen measurements from 12:12 LD entrained cry01 mutants (n > 540). All data are compiled from 4 biologically independent experiments (*p < 0.01, Student’s t test).
Figure 3.
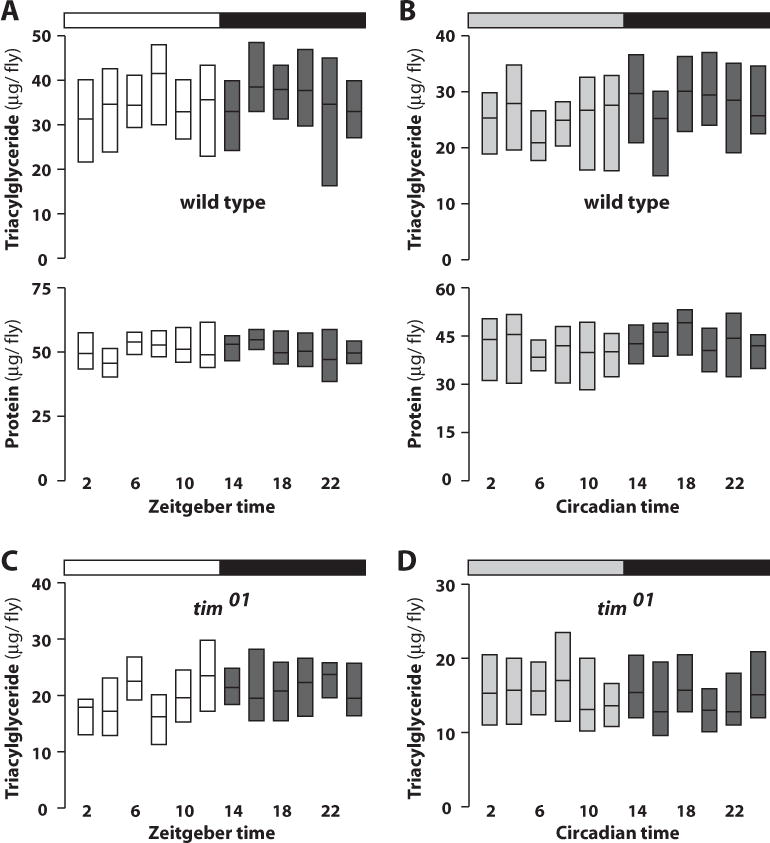
Triacylglyceride and protein concentrations do not oscillate in wild-type flies or tim01 mutants. Bars on top of each panel indicate light:dark (LD) (white/black) or dark:dark (DD) (gray/black) cycles, while zeitgeber time or circadian time in hours is shown at the bottom. (A) Amount of triacylglyceride (upper) and protein (lower) in 12:12 LD entrained individual wild-type flies (n > 540). (B) Triacylglyceride and protein measurements in individual wild-type flies collected during the second day of DD following entrainment at 12:12 LD (n > 540). (C) Triacylglyceride measurements in 12:12 LD entrained tim01 arrhythmic mutants (n > 540). (D) Triacylglyceride measurements in tim01 mutants during the second day of DD following entrainment at 12:12 LD (n > 540).
After observing the dramatic influence of light and cry on feeding behavior, we hypothesized that circadian photoperception may contribute to metabolic homeostasis. This connection has not been explored previously, although many mammalian species undergo photoperiod-dependent changes in physiology (Morgan and Hazlerigg, 2008). We therefore measured basic metabolic parameters in cry01 mutants entrained to 12:12 LD conditions and observed rhythmic changes in trehalose and glycogen concentration, with peaks at ZT 4 to 6 (Fig. 2F). This is consistent with the presence of an intact neuronal circadian clock in cry01 flies (Dolezelova et al., 2007). Interestingly, however, glycogen levels peak earlier in cry01 mutants (Fig. 2E, bottom). This conclusion is supported by ANOVA analysis of the data (Suppl. Fig. S2C). These results indicate that CRY is necessary to set the phase of glycogen accumulation. The effect on glycogen may also explain the dampened peak seen in wild-type flies in DD (Fig. 2B), when CRY is not activated by light.
Mice carrying circadian mutations can display significantly altered fat storage and mobilization phenotypes (Green et al., 2008). To test if this level of control is conserved through evolution, we compared triacylglyceride levels obtained throughout the day under LD and DD conditions. We found these concentrations to be significantly reduced in tim01 mutants, consistent with the previously described role for the circadian clock in mammalian fat homeostasis (Turek et al., 2005) (Fig. 4). Unexpectedly, wild-type animals also display reduced triacylglyceride levels after 2 days of DD relative to LD cycles. cry01 mutants display a similar phenotype, indicating that both the circadian clock and light input into the clock are necessary for proper fat homeostasis. It is also possible that CRY plays a core clock role in peripheral tissues, as has been previously reported (Ivanchenko et al., 2001; Levine et al., 2002; Collins et al., 2006), and that clock disruption in these peripheral tissues is responsible for the observed phenotype.
Figure 4.
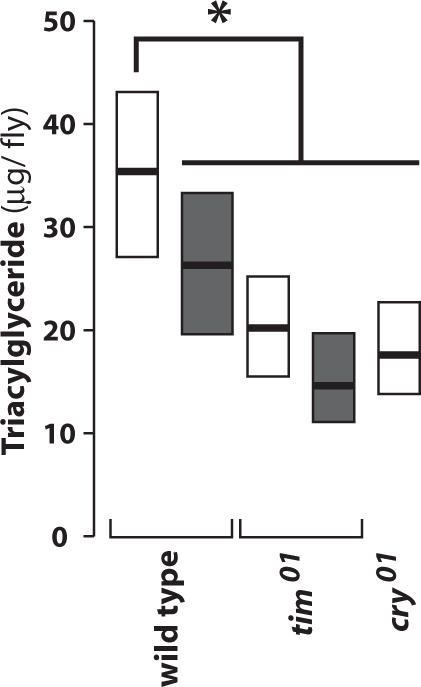
Triacylglycerol levels are reduced in tim01 and cry01 mutants. The amounts of triacylglycerol were determined in animals of the specified genotype (bottom) collected during 12:12 LD (white boxes) or the second day of DD after entrainment to 12:12 LD (gray boxes). All data are compiled from 4 biologically independent experiments (*p < 0.01, Student’s t test; n > 540).
Metabolite Levels Are Coordinated in Individual Flies by the Circadian Clock
Homeostatic regulation normally maintains a proper balance between metabolite levels in animals. While examining the data from the above experiments, we noticed that metabolite levels appeared to be coordinated in wild-type flies in LD but not DD. This suggested that an analysis of metabolite coordination in wild-type and mutant flies might provide a means of assessing their state of metabolic homeostasis. Accordingly, we calculated correlations between all metabolite levels measured in this study. Indeed, wild-type individuals with the most protein, a proxy for overall size, also had the highest levels of stored fat when entrained to LD conditions (Fig. 5A). This correlation is lost in DD, when the population has a negative and small correlation between these 2 parameters (Fig. 5B). Interestingly, a comparison of all metabolite levels in wild-type flies revealed that each set has a significant reduction in correlation in DD compared to LD (Fig. 5C and Suppl. Fig. S4), suggesting a role for light signaling and the circadian clock in the maintenance of homeostasis. Arrhythmic tim01 mutant animals have lower correlations between all metabolites measured under LD conditions, defining a role for the circadian clock in this process (Fig. 5C). Metabolite correlations in tim01 mutants are also significantly reduced under DD conditions, with the exception of trehalose and glycogen (Fig. 5C and Suppl. Fig. S4). These data reveal that the circadian clock is necessary for proper homeostatic coordination of specific energy forms and that light signaling can affect these correlations independent of the circadian clock.
Figure 5.
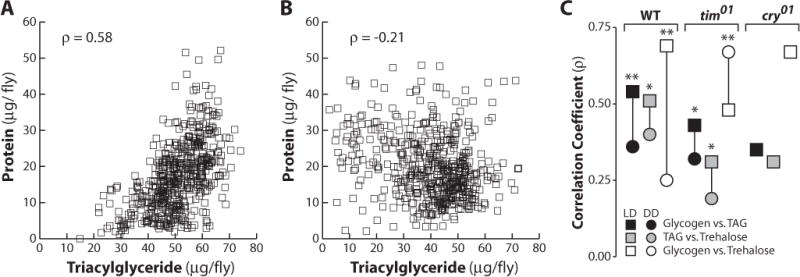
Metabolic levels are coordinated in individual flies under 12:12 LD environmental conditions. (A) Scatter plot showing glycogen and protein concentrations in individual wild-type flies (n > 540) entrained under 12:12 LD conditions. Correlation was determined using Spearman r. (B) Scatter plot showing glycogen and protein concentrations during the second day of DD following entrainment at 12:12 LD (n > 540). (C) Plot depicting changes in Spearman correlation coefficients from LD (circle) to DD (square) conditions, comparing glycogen to triacylglycerol (TAG) (black), trehalose to TAG (gray), or trehalose to glycogen (white) in wild-type, tim01, or cry01 mutants (*p < 0.01, **p < 0.001, Fisher z transformation). See Supplementary Figure S2 for primary data.
A role for light in metabolite coordination suggests that this process may be regulated by the CRY photoreceptor. Consistent with this proposal, cry01 mutants raised under LD conditions had very low correlations between either trehalose or glycogen and stored fat (Fig. 5C and Suppl. Fig. S4). However, trehalose and glycogen levels are highly correlated (ρ = 0.67), suggesting that CRY is dispensable for this homeostatic process. This indicates that CRY, at least partially, mediates the storage and/or utilization of energy and is consistent with a critical role for light in maintaining metabolic homeostasis.
DISCUSSION
The circadian clock and metabolic control are dependent upon one another for accurate anticipation of daily changes and physiological optimization for organismal fitness. Here, we have shown that the Drosophila circadian clock regulates feeding behavior, carbohydrate uptake and storage, lipid homeostasis, and metabolite coordination. Our studies also revealed an unexpected role for light exposure and CRY on feeding behavior and metabolism.
The Circadian Clock Regulates a Postprandial Response and Metabolite Coordination
The rapid and transient increase in circulating sugar concentrations following a meal is one of the best-characterized links between nutrition and metabolic homeostasis. Our studies have revealed that this classic postprandial response is conserved through evolution in Drosophila (Fig. 2A). We also observe a subsequent >2-fold increase in glycogen concentration, suggesting that circulating sugar is rapidly converted into stored energy. Arrhythmic flies do not display detectable periodicity in their levels of trehalose and glycogen under LD conditions, even though their feeding behavior is similar to wild-type. This indicates that the postprandial increases in circulating and stored carbohydrates are dependent upon a functional circadian clock. Similarly, no significant increases in trehalose or glycogen are seen in response to the late day feeding bout in either wild-type flies or cry01 mutants, demonstrating a lack of coordination between feeding and metabolism under these conditions (Figs. 1B and 1C and 2A and 2E). This loss of coupling between feeding behavior and metabolism has been reported in mammalian systems and is likely due to a disruption of the interactions between central and peripheral oscillators (Maury et al., 2010). Interestingly, measurements of glycogen levels in the livers of rabbits, rats, mice, and chickens have revealed daily approximately 2-fold increases with circadian periodicity, similar to the response we observe in flies (Forsgren, 1928; Bunning, 1973). Moreover, clock disruption specifically in mouse pancreatic b cells leads to hyperglycemia and diabetes without affecting activity or feeding rhythms (Marcheva et al., 2010). Taken together, these studies define the circadian clock as a central regulator of the normal postprandial response to dietary sugar uptake and the subsequent transient storage of this energy in the form of glycogen.
Although the complex biochemistry of carbohydrate metabolism makes it difficult to predict which gene or genes are responsible for the observed oscillation in glycogen levels, 2 candidates are promising. Shaggy is an ortholog of glycogen synthase kinase-3 (GSK-3), which inhibits glycogen synthesis by phosphorylating and inactivating glycogen synthase (Bourouis et al., 1990). Shaggy also regulates the speed of the circadian clock by phosphorylating Timeless protein and controlling its nuclear localization (Martinek et al., 2001). The direct connection of Shaggy with both clock function and glycogen homeostasis suggests that it may provide communication between these 2 systems. A second candidate, glycogen synthase 2, appears to be directly regulated by CLOCK in the rat liver (Doi et al., 2010), although its clock regulation remains unstudied in Drosophila.
Our approach of analyzing metabolite levels in individual animals also provided a unique opportunity to assess their coordination under LD and DD conditions and in different mutant backgrounds. We found highly significant pairwise correlations between trehalose, glycogen, and triacylglycerol levels in wild-type flies under LD conditions, reflecting the ability of these animals to maintain normal metabolic homeostasis. These correlations, however, are all reduced under DD conditions (Fig. 5C). They are also reduced in tim01 mutants under LD conditions, demonstrating that the critical role for the circadian clock in maintaining homeostasis is conserved through evolution (Green et al., 2008). Glycogen and trehalose coordination, however, appears distinct in several ways. First, it is the only metabolite comparison that displays an increased correlation in tim01 mutants when comparing LD and DD conditions (Fig. 5C). Second, even though wild-type flies in DD display a decrease in their glycogen and trehalose correlation, these metabolites remain highly coordinated in cry01 mutants. The high level of correlation between glycogen and triacylglycerol is also worth noting, given that glycogen is found predominantly in the muscle while triacylglycerol is primarily in the fat body, suggesting that the tissue-specific reserves of stored energy are coordinated by the circadian clock. It will be interesting in future studies to address the roles of peripheral clocks in these pathways.
Novel Roles for Cryptochrome
CRY is the primary molecule responsible for entraining the circadian clock to light and is necessary for clock function in some peripheral tissues (Ivanchenko et al., 2001; Levine et al., 2002; Collins et al., 2006). Although cry transcripts are most abundant during the day, CRY protein accumulates during the night, likely due to light-sensitive degradation (Stanewsky et al., 1998; Emery et al., 1998). Short pulses of light can either advance or delay behavior rhythms depending upon when they are administered and CRY activity (Stanewsky et al., 1998). CRY is thought to exert these effects through light-dependent degradation of Timeless protein (Busza et al., 2004), specifically within the oscillator neurons. Currently, TIM is the only known target of CRY, although CRY has recently been shown to have neuronal functions independent of the circadian clock (Fogle et al., 2011). Our studies have revealed new roles for CRY in the control of both feeding behavior and metabolism.
Late evening feeding is suppressed under normal LD conditions by light signaling through CRY (Fig. 1). Thus, animals lacking CRY feed in the evening similar to wild-type flies in DD. This result is unexpected because CRY functions are thought to be restricted to the early morning, when its protein levels are most abundant. In addition, Timeless protein does not provide a likely mechanism to explain the ability of CRY to suppress late day feeding because it is undetectable at this time (Hunter-Ensor et al., 1996). This is consistent with the absence of late evening feeding in tim01 mutants under LD conditions (Fig. 1B). Rather, we propose that CRY regulates feeding behavior through a distinct mechanism that remains to be identified.
Metabolic processes are also dependent on cry function, likely independent of the circadian clock. Animals lacking CRY can still be entrained to light cycles and retain wild-type circadian rhythms in locomotor activity, eclosion, and molecular oscillations of clock components (Dolezelova et al., 2007; Stanewsky et al., 1998). In spite of a functioning clock, however, we found a significant change in the phase of glycogen accumulation in cry01 flies. This indicates that CRY delays the accumulation and utilization of glycogen independent of traditional clock function. It is likely that peripheral tissues regulate this oscillation, and it is possible that CRY plays a timekeeping rather than time-setting role in this response, as it may in olfactory neurons (Krishnan et al., 2001). It will be interesting to determine the tissue specificity of CRY function to better understand its roles in regulating feeding behavior and carbohydrate homeostasis.
This study shows that CRY, light signaling, and clock function play central roles in controlling feeding behavior and metabolic homeostasis. Adult flies are capable of interpreting seasonal changes in photoperiod and respond with striking alterations in both the frequency and timing of feeding bouts. In addition, fundamental mechanisms of metabolic control, such as the postprandial response and metabolite correlations, are dependent on light signaling and clock function. These studies provide further evidence that key aspects of metabolic control are conserved between Drosophila and mammals and provide a foundation for exploiting the strengths of this model organism toward defining the mechanisms by which this regulation is achieved.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Hall for cry01 animals, Dr. Mike Young for tim01 flies, the Bloomington Stock Center for the Berlin-K stock, FlyBase for information, and the Thummel and Mark Metzstein laboratories for insightful discussions. This work was supported by funding from the National Institute of General Medical Sciences (NIGMS) (F32-GM093572) to D.J.S. and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (5RC1DK086426) to C.S.T.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
NOTE
Supplementary material for this article is available on the Journal of Biological Rhythms website at http://jbr.sagepub.com/supplemental.
References
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouis M, Moore P, Ruel L, Grau Y, Heitzler P, Simpson P. An early embryonic product of the gene Shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990;9:2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning E. Heidelberg Science Library. 3rd. New York: Springer-Verlag; 1973. The Physiological Clock. [Google Scholar]
- Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Diangelo JR, Erion R, Crocker A, Sehgal A. The central clock neurons regulate lipid storage in Drosophila. PLoS One. 2011;6:e19921. doi: 10.1371/journal.pone.0019921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet. 2010;44:419–444. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R, Oishi K, Ishida N. CLOCK regulates circadian rhythms of hepatic glycogen synthesis through transcriptional activation of Gys2. J Biol Chem. 2010;285:22114–22121. doi: 10.1074/jbc.M110.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezelova E, Dolezel D, Hall JC. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Fogle KJ, Parson KG, Dahm NA, Holmes TC. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331:1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E. Mikroskopische untersuchungenüber die gallebildung in den leberzellen. Z Zellforsch Mikr Anat. 1928;6:647. [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Ivanchenko M, Stanewsky R, Giebultowicz JM. Circadian photoreception in Drosophila: functions of cryptochrome in peripheral and central clocks. J Biol Rhythms. 2001;16:205–215. doi: 10.1177/074873040101600303. [DOI] [PubMed] [Google Scholar]
- Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Advanced analysis of a cryptochrome mutation’s effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 2002;3:5. doi: 10.1186/1471-2202-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity gene Shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Hazlerigg DG. Photoperiodic signalling through the melatonin receptor turns full circle. J Neuroendocrinol. 2008;20:820–826. doi: 10.1111/j.1365-2826.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- Rosbash M. The implications of multiple circadian clock origins. PLoS Biol. 2009;7:e62. doi: 10.1371/journal.pbio.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaud A-F, Lam G, Thummel CS. The Drosophila NR4A nuclear receptor DHR38 regulates carbohydrate metabolism and glycogen storage. Mol Endocrinol. 2010;25:83–91. doi: 10.1210/me.2010-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A, Stainier D. Lessons from “lower” organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet. 2007;3:e199. doi: 10.1371/journal.pgen.0030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Price JL, Man B, Young MW. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


