Abstract
Objectives
To describe unique features of neurocritical illness that are relevant to provision of high-quality palliative care; To discuss key prognostic aids and their limitations for neurocritical illnesses; To review challenges and strategies for establishing realistic goals of care for patients in the neuro-ICU; To describe elements of best practice concerning symptom management, limitation of life support, and organ donation for the neurocritically ill.
Data Sources
A search of Pubmed and MEDLINE was conducted from inception through January 2015 for all English-language articles using the term “palliative care,” “supportive care,” “end-of-life care,” “withdrawal of life-sustaining therapy,” “limitation of life support,” “prognosis,” or “goals of care” together with “neurocritical care,” “neurointensive care,” “neurological,” “stroke,” “subarachnoid hemorrhage,” “intracerebral hemorrhage,” or “brain injury.”
Data Extraction and Synthesis
We reviewed the existing literature on delivery of palliative care in the neurointensive care unit setting, focusing on challenges and strategies for establishing realistic and appropriate goals of care, symptom management, organ donation, and other considerations related to use and limitation of life-sustaining therapies for neurocritically ill patients. Based on review of these articles and the experiences of our interdisciplinary/interprofessional expert Advisory Board, this report was prepared to guide critical care staff, palliative care specialists, and others who practice in this setting.
Conclusions
Most neurocritically ill patients and their families face the sudden onset of devastating cognitive and functional changes that challenge clinicians to provide patient-centered palliative care within a complex and often uncertain prognostic environment. Application of palliative care principles concerning symptom relief, goal setting, and family emotional support, will provide clinicians a framework to address decision-making at a time of crisis that enhances patient/family autonomy and clinician professionalism.
Keywords: neurocritical care, neuro ICU, intensive care unit, palliative care, end of life care
Introduction
Neurocritical illness, defined as critical illness primarily involving the brain, spinal cord, or neuromuscular system [1], is often a sudden, catastrophic event for patients and their families. Although advances in neurocritical care continue to improve outcomes, mortality rates for common conditions including intracerebral hemorrhage and anoxic brain injury range above 50%, and many patients never regain functional independence (Table 1). Patients often experience significant cognitive loss along with deterioration in quality of life.[2, 3] Even for those who survive without permanent disabilities, recovery from neurological injury can be prolonged and accompanied by physical and psychological distress for patients as well as practical and emotional burdens for families. For these reasons, palliative care, including communication about goals of care in relation to the patient's condition, prognosis, and preferences, is an important component of high quality care in the neurocritical care environment.
Table 1.
Common Adult Neuro ICU Diagnoses and Outcomes
| Condition | Incidence in U.S. (annual) | Mortality Rates (%) | Functional Independence at 3-12 months (%) | |
|---|---|---|---|---|
| In-Hospital | 30-Day | |||
| Traumatic Brain Injury | 2,500,000 [97] | 7.5% [98] | 21% [99] | 25-32%* [100-102] |
| Ischemic Stroke | 795,000 [97] | 4.3-70% [98, 103] | 16-23%[104, 105] | 50% [106-108] |
| Anoxic Brain Injury | 424,000 out-of-hospital cardiac arrests [109] | 52-90%^ [98, 110] | 25-40%** [111, 112] | 48-55%** [111, 112] |
| Status Epilepticus^^ | 200,000 [113] | 14-50% [114, 115] | 19-65% [116-118] | 42% [119] |
| Intracerebral Hemorrhage | 63,000 [120] | 30% [98] | 34-50% [120-123] | 12-39% [123] |
| Subarachnoid Hemorrhage | 25,000 [124] | 20-26% [98, 124-127] | 45% [98, 124-127] | 16-55% [128, 129] |
Among patients with severe traumatic brain injury
Overall 90% mortality including those who do not survive to hospital admission [109]
Among patients who underwent targeted temperature management. Mortality rates are higher and functional outcome worse in patients with PEA/asystole arrest compared to Vfib/Vtach arrest.
Patients with refractory status epilepticus (continued seizures after two anti-epileptic drugs have been administered) have higher mortality rates and worse functional outcomes.
Palliative care focuses on relief of symptoms, effective communication about goals of care, alignment of treatment with patient preferences, family support, and planning for transitions.[4, 5] Whereas hospice or end of life care is for patients approaching death, palliative care is appropriate in the context of any serious illness, regardless of stage or prognosis, and is optimally provided together with, not in lieu of, disease-directed or life-prolonging treatment. Palliative care is an interprofessional specialty, but also an approach for all clinicians caring for seriously ill patients who are expected to provide excellent “generalist”-level palliative care.[6]
In this article, The IPAL-ICU (Improving Palliative Care in the ICU) Project Advisory Board brings interdisciplinary and interprofessional expertise together to address challenges and strategies regarding prognostication, communication, and decision making for the neurocritical care patient and family.
Onset and Trajectory of Neurocritical Illness
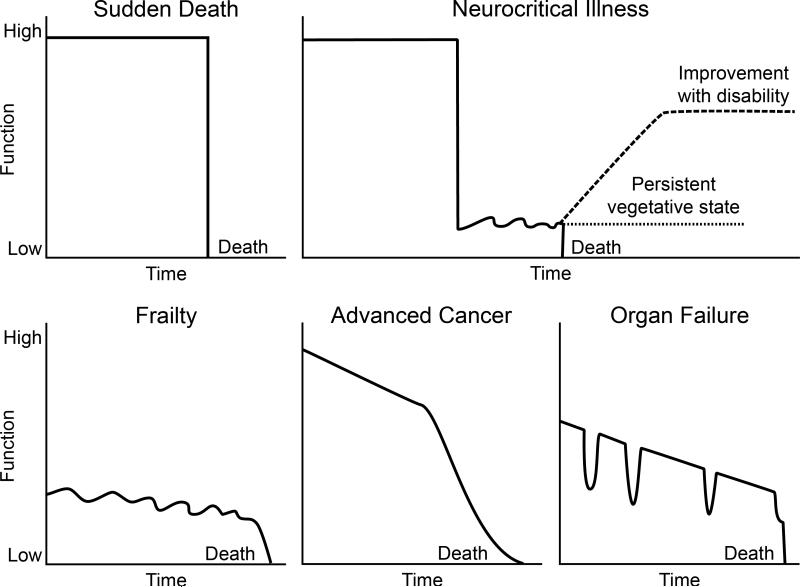
The onset of neurocritical illness is usually abrupt and follows a trajectory that is distinct from that of other patients with life-threatening illness (Figure 1).[7, 8] Neurocritically ill patients often face a sudden and total transformation from good health and independence to the prospect of death or serious and permanent disability. Over 70% of patients in the neuro-ICU have no premorbid symptoms and present with a precipitous loss of physical and cognitive function.[9, 10] Therefore, from the onset, clinicians face the challenge of helping families cope with the impact of an unexpected and devastating illness, making it more difficult to focus on the decision-making process.
Figure 1. Distinctive Trajectories of Neurocritical Illness.
This figure demonstrates trajectories for patients without limitation of life supporting therapies. Onset of neurocritical illness is often sudden, with precipitous decline from a normal baseline. However, most neurocritically ill patients do not progress to cardiovascular death or brain death, but survive with disability.
Decision-making is further complicated by the unpredictable and extended course of many forms of neurocritical illness. Often, patients do not progress to death (by cardiovascular or brain-based criteria) after neurological injury, but instead either improve slowly over several months or stagnate in a severely disabled state. Most deaths among the neurocritically ill occur after limitation of life-sustaining therapies. Nationally, 13.5% -15% of patients in neuro-ICUs have life-sustaining therapies withheld or withdrawn, and limitation of such therapies precedes up to 61% of all deaths in the neuro-ICU.[9-11] In those who do not die, recovery is prolonged. Brain-injured patients make their maximal spontaneous recovery over 3-6 months, though improvement can continue over the ensuing months with aggressive rehabilitation.[12-15] Therefore, neurological changes that would affect decision-making often occur well after the ICU and hospital stay, necessitating the use of prognostic scales that can assist in early discussions about goals of care.
Prognosis: Tools and Limitations
For some forms of neurocritical illness, specific clinical and radiographic features are predictive of poor neurological outcome across a number of studies (Table 2).[16] However, these predictors were derived primarily from observational studies, refer to a variety of time frames as outcomes, and have other limitations as outlined below. In general, the loss of brainstem reflexes, with radiographic confirmation of destruction of the brainstem or diffuse cortical infarction, is incompatible with meaningful functional recovery. Though survival is possible, the anticipated quality of life is often deemed unacceptable. In circumstances where the prognosis is less certain, it is important for clinicians to know the strengths and weaknesses of existing outcome data and prognostic models. Addressing uncertainty is an important part of communicating with patients and families to establish realistic goals of care.
Table 2.
Conditions that are Predictive of Poor Neurological Outcome [16]
| Neurological Insult | Features Predictive of Poor Prognosis | Time frame of outcome measurement | Level of Evidence |
|---|---|---|---|
| Hemispheric Ischemic Stroke* | Coma with loss of pontomesencephalic brainstem reflexes [130] Midline shift of the pineal gland >4 mm within 48 hours of onset [131] Age >60 years [103, 132] |
14 days-6 months | Prospective, observational and randomized controlled trials |
| Cerebellar Ischemic Stroke | Coma after decompressive neurosurgery[133] | 3 months | Prospective, observational |
| Lobar Intracerebral Hemorrhage | Coma with extensor posturing and absent pontomesencephalic brainstem reflexes [134-137] Coma with midline septum pellucidum shift >6 mm within 48 hours of onset [136] |
1-15 months | Prospective and retrospective, observational |
| Deep Basal Ganglion Intracerebral Hemorrhage | Coma with hydrocephalus and bleed volume > 60 mL [135, 138-142] | 1-3 months | Prospective and retrospective, observational |
| Pontine Hemorrhage | Coma with hyperthermia and tachycardia Coma with hemorrhage extension into the midbrain or thalamus and acute hydrocephalus[143, 144] |
3-12 months | Retrospective, observational |
| Cerebellar Hemorrhage | Absent oculocephalic reflexes with hydrocephalus Absent corneal reflexes [145-147] |
3 months | Retrospective, observational |
| Aneurysmal Subarachnoid Hemorrhage | Persistent deep coma or Hunt Hess grade 5 after attempts to lower ICP [129] | 12 months | Prospective, observational |
The risk of poor outcome in patients ≤60 years old with large hemispheric stroke is attenuated by decompressive hemicraniectomy. Mortality is reduced from 71% to 22% with hemicraniectomy versus medical management (P<0.0001) and the chances of moderate disability (able to walk without assistance or better) at 12 months was 43% with hemicraniectomy versus 21% without (P=0.014).[103]
A variety of prognostic scales offer useful outcome estimates for common neuro-ICU conditions (Table 3). The Glasgow Coma Scale [17] was developed for patients with traumatic brain injury (TBI). This scale (assessing eye opening, motor response, and verbal response, with lower scores reflecting more severe injury) is often used for serial abbreviated neurological exams and provides a globally recognized standard in neurological assessment. The GCS is a highly reliable predictor of in-hospital mortality, but has limited use for predicting long-term functional outcome of survivors. This scale has been applied across a wider spectrum of neurological diagnoses, including intracranial hemorrhage.[18] The NIH Stroke Scale is one of the most reliable instruments to predict outcome after ischemic stroke.[19] In North America, the Hunt-Hess Grade [20] for subarachnoid hemorrhage (SAH) is highly correlated with mortality and functional outcome. The Intracerebral Hemorrhage (ICH) [18] and FUNC [21] scores are used to predict mortality and functional outcomes, respectively, after intracerebral hemorrhage. These scales were developed to estimate the most likely outcome, though application on an individual level is more complex.
Table 3.
Selected Prognostic Scales Commonly Used in Neurocritical Illness
| Condition | Prognostic Scale | Scoring | Outcome Measure(s) | Pros and Cons |
|---|---|---|---|---|
| Traumatic Brain Injury | Glasgow Coma Scale[17] | 3 (worst)-15 (best) | Mortality, functional outcome | Widely used and simple, but the verbal score cannot be assessed in intubated patients; and brainstem reflexes and breathing patterns are not assessed as part of the GCS. |
| FOUR Score[90] (Full Outline of Unresponsiveness) |
0 (worst)- 16 (best) | In-hospital mortality | Has good intra- and inter-rater reliability and distinguishes among patients with the lowest GCS scores. Not widely used, and predicts only mortality, not functional outcome. | |
| Marshall Classification of Head Injury on Head Computed tomography[91] | I-VI | Intracranial Pressure, functional outcome | Widely used and has been found to predict increased intracranial pressure and outcome, but focuses primarily on CT findings and does not incorporate exam or other prognostic factors. | |
| Subarachnoid Hemorrhage | Hunt-Hess Grade[20] | I (best)-V (worst) | Mortality, functional outcome | Commonly used in the U.S., the Hunt-Hess grade is one of the strongest predictors of outcome after subarachnoid hemorrhage. It does not distinguish well between moderately injured grade 3 patients. |
| World Federation of Neurological Surgeons Scale[94] | 1 (best)-5 (worst) | Mortality, functional outcome | Commonly used in Canada and Europe, WFNS combines the GCS score with the presence or absence of a major neurological deficit. It is similar to Hunt-Hess scale in predicting outcome.[148] Does not distinguish outcome well among grade III patients and there is variable application of what constitutes a “major neurological deficit”. | |
| Intracerebral Hemorrhage | ICH Score[18] | 0 (best)- 6 (worst) | Mortality | Widely used and simple scoring system. Focuses on mortality only and confounded by withdrawal. Not validated in a separate cohort. |
| FUNC Score[21] | 0 (worst)-11 (best) | Functional Outcome | Incorporates premorbid cognitive function and strongly predicts long term functional outcome. In multiple cohorts, no patient with a FUNC score ≤4 achieved functional independence, while >80% of patients with a FUNC score of 11 were functionally independent at 3-months. Not widely used. | |
| Anoxic Brain Injury | AAN prognostic guideline[149]* | Poor outcome predicted by: Myoclonus status epilepticus (24 hours) Absent SSEP N20 bilaterally (24-72 hours) NSE>33 μg/L (24-72 hours) Exam with absent pupil or corneal responses; extensor or no motor response (72 hours) |
Mortality, functional outcome | Provides a time based guideline for prognostication with low false positive rates at each step. Does not account for the improved outcomes with hypothermia/induced normothermia. Guidelines are nearly a decade old.[149] |
| Spinal Cord Injury | American Spinal Injury Association Scale (ASIA)[96] | A (worst)- E (best) | Motor and Sensory Function | The ASIA scale was not originally developed as a prognostic scale, but does correlate with functional outcome. [96] |
Applies to patients who have not undergone therapeutic hypothermia/induced normothermia.
ICH=intracerebral hemorrhage; AAN=American Academy of Neurology; SSEP=median somatosensory evoked potentials; NSE=neuronal specific enolase
Impact of withdrawing life-sustaining treatment
Most studies describing the outcome of patients with severe brain injury have included patients from whom life-sustaining therapy was withheld or withdrawn. Yet, studies of ICH, SAH and TBI patients have found that an early “do not attempt resuscitation” (DNAR or DNR) directive and/or withdrawal of life-sustaining treatment as much as doubled the short- and long-term mortality, even after adjusting for known predictors of mortality (age, gender, ethnicity, Glasgow Coma Scale, ICH volume, intraventricular hemorrhage and infratentorial hemorrhage).[22-26] Thus, reliance on existing mortality data for prognostication can give rise to a “self-fulfilling prophecy” and perpetuate high mortality rates. Inclusion of patients undergoing early limitation of life support can confound the development of new prognostic models and hamper clinical trials to develop life-saving interventions for neurocritically ill patients. However, it is ethically important to offer families the option of withdrawal of life-support in keeping with the patient's right to autonomy and right to make an informed decision. A possible approach to this conundrum would be to use functional outcomes rather than mortality as primary endpoints. The development of new technologies and methodologies to predict outcome [27] after neurological injury is essential to improving the design of clinical trials and strengthening the ability of clinicians and families to make informed decisions about appropriate goals of care.
Model Characteristics
Models that predict functional outcome and quality of life, rather than mortality alone, would be more valuable for both clinicians and families. However, existing functional outcome scores (such as the Glasgow Outcome Score [28] or the modified Rankin Score [29, 30]) are heavily weighted toward motor ability and do not take into account other important outcomes such as cognitive and emotional function and quality of life, which are of utmost importance to patients and families.[31, 32] Prediction models for quality of life after neurocritical care have not been robustly developed, and proxy assessment tools to measure quality of life are scant and not well validated. Many studies use dichotomized outcomes, with arbitrary and varying cut-points that may not be meaningful for patients and their families. For example, the modified Rankin Scale (mRS) [29, 30], which ranges from 0 (no symptoms) to 6 (death), is variably dichotomized as 0-3 (0=no symptoms, 3=moderate disability requiring some help but able to walk without assistance) versus 4-6 (4=moderately severe disability; unable to walk without assistance or attend to bodily needs, 6=death), or as 0-1 (0=no symptoms, 1=no significant disability despite symptoms) versus 2-6 (2=slight disability but able to carry out own affairs, 6=death). Depending on the cut-point used, study results may significantly differ. In addition, trials yielding data used to generate prognostic scales often exclude the most severely affected patients, for whom little data are then available regarding the impact of treatments. Finally, models used for prognostication project what happens to a population on average; they cannot predict the outcome of any given individual. Adjustment for case-mix and the use of patient or family-reported outcomes in future studies may help improve prognostication, but, importantly, such models will always suffer from this inherent limitation.[33, 34] Models that predict functional recovery are also limited by the challenges in predicting an individual patient's ability to adapt (“response shift”) to a neurologic deficit that the patient has not yet experienced, as well as reframing or changing perceptions of quality of life that can occur over time.[35, 36]
Models Based on Outdated Practice or Lacking Adequate Validation
Older prognostication tools may reflect outdated medical and surgical practices that have evolved to offer patients a chance of better neurological recovery.[37] In addition, certain commonly used scales have not been validated or rigorously tested in different cohorts.[38]
Practitioner Variability
Survey results suggest that many physicians are both overly pessimistic and inaccurate in predicting outcome, particularly within the first 72 hours of neurocritical illness.[22, 39] There is also significant variation in physician perception of prognosis in case-based scenarios.[40] In addition, early medical and surgical interventions in many neurocritical illnesses can substantially alter a patient's clinical course. In recognition of these and other factors, the American Heart Association together with the American Stroke Association recommends that new DNR orders be deferred and full intensive care therapy be administered after ICH at least until the second day of hospitalization.[41] An initial trial of full intensive care therapy is also generally appropriate for the first 24-72 hours in other types of neurocritical illness, unless the patient has a condition outlined in Table 2 or clearly expressed a different preference through advance care planning. A longer period of observation would be appropriate when functional recovery is particularly difficult to predict and may continue over weeks to months, as for a young patient with traumatic brain injury.
Conversely, some physicians are overly optimistic when discussing prognosis with patients and families.[42] The motivation for this optimism may be to maintain hope for recovery, and/or avoid emotionally-laden encounters with patients or surrogates. Physicians also experience feelings of professional failure that make it difficult to acknowledge that the patient faces almost certain death or devastating neurologic impairment.[43] Although hope may help patients and surrogates cope with a serious illness, false hope tends to undermine the clinician-patient relationship, as well as delay appropriate decision-making.[44]
Public perceptions of recovery
Patients’ and families’ perceptions of neurocritical illness may be influenced by portrayals in film and other media that distort the reality of coma, persistent vegetative state, or minimally conscious state and tend to exaggerate the recovery prospects. In a review of 30 movies between 1970 and 2004, only two were found to represent coma realistically and 60% of coma patients portrayed in films awoke suddenly, even after a prolonged period of time, with intact cognition and physical function.[45] Although 15-50% of patients with persistent vegetative state regain consciousness over one year, depending on the mechanism of injury, the majority of these patients remain moderately-severely disabled and only 7% make a functional recovery, defined as mobile, able to communicate, and perform activities of daily living.[46, 47] Overall, the life expectancy for patients in persistent vegetative state is 2-5 years.[48] Patients with minimally conscious state (defined by intermittent command following and/or some ability to interact with their environment) have better long term outcomes than those with persistent vegetative state, but still, most remain disabled and dependent.[49] “Medically-induced coma” has a very different prognosis for recovery than coma resulting from illness or injury, but this distinction is frequently blurred in the media and poorly understood by the public.[50] To address potential misperceptions, clinicians need to explore the family's understanding of the nature and likely outcomes of the patient's condition, focus on the unique clinical circumstances, and endeavor to bring the family's perceptions and expectations into closer alignment with medical realities. A public education effort addressing the realities of brain injury is needed.
Strategies for establishing goals of care
Integrating the Interdisciplinary, Interprofessional Team
The expertise of a variety of clinicians can contribute to optimal care. We recommend that the clinical team (including intensivists, neurologists, and neurosurgeons) huddle at each critical discussion juncture to present a common message to the family, particularly when presenting prognosis and the uncertainty surrounding it. Physical therapists and neuro-rehabilitation specialists may be able to provide specific insight into expected long-term disabilities and the recovery process. Specialists in palliative care can support both the primary team and the family to address difficult decisions and complex symptoms. Ideally, involvement of such specialists is not deferred until the patient is imminently dying, since they are most effective when engaged early enough to build a trusting relationship with the family. At the same time, basic, generalist-level palliative care, remains the responsibility of the primary team, and should be part of daily practice. Both “specialist” and “generalist” models of palliative care are effective and a combined approach may represent the optimal model.[51, 52]. Strategies for developing such models have been reviewed.[52] ICU staff can be trained to manage distressing symptoms and conduct interprofessional/interdisciplinary family meetings, while involvement of palliative care specialists can support the primary team, particularly in the most challenging situations, and provide continuity of care to the patient and family.[52] Bedside ICU nurses can offer significant contributions to communication about appropriate and realistic goals of care, especially when physician-nurse communication has been optimized.[53] Social work, psychology, ethics services, pastoral care, and case management services all have a role to play in helping families.
Timing of Goals of Care Discussions
Effective communication with patients and families balances honesty with empathy and hope [54], and proceeds in the context of the condition and prognosis as well as the values, goals and preferences of the patient.[55] From the time of admission, available prognostic information, and all indicated treatment options should be openly discussed, along with a review of prior advance care planning so that the patient's wishes are respected. For patients without an advance directive, it is reasonable to initiate a time-limited trial of full intensive care therapy, except in extreme circumstances (e.g., brain death, poor premorbid function, low probability of successful intervention, and factors listed in Table 2). Early intervention may directly alter the patient's prognosis.
Shared, value-based, decision-making
The patient's values, goals and preferences provide the touchstone for communication and decision-making. Whether or not the patient specifically articulated such wishes, shared decision-making about appropriate goals of care entails discussion of the patient's values, including the quality of life that the patient would find acceptable. Especially in the context of neurocritical illness, in which patients may survive but with significant loss of function and cognition, the scope of discussion between clinicians and families must extend beyond a patient's chances for survival, to a fuller conversation about achievable goals in relation to the patient's condition, prognosis, and values. In these discussions the focus must remain on the values of the patient, rather than concerns of the family, or views projected by the clinician. Table 4 outlines an approach that facilitates discussion of a patient's values as the basis for decision-making.[55] Putting the physician's prognostic estimate of neurological outcome together with the patient's goals and values allows for a process of shared decision-making to develop a plan of care (Figure 2).
Table 4.
Steps in Discussing Goals of Care [54, 150]
| Step 1 | Introduce | After ensuring a quiet setting where all participants can sit down, introduce the members of the clinical team and their roles. Ask family to introduce themselves and their relationship to the patient. |
| Step 2 | Empathize | Express empathy and acknowledge that this is a difficult time and a challenging conversation. If the family is too emotionally overwhelmed to absorb and use information, continue responding empathically to the emotion before presenting information and decisions. |
| Step 3 | Inquire, Inform, Process Emotional Reactions | Inquire into the family's current understanding of the patient's condition. Clarify any gaps in understanding and update the family. Use of brain images may be useful for receptive families. Use of lay-person terminology is essential (e.g. “bleeding in the brain”, “dead brain tissue”). Allow families time to absorb information and ask questions. |
| Step 4 | Understand the patient's values | Review advance care planning discussions and written advance directives, if available, with the family, and seek out information about patient and family values that can guide decision-making. |
| Step 5 | Present Prognosis | Present the medical team's assessment of the patient's most likely prognosis in terms of future cognitive and functional outcome, acknowledging limitations and uncertainty in prognostication. Acknowledge the resulting emotional reactions to this information. |
| Step 6 | Present Broad Care Options | Offer possible pathways of care that are clearly delineated. In the right context, care focused entirely on comfort should be presented as an alternative to continuation of intensive care therapies. This approach may become more acceptable over a series of iterative discussions. |
| Step 7 | Family Decision-Making | Given the clinician's best estimate of the patient's long-term cognitive/functional outcome, and understanding the patient's values, ask the family to reflect on how the patient, if able, would decide in the present circumstances. If the family is receptive, the clinician can offer a professional recommendation based on best medical evidence and experience. |
| Step 8 | Match Care Goals to Medical Plan | Adjust the care plan to match goals including review of current interventions, medications, and CPR/DNR status. Ask about specific goals of importance, such as living until an upcoming life event, or returning to home rather than a facility. |
| Step 9 | Reflection and Questions | Ask the family to summarize their understanding of the conversation. Reflect back what you hear the family saying and summarize. Allow time for questions and offer the family time to discuss and consider the options. |
| Step 10 | Follow-up and Document | Make yourself available for follow-up conversations and questions. Document the results of the meeting in the medical record. Discuss plans with team members not present for the meeting. |
Figure 2. Shared Decision Making in Goals of Care Discussions.
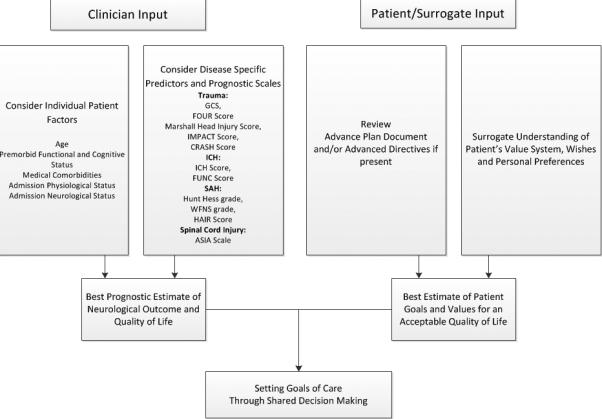
Both physicians and the patient/surrogate contribute to goals of care discussions. The physician should consider how individual patient factors and published prognostic scales contribute to the patient's overall prognosis. The patient/surrogate should contemplate the patient's advance directive and value system to determine if the best estimated prognosis is aligned with the patient's perception of an acceptable quality of life. Based on this balance, a shared-decision for appropriate goals of care can be reached.
GCS=Glasgow Coma Score[17]; FOUR Score[90]; Marshall Head Injury Score[91]; IMPACT score[92]; CRASH score [93]; ICH=intracerebral hemorrhage; ICH Score[18]; FUNC Score[21]; SAH=subarachnoid hemorrhage; Hunt Hess grade[20]; WFNS=World Federation of Neurosurgeons Score[94]; HAIR score[95]; ASIA= American Spinal Injury Association Scale[96]
Communicating Prognosis
When offering an estimated prognosis for neurological function, it can be helpful to communicate concrete skills, including activities of daily living, which the patient may or may not have a reasonable chance of achieving. For example, “the most likely outcome, should full, aggressive life support be applied, is that the patient will be completely dependent for all basic activities such as bathing, dressing and toileting, and likely require a machine to breathe, a feeding tube, and placement in a nursing home.” Or, “the patient will be weak on one side, and unable to walk, but still able to communicate and interact meaningfully with loved ones.” It is important to describe not only potential deficits, but also skills that the patient is likely to retain, since certain deficits may be acceptable based on the patient's values or prior expressed wishes.
Families often inquire about signs of neurological improvement that may alter prognosis. In general, for comatose patients, saccading (quick movements of both eyes in the same direction) to voice, tracking, and command-following are signs of improvement. One commonly misinterpreted interaction with the comatose patient is the grasp reflex. Since primitive reflexes can reemerge in the comatose state, families may believe that the patient is squeezing a loved one's hand purposefully. Careful explanation of reflexive movements is important for addressing family expectations, with recognition that the family may continue to interpret patients’ movements differently.[56]
Addressing Prognostic Limitations
The limitations of current prognostic models should not preclude clinicians from sharing an opinion on the patient's potential outcome. Communication that acknowledges the uncertainty of outcome predictions can actually build trust and is consistent with a shared-decision model of patient-clinician communication and with empirical data about patients’ and families’ interest in prognostic information, even if there is uncertainty.[57] In addition, communication can acknowledge that prognosis is not based on the admission neurological assessment alone, but may be altered by early treatment decisions, such as a decision to proceed with surgery. Besides consideration of disease-specific prognosis, clinicians should account for individual patient factors such as age, premorbid functional status, medical comorbidities and admission neurological and physiological status that interact to affect prognosis (Figure 2).[58] Some studies suggest that the combination of model-based prediction with expert clinician outcome estimates may be superior to either approach alone.[59]
Addressing family guilt
Surrogates may feel guilt, doubt, and regret about their decisions, especially when the patient was healthy prior to a sudden neurological catastrophe. Many surrogates carry these negative emotions for months to years.[60] To address and attempt to alleviate such distress, discussions may be framed in terms of the obligation to respect the patient's wishes, and the opportunity to help the patient preserve dignity when death is inevitable.[61] Intensive care support may only prolong the dying process, causing more suffering with the same eventual result.
Presenting Goals of Care Options
Communication about goals of care may be most effective when discussions are iterative, with continued discussion that takes account of the evolving clinical situation. Clear pathways of care, i.e., full life support including tracheotomy and feeding tube placement versus care focused exclusively on comfort (often referred to as “comfort measures only”), are the two most typical treatment approaches for neurocritically ill patients. Although some physicians may offer partial treatment options rather than directly addressing realistic goals of care, this approach can confuse the family, and partial treatment plans can lead to the same outcome as a “comfort measures only” approach, but with prolongation of the dying process and preventable distress. A time-limited trial of full intensive care with observation for improvement may be helpful when the outcome is unclear.[62] The use of approaches such as “no escalation of treatment,” which is another form of partial treatment, is more controversial.[63, 64] Families should be given adequate time to consider and modify goals of care, and to place increasing emphasis on palliative care at any stage in the patient's course.
Considerations in Withdrawal of Life-Sustaining Therapies
Symptom Management During Withdrawal of Life Support
The withdrawal of life-sustaining therapies should follow a written protocol to ensure attention to all key elements, including documentation of the goals of care and the resuscitation status.[65, 66] Consideration to family and visitor needs should be given when determining the timing of life support withdrawal. Although stroke and other neurocritically ill patients tend to have a lower symptom burden than other seriously ill patients,[67] pain, discomfort, and anxiety may cause distress even for a brain-injured patient. Patients with spinal cord injury, terminal neuromuscular disease, stroke, brain tumor, and other conditions may also suffer from thirst, dyspnea, fatigue, sleep deprivation, incontinence, depression, anxiety, delirium and emotional lability.[68] Because neuroICU patients are often unable to communicate their needs, clinicians must be especially vigilant for signs of discomfort.[69] Tachycardia and tachypnea may be the only manifestations of distress, although they are non-specific. Treatment of these symptoms in patients with neurocritical illness is similar to that in the general critical care population.[69]
Preparation for withdrawal of life-sustaining therapy includes having opioids available at the bedside prior to extubation to manage agonal breathing patterns and other signs of air hunger or distress. Anticholinergics (e.g., glycopyrrolate) can reduce tracheobronchial sections, and gurgling (death rattle) for some patients, although no large-scale, placebo-controlled study has been conducted to confirm this effect,[70] and death rattle may not be correlated with patient respiratory distress.[71] Anxiolytics and other sedatives should be immediately available to treat agitation or anxiety. Some ICUs use specific titration goals, such as increasing the dose of opioids and/or sedatives to maintain a heart rate less than 100 beats per minute or respiratory rate less than 20 breaths per minute. Such guidelines offer concrete guidance for nursing staff and may help prevent under-treatment of dyspnea and agitation.
Medications and interventions that do not offer symptom relief should be discontinued, including antibiotics, vasopressors/inotropes, antithrombotics, intravenous hydration, artificial feeding and patient monitors. Anti-epileptic medications may be continued since a seizure is considered to be an uncomfortable complication.
Long-acting sedatives
It is unnecessary to wait for a washout of long-acting sedative medications (e.g., barbiturates) prior to withdrawal of life-sustaining therapy. A prolonged delay to withdrawal in this context can be emotionally difficult for families and staff. The concept of autonomy supports a patient's ability to limit life support measures at any time, even if there is residual medication effect.[72] Withdrawal in the context of long-acting sedatives is not causing death, but is “allowing the patient to die” from the underlying illness.[73, 74] In addition, the “principle of double effect” supports the use of a therapy that is intended to provide symptom relief (such as pentobarbital and other long-acting sedatives for delirium or sleep disruption at end of life),[75-80] even if death is hastened as a secondary effect of the therapy.[81]
Organ Donation- Donation after Cardiac Death
Organ donation after cardiac death (DCD) and donation following brain death (death by neurological criteria [“DNC”]) are important considerations for neurocritically ill patients. The opportunity to save lives through organ donation can provide solace to some grieving families.[74, 82] Late or missed referrals to the organ procurement organization (OPO) deprive the family and patient of the opportunity to donate organs. In the United States, all hospitals are required to notify their assigned OPO when specific criteria are met such as loss of brainstem reflexes in a patient who may be approaching brain death, or consideration of withdrawal of life-sustaining therapies. Early notification is essential to allow time for OPO determination of donor suitability and for family decision-making. The decision to withdraw life-sustaining therapy should be made independently of discussions regarding organ or tissue donation, and withdrawal of life support should occur only after the family has had the opportunity to make an informed decision.
Following consent for DCD, extubation occurs in the operating room. The family should be offered the opportunity to be present and briefed on the organ recovery process, which entails antiseptic measures including prepping and draping prior to extubation. An OPO family counselor may be present in the operating room for additional family support. Valuable input may also be provided by a palliative care specialist/team [83], a social worker, and/or another clinician with appropriate training to support the family. DCD involves the use of some therapies (such as intravenous fluids, intravenous heparin, hemodynamic monitoring) that support the donation process.[74] To set appropriate expectations, the family should be informed that not all patients are suitable donors. If cardiopulmonary death does not occur after extubation (generally within an hour) and the OPO decides that the patient is no longer a suitable donor, the patient will be returned to the ICU or transition to a palliative care unit or other hospital bed for end-of-life care.[74]
Organ Donation: Death by Neurological Criteria
When brain death is expected and imminent, the use of aggressive therapies (such as osmotic therapy) may be futile, and lead to complications that can compromise organ donation. It is then reasonable to transition from “brain protective” strategies of care to “organ protective” strategies. While this approach still involves intensive medical management (e.g. treatment of hypotension, diabetes insipidus), it allows time for families to come to terms with the concept of brain death, and preserves organ homeostasis for possible donation.
Communicating the concept of death by neurologic criteria presents unique challenges as this concept is not accepted in certain cultures or religions.[84] Concrete communication, such as “brain death means the patient is declared dead,” is essential to avoid misunderstanding. In circumstances in which brain death is not accepted by the family, it is reasonable to continue mechanical ventilation for a limited time while supporting the family. Eliminating blood draws, electrolyte correction and vasopressor use and allowing the patient to progress to cardiopulmonary death may be necessary. When the patient/family has consented to donation, medical management may involve invasive diagnostic procedures to investigate donor suitability, and therapies aimed at restoring perfusion, such as hormonal replacement, and vasopressor support.[82]. Families should be counseled as to these possibilities by the OPO and supported throughout the donation process.
Time to death after palliative extubation
A common question raised by family members is, when will death occur following extubation? It is important to describe to the family what is likely to happen over the minutes, hours or days following extubation. For example: “the patient may not be awake, and may make sounds similar to snoring. We expect that the patient will drift into a deeper coma and not respond over the next few hours/days. Eventually the breaths will get further apart until they stop and death comes peacefully”. Expected timing of death is relevant in the context of DCD because cessation of cardiopulmonary activity within 60 minutes of extubation is a requirement for DCD in most states. In a multicenter study of comatose patients with irreversible brain injury undergoing palliative extubation, 46% died within 60 minutes. Major predictors of death within 60 minutes included: absent corneal reflex, absent cough, extensor or absent motor response and oxygenation index >3.0 (where oxygenation index= [FiO2 × mean airway pressure in cmH2O/PaO2 in torr] × 100).[85] Table 5 shows the DCD-N scoring system to predict the likelihood of cardiopulmonary arrest after withdrawal of life support. Other authors have produced similar results using equivalent models.[86] ICU specialist opinion regarding the probability of death within 60 minutes after withdrawal of life-sustaining therapy has been found to be very accurate along with variables such as pH, Glasgow Coma Score, spontaneous respiratory rate, PEEP level and systolic blood pressure.[87] Withdrawal of life sustaining therapies does not necessarily lead to imminent death. In studies based on the Nationwide Inpatient Sample, nearly 40% of patients with ischemic stroke who received thrombolysis and nearly 20% of patients with subarachnoid hemorrhage who underwent withdrawal of life support survived to hospital discharge. The discharge destination may be a long-term care facility or hospice. Additionally, length of stay and mean hospital costs were lower in patients who underwent withdrawal.[88, 89]
Table 5.
Prediction of Cardio-pulmonary Arrest After Withdrawal of Life Sustaining Therapy (DCD-N Score).[85]
| Component | Score |
|---|---|
| Cough | |
| Present | 0 |
| Absent | 2 |
| Corneal Reflex | |
| Present | 0 |
| Absent | 1 |
| Motor response | |
| Flexor or better | 0 |
| Extensor or absent | 1 |
| Oxygenation Index | |
| ≤3.0 | 0 |
| >3.0 | 1 |
| Total Score | Death within 60 minutes |
|---|---|
| 0 | 5% |
| 1 | 27% |
| 2 | 29% |
| 3 | 52% |
| 4 | 80% |
| 5 | 89% |
A score of ≥3 was associated with a 74% probability of death within 60 minutes, while a score of 0-2 was associated with a 77% probability of survival beyond 60 minutes.
Conclusions
Neurocritically ill patients and their families have needs that require integration of palliative care principles, practices, and services. Attention to the trajectory of neurocritical illness, utility and limitations of neuro-prognostic tools, and the public perceptions of coma and survival, can help inform effective communication and decision making. A shared, value-based decision making model for establishing goals of care, and special consideration of symptom management and family support during withdrawal of life-sustaining therapy can help ensure the delivery of high-quality palliative care to neurocritically ill patients.
Acknowledgments
Dr. Nelson received support for article research from the National Institutes of Health (NIH).Her institution received grant support from the National Institute on Aging (Academic Career Leadership Award). Dr. Campbell is employed by Wayne State University and received royalties from Wiley- Blackwell. Her institution received grant support from the National Institute of Nursing Research. Dr. Mosenthal received support for article research from the Heathcare Foundation of New Jersey. Dr. Puntillo is employed by University of California, San Francisco; received grant support from the Center for Health Quality and Innovation Quality Enterprise Risk Management (CHQIQERM) program, a joint venture of the University of California Center for Health Quality and Innovation and Office of Risk Services; and received royalties from Elsevier for the book, “Critical Care Nursing Secrets”. Dr. Lustbader provided expert testimony for MLMIC, PRI (Medical malpractice defense expert). Dr. Weiss’ institution received grant support from the National Institute on Aging (K07 Academic Career Leadership Award for Dr. Judith Nelson).
Financial Support: The IPAL-ICU Project was created with support from the National Institute on Aging (K07-034234 Academic Career Leadership Award to Dr. Nelson) and the Center to Advance Palliative Care.
Footnotes
Disclosures: None
Copyright form disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Jennifer A. Frontera, Cerebrovascular Center of the Neurological Institute, Cleveland Clinic.
J. Randall Curtis, University of Washington School of Medicine.
Judith E. Nelson, Icahn School of Medicine at Mount Sinai.
Margaret Campbell, Wayne State University.
Michelle Gabriel, VA Palo Alto Health Care System.
Ross M. Hays, University of Washington School of Medicine.
Anne C. Mosenthal, Rutgers New Jersey Medical School.
Colleen Mulkerin, Hartford Hospital.
Kathleen A. Puntillo, University of California, San Francisco.
Daniel E. Ray, Lehigh Valley Health Network.
Rick Bassett, St. Luke's Hospital.
Renee D. Boss, John Hopkins University School of Medicine.
Dana R. Lustbader, ProHEALTH, Hofstra North Shore-Long Island Jewish Health System.
Karen J. Brasel, Oregon Health & Science University.
Stefanie P. Weiss, Icahn School of Medicine at Mount Sinai.
REFERENCES
- 1. [ http://neurocriticalcare.org/patients-families/what-neurocritical-care]
- 2.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. The New England journal of medicine. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 3.Fried TR, Van Ness PH, Byers AL, Towle VR, O'Leary JR, Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. Journal of general internal medicine. 2007;22(4):495–501. doi: 10.1007/s11606-007-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson JE, Puntillo KA, Pronovost PJ, Walker AS, McAdam JL, Ilaoa D, Penrod J. In their own words: patients and families define high-quality palliative care in the intensive care unit. Critical care medicine. 2010;38(3):808–818. doi: 10.1097/ccm.0b013e3181c5887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke EB, Curtis JR, Luce JM, Levy M, Danis M, Nelson J, Solomon MZ, Robert Wood Johnson Foundation Critical Care End-Of-Life Peer Workgroup M Quality indicators for endof-life care in the intensive care unit. Critical care medicine. 2003;31(9):2255–2262. doi: 10.1097/01.CCM.0000084849.96385.85. [DOI] [PubMed] [Google Scholar]
- 6.Unroe KT, Ersek M, Cagle J. The IOM Report on Dying in America: A Call to Action for Nursing Homes. Journal of the American Medical Directors Association. 2014 doi: 10.1016/j.jamda.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. Bmj. 2005;330(7498):1007–1011. doi: 10.1136/bmj.330.7498.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA : the journal of the American Medical Association. 2003;289(18):2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 9.Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Critical care medicine. 2001;29(9):1792–1797. doi: 10.1097/00003246-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Naidech AM, Bernstein RA, Bassin SL, Garg RK, Liebling S, Bendok BR, Batjer HH, Bleck TP. How patients die after intracerebral hemorrhage. Neurocritical care. 2009;11(1):45–49. doi: 10.1007/s12028-009-9186-z. [DOI] [PubMed] [Google Scholar]
- 11.Varelas PN, Abdelhak T, Hacein-Bey L. Withdrawal of life-sustaining therapies and brain death in the intensive care unit. Seminars in neurology. 2008;28(5):726–735. doi: 10.1055/s-0028-1105969. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Physical medicine and rehabilitation clinics of North America. 1999;10(4):887–906. [PubMed] [Google Scholar]
- 13.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68(19):1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 14.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Annals of neurology. 2008;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 15.Kong KH, Lee J. Temporal recovery of activities of daily living in the first year after ischemic stroke: A prospective study of patients admitted to a rehabilitation unit. NeuroRehabilitation. 2014;35(2):221–226. doi: 10.3233/NRE-141110. [DOI] [PubMed] [Google Scholar]
- 16.Wijdicks EF, Rabinstein AA. Absolutely no hope? Some ambiguity of futility of care in devastating acute stroke. Critical care medicine. 2004;32(11):2332–2342. [PubMed] [Google Scholar]
- 17.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2001;32(4):891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, Saver JL, Hernandez AF, Peterson ED, Fonarow GC, Schwamm LH. Risk score for in-hospital ischemic stroke mortality derived and validated within the Get With the Guidelines-Stroke Program. Circulation. 2010;122(15):1496–1504. doi: 10.1161/CIRCULATIONAHA.109.932822. [DOI] [PubMed] [Google Scholar]
- 20.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. Journal of neurosurgery. 1968;28(1):14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 21.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke; a journal of cerebral circulation. 2008;39(8):2304–2309. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 22.Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, Winn HR, Longstreth WT., Jr. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56(6):766–772. doi: 10.1212/wnl.56.6.766. [DOI] [PubMed] [Google Scholar]
- 23.Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocritical care. 2013;19(3):269–275. doi: 10.1007/s12028-013-9929-8. [DOI] [PubMed] [Google Scholar]
- 24.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, Garcia NM, Morgenstern LB. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68(20):1651–1657. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 25.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocritical care. 2013;19(3):347–363. doi: 10.1007/s12028-013-9925-z. [DOI] [PubMed] [Google Scholar]
- 26.Turgeon AF, Lauzier F, Simard JF, Scales DC, Burns KE, Moore L, Zygun DA, Bernard F, Meade MO, Dung TC, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011;183(14):1581–1588. doi: 10.1503/cmaj.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [ http://www.tbi-prognosis.ca/]
- 28.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 29.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scottish medical journal. 1957;2(5):200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 30.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke; a journal of cerebral circulation. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 31.Benejam B, Sahuquillo J, Poca MA, Frascheri L, Solana E, Delgado P, Junque C. Quality of life and neurobehavioral changes in survivors of malignant middle cerebral artery infarction. Journal of neurology. 2009;256(7):1126–1133. doi: 10.1007/s00415-009-5083-9. [DOI] [PubMed] [Google Scholar]
- 32.Weil AG, Rahme R, Moumdjian R, Bouthillier A, Bojanowski MW. Quality of life following hemicraniectomy for malignant MCA territory infarction. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2011;38(3):434–438. doi: 10.1017/s0317167100011835. [DOI] [PubMed] [Google Scholar]
- 33.Teale E, Young J, Dennis M, Sheldon T. Predicting patient-reported stroke outcomes: a validation of the six simple variable prognostic model. Cerebrovascular diseases extra. 2013;3(1):97–102. doi: 10.1159/000351142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehlenbach WJ, Cooke CR. Making ICU prognostication patient centered: is there a role for dynamic information? Critical care medicine. 2013;41(4):1136–1138. doi: 10.1097/CCM.0b013e31827c03eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis CG, Egan M, Dubouloz CJ, Kubina LA, Kessler D. Adaptation following stroke: a personal projects analysis. Rehabilitation psychology. 2013;58(3):287–298. doi: 10.1037/a0033400. [DOI] [PubMed] [Google Scholar]
- 36.Korfage IJ, de Koning HJ, Essink-Bot ML. Response shift due to diagnosis and primary treatment of localized prostate cancer: a then-test and a vignette study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2007;16(10):1627–1634. doi: 10.1007/s11136-007-9265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. Bmj. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- 38.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. Bmj. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 39.Goettler CE, Waibel BH, Goodwin J, Watkins F, Toschlog EA, Sagraves SG, Schenarts PJ, Bard MR, Newell MA, Rotondo MF. Trauma intensive care unit survival: how good is an educated guess? The Journal of trauma. 2010;68(6):1279–1287. doi: 10.1097/TA.0b013e3181de3b99. discussion 1287-1278. [DOI] [PubMed] [Google Scholar]
- 40.Turgeon AF, Lauzier F, Burns KE, Meade MO, Scales DC, Zarychanski R, Moore L, Zygun DA, McIntyre LA, Kanji S, et al. Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Critical care medicine. 2013;41(4):1086–1093. doi: 10.1097/CCM.0b013e318275d046. [DOI] [PubMed] [Google Scholar]
- 41.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr., Greenberg SM, Huang JN, MacDonald RL, Messe SR, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2010;41(9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christakis N. Death Foretold: Prophecy and Prognosis in Medical Care. U Chicago Press; 1999. [Google Scholar]
- 43.Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. Jama. 2001;286(23):3007–3014. doi: 10.1001/jama.286.23.3007. [DOI] [PubMed] [Google Scholar]
- 44.Brody H. Hope. JAMA : the journal of the American Medical Association. 1981;246(13):1411–1412. [PubMed] [Google Scholar]
- 45.Wijdicks EF, Wijdicks CA. The portrayal of coma in contemporary motion pictures. Neurology. 2006;66(9):1300–1303. doi: 10.1212/01.wnl.0000210497.62202.e9. [DOI] [PubMed] [Google Scholar]
- 46.Luaute J, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, Boisson D, Fischer C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010;75(3):246–252. doi: 10.1212/WNL.0b013e3181e8e8df. [DOI] [PubMed] [Google Scholar]
- 47.Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75(3):239–245. doi: 10.1212/WNL.0b013e3181e8e8cc. [DOI] [PubMed] [Google Scholar]
- 48.Practice parameters: assessment and management of patients in the persistent vegetative state (summary statement). The Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1995;45(5):1015–1018. doi: 10.1212/wnl.45.5.1015. [DOI] [PubMed] [Google Scholar]
- 49.Giacino JT. The vegetative and minimally conscious states: consensus-based criteria for establishing diagnosis and prognosis. NeuroRehabilitation. 2004;19(4):293–298. [PubMed] [Google Scholar]
- 50.Wijdicks EF, Wijdicks MF. Coverage of coma in headlines of US newspapers from 2001 through 2005. Mayo Clinic proceedings. 200681(10):1332–1336. doi: 10.4065/81.10.1332. [DOI] [PubMed] [Google Scholar]
- 51.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. The New England journal of medicine. 2013;368(13):1173–1175. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 52.Nelson JE, Bassett R, Boss RD, Brasel KJ, Campbell ML, Cortez TB, Curtis JR, Lustbader DR, Mulkerin C, Puntillo KA, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: a report from the IPAL-ICU Project (Improving Palliative Care in the ICU). Critical care medicine. 2010;38(9):1765–1772. doi: 10.1097/CCM.0b013e3181e8ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson JE, Cortez TB, Curtis JR, Lustbader DR, Mosenthal AC, Mulkerin C, Ray DE, Bassett R, Boss RD, Brasel KJ, et al. Integrating Palliative Care in the ICU: The Nurse in a Leading Role. Journal of hospice and palliative nursing : JHPN : the official journal of the Hospice and Palliative Nurses Association. 2011;13(2):89–94. doi: 10.1097/NJH.0b013e318203d9ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Back A AR, Tulsky J. “Mastering Communication With Seriously Ill Patients.”. Cambridge University Press; New York: 2009. [Google Scholar]
- 55.Scheunemann LP, Arnold RM, White DB. The facilitated values history: helping surrogates make authentic decisions for incapacitated patients with advanced illness. American journal of respiratory and critical care medicine. 2012;186(6):480–486. doi: 10.1164/rccm.201204-0710CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engelberg RA, Wenrich MD, Curtis JR. Responding to families' questions about the meaning of physical movements in critically ill patients. Journal of critical care. 2008;23(4):565–571. doi: 10.1016/j.jcrc.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Apatira L, Boyd EA, Malvar G, Evans LR, Luce JM, Lo B, White DB. Hope, truth, and preparing for death: perspectives of surrogate decision makers. Annals of internal medicine. 2008;149(12):861–868. doi: 10.7326/0003-4819-149-12-200812160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McIntyre A, Mehta S, Aubut J, Dijkers M, Teasell RW. Mortality among older adults after a traumatic brain injury: a meta-analysis. Brain injury : [BI] 2013;27(1):31–40. doi: 10.3109/02699052.2012.700086. [DOI] [PubMed] [Google Scholar]
- 59.Knaus WA, Harrell FE, Jr., Lynn J, Goldman L, Phillips RS, Connors AF, Jr., Dawson NV, Fulkerson WJ, Jr., Califf RM, Desbiens N, et al. The SUPPORT prognostic model. Objective estimates of survival for seriously ill hospitalized adults. Study to understand prognoses and preferences for outcomes and risks of treatments. Annals of internal medicine. 1995;122(3):191–203. doi: 10.7326/0003-4819-122-3-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 60.Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Annals of internal medicine. 2011;154(5):336–346. doi: 10.7326/0003-4819-154-5-201103010-00008. [DOI] [PubMed] [Google Scholar]
- 61.Cook D, Rocker G. Dying with dignity in the intensive care unit. The New England journal of medicine. 2014;370(26):2506–2514. doi: 10.1056/NEJMra1208795. [DOI] [PubMed] [Google Scholar]
- 62.Quill TE, Holloway R. Time-limited trials near the end of life. JAMA : the journal of the American Medical Association. 2011;306(13):1483–1484. doi: 10.1001/jama.2011.1413. [DOI] [PubMed] [Google Scholar]
- 63.Curtis JR, Rubenfeld GD. “No escalation of treatment” as a routine strategy for decision-making in the ICU: con. Intensive care medicine. 2014;40(9):1374–1376. doi: 10.1007/s00134-014-3421-6. [DOI] [PubMed] [Google Scholar]
- 64.Thompson DR. “No escalation of treatment” as a routine strategy for decision-making in the ICU: pro. Intensive care medicine. 2014;40(9):1372–1373. doi: 10.1007/s00134-014-3422-5. [DOI] [PubMed] [Google Scholar]
- 65.Treece PD, Engelberg RA, Crowley L, Chan JD, Rubenfeld GD, Steinberg KP, Curtis JR. Evaluation of a standardized order form for the withdrawal of life support in the intensive care unit. Critical care medicine. 2004;32(5):1141–1148. doi: 10.1097/01.ccm.0000125509.34805.0c. [DOI] [PubMed] [Google Scholar]
- 66. [ http://www.capc.org/ipal/ipal-icu/improvement-and-clinical-tools]
- 67.Holloway RG, Ladwig S, Robb J, Kelly A, Nielsen E, Quill TE. Palliative care consultations in hospitalized stroke patients. Journal of palliative medicine. 2010;13(4):407–412. doi: 10.1089/jpm.2009.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holloway RG, Arnold RM, Creutzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, Rabinstein AA, Saposnik G, Sheth KN, Zahuranec DB, et al. Palliative and End-of-Life Care in Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2014 doi: 10.1161/STR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 69.Puntillo K, Nelson JE, Weissman D, Curtis R, Weiss S, Frontera J, Gabriel M, Hays R, Lustbader D, Mosenthal A, et al. Palliative care in the ICU: relief of pain, dyspnea, and thirst--a report from the IPAL-ICU Advisory Board. Intensive care medicine. 2014;40(2):235–248. doi: 10.1007/s00134-013-3153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wee B, Hillier R. Interventions for noisy breathing in patients near to death. The Cochrane database of systematic reviews. 2008;(1):CD005177. doi: 10.1002/14651858.CD005177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campbell ML, Yarandi HN. Death rattle is not associated with patient respiratory distress: is pharmacologic treatment indicated? Journal of palliative medicine. 2013;16(10):1255–1259. doi: 10.1089/jpm.2013.0122. [DOI] [PubMed] [Google Scholar]
- 72.Luce JM, Prendergast TJ. The changing nature of death in the ICU. In: Managing death in the intensive care unit: the transition from cure to comfort. 19-29. Oxford University Press; 2001. [Google Scholar]
- 73.Levine DZ, Truog RD. Discontinuing immunosuppression in a child with a renal transplant: are there limits to withdrawing life support? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;38(4):901–915. doi: 10.1053/ajkd.2001.27855. [DOI] [PubMed] [Google Scholar]
- 74.Frontera JA. How I manage the adult potential organ donor: donation after cardiac death (part 2). Neurocritical care. 2010;12(1):111–116. doi: 10.1007/s12028-009-9294-9. [DOI] [PubMed] [Google Scholar]
- 75.Kerr CW, Luczkiewicz DL, Holahan T, Milch R, Hang PC. The use of pentobarbital in cases of severe delirium: a case series. The American journal of hospice & palliative care. 2014;31(1):105–108. doi: 10.1177/1049909112474111. [DOI] [PubMed] [Google Scholar]
- 76.Greene WR, Davis WH. Titrated intravenous barbiturates in the control of symptoms in patients with terminal cancer. Southern medical journal. 1991;84(3):332–337. doi: 10.1097/00007611-199103000-00009. [DOI] [PubMed] [Google Scholar]
- 77.Truog RD, Berde CB, Mitchell C, Grier HE. Barbiturates in the care of the terminally ill. The New England journal of medicine. 1992;327(23):1678–1682. doi: 10.1056/NEJM199212033272311. [DOI] [PubMed] [Google Scholar]
- 78.Cherny NI, Portenoy RK. Sedation in the management of refractory symptoms: guidelines for evaluation and treatment. Journal of palliative care. 1994;10(2):31–38. [PubMed] [Google Scholar]
- 79.Rousseau P. Terminal sedation in the care of dying patients. Archives of internal medicine. 1996;156(16):1785–1786. doi: 10.1001/archinte.156.16.1785. [DOI] [PubMed] [Google Scholar]
- 80.Smith GP., 2nd Terminal sedation as palliative care: revalidating a right to a good death. Cambridge quarterly of healthcare ethics : CQ : the international journal of healthcare ethics committees. 1998;7(4):382–387. doi: 10.1017/s0963180198704074. [DOI] [PubMed] [Google Scholar]
- 81.J. M . Law and Bioethics: An Introduction. Georgetown University Press; Washington, D.C.: 2001. [Google Scholar]
- 82.Frontera JA, Kalb T. How I manage the adult potential organ donor: donation after neurological death (part 1). Neurocritical care. 2010;12(1):103–110. doi: 10.1007/s12028-009-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelso CM, Lyckholm LJ, Coyne PJ, Smith TJ. Palliative care consultation in the process of organ donation after cardiac death. Journal of palliative medicine. 2007;10(1):118–126. doi: 10.1089/jpm.2006.0118. [DOI] [PubMed] [Google Scholar]
- 84.Da Silva IR, Frontera JA. Worldwide Barriers to Organ Donation. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2014.3083. [DOI] [PubMed] [Google Scholar]
- 85.Rabinstein AA, Yee AH, Mandrekar J, Fugate JE, de Groot YJ, Kompanje EJ, Shutter LA, Freeman WD, Rubin MA, Wijdicks EF. Prediction of potential for organ donation after cardiac death in patients in neurocritical state: a prospective observational study. Lancet neurology. 2012;11(5):414–419. doi: 10.1016/S1474-4422(12)70060-1. [DOI] [PubMed] [Google Scholar]
- 86.de Groot YJ, Lingsma HF, Bakker J, Gommers DA, Steyerberg E, Kompanje EJ. External validation of a prognostic model predicting time of death after withdrawal of life support in neurocritical patients. Critical care medicine. 2012;40(1):233–238. doi: 10.1097/CCM.0b013e31822f0633. [DOI] [PubMed] [Google Scholar]
- 87.Brieva J, Coleman N, Lacey J, Harrigan P, Lewin TJ, Carter GL. Prediction of death in less than 60 minutes following withdrawal of cardiorespiratory support in ICUs. Critical care medicine. 2013;41(12):2677–2687. doi: 10.1097/CCM.0b013e3182987f38. [DOI] [PubMed] [Google Scholar]
- 88.Qureshi AI, Adil MM, Suri MF. Rate of use and determinants of withdrawal of care among patients with subarachnoid hemorrhage in the United States. World neurosurgery. 2014;82(5):e579–584. doi: 10.1016/j.wneu.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Qureshi AI, Adil MM, Suri MF. Rate of utilization and determinants of withdrawal of care in acute ischemic stroke treated with thrombolytics in USA. Medical care. 2013;51(12):1094–1100. doi: 10.1097/MLR.0b013e3182a95db4. [DOI] [PubMed] [Google Scholar]
- 90.Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: The FOUR score. Annals of neurology. 2005;58(4):585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 91.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. Journal of neurotrauma. 1992;9(Suppl 1):S287–292. [PubMed] [Google Scholar]
- 92.IMPACT TBI Calculator. [ http://www.tbi-impact.org/?p=impact/calc]
- 93.CRASH TBI Calculator. [ http://www.trialscoordinatingcentre.lshtm.ac.uk/Risk%20calculator/index.html]
- 94.Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. Journal of neurosurgery. 1988;68(6):985–986. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- 95.Lee VH, Ouyang B, John S, Conners JJ, Garg R, Bleck TP, Temes RE, Cutting S, Prabhakaran S. Risk stratification for the in-hospital mortality in subarachnoid hemorrhage: the HAIR score. Neurocritical care. 2014;21(1):14–19. doi: 10.1007/s12028-013-9952-9. [DOI] [PubMed] [Google Scholar]
- 96.Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Hurlbert RJ, Rozzelle CJ, Ryken TC, Theodore N. Clinical assessment following acute cervical spinal cord injury. Neurosurgery. 2013;72(Suppl 2):40–53. doi: 10.1227/NEU.0b013e318276edda. [DOI] [PubMed] [Google Scholar]