Abstract
Despite decades of concerted epidemiological research, relatively little is known about the etiology of prostate cancer. As genome‐wide association studies have identified numerous genetic variants, so metabolomic profiling of blood and other tissues represents an agnostic, “broad‐spectrum” approach for examining potential metabolic biomarkers of prostate cancer risk. To this end, we conducted a prospective analysis of prostate cancer within the Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study cohort based on 200 cases (100 aggressive) and 200 controls (age‐ and blood collection date‐matched) with fasting serum collected up to 20 years prior to case diagnoses. Ultrahigh performance liquid chromatography/mass spectroscopy and gas chromatography/mass spectroscopy identified 626 compounds detected in >95% of the men and the odds ratio per 1‐standard deviation increase in log‐metabolite levels and risk were estimated using conditional logistic regression. We observed strong inverse associations between energy and lipid metabolites and aggressive cancer (p = 0.018 and p = 0.041, respectively, for chemical class over‐representation). Inositol‐1‐phosphate showed the strongest association (OR = 0.56, 95% CI = 0.39–0.81, p = 0.002) and glycerophospholipids and fatty acids were heavily represented; e.g., oleoyl‐linoleoyl‐glycerophosphoinositol (OR = 0.64, p = 0.004), 1‐stearoylglycerophosphoglycerol (OR=0.65, p = 0.025), stearate (OR=0.65, p = 0.010) and docosadienoate (OR = 0.66, p = 0.014). Both alpha‐ketoglutarate and citrate were associated with aggressive disease risk (OR = 0.69, 95% CI = 0.51–0.94, p = 0.02; OR = 0.69, 95% CI = 0.50–0.95, p = 0.02), as were elevated thyroxine and trimethylamine oxide (OR = 1.65, 95% CI = 1.08–2.54, p = 0.021; and OR = 1.36, 95% CI = 1.02–1.81, p = 0.039). Serum PSA adjustment did not alter the findings. Our data reveal several metabolomic leads that may have pathophysiological relevance to prostate carcinogenesis and should be examined through additional research.
Keywords: metabolomics, prostate cancer, serology, energy metabolism, lipid metabolism, biomarkers, thyroxine, TMAO, TCA cycle, Warburg
Short abstract
What's new?
Prostate cancer is the second most common cancer in men, yet few modifiable risk factors are known. A window to the study of modifiable risk factors is the metabolome, with prospective metabolomic serum profiling being a promising new investigative approach. In prostate cancer, use of this approach had resulted in the identification of numerous circulating lipids associated with risk of aggressive disease. Here, the authors report inverse associations between aggressive disease and energy and lipid metabolites, including alpha‐ketoglutarate, citrate, inositol‐1‐phosphate and several glycerophospholipids and fatty acids. Some of the metabolites may be relevant to prostate cancer development and progression.
Abbreviations
- Acetyl‐CoA
acetyl coenzyme A
- AJCC
American Joint Committee on Cancer
- AMP
adenosine monophosphate
- ATBC
Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study
- ATP
adenosine triphosphate
- BPH
benign prostatic hyperplasia
- CI
confidence interval
- CV
coefficient of variation
- GSA
gene set analysis
- I1P
inositol‐1‐phosphate
- ICD
international classification of diseases
- IP
inositol‐phosphate
- LCFA
long chain fatty acid
- MCFA
medium chain fatty acid
- OR
odds ratio
- PSA
prostate‐specific antigen
- PUFA
polyunsaturated fatty acid
- QC
quality control
- TCA
tricarboxylic acid
- TMAO
trimethylamine oxide
- TNM
tumor nodes metastasis
Despite being the most commonly diagnosed malignancy and the second leading cause of cancer death among men in most developed populations,1 hypothesis‐driven research has yet to elucidate established modifiable risk factors for prostate cancer. Similar to agnostic methods such as genome‐wide association studies that have successfully identified novel biological pathways relevant to prostate and other cancers,2 metabolomic technologies measuring a wide array of low molecular weight biochemicals in blood and other tissues are a powerful new tool that may help identify compounds relevant to cancer etiology, early detection or progression.3, 4 For example, metabolomic profiles of tissue, urine and blood from men with prostate cancer have been examined, including by disease aggressiveness.5, 6 Our previous serum metabolomic analysis of prostate cancer risk in 74 nested case‐control pairs with up to 23 years of follow‐up within the Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention (ATBC) Study of male smokers found strong inverse risk signals for circulating 1‐stearoylglycerol (or 1‐monostearin), glycerol and alpha‐ketoglutarate along with several other lipid metabolites, supporting a role for lipid and energy dysregulation in prostate carcinogenesis.7 The associations persisted among cases diagnosed >5 years after blood collection and appeared stronger for cases that were aggressive at diagnosis. The novel findings were based on a relatively small sample, however and required reexamination. We therefore endeavored in the present study to replicate the initial results in a larger prospective study sample.
Material and Methods
Study population
Details of the Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention (ATBC) Study have been described.8 Male Caucasian 50–69 year old smokers from southwestern Finland (n = 29,133) were recruited from 1985 to 1988. They were assigned to one of the following intervention groups based on a 2 × 2 factorial trial design: (i) α‐tocopherol (dl‐α‐tocopheryl‐acetate, 50 mg/day), (ii) β‐carotene (20 mg/day), (iii) both supplements or (iv) placebo. Participants took the study capsules for 5–8 years (median 6.1 years) through April 30, 1993 or until they left the trial. Questionnaires about general risk factors, smoking and medical history as well as a validated food‐frequency questionnaire were completed at entry, height and weight were measured and serum was collected (after an overnight fast) protected from light and stored at −70 °C until assayed. Written informed consent was obtained from all participants and the trial was approved by institutional review boards at both the Finnish National Public Health Institute and the US National Cancer Institute.
Case identification and control selection
The present analysis includes 200 confirmed cases of incident prostate adenocarcinoma (ICD‐9 185) matched 1:1 with controls on age (±1 year) and date of baseline blood collection (± 30 days). These cases and controls were independent of our original study,7 having no overlap. To enable us to examine aggressive cases separately and to examine associations stratifying by time between blood collection and diagnosis, cases were randomly selected such that 25 nonaggressive and 25 aggressive cases were sampled from each of four periods between blood collection and diagnosis: <5 years, 5‐<10 years, 10‐<15 years and 15‐20 years. Aggressive cases were defined as TNM stage III‐IV, AJCC stage ≥3 or Gleason sum ≥8. Only two cases (stage 2) were included as “aggressive” based solely on a high Gleason sum.
Laboratory analyses
Serum metabolomic profiling was conducted at Metabolon (Durham, N.C.) using ultrahigh performance liquid chromatography/mass spectroscopy and gas chromatography/mass spectroscopy. Sixteen batches of case‐control pairs and quality control (QC) replicate samples (8%) were analyzed. Raw data were extracted, peak‐identified and QC processed on the assay platform as previously described.9, 10 After excluding metabolites that had >20 (5%) missing values, 626 identified compounds remained for analysis. Based on existing literature, metabolites were categorized as eight mutually exclusive chemical classes (amino acids, carbohydrates, cofactors and vitamins, energy metabolites, lipids, nucleotides, peptides and xenobiotics). The median and interquartile range of the CV% across the metabolites was 9% (4–20%). These CVs are similar to those previously observed for blood samples analyzed by the same laboratory.11
Serum total prostate specific antigen (PSA) concentration was measured in the clinical chemistry laboratory of Dr. Alan Remaley in the NIH Clinical Center. Each batch included blinded duplicate QC samples, with the CVs ranging from 5 to 10%.
Statistical analyses
To standardize for batch variability, the signal strength of each metabolite was divided by the median value of participants in a given batch; these normalized levels were then log‐transformed and missing values were assigned the minimum nonmissing value. We estimated the association between log‐metabolite levels and prostate cancer by conditional logistic regression. In addition to the matching factors, the following variables were evaluated as potential confounders: trial treatment group, family history of prostate cancer, history of benign prostatic hyperplasia (BPH), physical activity, body mass index, smoking (cigarettes per day), serum total and HDL cholesterol, serum retinol and serum α‐tocopherol. Inclusion of any of these variables in the model did not change the effect of any metabolite by 10% or more. Thus, our final model is conditioned on the matching factors only. Subgroup analyses were conducted based on trial α‐tocopherol and β‐carotene supplementation (yes/no for each), smoking intensity (split at 20 cigarettes daily) and BMI, serum total and HDL cholesterol (all based on medians). The threshold for statistical significance in our primary analysis is p = 0.00008, based on a Bonferroni correction for 626 tests; the threshold is 0.003 for the most significant metabolites in the main analysis, based on correction for 170 tests. It should be noted that this is a highly conservative (i.e., stringent) threshold because of intercorrelation among many of the metabolites. Key findings of this analysis were meta‐analyzed with data from our initial study in this cohort7 using a random effects modeling approach. We evaluated the association between chemical classes of metabolites and prostate cancer using Gene Set Analysis (GSA) as described in detail by Efron and Tibshirani,12 a potentially more robust and powerful test than the Gene Set Enrichment Analysis described by Subramanian et al.13 Letting {z 1, …, z s}be the Z values from testing the S metabolites in a pathway, GSA evaluates the “maxmean” statistic max (‐z +, ‐z −), where ‐z +(‐z −) is the average of all positive (negative) values and calculates the p‐values by 10,000 permutations.
All analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, N.C.), with the exception of the GSA, which was conducted using R; all statistical tests were 2‐sided.
Results
Characteristics of the study population are shown in Table 1. Only serum retinol and total PSA differed significantly between cases and controls, both being higher among cases.
Table 1.
Selected characteristics1 of the cases and controls
| Controls | Cases | p‐Value | |
|---|---|---|---|
| N | 200 | 200 | |
| Age (years) | 59.3 | 59.4 | Matched |
| Height (cm) | 173 | 173 | 0.94 |
| Weight (kg) | 78.1 | 80.0 | 0.29 |
| BMI (kg/m2) | 26.0 | 26.6 | 0.18 |
| Serum total cholesterol (mmol/L) | 6.2 | 6.1 | 0.89 |
| Serum α‐tocopherol (mg/L) | 11.8 | 11.6 | 0.33 |
| Serum β‐carotene (μg/L) | 200 | 201 | 0.82 |
| Serum retinol (μg/L) | 587 | 611 | 0.02 |
| Serum total PSA (ng/mL) | 1.4 | 8.0 | <0.0001 |
| Cigarettes per day | 19.4 | 19.3 | 0.87 |
| Years of cigarette smoking | 37.5 | 37.5 | 0.81 |
| Family history of prostate cancer (%) | 3.5 | 5.8 | 0.36 |
| Physically active (%) | 19.0 | 13.5 | 0.14 |
| Dietary intake per day | |||
| Total energy (kcal) | 2,667 | 2,662 | 0.96 |
| Fruit (g) | 216 | 205 | 0.52 |
| Vegetables (g) | 293 | 289 | 0.54 |
| Red meat (g) | 70.5 | 70.7 | 0.85 |
| Alcohol (ethanol, g) | 17.4 | 16.8 | 0.53 |
| Supplement use | |||
| Vitamin A (%) | 10.5 | 11.0 | 0.87 |
| Vitamin D (%) | 7.0 | 7.0 | 1.0 |
| Calcium (%) | 13.5 | 13.0 | 0.88 |
1Values are means unless otherwise indicated. All characteristics are from the baseline questionnaire except family history, which was collected during follow‐up and is available for 264 men in this analysis.
Serum metabolites associated with risk of total, nonaggressive and aggressive prostate cancer at a p < 0.05 level of statistical significance are shown sorted by chemical class and then by p values in Tables 2, 3, 4 (none achieved p = 0.00008 level of significance). Although not found for all cases combined or for nonaggressive disease (Tables 2 and 3, respectively), we observed strong risk associations between the energy and lipid metabolite chemical classes and aggressive cancer, with both being statistically significantly over‐represented among our top metabolites (Table 4, energy p = 0.018, lipids p = 0.041). With the exception of erucoyl‐sphingomyelin (OR = 1.53) and trimethylamine oxide (TMAO; OR 1.36), all other lipids were inversely related to aggressive prostate cancer (Table 4). Inositol‐1‐phosphate showed the strongest association (OR = 0.56, 95% CI‐0.39 – 0.81, p = 0.002), although glycerophospholipids (e.g., oleoyl‐linoleoyl‐glycerophosphoinositol and 1‐stearoylglycerophosphoglycerol) and fatty acids (e.g., stearate and docosadienoate) were heavily represented. The lipid signal for overall prostate cancer was weaker (chemical class test, p = 0.27; Table 2). The data in Tables 2, 3, 4 were not materially altered by adjustment for serum total PSA, total cholesterol or retinol (data not shown). Mutual adjustment for each other metabolite led to relatively little overall attenuation of the ORs, although some attenuation was evident when adjusting within chemical classes (e.g., within lipids, stearate did attenuate the ORs for many of the other fatty acids), while for some metabolites, associations were strengthened (data not shown). A heat‐map of the aggressive prostate cancer metabolite intercorrelations appears in Figure 1. Higher positive correlations were seen among fatty acids and glycerophospholipids and PSA was weakly correlated with the metabolites.
Table 2.
Serum metabolites associated with overall prostate cancer (p < 0.05)
| Metabolite | Subpathway | Odds Ratio a | 95% CI | p‐Value | p for Chemical Class |
|---|---|---|---|---|---|
| Lipids | |||||
| 3‐Hydroxylaurate | Fatty acid, monohydroxy | 0.78 | 0.63 − 0.96 | 0.0169 | 0.27 |
| Oleoyl‐linoleoyl‐glycerophosphoinositol | Glycerophospholipid | 0.81 | 0.65 − 1.00 | 0.0462 | |
| Erucoyl‐sphingomyelin | Sphingolipid metabolism | 1.35 | 1.07 − 1.70 | 0.0101 | |
| Stearoyl‐arachidonoyl‐glycerophosphoethanolamine | Glycerophospholipid | 0.76 | 0.61 − 0.94 | 0.0109 | |
| Palmitoyl‐linoleoyl‐glycerophosphocholine | Glycerophospholipid | 0.78 | 0.64 − 0.96 | 0.0166 | |
| Glycolithocholate sulfate | Secondary bile acid metabolism | 1.28 | 1.04 − 1.57 | 0.0181 | |
| Docosadienoate (22:2n6) | LCFA; polyunsaturated fatty acid | 0.77 | 0.62 − 0.96 | 0.0220 | |
| Inositol‐1‐phosphate (I1P) | Inositol metabolism | 0.77 | 0.62 − 0.97 | 0.0231 | |
| Stearoyl‐linoleoyl‐glycerophosphoethanolamine | Glycerophospholipid | 0.80 | 0.66 − 0.98 | 0.0329 | |
| 3‐Hydroxydecanoate | Fatty acid, monohydroxy | 0.80 | 0.65 − 0.98 | 0.0335 | |
| Stearoyl‐linoleoyl‐glycerophosphocholine | Glycerophospholipid | 0.82 | 0.67 − 0.99 | 0.0382 | |
| 16a‐Hydroxy‐DHEA 3‐sulfate | Sterol/steroid | 0.81 | 0.66 − 1.00 | 0.0469 | |
| Tauro‐beta‐muricholate | Primary bile acid metaolism | 0.80 | 0.64 − 1.00 | 0.0481 | |
| Amino Acids | |||||
| 4‐Imidazoleacetate | Histidine metabolism | 1.33 | 1.08 − 1.63 | 0.0074 | 0.40 |
| Phenylpyruvate | Phenylalanine and tyrosine metabolism | 1.28 | 1.03 − 1.57 | 0.0227 | |
| Beta‐hydroxyisovalerate | Valine, leucine and isoleucine metabolism | 1.26 | 1.02 − 1.54 | 0.0283 | |
| 2‐Hydroxybutyrate (AHB) | Cysteine, methionine, SAM, taurine metabolism | 0.81 | 0.67 − 0.99 | 0.0380 | |
| Indolebutyrate | Tryptophan metabolism | 1.22 | 1.00 − 1.48 | 0.0445 | |
| Xenobiotics | |||||
| Homostachydrine | Food component/plant | 0.81 | 0.67 − 0.99 | 0.0380 | 0.46 |
| Propyl‐4‐hydroxybenzoate sulfate | Benzoate metabolism | 0.79 | 0.63 − 0.99 | 0.0406 | |
| S‐(3‐hydroxypropyl)mercapturic acid (HPMA) | Chemical | 0.81 | 0.66 − 0.99 | 0.0417 | |
| Nucleotides | |||||
| 2′‐Deoxyuridine | Pyrimidine metabolism, uracil containing | 1.42 | 1.09 − 1.85 | 0.0084 | 0.48 |
The odds ratio per 1 standard deviation increase in metabolite level.
Table 3.
Serum metabolites associated with nonaggressive prostate cancer (p < 0.05)
| Metabolite | Subpathway | Odds ratio a | 95% CI | p‐Value | p for chemical class |
|---|---|---|---|---|---|
| Lipids | |||||
| 11‐Dehydrocorticosterone | Sterol/steroid | 0.62 | 0.44 − 0.88 | 0.0069 | 0.66 |
| 21‐Hydroxypregnenolone monosulfate | Sterol/steroid | 1.46 | 1.03 − 2.06 | 0.0344 | |
| Glycerol 3‐phosphate (G3P) | Glycerolipid metabolism | 0.70 | 0.51 − 0.98 | 0.0391 | |
| Amino Acids | |||||
| N‐acetyl‐3‐methylhistidine | Histidine metabolism | 1.52 | 1.14 − 2.02 | 0.0048 | 0.33 |
| N‐acetylleucine | Valine, leucine and isoleucine metabolism | 1.37 | 1.02 − 1.85 | 0.0379 | |
| Beta‐hydroxyisovalerate | Valine, leucine and isoleucine metabolism | 1.38 | 1.01 − 1.89 | 0.0419 | |
| Indolepropionate | Tryptophan metabolism | 1.36 | 1.00 − 1.85 | 0.0498 | |
| Xenobiotics | |||||
| Cotinine | Tobacco metabolite | 0.59 | 0.38 − 0.90 | 0.0140 | 0.27 |
| Hydroxycotinine | Tobacco metabolite | 0.67 | 0.47 − 0.96 | 0.0291 | |
| Saccharin | Food component/plant | 0.70 | 0.50 − 0.98 | 0.0354 | |
| Nucleotides | |||||
| 2′‐Deoxyuridine | Pyrimidine metabolism, uracil containing | 1.81 | 1.15 − 2.85 | 0.0105 | 0.26 |
| Adenosine 5′‐monophosphate (AMP) | Purine metabolism, adenine containing | 1.50 | 1.03 − 2.16 | 0.0328 | |
| Dihydroorotate | Pyrimidine metabolism, orotate containing | 1.36 | 1.01 − 1.83 | 0.0401 | |
| Cofactors and Vitamins | |||||
| l‐Urobilin | Hemoglobin and porphyrin metabolism | 1.45 | 1.03 − 2.04 | 0.0321 | 0.30 |
The odds ratio per 1 standard deviation increase in metabolite level.
Table 4.
Metabolites associated with aggressive prostate cancer (p < 0.05).
| Metabolite | Subpathway | Odds ratio a | 95% CI | p‐Value | p for chemical class |
|---|---|---|---|---|---|
| Energy | |||||
| Alpha‐ketoglutarate | TCA/Krebs cycle | 0.69 | 0.51 − 0.94 | 0.0201 | 0.018 |
| Citrate | TCA/Krebs cycle | 0.69 | 0.50 − 0.95 | 0.0220 | |
| Lipids | |||||
| Inositol‐1‐phosphate (I1P) | Inositol metabolism | 0.56 | 0.39 − 0.81 | 0.0024 | 0.041 |
| Oleoyl‐linoleoyl‐glycerophosphoinositol | Glycerophospholipid | 0.64 | 0.47 − 0.87 | 0.0042 | |
| Stearate (18:0) | LCFA | 0.65 | 0.47 − 0.90 | 0.0102 | |
| Docosadienoate (22:2n6) | LCFA; polyunsaturated fatty acid | 0.66 | 0.48 − 0.92 | 0.0141 | |
| 3‐Hydroxylaurate | Fatty acid, monohydroxy | 0.70 | 0.52 − 0.94 | 0.0162 | |
| Margarate (17:0) | LCFA | 0.67 | 0.48 − 0.93 | 0.0185 | |
| Linoleate (18:2n6) | Essential fatty acid; polyunsaturated fatty acid | 0.68 | 0.49 − 0.94 | 0.0204 | |
| Erucoyl‐sphingomyelin | Sphingolipid metabolism | 1.53 | 1.07 − 2.20 | 0.0205 | |
| Palmitoyl‐linoleoyl‐glycerophosphoinositol | Glycerophospholipid | 0.68 | 0.49 − 0.95 | 0.0220 | |
| Laurate (12:0) | MCFA | 0.70 | 0.51 − 0.95 | 0.0230 | |
| Dihomo‐linoleate (20:2n6) | LCFA; polyunsaturated fatty acid | 0.71 | 0.52 − 0.95 | 0.0232 | |
| 1‐Stearoylglycerophosphoglycerol | Glycerophospholipid | 0.69 | 0.50 − 0.95 | 0.0245 | |
| 16‐Hydroxypalmitate | Fatty acid, monohydroxy | 0.71 | 0.53 − 0.97 | 0.0288 | |
| Stearoyl‐linoleoyl‐glycerophosphoethanolamine | Glycerophospholipid | 0.72 | 0.53 − 0.97 | 0.0308 | |
| Nonadecanoate (19:0) | LCFA | 0.71 | 0.51 − 0.97 | 0.0310 | |
| Palmitate (16:0) | LCFA | 0.69 | 0.50 − 0.97 | 0.0310 | |
| Stearoyl‐arachidonoyl‐glycerophosphoethanolamine | Glycerophospholipid | 0.69 | 0.50 − 0.97 | 0.0341 | |
| Trimethylamine N‐oxide | Glycerolipid metabolism; phospholipid metabolism | 1.36 | 1.02 − 1.81 | 0.0389 | |
| 1‐Linoleoylglycerophosphoethanolamine | Glycerophospholipid | 0.73 | 0.54 − 0.99 | 0.0401 | |
| 17‐Methylstearate | Fatty acid, branched | 0.72 | 0.53 − 0.99 | 0.0417 | |
| 1‐Linoleoylglycerophosphoinositol | Glycerophospholipid | 0.74 | 0.56 − 0.99 | 0.0436 | |
| Palmitoyl‐linoleoyl‐glycerophosphocholine | Glycerophospholipid | 0.74 | 0.55 − 1.00 | 0.0487 | |
| 1‐Arachidonoylglycerophosphoethanolamine | Glycerophospholipid | 0.72 | 0.52 − 1.00 | 0.0494 | |
| Amino Acids | |||||
| 3‐Methylglutarylcarnitine | Lysine metabolism | 1.60 | 1.13 − 2.28 | 0.0082 | 0.87 |
| Thyroxine | Phenylalainine and tyrosine metabolism | 1.65 | 1.08 − 2.54 | 0.0216 | |
| Alpha‐ketobutyrate | Cystine, methionine, SAM, taurine metabolism | 0.69 | 0.51 − 0.95 | 0.0232 | |
| 4‐Imidazoleacetate | Histidine metabolism | 1.40 | 1.04 − 1.89 | 0.0272 | |
| Glutarate (pentanedioate) | Lysine metabolism | 0.75 | 0.57 − 0.99 | 0.0435 | |
| Arginine | Urea cycle; arginine and proline metabolism | 1.31 | 1.00 − 1.72 | 0.0488 | |
| Xenobiotics | |||||
| Salicyluric glucuronide | Drug | 0.65 | 0.46 − 0.90 | 0.0109 | 0.87 |
| Isoeugenol sulfate | Food component/plant | 1.38 | 1.01 − 1.89 | 0.0410 | |
| Peptides | |||||
| Gamma‐glutamylhistidine | Gamma‐glutamyl amino acid | 0.75 | 0.56 − 1.00 | 0.0465 | 0.91 |
The odds ratio per 1 standard deviation increase in metabolite level.
Figure 1.
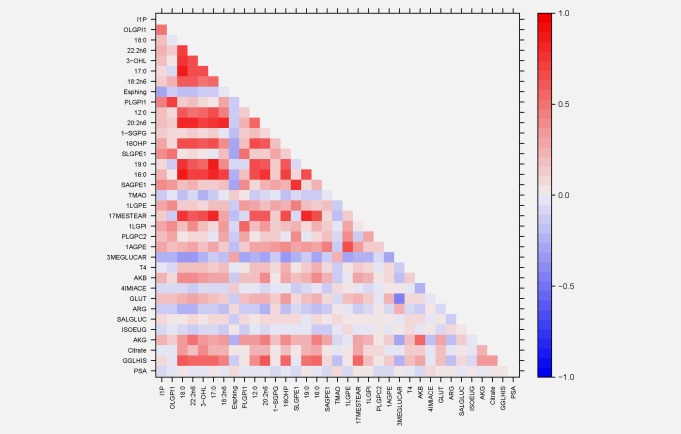
Heat map of correlation coefficients among metabolites associated with aggressive prostate cancer.
Of the metabolites most strongly associated with prostate cancer in our initial study,7 the association between alpha‐ketoglutarate and aggressive prostate cancer replicated in the present data (OR = 0.69, 95% CI‐0.51 – 0.94, p = 0.02, Table 4). Another TCA cycle (i.e., citric acid or Krebs cycle) metabolite, citrate, was similarly but independently associated with aggressive cancer risk (OR = 0.69; Table 4).
Even though we identified a large number of glycerophospholipids and long chain fatty acids (LCFA) among the top metabolites, 1‐stearoylglycerol and glycerol (i.e., the two other top signals from our prior study7) specifically were not associated with prostate cancer risk in the present analysis (1‐stearoylglycerol: OR = 1.03, 95% CI = 0.84–1.26, p = 0.79; glycerol: OR = 0.96, 95% CI = 0.78–1.19, p = 0.71), with no difference based on disease aggressiveness (data not shown). Meta‐analysis with our prior data also showed nonstatistically significant inverse associations for overall prostate cancer: 1‐stearoylglycerol OR = 0.61, 95% CI = 0.21–1.79, p = 0.37; glycerol OR = 0.77, 95% CI = 0.45–1.31, p = 0.33. Stratifying our cases and their controls by the median time from serum collection to diagnosis revealed more abundant glycerophospholipid, sphingolipid and steroid hormone metabolites inversely associated with aggressive prostate cancer diagnosed within 10 years of serum collection, whereas medium chain fatty acid (MCFA), LCFA and polyunsaturated fatty acid (PUFA) metabolites were prominently and inversely associated in serum sampled from cases 10–20 years prior to diagnosis (data not shown). We observed no statistically significant interaction between any of the 34 metabolites associated with aggressive prostate cancer at p < 0.05 and the following factors: the ATBC trial α‐tocopherol or β‐carotene supplementation, BMI, cigarettes per day and serum total or HDL cholesterol.
We found other notable, biologically relevant metabolite signals for prostate cancer. For aggressive disease, elevated thyroxine and TMAO were associated with higher risk: respectively, OR = 1.65, 95% CI = 1.08 – 2.54, p = 0.021; and OR = 1.36, 95% CI = 1.02 – 1.81, p = 0.039. Metabolites associated with nonaggressive cancers were nucleotides (2′‐deoxyuridine and adenosine 5′‐monophosphate (AMP): OR = 1.81, CI = 1.15 – 2.85, p = 0.010; and, OR = 1.50, CI = 1.03 − 2.16, p = 0.032, respectively; p = 0.03 for nucleotide class), two steroid hormones (11‐dehydrocorticosterone and 21‐hydroxypregnenolone monosulfate: OR = 0.62, CI = 0.44 − 0.88, p = 0.0069; and OR = 1.46, CI = 1.03 − 2.06, p = 0.034, respectively) and two tobacco metabolites (cotinine and hydroxycotinine: OR = 0.59, CI = 0.38 − 0.90, p = 0.014; and, OR = 0.67, CI = 0.47 − 0.96, p = 0.029, respectively). It should also be noted that we did not observe a positive risk association for the branched‐chain amino acids leucine, isoleucine and valine (data not shown), as was recently reported for pancreatic cancer risk.14
Discussion
The present metabolomics analysis reveals associations between aggressive prostate cancer and a large number serum glycerophospholipids, LCFAs, PUFAs and TCA cycle compounds, consistent with our recent preliminary data.7 Specifically, we replicated the original inverse association with alpha‐ketoglutarate (meta‐analysis of the two sets reveals alpha‐ketoglutarate as the top metabolite associated with aggressive disease: OR = 0.62, 95% CI = 0.46 – 0.82, p = 0.0009) and identified several additional biologically relevant diacyl‐glycerophospholipids. These findings were independent of BPH and serum total PSA, as evidenced by the low PSA‐metabolite correlations and the lack of metabolite association attenuation upon model adjustment for PSA or BPH.
Other than our initial investigation,7 this may be the only report of a prospective metabolomics profiling study of prostate cancer risk having long follow‐up. Cross‐sectional clinical studies recently reviewed5, 6 have primarily focused on metabolomic profiles of tumor tissue (primaries and metastases; biopsies and prostatectomies) and urine in men with prostate cancer. Although a large number of metabolites were detected in relation to disease aggressiveness, metastases and biochemical recurrence, evidence to support the associations with increased glycerophospholipids, cholines and sarcosine and decreased citrate and polyamines is more preponderant (albeit, with inconsistent findings).6 These data do in fact indicate that metabolite associations vary by cancer aggressiveness, mostly defined by stage at diagnosis, and we might expect the serologic molecular patterns for aggressive and advanced disease to be more similar to prostate cancer cases profiled at the time of diagnosis than to early, nonaggressive cancers. For example, we did identify several glycerophospholipids and citrate as being inversely associated with aggressive disease. Studies of serial prospective metabolomic profiles leading up to clinical diagnosis will be useful in this regard.
The strong association signals for lipids and TCA cycle members are consistent with current knowledge from abundant basic research regarding cancer cell metabolic changes related to energy, biosynthetic capacity and cell signaling.15, 16, 17, 18 That rapidly proliferating malignant cells derive much of their energy requirements from cytosolic anaerobic glycolysis, possibly in response to hypoxic intra‐tumor conditions, was originally proposed by Warburg several decades ago.19 More recently, laboratory studies support some degree of functional TCA cycle and electron transport‐oxidative phosphorylation production of ATP in malignancies.20 Fatty acids from de novo biosynthesis or through monoacylglycerol or diacylglycerol lipase activity—reported to be overexpressed in aggressive but not nonaggressive prostate cancers15, 17, 21, 22—provide a key molecular source of acetyl‐CoA (beyond pyruvate from glycolysis) through successive two‐carbon beta‐oxidation in mitochondria.16, 17 This is facilitated by carnitine palmitoyl transferase‐1 transport of fatty acids into the mitochondrial matrix, including specifically in prostate cancer.23 Acetyl‐CoA then enters the TCA cycle via citrate synthase and oxaloacetic acid to yield citrate. These additional cellular perturbations of fatty acid metabolism involving LCFAs, acetyl‐CoA and citrate provide energy and other macromolecules for malignant cell proliferation and tumor anabolic requirements and they potentially account for the lower lipid metabolite profiles in the prostate cancer cases.
Beyond energy and anabolic requirements, altered lipid regulation also supports membrane macromolecular species needed during rapid cell proliferation, including for lipid rafts and other key signaling constituents.24, 25 Consistent with this, we identified a large number of glycerophospholipids (including monoacyl‐ and diacyl‐ glycerophosphoinositols, ‐glycerophosphoethanolamines and ‐glycerophosphocholines), LCFA, PUFA and erucoyl‐sphingomyelin among the top metabolites associated with aggressive prostate cancer, with the phosphorylated derivative of myo‐inositol, inositol‐1‐phosphate or I1P, ranking highest. I1P is a member of the phosphatidylinositide family of compounds that have become increasingly recognized as integral plasma membrane, protein phosphorylation and second messenger signaling components.26 I1P and related IP species such as IP4,5 (i.e., phosphorylated at carbons 4 and 5 of the inositol ring) are functionally related to the numerous diacylglycerophospoinositol lipid metabolites we found associated with aggressive prostate cancer. These compounds serve as substrates for plasma membrane phospholipase C that produces, (i) IP1,4,5 which is released into the cytosol where it mobilizes ionized calcium from endoplasmic reticulum and (ii) diacylglycerol, which remains membrane‐associated to transactivate protein kinase C.15, 18, 27 Triggered by plasma membrane hormone receptor binding and related to prostate cancer aggressiveness, both IP1,4,5 and diacylglycerol can therefore serve as second messengers for signal transduction through protein phosphorylation.21, 27, 28
Similar in association strength to our finding for alpha‐ketoglutarate, but independent of it, we found lower risk of aggressive prostate cancer in men with higher serum citrate status. Citrate is concentrated in the normal prostate peripheral zone glandular epithelium owing to high zinc ion concentration inhibition of mitochondrial aconitase conversion of citrate to isocitrate.29 In turn, citrate is secreted in prostatic fluid in high concentration, a process referred to as “net citrate production”,29 possibly to maintain a lower semen pH or provide an energy source for sperm motility or oocyte fertilization. BPH tissue also exhibits elevated citrate content.30, 31 By contrast, malignant prostate cells exhibit low zinc and low citrate concentrations, apparently the result of a biochemical shift to citrate oxidation (along with alpha‐ketoglutarate) through the TCA cycle to provide greater ATP production for tumor cell anabolism and proliferation. Such a metabolic transformation of malignant prostate cells could account for our finding of lower citrate status in aggressive cases versus noncases and be reflective of the higher tumor energy requirements. Alternatively, tumor cell conversion of alpha‐ketoglutarate to citrate through “reductive carboxylation” as a result of isocitrate dehydrogenase isoforms and NADP+/NADPH, with subsequent acetyl‐CoA formation and fatty acid synthesis, has also been suggested in the setting of mitochondrial dysregulation.32 Citrate and alpha‐ketoglutarate might also be metabolized to biosynthetic precursors for fatty acids and sterols (citrate) and glutamate (alpha‐ketoglutarate). It is also relevant and of interest that recently developed prostate cancer magnetic resonance spectroscopic metabolic imaging includes targeting decreased citrate as well as increased choline, creatine, polyamines and myo‐inositol.30, 31, 33
Beyond the strong signals we detected for lipid and energy metabolites, other specific metabolites appeared associated with prostate cancer, including TMAO and thyroxine for aggressive disease. The choline derivative TMAO has been linked to atherosclerosis and cardiovascular disease,34 and most recently to elevated colorectal cancer risk.35 Given the role of intestinal microbiota in human TMAO production and the potential for local luminal epithelial effects, the higher risk for prostate cancer suggested here deserves further study. Thyroid hormone status has been previously associated with prostate cancer, including in a recent study from the ATBC cohort showing lower risk for hypothyroid men.36 The positive associations between some nucleotides, including AMP and nonaggressive disease (chemical class p‐value = 0.03) require reevaluation in other studies, particularly given the number of known metabolites examined. That the branched‐chain amino acids did not show positive associations with prostate cancer similar to those observed for pancreatic cancer14 supports the likelihood that organ site differences in metabolomic changes reflect underlying tissue‐specific biological changes that should be further investigated.
Our study has several important strengths, primary among which are: (i) having up to two decades between blood sampling and prostate cancer diagnosis, (ii) analysis of serum collected after an overnight fast and (iii) complete, population registry‐based case ascertainment. Long follow‐up affords us the opportunity to examine reverse causality and possible temporal changes in circulating metabolites ranging from up to 20 years before clinical diagnosis to within 10 years. Qualitative differences in lipid subclasses based on time from blood collection to diagnosis were detected (e.g., fatty acids vs. glyceropholipids) and these patterns may provide biologically based leads regarding transformations during prostate cancer development and progression. Fasting serum should in theory reduce metabolite variation related to recent food consumption and may offer a snapshot of “basal” metabolomic profiles less influenced by recent food/beverage consumption. Prostate cancers were for the most part diagnosed clinically and not from population PSA screening. The high‐quality laboratory platform provided low operational variation in metabolite detection. Study limitations include the relatively homogeneous population (i.e., Finnish smokers of five or more cigarettes daily who were of Caucasian descent) and modest sample size. Whether our findings for cotinine and hydroxycotinine in nonaggressive prostate cancer were influenced by metabolite changes related to tobacco smoking is possible but unlikely given that all cases and controls were smokers at the time of serum collection and those compounds are but two of several known tobacco‐related metabolites.37 Further, our results were unchanged when adjusting for cigarettes smoked per day and were similar for lighter and heavier smokers, suggesting that cigarette smoking did not strongly influence the results. Given that we identified 626 known biochemicals, the findings for some of the metabolites may be due to chance.
How the numerous metabolites identified in the present study might biologically impact prostate cancer risk or relate to known biochemical changes during its development and progression can only be speculated. The specificity and subpathway interrelatedness of the metabolites we identified are compelling in light of well‐established metabolic changes in prostate malignancies, however. To our knowledge, alpha‐ketoglutarate, citrate, I1P and most of the other metabolites we found associated with aggressive prostate cancer have not been similarly examined in epidemiologic studies to date. Lipid‐lowering statin use is associated with lower risk of aggressive prostate cancer38, 39 and a recent meta‐analysis of cohorts with an average follow‐up of five years found that lower plasma/serum stearic acid and higher eicosapentaenoic and docosapentaenoic acid concentrations, were related to higher risk (with no other fatty acid or phospholipid associations).40 The case‐control differences in the circulating metabolites we observed were present up to 20 years before clinical diagnosis. Given the widely accepted pathobiology of latent, slow‐growing, sub‐clinical prostate cancers that is supported by autopsy studies41 and registry analyses,42 our findings for aggressive disease could be interpreted as reflecting the impact of metabolic changes in larger and disseminated malignancies on circulating metabolites. Serum‐tissue correlative studies would be useful in this regard. Alternatively, greater availability of lipid and energy metabolites in circulation resulting from lifestyle exposures or genetic predisposition may inhibit the initiation or growth of prostate cancers. It remains unclear whether the magnitude of the differences we observed in metabolite signals between cases and controls would be sufficient to affect prostate cancer growth or initiation. Additional basic and clinical research should address the questions raised by these data.
In conclusion, our prospective study data indicate that several circulating glycerophospholipid, fatty acid, energy and related metabolites are inversely associated with aggressive prostate cancer up to 20 years prior to diagnosis. The findings may provide valuable and timely prospective human validation of substantial prior basic and clinical research relevant to lipid and energy metabolic transformations during prostate carcinogenesis. Further replication in other prospective studies, including, where possible, metabolomic profiling of serial pre‐diagnostic blood samples and in prostate tissue (tumor and adjacent normal), will be informative.
Dr. Mondul was affiliated with the Division of Cancer Epidemiology and Genetics, National Cancer Institute when this research was begun.
Dr. Karoly is an Associate Director of Project Management with Metabolon, Inc.
This article was published online on 9 May 2015. A typo in the title was subsequently identified and corrected 1 March 2016.
References
- 1. Brawley OW. Prostate cancer epidemiology in the United States. World J Urol 2012;30:195–200. [DOI] [PubMed] [Google Scholar]
- 2. Al Olama AA, Kote‐Jarai Z, Berndt SI, et al. A meta‐analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 2014;46:1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature 2008;455:1054–6. [DOI] [PubMed] [Google Scholar]
- 4. O'Connell TM. Recent advances in metabolomics in oncology. Bioanalysis 2012;4:431–51. [DOI] [PubMed] [Google Scholar]
- 5. Lokhov PG, Dashtiev MI, Moshkovskii SA, et al. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics 2010; 6:156–63. [Google Scholar]
- 6. Trock BJ. Application of metabolomics to prostate cancer. Urol Oncol 2011;29:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mondul AM, Moore SC, Weinstein SJ, et al. 1‐Stearoylglycerol is associated with risk of prostate cancer: results from serum metabolomic profiling. Metabolomics 2014;10:1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The ATBC Cancer Prevention Study Group. The alpha‐tocopherol, beta‐carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 9. Evans AM, DeHaven CD, Barrett T, et al. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small‐molecule complement of biological systems. Anal Chem 2009;81:6656–67. [DOI] [PubMed] [Google Scholar]
- 10. Dehaven CD, Evans AM, Dai H, et al. Organization of gc/ms and lc/ms metabolomics data into chemical libraries. J Cheminform 2010;2:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sampson JN, Boca SM, Shu XO, et al. Metabolomics in epidemiology: sources of variability in metabolite measurements and implications. Cancer Epidemiol Biomarkers Prev 2013;22:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Efron B, Tibshirani R. On testing the significance of sets of genes. Ann Appl Stat 2006; 1:107–29. [Google Scholar]
- 13. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched‐chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 2014;20:1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nomura DK, Long JZ, Niessen S, et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010;140:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J 2012;279:2610–23. [DOI] [PubMed] [Google Scholar]
- 17. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 2013;13:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Freter C. Lipid metabolism, apoptosis and cancer therapy. Int J Mol Sci 2015;16:924–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warburg O. On the origin of cancer cells. Science 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- 20. Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion 2005;5:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nomura DK, Lombardi DP, Chang JW, et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol 2011;18:846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito K, Suda T. Metabolic requirements for the maintenance of self‐renewing stem cells. Nat Rev Mol Cell Biol 2014;15:243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlaepfer IR, Rider L, Rodrigues LU, et al Lipid catabolism via cpt1 as a therapeutic target for prostate cancer. Mol Cancer Ther 2014;13:2361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freeman MR, Cinar B, Lu ML. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab 2005;16:273–9. [DOI] [PubMed] [Google Scholar]
- 25. Menendez JA. Fine‐tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta 2010;1801:381–91. [DOI] [PubMed] [Google Scholar]
- 26. Tsui MM, York JD. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv Enzyme Regul 2010;50:324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scott SA, Mathews TP, Ivanova PT, et al. Chemical modulation of glycerolipid signaling and metabolic pathways. Biochim Biophys Acta 2014;1841:1060–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cinar B, Mukhopadhyay NK, Meng G, et al. Phosphoinositide 3‐kinase‐independent non‐genomic signals transit from the androgen receptor to akt1 in membrane raft microdomains. J Biol Chem 2007;282:29584–93. [DOI] [PubMed] [Google Scholar]
- 29. Costello LC, Franklin RB. The intermediary metabolism of the prostate: A key to understanding the pathogenesis and progression of prostate malignancy. Oncology 2000;59:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang XZ, Wang B, Gao ZQ, et al. 1H‐MRSI of prostate cancer: The relationship between metabolite ratio and tumor proliferation. Eur J Radiol 2010;73:345–51. [DOI] [PubMed] [Google Scholar]
- 31. Riches SF, Payne GS, Morgan VA, et al. Multivariate modelling of prostate cancer combining magnetic resonance derived t2, diffusion, dynamic contrast‐enhanced and spectroscopic parameters. Eur Radiol 2015;25:1247–1256. [DOI] [PubMed] [Google Scholar]
- 32. Mullen AR, Hu Z, Shi X, et al. Oxidation of alpha‐ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep 2014;7:1679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas MA, Nagarajan R, Huda A, et al. Multidimensional mr spectroscopic imaging of prostate cancer in vivo. NMR Biomed 2014;27:53–66. [DOI] [PubMed] [Google Scholar]
- 34. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bae S, Ulrich CM, Neuhouser ML, et al. Plasma choline metabolites and colorectal cancer risk in the women's health initiative observational study. Cancer Res 2014;74:7442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mondul AM, Weinstein SJ, Bosworth T, et al. Circulating thyroxine, thyroid‐stimulating hormone, and hypothyroid status and the risk of prostate cancer. PLoS One 2012;7:e47730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cross AJ, Boca S, Freedman ND, et al. Metabolites of tobacco smoking and colorectal cancer risk. Carcinogenesis 2014;35:1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer 2008;123:899–904. [DOI] [PubMed] [Google Scholar]
- 39. Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf 2010;9:603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crowe FL, Appleby PN, Travis RC, et al. Circulating fatty acids and prostate cancer risk: individual participant meta‐analysis of prospective studies. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann Intern Med 1993;118:793–803. [DOI] [PubMed] [Google Scholar]
- 42. Etzioni R, Cha R, Feuer EJ, et al. Asymptomatic incidence and duration of prostate cancer. Am J Epidemiol 1998;148:775–85. [DOI] [PubMed] [Google Scholar]


