Abstract
Objectives
To compare changes in vitamin D status and cathelicidin (LL-37) levels in septic ICU patients treated with placebo versus cholecalciferol.
Design
Randomized, placebo-controlled, trial.
Setting
Medical and surgical ICUs of a single teaching hospital in Boston, MA.
Patients
30 adult ICU patients.
Interventions
Placebo (n=10) versus 200,000 IU cholecalciferol (n=10) versus 400,000 IU cholecalciferol (n=10), within 24 hours of new-onset severe sepsis or septic shock.
Measurements and Main Results
Blood samples were obtained at baseline (day 1) and on days 3, 5, and 7, to assess total 25-hydroxyvitamin D (25OHD), as well as vitamin D binding protein and albumin to calculate bioavailable 25OHD. Plasma LL-37 and high sensitivity C-reactive protein (hsCRP) levels were also measured. At baseline, median (IQR) plasma 25OHD was 17 (13 to 22) ng/mL and peaked by day 5 in both intervention groups. Groups were compared using Kruskal-Wallis tests. Relative to baseline, on day 5, median change in biomarkers for placebo, 200,000 IU cholecalciferol, and 400,000 IU cholecalciferol groups, respectively, were: 1) total 25OHD: 3 (-3 to 8)%, 49 (30 to 82)%, and 69 (55 to 106)%, (P<0.001); 2) bioavailable 25OHD: 4 (-8 to 7)%, 45 (40 to 70)%, and 96 (58 to 136)%, (P<0.01); and 3) LL-37: -17 (-9 to -23)%, 4 (-10 to 14)%, and 30 (23 to 48)%, (P=0.04). Change in hsCRP levels did not differ between groups. A positive correlation was observed between bioavailable 25OHD and LL-37 (Spearman's rho=0.44, P=0.03), but not for total 25OHD and LL-37.
Conclusions
High-dose cholecalciferol supplementation rapidly and safely improves 25OHD and bioavailable 25OHD levels in patients with severe sepsis or septic shock. Changes in bioavailable 25OHD are associated with concomitant increases in circulating LL-37 levels. Larger trials are needed to verify these findings and to assess whether optimizing vitamin D status improves sepsis-related clinical outcomes.
Keywords: vitamin D, 25-hydroxyvitamin, ICU, cholecalciferol, cathelicidin, LL-37
Introduction
Sepsis is a clinical entity that complicates severe infections [1]. It is characterized by the cardinal signs of inflammation (e.g., vasodilation, leukocytosis, increased microvascular permeability) occurring in tissues that are remote from the site of an infection [2]. The spectrum of disease severity can range from sepsis to severe sepsis, septic shock, and multiple organ dysfunction syndrome [3]. While recent data suggest that survival rates have improved modestly over the last two decades [4-7], in general, the mortality associated with each of these is estimated to be 16%, 20%, 46%, and >80%, respectively [8-10]. The annual incidence of sepsis exceeds 1.6 million cases in the United States alone and results in costs exceeding $24 billion every year [10,11].
Current theories about the onset and progression of sepsis focus on dysregulation of inflammatory responses, including the possibility that a massive and uncontrolled release of pro-inflammatory mediators initiates a chain of events that lead to widespread tissue injury [12]. The degree of immune dysfunction is thought to correlate with the severity of sepsis [13]. Recently, cells of the innate and adaptive immune system were shown to express the vitamin D receptor [14]. In contrast to its inhibitory role in adaptive immunity, vitamin D is a potent activator of the innate immune system [15] and may be integral for natural defense mechanisms against microbial invasion [16].
In humans, circulating 25-hydroxyvitamin D (25OHD) is the most abundant vitamin D metabolite [17]. As such, it is often used as a proxy for total body vitamin D status [18]. Growing evidence suggests that a significant proportion (50-90%) of critically ill patients have low 25OHD levels during ICU admission [19-23]. Suboptimal 25OHD levels, in turn, appear to be associated with a higher risk of mortality in critically ill patients [21-23]. And while it is known that septic patients typically have low 25OHD levels [24,25], and that vitamin D status is inversely associated with the severity of sepsis [26,27], little is known regarding the effects of vitamin D supplementation in this patient cohort. Therefore, our primary goal was to determine whether high-dose cholecalciferol (vitamin D3) supplementation in patients with severe sepsis or septic shock can: 1) rapidly influence vitamin D status; and 2) affect systemic expression of cathelicidin (LL-37), a vitamin D-dependent, endogenous, anti-microbial peptide [28]. Our secondary goal was to determine whether high-dose cholecalciferol supplementation modifies systemic cytokine levels in patients with severe sepsis or septic shock.
Materials and Methods
Study Cohort
Patients were recruited from a single medical ICU and two surgical ICUs at the Massachusetts General Hospital (MGH) in Boston, MA. These three, 18-bed ICUs, receive admissions from all medical and surgical services, except for Cardiology and Cardiac Surgery. MGH is a large teaching hospital, and a level-one trauma center, which serves a diverse population in eastern Massachusetts. The Partners Human Research Committee (Institutional Review Board) approved the study protocol and the trial was registered on ClinicalTrials.gov (NCT01896544). All subjects were enrolled between 01/01/2014 and 03/01/2014.
Inclusion and exclusion criteria
All adult men and women, ≥18 years of age, admitted to the medical or surgical ICUs at MGH, and within 24 hours of new-onset sepsis, were potentially eligible to participate. During the initial screen, for patients transferred from within the hospital (but not the emergency department) or another healthcare facility, we performed a detailed review of the electronic medical records to determine an approximate time of when the patients met sepsis criteria according to the 2012 Surviving Sepsis Campaign [29]. For patients who presented from home and were admitted to the ICU through the emergency department, we reviewed the initial documentation on arrival to MGH, and for those who met criteria for sepsis, we considered time of arrival to the emergency department as the time of new onset-sepsis. At the time of enrollment, only those patients who met criteria for severe sepsis or septic shock (according to the consensus definition [29]) were approached for study consideration. Informed consent was obtained from subjects if they were oriented to person, place, and time in addition to testing negative on the Confusion Assessment Method for the ICU test [30]; surrogate consent was obtained when subjects did not meet these criteria. Subjects were only included in the study if the baseline blood sample and subsequent cholecalciferol supplementation could be completed within 24 hours of new-onset sepsis.
Exclusion criteria included a known history of renal stones, diagnosis of hypercalcemia within the past year, baseline serum total calcium >10 mg/dL, established diagnosis associated with increased risk of hypercalcemia (e.g. metastatic cancer, sarcoidosis, multiple myeloma, primary hyperparathyroidism), and current vitamin D supplementation. Due to the need for repeated blood samples, we also excluded patients with a known history of anemia at the time of ICU admission (defined as hematocrit <25%) as well as any pregnant or immediately post-partum women. We also excluded any patients with high gastrointestinal output requiring continuous decompression since cholecalciferol administration through the naso/oro-gastric tube would not be possible. Lastly, patients were also excluded if they did not have a suitable healthcare proxy (when they were not able to provide consent), if they had a high likelihood of dying within the first 48 hours of ICU admission (as determined by the treating ICU team), if they had transitioned to a “comfort care only” status, or if they refused to participate.
Blood sample processing and biomarker assays
Following informed consent, fresh blood from an indwelling arterial or central venous catheter was collected directly into an EDTA-containing tube (this was designated as day 1 of the study). The sample was immediately stored on ice and then centrifuged within 30 minutes to separate out plasma. All samples were centrifuged at 2,300 rpm for 15 minutes at a temperature of 4°C. The separated plasma was immediately transferred to polypropylene tubes and stored at -80°C until biomarker testing was ready to be initiated. Follow-up blood samples were collected and processed in a similar manner on days 3, 5, and 7.
Assays were performed at the Harvard Medical School Clinical and Translational Science Award core laboratory at MGH. 25OHD (combined D2 and D3) and vitamin D binding protein (DBP) were measured by enzyme-linked immunoabsorbent assay (ELISA), using commercially available kits (Abbott Laboratories, Abbott Park, IL, and Hycult Biotech, Plymouth Meeting, PA, respectively). Intra- and inter-assay coefficients of variation (CV) were both <8% for 25OHD and <12% for DBP, respectively. LL-37 was also measured by ELISA, using a commercially available kit (Hycult Biotech, Plymouth Meeting, PA), with intra- and inter-assay CVs of <10% for both, respectively. The MGH core laboratory is a Clinical Laboratory Improvement Amendments (CLIA)-certified facility, which uses rigorous methods and continuously updated reference standards for the assessment of albumin and high sensitivity C-reactive protein (hsCRP). Bioavailable 25OHD was calculated using previously described formulas using total 25OHD, DBP, and albumin levels [31]. Plasma cytokine levels of interleukin (IL)-1β, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ were measured using a commercially available kit (Cytokine Human Magnetic 10-Plex Panel, Life Technologies, Grand Island, NY) using a Luminex FLEXMAP 3D (Luminex, Austin, TX) multiplex assay analyzer.
Vitamin D supplementation
Cholecalciferol and matched placebo were both supplied in a clear liquid form by a commercial vendor (Ddrops Company, Toronto, Canada) directly to the MGH Research Pharmacy. The Research Pharmacy was responsible for establishing the study code using a computer-generated block randomization algorithm, preparing syringes containing the study drug (each syringe contained 7 mL of liquid), and dispensing the coded syringes directly to a member of the research team after verifying subject enrollment in the study. Once dispensed by the research pharmacy, a member of the research team witnessed the administration of the study drug as a single bolus to all enrolled subjects. The study drug was administered either orally (when clinically appropriate), or via an indwelling naso/oro-gastric tube by a registered nurse responsible for the care of each patient. The standard MGH protocol involves flushing all gastric tubes with 20 mL of saline before and after drug administration. Suctioning through the gastric tube was held for at least one hour after study drug administration.
Clinical data collection
Partners Healthcare electronic medical records system was used to obtain baseline information related to: age, sex, race, body mass index (BMI), acute physiology and chronic health evaluation II (APACHE II) score, and type of patient (medical versus surgical ICU). Microbiology results were also abstracted from the electronic medical records system. Total body fluid balance (TBB) from admission to the time of study-related blood sample acquisition was calculated from the bedside ICU flow sheet for each subject. The 30-day mortality rate for the analytic cohort was confirmed by reviewing individual medical records and cross-referencing each case using the Social Security Death Index Master File up to 90 days following hospital discharge.
Statistical Analysis
Descriptive statistics were tabulated in aggregate for the groups who received placebo versus 200,000 IU cholecalciferol versus 400,000 IU cholecalciferol. Continuous data were reported as medians with interquartile ranges (IQRs) and compared using Kruskal-Wallis tests. Categorical values were expressed as proportions and compared using χ2 tests. Length-of-stay data were compared using Log-rank tests. We performed Spearman correlation testing to assess the relationship between the change in 25OHD and bioavailable 25OHD with change in LL-37 levels.
The study was powered to investigate our primary goal. Based on previous studies, we assumed that the mean 25OHD level in severely septic or septic shock patients at MGH would be 17 ng/mL [31]. We also assumed that by day 7, 25OHD levels in the placebo group would decease by 2 ng/mL, while a single dose of 400,000 IU cholecalciferol would raise levels by 15 ng/mL [32]. Assuming a common standard deviation of 10 ng/mL and alpha=0.05, a sample size of 6 patients in each group (placebo versus intervention) would allow us to detect this difference with a power of 80%. Similarly, based on preliminary data, we assumed that the baseline LL-37 levels in ICU patients would be 50 ng/mL. We also assumed that by day 7, LL-37 levels in the placebo group would decrease by 10 ng/mL, while supplementation with 400,000 IU cholecalciferol would raise levels to 100 ng/mL. Assuming a common standard deviation of 40 ng/mL and alpha=0.05, a sample size of 7 patients in each group (placebo versus intervention) would allow us to detect this difference with a power of 80%. To compensate for potential study withdrawal, drug intolerance, or death before completing the study, we enrolled 10 patients in each arm. All analyses were performed using STATA 12.0 (StataCorp LP, College Station, Texas). A two-tailed P <0.05 was considered to statistically significant.
Results
A total of 87 patients were screened for study inclusion. 51 patients did not meet criteria for severe sepsis or septic shock according to the 2012 Surviving Sepsis Campaign guidelines [29], and were therefore excluded. Furthermore, 4 patients were expected to die within 48 hours of ICU admission (transitioned to “comfort care only” measures), while 2 patients died within 24 hours of new onset sepsis, and therefore could not be approached for study participation. This left 30 patients in the analytic cohort. Their baseline characteristics and clinically relevant outcomes are shown in Table 1.
Table 1.
Demographic factors, baseline clinical information, and major clinical outcomes among 30 patients with severe sepsis or septic shock, according to randomly-assigned treatment group.
| Treatment Group | P-value | |||
|---|---|---|---|---|
| Placebo (n=10) |
200,000 IU (n=10) |
400,000 IU (n=10) |
||
| Age (years) | 65 (58-70) | 64 (55-66) | 62 (59-67) | 0.91 |
| Sex (%) | 1.00 | |||
| Female | 40 | 40 | 40 | |
| Male | 60 | 60 | 60 | |
| BMI (kg/m2) | 28 (26-35) | 28 (27-33) | 30 (28-37) | 0.40 |
| APACHE II | 22 (16-28) | 21 (19-32) | 23 (16-25) | 0.62 |
| ICU Type (%) | 0.26 | |||
| Medical | 60 | 50 | 50 | |
| Surgical | 40 | 50 | 50 | |
| Positive culture (%)* | 0.74 | |||
| Pulmonary | 40 | 50 | 40 | |
| Bloodstream | 60 | 60 | 70 | |
| Urinary | 10 | 10 | 10 | |
| Day 1: 25OHD (ng/mL) | 19 (13-22) | 15 (12-20) | 17 (13-25) | 0.76 |
| Day 5: 25OHD (ng/mL) | 19 (11-23) | 22 (16-25) | 29 (23-41) | 0.04 |
| Day 1: B25OHD (ng/mL) | 1.4 (0.8-2.1) | 1.9 (1.2-2.5) | 2.5 (1.7-2.9) | 0.16 |
| Day 5: B25OHD (ng/mL) | 1.6 (1.2-2.1) | 1.8 (1.6-3.7) | 4.0 (3.2-4.5) | 0.04 |
| Day 1: LL-37 (ng/mL) | 53 (43-62) | 52 (48-59) | 51 (41-52) | 0.52 |
| Day 5: LL-37 (ng/mL) | 50 (35-54) | 54 (40-64) | 67 (59-68) | 0.02 |
| Day 1: hsCRP | 106 (60-267) | 208 (90-359) | 119 (85-247) | 0.62 |
| Day 5: hsCRP | 53 (38-72) | 98 (12-130) | 31 (26-160) | 0.99 |
| Day 1: TBB (mL) | 1592 (1086-2117) | 1730 (925-3075) | 1518 (696-2088) | 0.55 |
| Day 5: TBB (mL) | 2996 (1980-4081) | 3241 (2228-6586) | 3060 (1355-3803) | 0.63 |
| ICU LOS (days) | 12 (7-15) | 4 (3-11) | 3 (2-11) | 0.83 |
| Hospital LOS (days) | 21 (18-31) | 13 (12-16) | 14 (8-21) | 0.03 |
| 30-day readmission (%) | <0.001 | |||
| No | 80 | 100 | 100 | |
| Yes | 20 | - | - | |
| 30-day mortality (%) | 0.18 | |||
| Dead | 30 | 30 | 20 | |
| Alive | 70 | 70 | 80 | |
Data presented as either median (interquartile range) or proportions and compared using Kruskal-Wallis tests or chi-squared tests, respectively. Length-of-stay data are compared using log-rank tests for trend. BMI = body mass index; APACHE = acute physiology and chronic health evaluation; ICU = intensive care unit; 25OHD = 25-hydroxyvitamin D; B25OHD = bioavailable 25-hydroxyvitamin D; LL-37 = cathelicidin; hsCRP = high-sensitivity C-reactive protein; TBB = total body fluid balance; LOS = length of stay.
Totals do not equal 100% since some patients had more than one location where a positive culture was obtained.
The median age of study participants was 64 (IQR 58-68) years and median baseline 25OHD level was 17 (IQR 13-22) ng/mL. Overall, subjects were predominantly male (60%) and white (93%). There was no difference between groups at baseline in terms of age, sex (ratio of women versus men), BMI, APACHE II score, type of patient (medical versus surgical ICU), and site(s) of positive microbial culture.
Peak change in 25(OH)D levels was achieved by day 5 in both intervention groups (Figure 1). To account for variations in the individual responses to acute stress and to cholecalciferol supplementation, changes in 25OHD, bioavailable 25OHD, LL-37, hsCRP, and cytokines were expressed as a percentage of baseline and compared between day 1 and 5 (Figure 2, Figure 3, and Figure 4). No adverse events related to cholecalciferol supplementation were observed in either intervention group.
Figure 1.
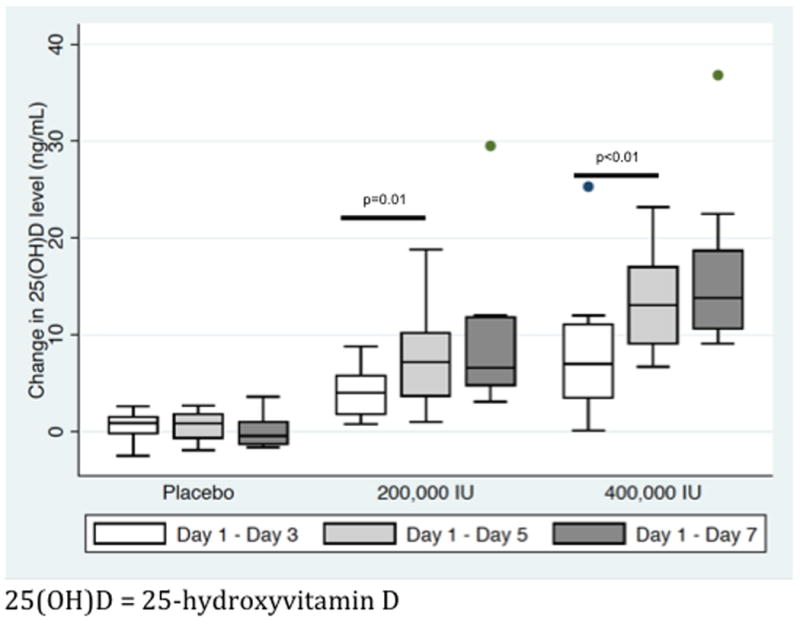
Median (with interquartile ranges) change in 25-hydroxyvitamin D levels on days 3, 5, and 7 relative to baseline (day 1): Peak plasma levels are achieved by day 5 in both the 200,000 IU cholecalciferol and 400,000 IU cholecalciferol groups.
Figure 2.
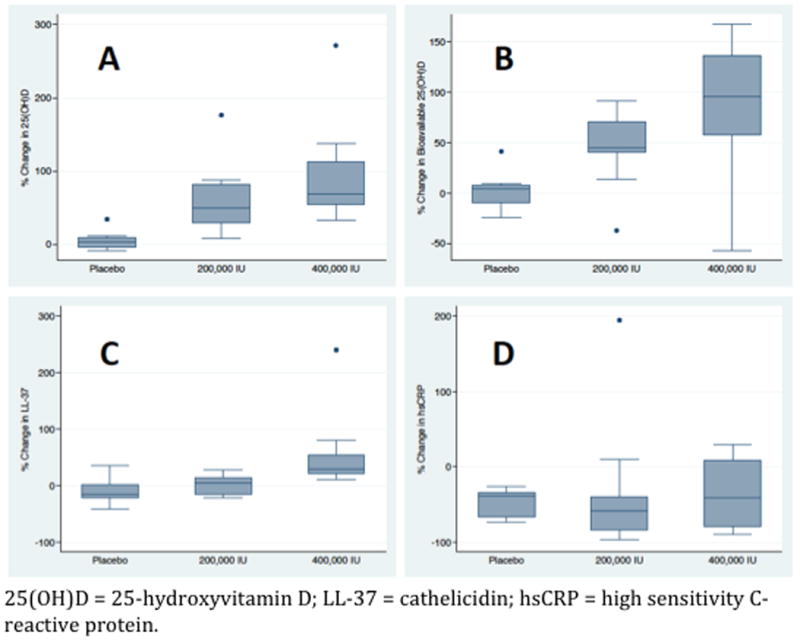
Change in biomarker levels on day 5 expressed as a percentage of baseline levels (day 1). Significant differences in 25-hydroxyvitamin D (A), bioavailable 25-hydroxyvitamin D (B), and cathelicidin (C) levels were observed between groups. No difference was observed in high-sensitivity C-reactive protein (D) levels.
Figure 3.
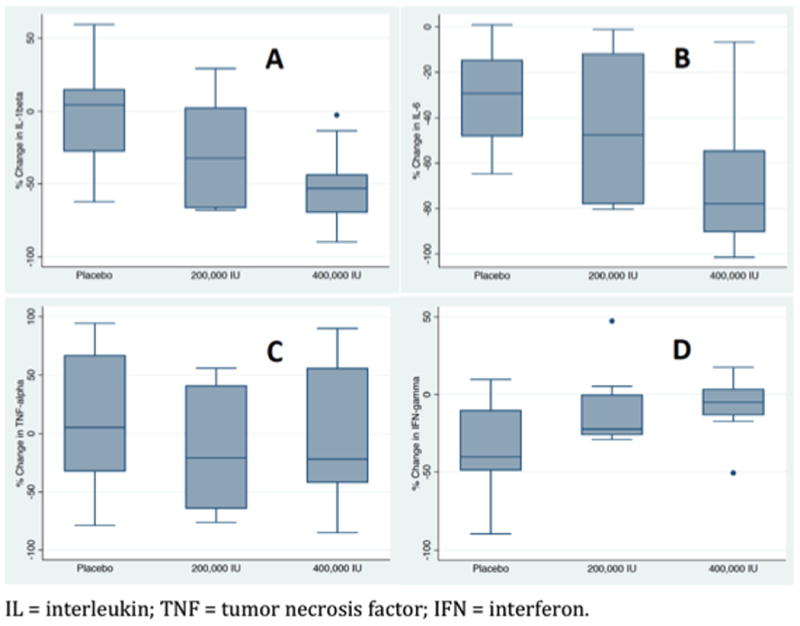
Change in pro-inflammatory cytokines on day 5 expressed as a percentage of baseline level (day 1). Significant differences in interleukin-1β (A) and interleukin-6 (B) levels were observed between groups. A trend towards significant difference in interferon-γ (D) was also observed between groups. No difference was observed in tumor necrosis factor-α (C) levels between groups.
Figure 4.
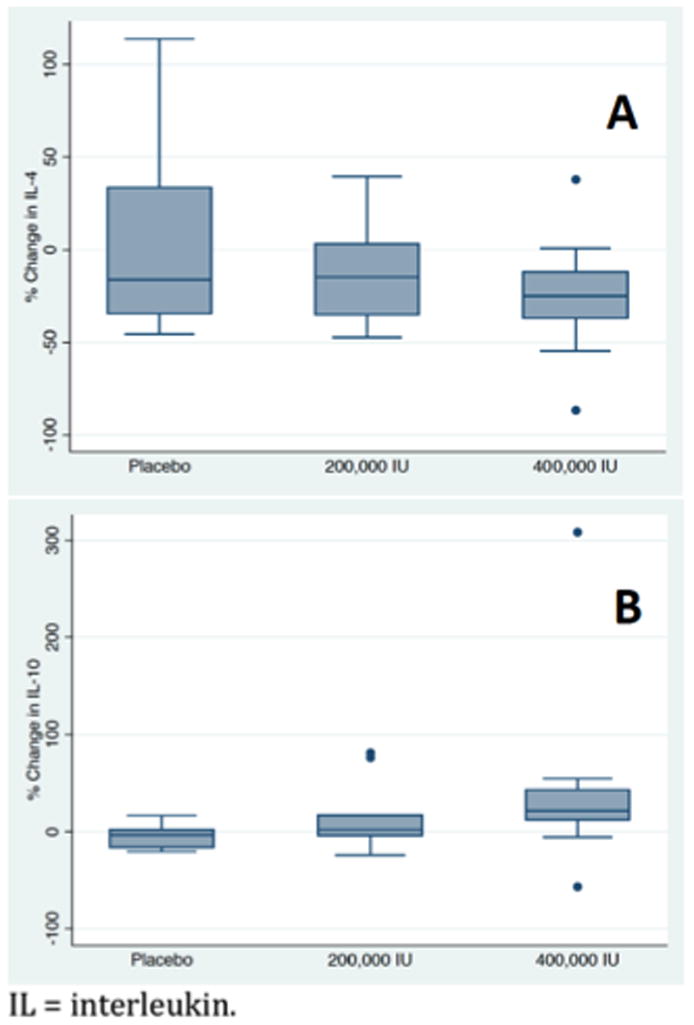
Change in anti-inflammatory cytokines on day 5 expressed as a percentage of baseline level (day1). A trend towards significant difference in interleukin-10 (B) levels was observed between groups. No difference was observed in interleukin-4 (A) levels between groups.
Between days 1 and 5, median change in plasma total 25OHD level was 3 (IQR -3 to 8)% for the placebo group, 49 (IQR 30-82)% for the 200,000 IU cholecalciferol group, and 69 (IQR 55-106)% for the 400,000 IU cholecalciferol group (P<0.001). The calculated median change in bioavailable 25OHD level between day 1 and 5 was 4 (IQR -8 to 7)%, 45 (IQR 40-70)%, and 96 (IQR 58-136)%, respectively (P<0.01). Relative to baseline, on day 5 median change in plasma LL-37 level was -17 (IQR -9 to -23)%, 4 (IQR -10 to 14)%, and 30 (IQR 23-48)%, respectively (P=0.04). Change in hsCRP levels did not differ between groups: -39 (IQR -36 to 64)%, -58 (IQR -40 to -77)%, and -41 (IQR -8 to -74)%, respectively (P=0.59). Correlation testing demonstrated a significant relationship between the change in bioavailable 25OHD and change in LL-37 levels (Spearman's rho=0.44; P=0.03), but not between the change in total 25OHD and change in LL-37 levels (Spearman's rho=0.23; P=0.23).
Between days 1 and 5, median change in plasma IL-1β was 4 (IQR -36 to 16)% for the placebo group, -32 (IQR -66 to 5)% for the 200,000 IU cholecalciferol group, and -53 (IQR -36 to -71)% for the 400,000 IU cholecalciferol group (P=0.02). The median change in IL-4 between day 1 and day 5 was -16 (IQR -40 to 51)%, -15 (IQR -36 to 5)%, and -25 (IQR -9 to -41)%, respectively (P=0.57). The median change in IL-6 between day 1 and day 5 was -29 (IQR -14 to -50)%, -48 (IQR -12 to -78)%, and -78 (IQR -53 to -93)%, respectively (P=0.02). The median change in IL-10 between day 1 and day 5 was -3 (IQR -18 to 3)%, 2 (IQR -7 to 31)%, and 16 (IQR -15 to 42)%, respectively (P=0.07). The median change in TNF-α between day 1 and day 5 was 5 (IQR -38 to 68)%, -20 (IQR -65 to 48)%, and -22 (IQR -46 to 61)%, respectively (P=0.61). And, relative to baseline, on day 5, median change in IFN- γ was -40 (IQR -6 to -50)%, -22 (IQR -26 to 2)%, and -5 (IQR -14 to 5)%, respectively (P=0.09).
With respect to key clinical outcomes, there was no difference between groups regarding ICU length of stay (LOS): 12 (IQR 7 to 15) days for the placebo group, 4 (IQR 3 to 11) days for the 200,000 IU cholecalciferol group, and 3 (IQR 2 to 11) days for the 400,000 IU cholecalciferol group (test for trend, p=0.83). However, there was a difference between groups regarding hospital LOS: 21 (IQR 18 to 31) days, 13 (IQR 12 to 16) days, and 14 (IQR 8 to 21) days, respectively (test for trend, p=0.03). While there was a significant difference in the 30-day ICU readmission rate between groups (both cases were for respiratory failure and sepsis): 20%, 0%, and 0%, respectively (p<0.001), there was no difference between groups regarding 30-day all-cause mortality (30%, 30%, and 20%, respectively; p=0.18). When the two cholecalciferol groups were combined into a single intervention arm and compared to the placebo group, there was no difference is ICU LOS: 12 (IQR 7 to 15) days for the placebo group versus 3 (IQR 3 to 12) days for the cholecalciferol group (p=0.77). However, hospital LOS was still significantly longer in the placebo group compared to the cholecalciferol group: 21 (18 to 31) days versus 13 (10 to 18) days (p=0.01), respectively. And while 30-day ICU readmission rate was still higher in the placebo group compared to the cholecalciferol group (20% versus 0%; p<0.001, respectively), there was no difference between them in terms of 30-day all-cause mortality (30% versus 25%; p=0.43, respectively).
Discussion
In this randomized controlled trial of critically ill patients, we compared the effects of placebo versus 200,000 IU cholecalciferol versus 400,000 IU cholecalciferol on vitamin D status and expression of an endogenous, vitamin D-dependent, antimicrobial peptide. We demonstrated that a single bolus dose of 400,000 IU cholecalciferol is safe and effective for rapidly improving circulating 25OHD levels, bioavailable 25OHD, and expression of LL-37 in patients with severe sepsis or septic shock. We also observed that cholecalciferol supplementation can modulate cytokine expression, resulting in a significant reduction in systemic levels of IL-1β and IL-6. Although the present study was underpowered to reliably detect differences in clinical outcomes, our results provide important mechanistic evidence to explain why rapidly improving vitamin D status in patients with severe sepsis or septic shock may improve clinical outcomes and support the design and implementation of larger, multicenter trials.
The primary results of our trial are consistent with evidence that cells of the innate and adaptive immune system express the vitamin D receptor (VDR) [14]. Vitamin D is an important link between Toll Like Receptor (TLR) activation and antibacterial responses [33]. In vitro studies have shown that in low vitamin D states, stimulation of human macrophages by TLR induces a series of important responses, which include: 1) increased expression of VDR [34]; 2) conversion of 25OHD to its most biologically active form of 1,25-dihydroxyvitamin D (1,25OH2D) [35]; and 3) enhanced production of LL-37 [28,36]. Our study builds on these findings and demonstrates in vivo, that improving bioavailable 25OHD in humans with overwhelming infections is associated with elevated systemic LL-37 levels. However, to fully appreciate the significance of these findings, it is important to discuss two fundamental concepts: total 25OHD versus bioavailable 25OHD; and the role of cathelicidins in innate immune responses.
In humans, cholecalciferol is either obtained through the diet or synthesized by skin upon exposure to ultraviolet B radiation [17]. Cholecalciferol is converted to 25OHD in the liver and then to 1,25OH2D by the kidneys and by cells of the immune system (amongst others) for paracrine use [37]. Circulating 25OHD levels are, however, approximately 500–1000 times higher than 1,25OH2D levels, and both 25OHD and 1,25OH2D are predominantly protein-bound in circulation [38]. Only 0.03% of 25OHD is free [24], with close to 88% bound to vitamin D binding protein (DBP) and the remainder to albumin [38]. Although free 25OHD is most readily available for conversion into 1,25OH2D [37], the albumin-bound component of total 25OHD can be easily mobilized in times of need and provides a larger pool of 25OHD to maintain optimal vitamin D-mediated autocrine and paracrine activity [39]. By contrast, the 25OHD-DBP complex is very stable, with an affinity of binding several orders of magnitude greater than that of 25(OH)D to albumin [37]. 25OHD bound to DBP is typically involved in the regulation of gene expression, requiring intracellular enzymatic cleavage of the 25OHD, and thus, it is thought to have limited biological activity during acute stress [39,40]. As such, the free and albumin-bound 25OHD are collectively referred to as “bioavailable” 25OHD and it has been hypothesized that assessment of the bioavailable forms of vitamin D may have greater predictive value for important ICU-related outcomes when compared with total serum 25OHD [41,42].
Cathelicidins are a family of antimicrobial and endotoxin-binding proteins largely found in peroxidase-negative granules of mammalian neutrophils [43]. The cathelicidins are synthesized as pre-pro-proteins and after removal of the signal peptide, they are stored in granules as inactive pro-forms [43]. The active biologic domains of the cathelicidins generally reside in the C-terminus [44]. The C-terminal antibacterial peptides are activated when cleaved from the proforms of the cathelicidins by serine proteases from azurophil granules [44]. While most mammals express several types of cathelicidins, hCAP-18 is the only human cathelicidin [45]. And although hCAP-18 is a major protein in specific granules of neutrophils [46], but it is also present in subpopulations of lymphocytes as well as monocytes [47], and in squamous epithelia at barrier sites such as skin, lungs, gut, and the genitourinary tract [48-50]. Plasma also contains a significant concentration of hCAP-18 bound to lipoproteins [51]. The antibacterial C-terminus of hCAP-18, LL-37, exhibits potent activity against a broad spectrum of infectious agents such as, both gram-positive and gram-negative bacteria, viruses, fungi, and mycobacteria [52]. LL-37 also has synergistic antibacterial effects with the defensins (another major class of endogenous, antimicrobial peptides) [53], and is a chemotactic agent for neutrophils, monocytes, and T cells [54]. As such, LL-37 expression by neutrophils and epithelial cells may represent an important first line of defense for the innate immune system [55].
In addition to upregulation of antimicrobial peptide production, our study demonstrates that high-dose cholecalciferol supplementation results in a significant reduction in IL-1β and IL-6, but not TNF-α levels. Although TNF-α is often regarded as the “master regulator” of pro-inflammatory cytokines [56], both IL-1β and IL-6 play important roles in the early inflammatory response that characterizes sepsis. IL-1β is a major mediator of inflammation-induced coagulation [57], lipid mediators, as well as reactive oxygen and nitrogen species [58]. IL-1β also amplifies the inflammatory cascade in an autocrine and paracrine manner by activating macrophages to secrete other pro-inflammatory cytokines (e.g. IL-6) [59]. All these actions have been shown to be critical in the development of sepsis-induced organ dysfunction [60]. On the other hand, a key function of IL-6 is the induction of fever [61] and the mediation of the acute phase response [62, 63]. IL-6 has also recently been shown to have an important role in sepsis-induced myocardial dysfunction [64] and therefore may play a critical role in the progression of sepsis to multi-organ failure [60]. It is worth noting that though not statistically significant, we observed a trend towards less suppression of IFN-γ and higher IL-10 levels in patients who received high dose cholecalciferol. IFN-γ production is tightly regulated and stimulated by macrophage-derived cytokines (especially TNF-α) [65]. It is normally not detectable in the plasma of healthy humans, but levels can be elevated in patients with sepsis [66]. Recent evidence suggests that IFN-γ may play an important role in the reversal of sepsis-induced immunoparalysis [67,68]. In contrast, expression of IL-10 is largely associated with anti-inflammatory functions, such as suppression of pro-inflammatory mediators (e.g. TNF-α, IL-1β, IL-6) [69,70]. Moreover, data suggest that IL-10 may be a critical regulator of the transition from early reversible sepsis to late irreversible septic shock and multi-organ failure [71,72]. And although IL-4 is a cytokine with many immunoregulatory functions in addition to regulation of cell proliferation, differentiation, and apoptosis, its role in sepsis remains unclear [60]. Our findings do not support a relationship between cholecalciferol supplementation and systemic IL-4 expression in septic patients. Taken all together, our results suggest that early administration of high dose cholecalciferol in patients with severe sepsis or septic shock may significantly influence cytokine expression compared to patients who received placebo.
Our study findings build upon two recently published randomized, placebo-controlled, clinical trials. Leaf et al. [73] reported that 1,25OH2D administration did not result in higher plasma LL-37 levels in patients with sepsis. However, it is important to recognize that the intervention group in this study received a single dose of 1,25OH2D, which has a half-life of only a few hours [37]. As such, 1,25OH2D resulted in a significant increase in leukocyte mRNA expression of LL-37 but did not significantly elevate systemic levels of the antimicrobial peptide. The use of cholecalciferol in our study likely provided substrate for continuous paracrine and/or autocrine production of 1,25OH2D and was therefore likely more effective in boosting systemic levels of LL-37. Additionally, Amrein et al. [74] reported that high dose cholecalciferol supplementation in critically ill patients with 25OHD levels ≤12 ng/mL resulted in a significant reduction of in-hospital mortality. Although the mechanisms underlying such an outcome remain unclear, the results of our study suggest that anti-microbial and immunomodulatory effects of vitamin D may play an important role during critical illness.
While our findings are novel and intriguing, we acknowledge the potential limitations of our trial. First, our a priori sample size calculation was only aimed at adequately powering the trial to assess whether two different doses of cholecalciferol could rapidly improve vitamin D status and enhance circulating LL-37 levels in patients with severe sepsis or septic shock. Although we did find a significant difference in cytokine levels and the 30-day readmission rate between groups, our current study was not specifically powered to assess these or other important clinical outcomes, such as ICU LOS, hospital LOS, and mortality. Second, we only measured a number of the cytokines most commonly associated with sepsis [60]. A deeper exploration of cytokine expression and immunophenotyping are likely needed to enhance our understanding of the immunomodulatory effects of vitamin D in acute illness. Third, we enrolled patients from both medical and surgical ICUs. Differences in patient-specific factors such as underlying co-morbidities, baseline vitamin D status, and variable absorption through the alimentary tract, may have influenced the observed outcomes. And fourth, fluid loading, renal loss of albumin, and inflammatory changes, which are typical of the sepsis syndrome, are known to affect circulating 25OHD levels. We attempted to control for these factors by calculating the bioavailable 25OHD (which takes into consideration alterations in albumin and DBP levels) in addition to measuring total body fluid balance and hsCRP. However, our calculations for bioavailable 25OHD are based on formulas validated in non-critically ill patients. And while a number of commercial tests for free 25OHD levels have recently become available [75], their role is not yet clear. Moreover, methods for direct measurement of bioavailable 25OHD are not readily available. These issues merit consideration in the design of future trials that aim to confirm and expand on our novel findings.
Conclusion
Administration of 400,000 IU cholecalciferol as a single bolus at the outset of severe sepsis or septic shock is a safe and effective intervention to rapidly improve 25OHD levels and bioavailable 25OHD in patients with low vitamin D status. In turn, the changes in bioavailable 25OHD level are associated with concomitant increases in circulating LL-37 levels. Cholecalciferol supplementation also significantly influenced cytokine expression in patients with severe sepsis or septic shock. Larger trials are needed to verify these findings and to assess whether optimizing vitamin D status may improve sepsis-related clinical outcomes.
Acknowledgments
Funding: SAQ received support from the National Institutes of Health grants 5T32GM007592 and department funding. MK received support from the National Institutes of Health grant P50GM02700. EKB received support from the American Thoracic Society Foundation and the National Institutes of Health grant R01 HL119344. CAC received support from the National Institutes of Health grants R01 AI093723 and U01 AI087881. IB received support from the National Institutes of Health grant 5K23DK081677.
Footnotes
Where work was performed: Vitamin D in Stress (ViDIS) Laboratory, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA
Copyright form disclosures: Dr. Quraishi received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NIH. Dr. Kaneki received support for article research from the NIH. His institution received grant support from the NIH grant P50GM02700 (Projects 1 and 4). Dr. Bajwa received support for article research from the NIH and the American Thoracic Society. His institution received grant support from the NIH and the American Thoracic Society. Dr. Camargo received support for article research from the NIH. His institution received grant support from the NIH. Dr. Bhan received support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS /SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Danai P, Martin GS. Epidemiology of sepsis: recent advances. Curr Infect Dis Rep. 2005;7:329–34. doi: 10.1007/s11908-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 4.Gaieski DF, Edwards JM, Kallan MJ, et al. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190:665–74. doi: 10.1164/rccm.201402-0289OC. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42:625–31. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 7.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–74. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 9.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 10.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–61. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Friedman B, Stranges E. Statistical Brief #122. Agency for Healthcare Research and Quality; Rockville, MD: 2009. [Accessed on May 15, 2013]. Septicemia in U.S. Hospitals. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. [PubMed] [Google Scholar]
- 12.Esper AM, Martin GS. Extending international sepsis epidemiology: the impact of organ dysfunction. Crit Care. 2009;13:120. doi: 10.1186/cc7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12:R158. doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kankova M, Luini W, Pedrazzoni M, et al. Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology. 1991;73:466–471. [PMC free article] [PubMed] [Google Scholar]
- 17.Quraishi SA, Camargo CA., Jr Vitamin D and chronic illness. J Restor Med. 2012;1:9–23. doi: 10.14200/jrm.2012.1.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick MF. Evidence-based D-bate on health benefits of vitamin D revisited. Dermatoendocrinol. 2012;4:183–90. doi: 10.4161/derm.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucidarme O, Messai E, Mazzoni T, et al. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36:1609–11. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360:1912–4. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 21.Venkatram S, Chilimuri S, Adrish M, et al. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15:R292. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39:671–7. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecchi A, Bonizzoli M, Douar S, et al. Vitamin D deficiency in septic patients at ICU admission is not a mortality predictor. Minerva Anestesiol. 2011;77:1184–9. [PubMed] [Google Scholar]
- 25.Kempker JA, Han JE, Tangpricha V, et al. Vitamin D and sepsis: An emerging relationship. Dermatoendocrinol. 2012;4:101–8. doi: 10.4161/derm.19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginde AA, Camargo CA, Jr, Shapiro NI. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad Emerg Med. 2011;18:551–4. doi: 10.1111/j.1553-2712.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro da Silva F, Machado MC. Antimicrobial peptides: clinical relevance and therapeutic implications. Peptides. 2012;36:308–14. doi: 10.1016/j.peptides.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 30.Gusmao-Flores D, Salluh JI, Chalhub RÁ, et al. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16:R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quraishi SA, Bittner EA, Blum L, et al. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42:1365–71. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu PT, Stenger S, Tang DH, et al. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 34.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 35.Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986;98:311–22. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 36.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15:625–34. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amrein K, Venkatesh B. Vitamin D and the critically ill patient. Curr Opin Clin Nutr Metab Care. 2012;15:188–93. doi: 10.1097/MCO.0b013e32834f0027. [DOI] [PubMed] [Google Scholar]
- 39.Leaf DE, Waikar SS, Wolf M, et al. Dysregulated mineral metabolism in patients with acute kidney injury and risk of adverse outcomes. Clin Endocrinol (Oxf) 2013;79:491–8. doi: 10.1111/cen.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–73. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Pascale G, Quraishi SA. Vitamin D status in critically ill patients: the evidence is now bioavailable! Crit Care. 2014;18:449. doi: 10.1186/cc13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 44.Scocchi M, Skerlavaj B, Romeo D, et al. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem. 1992;209:589–95. doi: 10.1111/j.1432-1033.1992.tb17324.x. [DOI] [PubMed] [Google Scholar]
- 45.Larrick JW, Michimasa H, Balint RF, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sørensen O, Arnljots K, Cowland JB, et al. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–803. [PubMed] [Google Scholar]
- 47.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 48.Nilsson MF, Sandstedt B, Sørensen O, et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and co-localizes with interleukin 6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 51.Sørensen O, Bratt T, Johnsen AH, et al. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J Biol Chem. 1999;274:22445–51. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- 52.Turner J, Cho Y, Dihn N-N, et al. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. J Antimicrob Chemother. 1998;42:2206–14. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagaoka I, Hirota S, Yomogida S, et al. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–9. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 54.Yang D, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophil, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tollin M, Bergman P, Svenberg T, et al. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides. 2003;24:523–30. doi: 10.1016/s0196-9781(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 56.Parameswaran N, Patial S. Tumor necrosis factor-a signaling in macrophages. Crit Rev Eukaryotic Gene Expression. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schouten M, Wiersinga WJ, Levi M, et al. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–45. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 58.Pittet D, Rangel-Frausto S, Li N, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21:302–9. doi: 10.1007/BF01705408. [DOI] [PubMed] [Google Scholar]
- 59.Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1β and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989;170:1627–33. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surbatovic M, Veljovic M, Jevdjic J, et al. Immunoinflammatory response in critically ill patients: severe sepsis and/or trauma. Mediators Inflamm. 2013;2013:362793. doi: 10.1155/2013/362793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chai Z, Gatti S, Toniatti C, et al. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1β: a study on IL- 6-deficient mice. J Exp Med. 1996;183:311–16. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 63.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–32. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 64.Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. The Lancet. 2004;363:203–9. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 65.Okamura H, Kashiwamura S, Tsutsui H, et al. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 66.Calandra T, Baumgartner JD, Grau GE, et al. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-α, and interferon-γ in the serum of patients with septic shock. J Infect Dis. 1990;161:982–7. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 67.Flohé SB, Agrawal H, Flohé, M, et al. Diversity of interferon γ and granulocyte-macrophage colony-stimulating factor in restoring immune dysfunction of dendritic cells and macrophages during polymicrobial sepsis. Mol Med. 2008;14:247–56. doi: 10.2119/2007-00120.Flohe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leentjens L, Kox M, Koch RM, et al. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled randomized pilot study. Am J Respir Crit Care Med. 2012;186:838–45. doi: 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 69.Malefyt RDW, Abrams J, Bennett B, et al. Interleukin 10(IL-10) inhibits cytokine synthesis by humanmonocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiorentino DF, Zlotnik A, Mosmann TR. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 71.Latifi SQ, O'Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–6. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manley MO, O'Riordan MA, Levine AD, et al. Interleukin 10 extends the effectiveness of standard therapy during late sepsis with serum interleukin 6 levels predicting outcome. Shock. 2005;23:521–6. [PubMed] [Google Scholar]
- 73.Leaf DE, Raed A, Donnino MW, et al. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–41. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amrein K, Schnedl C, Holl A, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–30. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 75.Rousseau AF, Damas P, Janssens M, et al. Critical care and vitamin D status assessment: what about immunoassays and calculated free 25OH-D? Clin Chim Acta. 2014;437:43–7. doi: 10.1016/j.cca.2014.07.007. [DOI] [PubMed] [Google Scholar]


