Abstract
Background
Children undergoing cardiac surgery may exhibit a pronounced inflammatory response to cardiopulmonary bypass (CPB). Inflammation is recognized as an important pathophysiologic process leading to acute kidney injury (AKI). The aim of this study was to evaluate the association of two inflammatory cytokines interleukin (IL)-6 and IL-10 with AKI and other adverse outcomes in children after CPB surgery.
Methods
This is a sub-study of the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) cohort, including 106 children from 1 month to 18 years old undergoing CPB. Plasma IL-6 and IL-10 were measured preoperatively and postoperatively on days 1 (within 6 hours after surgery) and 3.
Results
Stage 2/3 AKI, defined by atleast a doubling of baseline serum creatinine or dialysis, was diagnosed in 24 (23%) patients. Preoperative IL-6 was significantly higher in patients with stage 2/3 AKI vs. without stage 2/3 AKI (median (IQR), 2.6 (0.6-4.9) vs. 0.6 (0.6-2.2), p=0.03). After adjustment for clinical and demographic variables, the highest preoperative IL-6 tertile was associated with a six-fold increased risk for stage 2/3 AKI compared with the lowest tertile (adjusted OR 6.41 (CI: 1.16-35.35)). IL-6 and IL-10 increased significantly after surgery, peaking postoperatively on day 1. First postoperative IL-6 and IL-10 did not significantly differ between patients with vs. without stage 2/3 AKI. Elevated IL-6 on day 3 was associated with longer hospital stay (p=0.0001).
Conclusions
Preoperative plasma IL-6 is associated with development of stage 2/3 AKI and may be prognostic of resource utilization.
Keywords: IL-6, IL-10, inflammatory, cardiopulmonary bypass, CPB, children
Introduction
Pediatric acute kidney injury (AKI) has become a significant health concern due to its rising incidence [1, 2]. AKI develops in about 50% of children who undergo cardiac surgery with cardiopulmonary bypass (CPB) [3, 4]. Pediatric AKI is associated with adverse outcomes such as higher mortality and longer length of stay [5]. In addition, clinical studies have shown that pediatric AKI may be associated with higher risk of developing proteinuria, hypertension, and chronic kidney disease long-term [6, 7].
Serum creatinine is currently used to diagnose AKI in children. It is a useful indicator of glomerular clearance but is a late marker of AKI and is not involved in the process of AKI. Novel AKI biomarker candidates currently being evaluated include some of the cytokines that mediate inflammation. Pathophysiologically, these are relevant as AKI is an inflammatory process mediated by a number of cytokines [8]. Two cytokines that are believed to play a role in the pathophysiology of AKI are IL-6 and IL-10. IL-6 is one of the main proinflammatory cytokines, while IL-10 has anti-inflammatory and immunomodulatory functions regulating lymphocyte activity. Studies in children and adults have demonstrated that these cytokines may be potential biomarkers of AKI [9-12]. It also has been demonstrated in animal models that AKI itself, not just the pro-inflammatory state of sepsis or CPB, induces higher IL-6 and IL-10 levels [13, 14].
IL-6 and IL-10 are also known to play a key role in the systemic inflammatory response to cardiac surgery and CPB [15]. This heightened state of inflammation is partly due to surgical trauma, including sternotomy and incision into the myocardium. Most of the pro-inflammatory response is elicited as the patient's blood contacts the extracorporeal circuit leading to cytokine production, endothelial dysfunction, and neutrophil, platelet, and complement system activation [16]. There is evidence that these circulating cytokines and activated cells cause organ dysfunction particularly in the kidneys [17]. Children are particularly vulnerable to this inflammatory state as a greater percentage of their blood is exposed to the artificial circuit leading to a more pronounced inflammatory response [18]. There is a paucity of data in children on these inflammatory biomarkers and their role in clinical AKI. Hence, we conducted an analysis of the perioperative plasma IL-6 and IL-10 as part of the TRIBE-AKI (Translational Research Investigating Endpoints in Acute Kidney Injury) prospective multi-center cohort of children undergoing cardiac surgery. Our goal was to assess whether these two cytokines were associated with AKI or other adverse patient outcomes.
Methods
This is a sub-study of the TRIBE-AKI cohort in which children who were undergoing surgery for congenital heart disease were prospectively enrolled. Patients were enrolled from July 2007 to December 2010 at three medical centers within the TRIBE-AKI network [3].
The TRIBE-AKI parent study included patients older than 1 month and younger than 18 years who were scheduled to be placed on CPB during cardiac surgery. We excluded patients who had a history of kidney transplantation or prior receipt of dialysis. Written informed consent was obtained from all parents or legal guardians, along with an assent when appropriate. The study was approved by each center's research ethics board. Patients were only included in this sub-study if blood specimens were collected on all consecutive days after surgery. IL-6 and IL-10 were selected as candidate biomarkers of AKI by literature review. The literature review was performed on inflammatory mediators that have reported associations with AKI. The reporting of this study follows guidelines set out in the Strengthening the Reporting of Observational Studies in Epidemiology statement [19].
Sample Collection
Blood specimens were collected preoperatively and postoperatively on three consecutive days after surgery in the parent study. The first postoperative blood sample was collected 0 to 6 hours after admission to the intensive care unit and is referred to as the day 1 sample. The subsequent day 2 and day 3 blood samples were obtained at the time of routine morning blood collection done for clinical care. Blood was collected in EDTA tubes and was centrifuged to separate plasma. Barcoded aliquots of plasma were stored at -80°C until biomarker measurement. No additives or protease inhibitors were added.
Biomarker Measurements
Following one freeze-thaw (storage at -80°C), IL-6 and IL-10 samples were analyzed on the Randox Evidence InvestigatorTM using a Randox developed custom cytokine array (Randox Laboratories Ltd). The detection range for IL-6 is 0.6–790 pg/ml, whereas IL-10 is 0.9–840 pg/ml. The intra-assay coefficients of variation for IL-6 and IL-10 are 7% and 6% for quality control (QC) 1 and 7% and 3% for QC 2, respectively, and the inter-assay coefficients of variation are 16% and 17% for QC1 and 14% and 9% for QC2, respectively. We blinded the personnel measuring the biomarkers to clinical outcomes, and samples were analyzed according to manufacturer specifications. If a patient had insufficient plasma at a specific time point, the biomarker could not be measured and the patient was excluded from analyses for that time point.
Outcome definitions
The primary outcome, stage 2/3 AKI, was the development of at least stage 2 AKI, defined by Kidney Disease Improving Global Outcomes (KDIGO) [20] as at least a doubling of serum creatinine from the baseline pre-operative value or receipt of acute dialysis, during the first postoperative week. The secondary outcome was “any AKI” which includes all patients diagnosed with atleast stage 1 AKI (composite of stage 1, stage 2 and stage 3 AKI). Stage 1 AKI, defined by KDIGO, is a ≥ 50% rise of baseline serum creatinine or a ≥ 0.3mg/dL increase of baseline serum creatinine. We also analyzed resource utilization outcomes such as length of in-hospital, length of intensive care unit (ICU) stay, and time to extubation. Pre- and postoperative serum creatinine levels were measured in the same clinical laboratory for each patient at all sites.
Variable Definitions
We collected preoperative characteristics, operative details, and postoperative complications using the definitions of the Society of Thoracic Surgeons [21]. We utilized the risk adjustment for congenital heart surgery-1 (RACHS-1) consensus-based scoring system to categorize the complexity of surgery [22]. This method of risk stratification is a widely accepted tool for the evaluation of differences in outcomes of surgery for congenital heart disease. We determined the preoperative estimated glomerular filtration rate (eGFR) using the updated Schwartz equation [23] and determined eGFR percentiles based on published normal renal function data in children 24,25].
Statistical Analysis
Continuous variables were compared with two-sample t test or Wilcoxon rank sum test and dichotomous variables with the chi-squared test or Fisher's exact test. For calculation of p-values in Table 1, one-way ANOVA and Kruskal-Wallis tests were used to compare continuous variables between multiple groups, as appropriate. To evaluate the association of each biomarker with AKI, we divided the cohort into tertiles on the basis of IL-6 or IL-10 levels. Mixed logistic regression models with random intercepts for each center were used to determine the adjusted odds ratios of AKI. Linear regression was used to evaluate the relationship between pre-operative IL6 and postoperative tubular injury biomarkers (NGAL, KIM-1, IL-18, L-FABP). We adjusted for important covariates that predict AKI in the pediatric cardiac surgery setting including age, gender, race, surgical site, RACHS-1, and CPB time. Poisson regression with log link function was used to estimate the association between biomarkers and clinical outcomes: number of days to extubation, length of intensive care unit stay, and length of hospital stay. Biomarker values below the assay's detectable limit were assigned the lower limit of detection.
Table 1.
Patient characteristics by Acute Kidney Injury (AKI) status
| No AKI (n=50) | Stage 1 AKI (n=32) | Stage 2/3 AKI (n=24) | p-value | |
|---|---|---|---|---|
| Demographic Characteristics and Medical History | ||||
| Age (months), mean (SD) | 53 ± 64 | 33 ± 45 | 13 ± 23 | 0.009 |
| Male gender, % | 28 (55) | 20 (63) | 13 (57) | 0.8 |
| Weight (kg), mean (SD) | 19 ± 22 | 12 ± 12 | 7.0 ± 4.6 | 0.01 |
| Prior cardiothoracic surgery, % | 20 (42) | 16 (52) | 13 (57) | 0.4 |
| Preoperative Characteristics | ||||
| Preoperative eGFR (ml/min per 1.73 m2), mean (SD) | 82 ± 22 | 91 ± 35 | 101 ± 33 | 0.04 |
| Preoperative eGFR (percentile), mean (SD) | 45 ± 33 | 60 ± 35 | 76 ± 30 | 0.001 |
| Perioperative Characteristics | ||||
| Urgency of surgery, % | 0.2 | |||
| Elective | 41 (80) | 30 (94) | 19 (83) | |
| Urgent | 10 (20) | 2 (6) | 4 (17) | |
| Type of Surgery, % | 0.9 | |||
| Septal defect repair | 14 (28) | 9 (28) | 6 (26) | |
| Inflow/Outflow tract or valve procedure | 15 (29) | 10 (31) | 5 (22) | |
| Combined procedure | 22 (43.1) | 13 (40.6) | 12 (52.2) | |
| RACHS-1 score, % | 0.5 | |||
| 2 | 24 (48) | 15 (47) | 12 (52) | |
| 3 | 23 (46) | 17 (53) | 9 (39) | |
| 4 | 3 (6.0) | 0 (0) | 2 (8.7) | |
| CPB time (minutes), mean (SD) | 118 ± 64 | 101 ± 40 | 134 ± 80 | 0.1 |
| Cross-clamp time (minutes), mean (SD) | 57 ± 51 | 46 ± 41 | 61 ± 40 | 0.5 |
| Outcomes | ||||
| In-hospital mortality, % | 0 (0) | 0 (0) | 2 (8.7) | 0.05 |
| Length of ICU stay (days), median (IQR) | 3.0 (2.0, 4.0) | 3.0 (2.0, 5.5) | 5.5 (3.0, 9.0) | 0.003 |
| Length of hospital stay (days), median (IQR) | 6.0 (5.0, 10.0) | 5.5 (4.0, 12.5) | 12.0 (9.0, 15.0) | 0.002 |
Abbreviations: CPB, cardiopulmonary bypass; GFR, glomerular filtration rate; IQR, interquartile range; RACHS-1, risk adjustment for congenital heart surgery-1; SD, standard deviation
Results
Characteristics of the Study Cohort
106 pediatric patients were enrolled in the sub-study are listed in Table 1 by AKI status. The median age of the participants of this cohort was 0.7 years (IQR, 0.3 – 4.5). 58% of participants were male. All patients received CPB during cardiac surgery with an average CPB time of 116 minutes and average cross-clamp time of 55 minutes. 56 (53%) participants developed any AKI and 24 (23%) participants developed stage 2/3 AKI during their hospital stay. Two patients received acute dialysis, and two patients died before discharge.
Biomarker Results and Preoperative Risk of AKI
Median preoperative plasma IL-6 levels were significantly higher in patients with stage 2/3 AKI compared to those without stage 2/3 AKI (2.6 (IQR, 0.6 – 4.9) pg/mL vs. 0.6 (IQR, 0.6 – 2.2) pg/mL; p=0.03). There was no significant difference in preoperative plasma IL-10 levels (p=0.8) (Supplementary Table 2). Preoperative IL-6 and IL-10 levels comparing any AKI vs. no AKI patients were not significant and can be found in Supplementary Table 1. Of note, 78% of preoperative IL-10 values were undetectable by our laboratory assay.
Preoperative biomarker measurements were categorized into tertiles of IL-6 and IL-10 (Table 2). The third tertile of IL-6 was significantly associated with a six-fold higher odds (95% CI,1.16 to 35.35) (relative to the first tertile) of stage 2/3 AKI after adjusting for age, gender, race, study site, RACHS-1 score, and CPB time. None of the preoperative tertiles of IL-10 were associated with stage 2/3 AKI.
Table 2.
Association of Inflammatory Biomarkers with Stage 2/3 acute kidney injury (AKI)
| Unadjusted | Adjusted* | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| IL-6 pre-op | ||
| T1, n=41 | 1.0 (referent) | 1.0 (referent) |
| T2, n=14 | 1.54 (0.25, 9.50) | 0.95 (0.10, 8.87) |
| T3, n=27 | 5.44 (1.49, 19.85) | 6.41 (1.16, 35.35) |
| IL-6 day 1 | ||
| T1, n=35 | 1.0 (referent) | 1.0 (referent) |
| T2, n=35 | 1.43 (0.44, 4.67) | 1.64 (0.43, 6.26) |
| T3, n=35 | 1.67 (0.52, 5.34) | 2.40 (0.63, 9.07) |
| IL-6 day 3 | ||
| T1, n=31 | 1.0 (referent) | 1.0 (referent) |
| T2, n=32 | 0.96 (0.27, 3.38) | 0.26 (0.05, 1.28) |
| T3, n=31 | 1.98 (0.62, 6.37) | 0.40 (0.08, 2.12) |
| IL-10 pre-op | 1.0 (referent) | |
| T1, n=63 | 1.0 (referent) | 0.57 (0.07, 4.85) |
| T2, n=18 | 1.24 (0.35, 4.44) | 1.0 (referent) |
| IL-10 day 1 | ||
| T1, n=34 | 1.0 (referent) | 1.0 (referent) |
| T2, n=35 | 3.91 (1.11, 13.73) | 4.31 (1.00, 18.50) |
| T3, n=35 | 1.88 (0.50, 7.10) | 1.13 (0.26, 4.90) |
| IL-10 day 3 | ||
| T1, n=52 | 1.0 (referent) | 1.0 (referent) |
| T2, n=25 | 3.75 (1.29, 10.92) | 2.23 (0.61, 8.15) |
Adjusted for age, gender, race, surgical site, CPB time, RACHS-1 score
† IL-10 pre-op were undetectable in 78% of the sample and are in T1.
‡ IL-10 day 3 were undetectable in 68% of the sample and are in T1.
Pre-operative IL-6 was also evaluated as a diagnostic test for predicting stage 2/3 AKI. The area under the curve (AUC) for preoperative IL-6 to predict stage 2/3 AKI was 0.69. The sensitivity and specificity of the third tertile of IL-6 (2.2 pg/ml) is 0.63 and 0.76, respectively.
Biomarker Results and Postoperative Risk of AKI
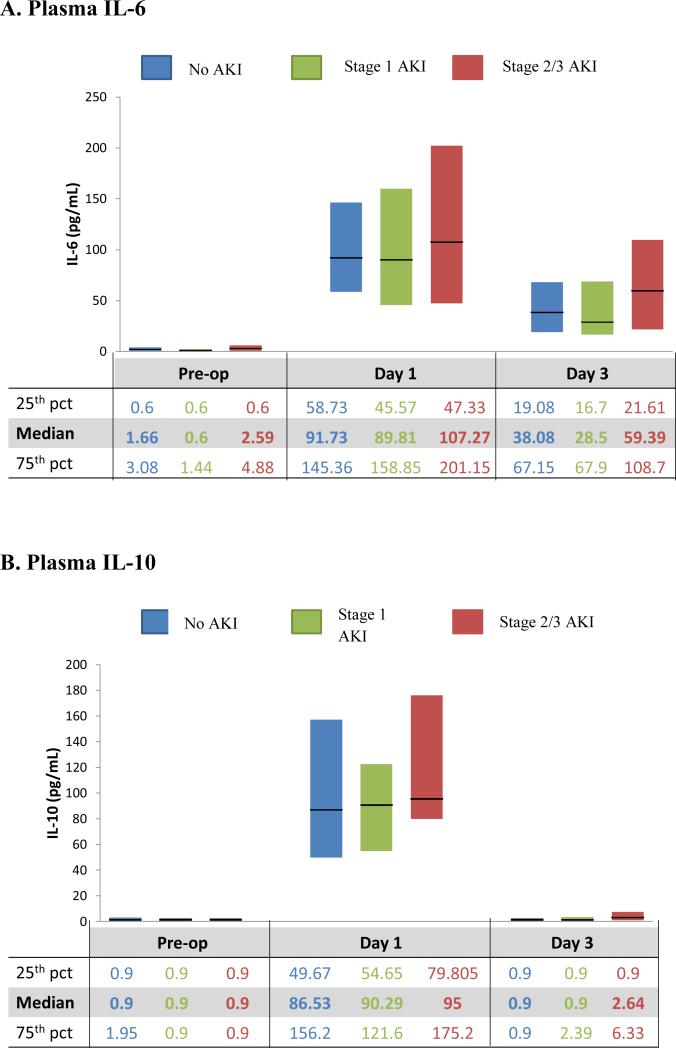
Plasma IL-6 and IL-10 concentrations, collected within 6 hours of arrival in the pediatric ICU (PICU), peaked at this first postoperative collection and then declined on day 3 (Figure 1). IL-6 remained elevated on day 3, whereas IL-10 had returned to preoperative levels. AKI was most frequently diagnosed on day 2 after surgery [4]. On day 1, there was no statistically significant difference between the patients with and without stage 2/3 AKI in plasma IL-6 (p=0.4) and IL-10 (p=0.3) levels (Supplementary Table 2).
Figure 1. Biomarker Distribution by acute kidney injury (AKI) Status.
Stage 1 AKI: includes patients with stage 1 AKI but not those with stage 2 or stage 3 AKI
Stage 2/3 AKI: only includes those patients with stage 2 and stage 3 AKI
On day 3, median plasma IL-10 was significantly higher in patients with stage 2/3 AKI (2.6 (IQR, 0.9 – 6.3) pg/mL) vs. patients without stage 2/3 AKI (0.9 (IQR, 0.9 – 0.9) pg/mL) (p=0.001) and in patients with any AKI (0.9 (IQR, 0.9 – 4.7) pg/mL) vs. no AKI (0.9 (IQR, 0.9 – 0.9) pg/mL) (p=0.02). Otherwise, IL-6 and IL-10 were not found to be significantly different in the AKI groups postoperatively. Of note, 68% of day 3 IL-10 values were undetectable.
Biomarker Results and Nonrenal Outcomes
The average lengths of stay in the PICU and hospital for the entire cohort were 5.5 days (SD, 7.3) and 10 days (SD, 9.9), respectively. The average length of mechanical ventilation for the entire cohort was 2.3 days (SD, 1.6). Postoperatively, on day 3, plasma IL-6 was linearly associated with a longer length of hospital stay (p=0.0001). Otherwise, neither IL-6 nor IL-10 was significantly associated across all time points with the nonrenal outcomes of length of PICU stay, length of hospital stay, and length of mechanical ventilation. We were unable to reliably analyze associations with in-hospital mortality as there were only two deaths in this cohort.
Preoperative IL-6 and Postoperative Tubular Biomarkers
Previously, perioperative tubular injury biomarkers (NGAL, KIM-1, IL-18, L-FABP) were measured and described in this pediatric cardiac surgery cohort [26,27]. The relationship between preoperative IL-6 and postoperative tubular biomarkers was statistically significant for NGAL on day 1, 2, and 3 and also for L-FABP on day 3. After multivariable adjustment, only day 1 NGAL remained significantly associated with IL-6 with a R2 of 0.13 (p= 0.03) (Supplementary Table 3).
Discussion
In our multicenter study of children undergoing cardiac surgery with cardiopulmonary bypass, we have demonstrated for the first time that high levels of preoperative IL-6 are associated with AKI such that the children in the third tertile exhibited a six-fold increased risk of stage 2/3 AKI. Preoperative IL-6 was also associated with tubular injury on day 1 such that there was a statistically significant relationship between pre-op IL-6 and day 1 NGAL. We did not find an association of IL-10 with AKI except that the levels were still elevated on day 3 in patients who had AKI. We also found that on day 3, IL-6 was associated with prolonged stay in the hospital after surgery. This linear association on day 3 was statistically significant and stronger than serum creatinine for predicting length of hospital stay [4]. To our surprise, there was only a weak correlation between the inflammatory biomarkers and CPB time compared with published reports in adults [28,29], suggesting that distinct pathways may mediate inflammation in children. This is the largest research study to examine perioperative IL-6 and IL-10 levels in children after cardiac surgery and their association with AKI.
IL-6 is mainly secreted by lymphocytes, fibroblasts, macrophages, mesangial cells, and endothelial cells and plays a major role in regulating inflammatory and immunologic processes [30, 31]. IL-6 can also be upregulated and released from renal tubular epithelial cells in response to injury and plays a fundamental role in the pathophysiology of AKI [32]. Additionally, IL-6 production is increased by the myocardium in states of congestive heart failure, cardiac ischemia, and congenital heart disease [33-35]. Preoperatively, we found that IL-6 was statistically different in patients who did and did not go on to develop stage 2/3 AKI. IL-6 likely served as a marker for preoperative severity of illness, an ongoing inflammatory process, or cardiac dysfunction. These findings suggest that timing of surgery and alleviation of inflammation before surgery may assist with reducing postoperative AKI. Alleviating preoperative inflammation may include treating any subclinical infection, heart failure, or myocardial dysfunction, and managing any systemic inflammatory process before surgery. Treating sources of inflammation may have been feasible in the pediatric cohort that we studied as 83% of the patients’ cardiac operations were considered elective.
Previous studies by Miklaszewska et al. and Liu et al. did not find a significant difference in IL-6 levels preoperatively but did find a significant difference in IL-6 levels postoperatively on the day of surgery in children with and without AKI [9,36]. However, Morgan et al. did not find any association of IL-6 with AKI on the day after surgery [37], similar to our findings. There are likely multiple factors contributing to these differing results. One is the different AKI definition used by Miklaszewska et al. [36] as AKI was defined by a 25% reduction in eGFR. In addition, Miklaszewska et al.[36] and Liu et al. [9] both measured IL-6 levels two hours after bypass. Both of these studies showed the most substantial difference in IL-6 concentrations between AKI and non-AKI patients at this earliest time point after surgery. Over time, the difference in IL-6 concentrations diminished. In contrast, our study's blood collection was performed within six hours of bypass. Likewise, Morgan et al. measured cytokine levels at a later time point of four hours after surgery [37]. In addition, different laboratory assays were used to measure IL-6 levels in these other studies. With these inconsistencies, as a central pro-inflammatory cytokine, further research is required to evaluate IL-6 as a candidate biomarker in AKI.
As an anti-inflammatory cytokine, IL-10 plays an important role in AKI. IL-10 limits inflammation and terminates inflammatory responses by decreasing secretion of proinflammatory cytokines and inhibiting lymphocytes, dendritic cells, NK cells, and marcophages[38]. As a testament to its importance in the anti-inflammatory pathway, several viral genomes including Epstein-Barr virus, cytomegalovirus, and herpes type 2 virus code for IL-10 homologs [39]. Additionally, several single nucleotide polymorphisms in the IL-10 promoter region have been associated with a faster decline in renal function in both IgA nephropathy and focal segmental glomerulosclerosis [40]. Abnormally high or low levels of IL-10 have been shown to increase proteinuria and decrease GFR in animal models [39]. IL-10 has also been shown to be elevated in several types of glomerulonephritides. Previous studies have presented mixed results when investigating IL-10 and its association with AKI [9, 12]. In a pediatric study by Liu et al., serum IL-10 did not idenitify AKI on the day of surgery. In an adult study on patients with septic shock by Payen et al., the serum IL-10 level on admission to the ICU was found to be associated with AKI [12]. Postoperatively, we found that IL-10 was statistically different on day 3 in patients with and without AKI. We suspect that IL-10 is higher in children with AKI on day 3 in order to regulate a more pronounced inflammatory response at tissue levels. Another possibility is that IL-10 is a direct or indirect mediator of AKI [39]. While our IL-10 results are intriguing, we are most interested in a biomarker that predicts AKI before it is diagnosed by serum creatinine.
The roles IL-6 and IL-10 play in AKI is not entirely clear. Previous research indicates that cytokines have a direct role in the pathogenesis of AKI and are elevated in AKI states. There is also evidence that cytokines are cleared by the kidney and elevated when there is decreased renal function [41]. One pediatric study reported that impaired proximal tubule metabolism of IL-6 may be the cause of increases in both plasma and urine IL-6 [42]. However, the degree to which elevated cytokine levels are causing AKI or simply surrogate markers of decreased renal clearance has not yet been determined.
This study has limitations. First, we lacked sufficient plasma volume to include all of the patients from our previously performed prospective study. A larger sample size would have improved our power to assess the performance of IL-6 and IL-10 in the perioperative setting. Another limitation was that we could not include oliguria as part of our AKI definition as we did not have an hourly record of urine output. Additionally, we were unable to evaluate mortality as an endpoint as there were only two deaths in this cohort. Finally, we used changes in serum creatinine to define AKI. Serum creatinine has limitations in its ability to estimate GFR, and we used it to define the utility of IL-6 and IL-10. The strengths of our study include the prospective multicenter design and the known cause and time of AKI.
We have demonstrated that IL-6 and IL-10 rise substantially after cardiac bypass surgery in children. In addition, we have presented a novel finding in that IL-6 can preoperatively predict stage 2/3 AKI and may be a useful biomarker to plan the timing of surgery. Preoperative IL-6 levels may also help with the design of prevention clinical trials for AKI by enriching the patients who develop AKI after cardiac surgery. Future studies should further investigate the role of these biomarkers in the immediate postoperative period within two hours after surgery.
Supplementary Material
Acknowledgements
The Evidence Investigator™ Cytokine Custom Array 4 kits were donated by Randox Laboratories Ltd. This study was supported by the National Institutes of Health (NIH) (R01HL085757 to C.R.P.) to fund the TRIBE-AKI Consortium to study novel biomarkers of AKI in cardiac surgery. J.H.G. is funded by the T32DK007276-35 training grant to the Yale University School of Medicine Section of Nephrology. C.R.P. is supported by the NIH (K24DK090203) and P30 DK079310-07 O'Brien Center Grant. A.X.G., and C.R.P. are also members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Consortium (U01DK082185).
The granting agency, Randox Laboratories Ltd., did not participate in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript. The results presented in this article have not been published previously in whole or part, except in abstract form.
Footnotes
Disclosures:
Dr. Kavsak has received grants/honorariums/consultant/advisor fees from Abbott Laboratories, Abbott Point of Care, Beckman Coulter, Ortho Clinical Diagnostics, Randox Laboratories, Roche diagnostics, and the Canadian Agency for Drugs and Technologies in Health. He is listed as an inventor on patents filed by McMaster University related to laboratory testing in acute cardiac care.
Dr. P.J. Devereaux is part of a group that has a policy of not accepting honorariums or other payments from industry for their own personal financial gain. They do accept honorariums or other payments from industry to support research endeavors and for reimbursement of costs to participate in meetings such as scientific or advisory committee meetings. Based on study questions he originated and grants he wrote, he has received grants from Abbott Diagnostics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Stryker, and Roche Diagnostics. He has participated in an advisory board meeting for GlaxoSmithKline and an expert panel meeting for Astra Zeneca. He has also participated on a Consultancy Board for Boehringer Ingelheim.
References
- 1.Ladapo TA, Esezobor CI, Lesi FE. Paediatric kidney diseases in an African country: Prevalence, Spectrum and Outcome. Saudi J Kidney Dis Transp. 2013;25:1110–1116. doi: 10.4103/1319-2442.139976. [DOI] [PubMed] [Google Scholar]
- 2.Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E. Childhood acute renal failure: 22- year experience in a university hospital in southern Thailand. Pediatrics. 2006;118:e786–791. doi: 10.1542/peds.2006-0557. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR, Consortium T-A. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, Kim RW, Koyner JL, Coca SG, Edelstein CL, Shlipak MG, Garg AX, Krawczeski CD, Consortium T-A. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8:1661–1669. doi: 10.2215/CJN.00270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3-5 year longitudinal follow- up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 7.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol. 2013;24:529–536. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 9.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennen P, Altmann C, Kaufman J, Klein CL, Andres-Hernando A, Ahuja NH, Edelstein CL, Cadnapaphornchai MA, Keniston A, Faubel S. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care. 2010;14:R181. doi: 10.1186/cc9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, Korpak A, Thompson BT, Chertow GM, Matthay MA, National Heart L, Blood Institute ANCTG Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 12.Payen D, Lukaszewicz AC, Legrand M, Gayat E, Faivre V, Megarbane B, Azoulay E, Fieux F, Charron D, Loiseau P, Busson M. A multicentre study of acute kidney injury in severe sepsis and septic shock: association with inflammatory phenotype and HLA genotype. PloS One. 2012;7:e35838. doi: 10.1371/journal.pone.0035838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005;16:3315–3325. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 14.Brochner AC, Dagnaes-Hansen F, Hojberg-Holm J, Toft P. The inflammatory response in blood and in remote organs following acute kidney injury. APMIS. 2013;122:399–404. doi: 10.1111/apm.12157. [DOI] [PubMed] [Google Scholar]
- 15.Chew MS, Brandslund I, Brix-Christensen V, Ravn HB, Hjortdal VE, Pedersen J, Hjortdal K, Hansen OK, Tonnesen E. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: a descriptive study. Anesthesiology. 2001;94:745–753. doi: 10.1097/00000542-200105000-00010. discussion 745A. [DOI] [PubMed] [Google Scholar]
- 16.Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand. 2001;45:671–679. doi: 10.1034/j.1399-6576.2001.045006671.x. [DOI] [PubMed] [Google Scholar]
- 17.Seghaye MC, Duchateau J, Grabitz RG, Faymonville ML, Messmer BJ, Buro-Rathsmann K, von Bernuth G. Complement activation during cardiopulmonary bypass in infants and children. Relation to postoperative multiple system organ failure. J Thorac Cardiovasc Surg. 1993;106:978–987. [PubMed] [Google Scholar]
- 18.el Habbal MH, Carter H, Smith LJ, Elliott MJ, Strobel S. Neutrophil activation in paediatric extracorporeal circuits: effect of circulation and temperature variation. Cardiovasc Res. 1995;29:102–107. [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury N. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavroudis C, Gevitz M, Ring WS, McIntosh CL, Schwartz M. The Society of Thoracic Surgeons National Congenital Heart Surgery Database Report: analysis of the first harvest (1994-1997). Ann Thorac Surg. 1999;68:601–624. doi: 10.1016/s0003-4975(99)00631-1. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg y. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piepsz A, Tondeur M, Ham H. Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging. 2006;33:1477–1482. doi: 10.1007/s00259-006-0179-2. [DOI] [PubMed] [Google Scholar]
- 25.Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, Li S, Bennett MR, Ma Q, Shlipak MG, Garg AX, Parikh CR, consortium T-A. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80:655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, Consortium T-A. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG, Consortium T-A. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8:1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Azab SR, Doha N, Rady A, El-Sayed AE, Abd-Rabo M. The cytokine balance during CABG surgery with and without cardiopulmonary bypass. Egyptian J Anaes. 2010;26:281–286. [Google Scholar]
- 29.Gasz B, Lenard L, Racz B, Benko L, Borsiczky B, Cserepes B, Gal J, Jancso G, Lantos J, Ghosh S, Szabados S, Papp L, Alotti N, Roth E. Effect of cardiopulmonary bypass on cytokine network and myocardial cytokine production. Clin Cardiol. 2006;29:311–315. doi: 10.1002/clc.4960290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ataie-Kachoie P, Pourgholami MH, Morris DL. Inhibition of the IL-6 signaling pathway: a strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev. 2013;24:163–173. doi: 10.1016/j.cytogfr.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL. Gene of the month: Interleukin 6 (IL-6). J Clin Pathol. 2014;k67:932–937. doi: 10.1136/jclinpath-2014-202493. [DOI] [PubMed] [Google Scholar]
- 32.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afify MF, Mohamed GB, El-Maboud MA, Abdel-Latif EA. Serum levels of ghrelin, tumor necrosis factor-alpha and interleukin-6 in infants and children with congenital heart disease. J Trop Pediatr. 2009;55:388–392. doi: 10.1093/tropej/fmp036. [DOI] [PubMed] [Google Scholar]
- 34.Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183–193. doi: 10.1536/jhj.45.183. [DOI] [PubMed] [Google Scholar]
- 35.Kavsak PA, Ko DT, Newman AM, Palomaki GE, Lustig V, MacRae AR, Jaffe AS. Risk stratification for heart failure and death in an acute coronary syndrome population using inflammatory cytokines and N-terminal pro-brain natriuretic peptide. Clin Chem. 2007;53:2112–2118. doi: 10.1373/clinchem.2007.090613. [DOI] [PubMed] [Google Scholar]
- 36.Miklaszewska M, Korohoda P, Zachwieja K, Mroczek T, Drozdz D, Sztefko K, Moczulska A, Pietrzyk JA. Serum interleukin 6 levels as an early marker of acute kidney injury on children after cardiac surgery. Adv Clin Exp Med. 2013;2:377–386. [PubMed] [Google Scholar]
- 37.Morgan CJ, Gill PJ, Lam S, Joffe AR. Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study. Intensive Care Med. 2013;39:934–941. doi: 10.1007/s00134-013-2849-4. [DOI] [PubMed] [Google Scholar]
- 38.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 39.Sinuani I, Beberashvili I, Averbukh Z, Sandbank J. Role of IL-10 in the progression of kidney disease. World J Transplant. 2013;3:91–98. doi: 10.5500/wjt.v3.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bantis C, Heering PJ, Aker S, Klein-Vehne N, Grabensee B, Ivens K. Association of interleukin-10 gene G-1082A polymorphism with the progression of primary glomerulonephritis. Kidney Int. 2004;66:288–294. doi: 10.1111/j.1523-1755.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- 41.Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 42.Dennen P, Altmann C, Kaufman J, Klein C, Andres-Hernando A, Ahuja N, Edelstein C, Cadnapaphornchai M, Keniston A, Faubel S. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care. 2010;14:R181. doi: 10.1186/cc9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.