Abstract
Currently, non-small cell lung carcinomas are primarily classified by light microscopy. However, recent studies have shown that poorly-differentiated tumors are more accurately classified by immunohistochemistry. In this study, we investigated the use of immunohistochemical analysis in reclassifying lung carcinomas that were originally diagnosed as squamous cell carcinoma. Tumor slides and blocks were available for histologic evaluation, and tissue microarrays were constructed from 480 patients with resected lung carcinomas originally diagnosed as squamous cell carcinoma between 1999 and 2009. Immunohistochemistry for p40, p63, thyroid transcription factor-1 (TTF-1; clone SPT24 and 8G7G3/1), Napsin A, Chromogranin A, Synaptophysin, and CD56 were performed. Staining intensity (weak, moderate, or strong) and distribution (focal or diffuse) were also recorded. Of all, 449 (93.5%) patients were confirmed as having squamous cell carcinomas; the cases were mostly diffusely positive for p40 and negative for TTF-1 (8G7G3/1). Twenty cases (4.2%) were reclassified as adenocarcinoma since they were positive for TTF-1 (8G7G3/1 or SPT24) with either no or focal p40 expression, and all of them were poorly-differentiated with squamoid morphology. In addition, 1 case was reclassified as adenosquamous carcinoma, 4 cases as large cell carcinoma, 4 cases as large cell neuroendocrine carcinoma, and 2 cases as small cell carcinoma. In poorly-differentiated non-small cell lung carcinomas, an accurate distinction between squamous cell carcinoma and adenocarcinoma cannot be reliably determined by morphology alone and requires immunohistochemical analysis, even in resected specimens. Our findings suggest that TTF-1 8G7G3/1 may be better suited as the primary antibody in differentiating adenocarcinoma from squamous cell carcinoma.
Keywords: squamous cell carcinoma, lung, immunohistochemistry, classification
Introduction
Over the past few decades, the simple histological division of lung carcinomas between small cell carcinoma and other major histologic types such as adenocarcinoma or squamous cell carcinoma was clinically adequate for determining the appropriate therapy for patients. However, with recent major advances in thoracic medical oncology, the precise distinction between adenocarcinoma and squamous cell carcinoma—the 2 most common types of lung cancer—have become important in clinical practice. First, activating mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) can predict sensitivity to EGFR tyrosine kinase inhibitors, such as erlotinib and gefitinib, in patients primarily with lung adenocarcinomas.1–3 Second, pemetrexed—a potent inhibitor of thymidylate synthase and other folate-dependent enzymes—has greater efficacy in patients with lung adenocarcinoma than it does in patients with squamous cell carcinoma.4, 5 Third, bevacizumab—a recombinant humanized version of the murine antihuman vascular endothelial growth factor monoclonal antibody—was excluded from use in patients with squamous cell carcinomas because of the potential risk of life-threatening pulmonary hemorrhage.6, 7 Lastly, the recently recognized anaplastic lymphoma kinase (ALK) rearrangement predicted sensitivity to the targeted agent, Crizotinib, and it also specifically occurred in adenocarcinoma.8, 9 These findings have increased the requirement for accurate pathological classification (i.e., adenocarcinoma vs. squamous cell carcinoma) in the personalized selection of patients for appropriate histology-specific evaluation for targeted therapies.
In the 2004 World Health Organization (WHO) lung carcinoma classification, histological identification between adenocarcinoma and squamous cell carcinoma was still based on morphologic criteria that used standard hematoxylin and eosin (H&E) staining, with the option of histochemical staining of mucin to identify the presence of intraluminal and cytoplasmic mucin.10 Squamous cell carcinoma was characterized by keratinization and intercellular bridges, while adenocarcinoma was characterized by glandular structures and the presence of mucin. However, the histological distinction between them was difficult because those features were not always prominent in poorly-differentiated carcinomas. Recently, there have been various studies that have investigated the ability of a number of immunohistochemical markers to differentiate between squamous cell carcinoma and adenocarcinoma of the lung.11–17 Among these markers, p40 and thyroid transcription factor-1 (TTF-1) proved to be the two best squamous cell carcinoma and adenocarcinoma markers.12, 16 More importantly, the majority (80%) of large cell carcinomas can be reclassified into squamous cell carcinoma or adenocarcinoma using these 2 markers.18 For squamous cell carcinoma, p40, which is an isoform of p63, has equivalent sensitivity and higher specificity than p63.16, 17 Regarding TTF-1, two monoclonal antibodies (8G7G3/1 and SPT24) have been commercially available for immunohistochemistry. When differentiating adenocarcinoma and squamous cell carcinoma, 8G7G3/1 was more specific to but less sensitive while SPT24 was less specific to but more sensitive for lung adenocarcinoma.19, 20 In addition to TTF-1, Napsin A has been also recognized as a promising lung adenocarcinoma marker.13, 14
In a recent study from our institution, immunohistochemical analysis with p40 and TTF-1 using whole sections from 98 resected tumors which were originally diagnosed as squamous cell carcinoma revealed that 4 cases had an immunoprofile supporting the diagnosis of solid subtype adenocarcinoma rather than squamous cell carcinoma.21 To expand on these findings, in this study, we reviewed and reclassified a larger series of resected lung carcinomas, which were originally diagnosed as squamous cell carcinomas, using an expanded immunohistochemical analysis in an effort to distinguish them from other lung cancers such as adenocarcinoma, large cell neuroendocrine carcinoma, small cell carcinoma, and large cell carcinoma. We also compared the specificity –positive rate in squamous cell carcinomas—of the two monoclonal antibodies as we used immunohistochemistry to reclassify this series of squamous cell carcinomas.
Materials and methods
Patients
This retrospective study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. We reviewed patients with solitary lung carcinomas that were surgically resected and originally diagnosed as squamous cell carcinoma at Memorial Sloan Kettering Cancer Center between 1999 and 2009. Other clinical and pathologic aspects of these tumors have been published elsewhere.22 Tumor slides and blocks were available for histologic evaluation and tissue microarray construction from 480 patients. This series included a subset of our previous study (n=98) analyzing squamous cell carcinoma cases with immunohistochemistry.21 Clinical data were collected from our prospectively maintained lung carcinoma database.
Histologic Evaluation
All available H&E stained slides were reviewed by two pathologists (K.K. and W.D.T.) using an Olympus BX51 microscope (Olympus Optical Co., Tokyo, Japan) with a standard 22-mm diameter eyepiece. Both pathologists had no knowledge of the clinical information of those cases during the review.
Tumors were graded by a degree of squamous differentiation—well, moderately, and poorly-differentiated—in accordance with the 2004 WHO classification of lung carcinomas.10 In the well-differentiated tumors, tumor nests were composed of differentiated keratinocyte-like tumor cells with prominent keratinization (layered and cytoplasmic keratin) and intercellular bridges (Figures 1A and 1B). In the poorly-differentiated tumors, squamous morphology was only noticeable in a small area of the tumor and the vast majority of tumor cells showed nonkeratinizing pattern (Figure 1C). Moderately-differentiated tumors showed an intermediate degree of squamous differentiation between well-differentiated and poorly-differentiated tumors.
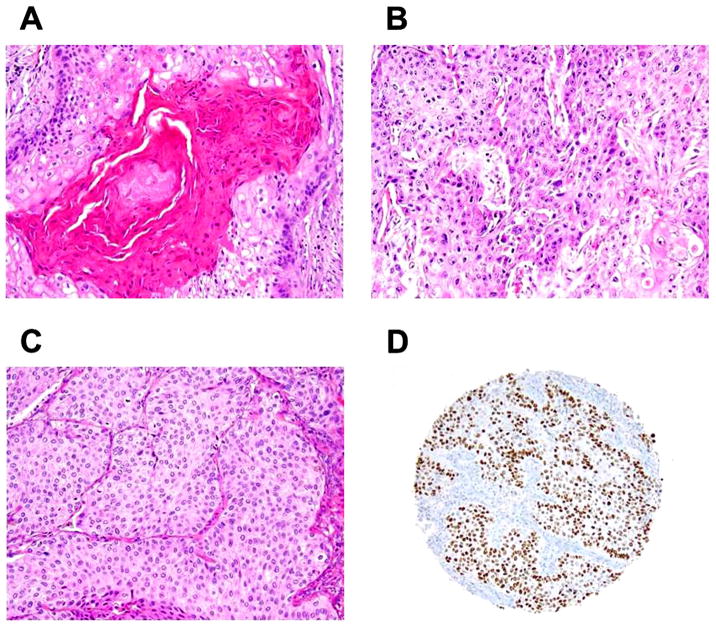
Figure 1. Histologic findings of squamous cell carcinoma (original magnification, x100: A–D).
(A) Well-differentiated tumor cells with layered keratinization. (B) Well-differentiated tumor cells with cytoplasmic keratinization. (C) Nonkeratinizing, poorly-differentiated tumor cells. (D) Nonkeratinizing, poorly-differentiated tumor cells positive for p40.
Histologic subtyping was performed in a similar fashion for nasopharyngeal carcinomas in the 2005 WHO Classification, Pathology and Genetics of Head and Neck Tumours; they were classified as nonkeratinizing, keratinizing, and basaloid squamous cell carcinomas.23 The percentage of keratinizing pattern, including layered and cytoplasmic keratinization, was recorded and then tumors were classified as either keratinizing subtype, which was defined as having any amount of keratinizing pattern in the tumor, or nonkeratinizing subtype, which was defined as having no keratinizing pattern. Basaloid pattern was defined as tumor nests that showed prominent peripheral palisading of tumor cells with scant cytoplasm (high nuclear/cytoplasmic ratio) and had a greater amount of hyperchromatic nuclei (Figure 2A).10 The percentage of basaloid pattern was recorded in each tumor; the tumor was classified as basaloid subtype if there was ≥50% basaloid pattern.24
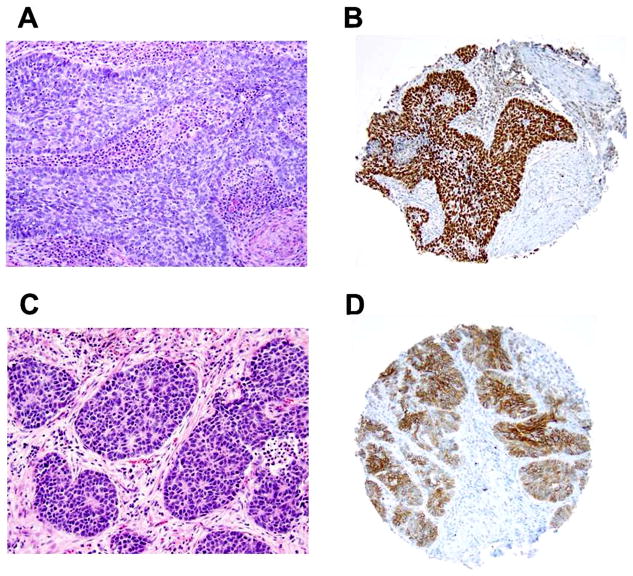
Figure 2. Basaloid pattern in squamous cell carcinoma and large cell neuroendocrine carcinoma (original magnification, x100: A–B).
(A) Basaloid squamous cell carcinoma showing prominent peripheral palisading of tumor cells with scanty cytoplasm. (B) Basaloid squamous cell carcinoma positive for p40. (C) Large cell neuroendocrine carcinoma showing basaloid-like pattern with rosette-like features and nuclear palisading. (D) Large cell neuroendocrine carcinoma positive for CD56.
Tissue Microarray Construction and Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor specimens were used for tissue microarray construction. We marked 3 representative tumor areas on H&E stained slides and, using an Automated Tissue Arrayer ATA-27 (Beecher Instruments inc., Sun Prairie, WI, USA), we arrayed cylindrical 0.6 mm tissue cores from corresponding paraffin blocks into a recipient block; this resulted in 5 tissue microarray blocks.
We took 4-μm sections from the tissue microarray blocks and briefly deparaffinized them in xylene and dehydrated them in graded alcohols. The standard avidin-biotin-peroxidase complex technique was used for immunohistochemical stains of anti-p40 antibody (polyclonal 5–17, CalBiochem; diluted at 1:2000), anti-p63 antibody (clone 4A4, Dako; diluted at 1:600), anti-TTF-1 antibody (clone SPT24, NovoCastra; diluted at 1:100), anti-TTF-1 antibody (clone 8G7G3/1, Dako; diluted at 1:100), anti-Napsin A antibody (clone IP64, NovoCastra; diluted at 1:200), anti-Chromogranin A antibody (polyclonal, Dako; diluted at 1:2000), anti-Synaptophysin antibody (polyclonal, Dako; diluted at 1:1000), and anti-CD56 antibody (clone 123C3.D5, Lab Vision; diluted at 1:25). Sections were stained using a Ventana Discovery XT Automated Immunohistochemical Stainer (Ventana Medical Systems Inc., Tucson, AZ, USA), according to manufacturer guidelines. Diaminobenzidine and hematoxylin were used as the chromogen and the nuclear counterstain, respectively. Positive control tissues were stained in parallel with the study cases.
For each marker, both maximal intensity immunostaining (weak, moderate, and strong) and distribution of positive tumor cells (percentage) were recorded for each core.25, 26 The average percentage of positive tumor cells within the tumor cores was used as the immunoreactive distribution for each patient. Diffuse reactivity was defined as ≥50% positive tumor cells and 1–49% as focal reactivity.12
Results
Histologic Evaluation with Immunohistochemical Analysis
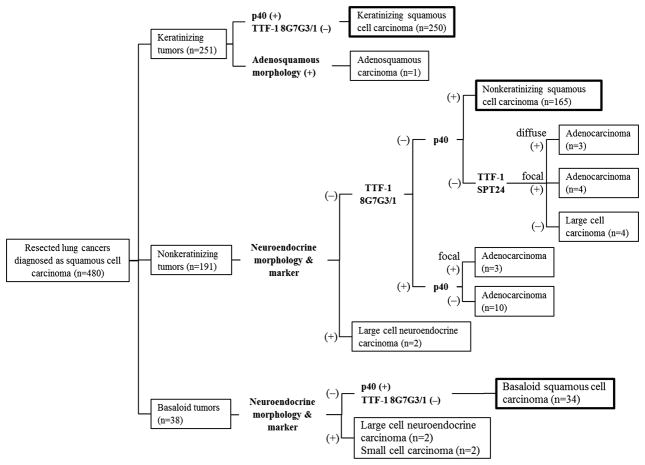
Figure 3 represents a flowchart to show how the tumors that were originally diagnosed as squamous cell carcinoma were reclassified based on slide review and immunohistochemistry. This is not a proposed algorithm for how to classify adenocarcinoma versus squamous cell carcinomas. After slide review, we identified 251 keratinizing tumors, 191 nonkeratinizing tumors, and 38 basaloid tumors.
Figure 3. Flowchart reclassifying tumors originally diagnosed as squamous cell carcinoma (n=480) with histologic review and immunohistochemical analysis.
Among keratinizing tumors (n = 251), 250 were confirmed as keratinizing squamous cell carcinoma—p40 positive but TTF-1 (8G7G3/1) negative—and one tumor was reclassified as adenosquamous carcinoma since it had both squamous cell carcinoma and adenocarcinoma morphology. Among nonkeratinizing tumors (n = 191), 165 cases were confirmed as nonkeratinizing squamous cell carcinoma—p40 positive but TTF-1 (8G7G3/1) negative—and there were 20 cases reclassified as adenocarcinoma since they were TTF-1 (8G7G3/1 or SPT24) positive but p40 only very focally positive or negative. The two cases reclassified as large cell neuroendocrine carcinoma exhibited neuroendocrine morphology and differentiation. Among basaloid tumors (n = 38), 34 were confirmed as basaloid squamous cell carcinoma—p40 positive but TTF-1 (8G7G3/1) negative—and 4 tumors were reclassified as high-grade neuroendocrine carcinoma (2 large cell neuroendocrine carcinomas and 2 small cell carcinomas) because they exhibited neuroendocrine morphology and differentiation.
All tumors were first classified on the basis of p40 and TTF-1 (8G7G3/1) expression because of their high specificity in differentiating squamous cell carcinoma from adenocarcinoma.16, 20 However, if the tumors were negative for both of these markers without diffuse TTF-1 (SPT24) expression in tissue microarray analysis (n = 11), squamous and adenocarcinoma differentiation was confirmed via immunohistochemistry (p40 and TTF-1 [SPT24]) and mucicarmine stain using whole blocks. However, not all cases were systemically stained for mucin staining using whole blocks. In addition, if that tumor showed neuroendocrine morphology and differentiation in tissue microarray analysis (n = 6), neuroendocrine marker expression (Chromogranin A and CD56) was confirmed with immunohistochemistry using whole blocks.
Among keratinizing tumors (n = 251), 250 were positive for p40 but negative for TTF-1 (8G7G3/1); and they were confirmed as keratinizing squamous cell carcinoma. One tumor was identified as having both squamous cell carcinoma and adenocarcinoma morphology (more than 10% for each component). After immunohistochemistry and mucicarmine histochemistry, using a whole tumor block, this tumor was reclassified as adenosquamous carcinoma with both p40 and TTF-1 expression in distinct areas and focal positivity for mucin stain in the area with adenocarcinoma morphology.
Among nonkeratinizing tumors (n = 191), 2 tumors showed neuroendocrine morphology and differentiation and they were reclassified as large cell neuroendocrine carcinomas. Of the tumors showing no neuroendocrine morphology (n = 189), 165 were positive for p40 (Figure 1D) but negative for TTF-1 (8G7G3/1), and they were confirmed as nonkeratinizing squamous cell carcinoma. Among them, there were two cases where p40 was negative on tissue microarray analysis but positive on whole block analysis; these tumors were negative for mucicarmine stain. Ten cases were positive for TTF-1 (8G7G3/1) and negative for p40, and they were reclassified as adenocarcinoma. Three cases were all focally positive for p40, but focally (n = 2) or diffusely (n = 1) positive for TTF-1 (8G7G3/1), all diffusely positive for TTF-1 (SPT24), and focally (n = 1) or diffusely (n = 2) positive for Napsin A, and they were reclassified as adenocarcinoma. Eleven cases were completely negative for both p40 and TTF-1 (8G7G3/1), and they were further classified using TTF-1 (SPT24). There were 3 cases that were diffusely positive for TTF-1 (SPT24), and one of them was positive for Napsin A as well; those 3 cases were subsequently reclassified as adenocarcinoma. One of them was very focally positive for TTF-1 (SPT24) on tissue microarray analysis but was diffusely positive for TTF-1 (SPT24) and mucicarmine stain on whole block analysis. Eight cases were only focally positive (n = 3) or negative (n = 5) for TTF-1 (SPT24) in tissue microarray analysis, and were all negative for Napsin A. After reevaluation of these 8 cases with whole block analysis, 4 cases were focally positive for TTF-1 (SPT24), and one of them was positive for mucicarmine stain; those 4 cases were reclassified as adenocarcinoma. The other 4 cases remained negative for TTF-1 (SPT24) and mucicarmine stain in whole block analysis, and were classified as large cell carcinoma. In 11 cases tested for mucicarmine stain, none of the squamous cell carcinomas (n=2; positive for p40 but negative for TTF-1 [8G7G3/1 and SPT24]) or large cell carcinomas (n=4; negative for p40 and TTF-1 [8G7G3/1 and SPT24]) were positive for mucicarmine stain, and 2 out of 5 adenocarcinomas (negative for p40 but positive for TTF-1 [SPT24]) were positive for mucicarmine stain. Of all nonkeratinizing tumors, 20 cases (10%) were reclassified as adenocarcinoma.
Among basaloid tumors (n=38), 4 tumors showed neuroendocrine morphology and differentiation (Figure 2C and 2D), and they were reclassified as high grade neuroendocrine carcinoma (2 large cell neuroendocrine carcinomas and 2 small cell carcinomas). Those cases exhibited rosette-like structures and nuclear palisading (Figure 2C) that probably contributed to their misidentification as basaloid squamous cell carcinoma. Among them, there was one small cell carcinoma that had focal large cell morphology and was classified as combined small cell carcinoma and large cell carcinoma. The rest of the tumors (n = 34) were positive for p40 (Figure 2B) but negative for TTF-1 (8G7G3/1), and they were confirmed as basaloid squamous cell carcinoma.
Distribution of Histologic Types Confirmed with Immunohistochemistry
Of all 480 cases originally diagnosed as squamous cell carcinoma, 449 (93.5%) were positive for p40 and negative for TTF-1 (8G7G3/1), and were confirmed as squamous cell carcinomas including 250 keratinizing, 165 nonkeratinizing, and 34 basaloid tumors. The 20 cases (4.2%) that were positive for TTF-1 (8G7G3/1 or SPT24) with no or focal p40 expression were reclassified as adenocarcinoma. In addition, 1 case (0.2%) was reclassified as adenosquamous carcinoma, 4 (0.8%) cases as large cell carcinoma, 4 (0.8%) cases as large cell neuroendocrine carcinoma, and 2 (0.4%) cases as small cell carcinoma.
Immunohistochemical and Morphological Profiles of Squamous Cell Carcinomas Confirmed with Immunostaining
Table 1 summarizes the immunohistochemical profile of squamous cell carcinomas confirmed by immunohistochemistry (n = 449; p40 positive and TTF-1 [8G7G3/1] negative). Of all, p40 was diffusely positive in 97% and strongly positive in 91% of cases. p63 was diffusely positive in 97% and strongly positive in 91%. Focal positivity for p40 and p63 was identified in 13 (3%) and 14 (3%) cases, respectively, and every case was negative for TTF-1 (SPT-24). In tissue microarray analysis for p63, 3 cases lacked tumor cores for evaluation but all were positive for p40 where the tumor cores were available. TTF-1 (SPT24) was positive in 27 (6%) squamous cell carcinomas cases, but most of them were focally (70%) and weakly (63%) positive. Importantly, all squamous cell carcinomas cases that were positive for TTF-1 (SPT24) were diffusely positive for p40.
Table 1.
Immunohistochemical profile in lung squamous cell carcinomas confirmed with immunohistochemistry (n = 449)
| Marker | Positive | Distribution
|
Intensity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Focal | Diffuse | Weak | Moderate | Strong | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| p40 | 449 | 100% | 13 | 3% | 436 | 97% | 3 | 1% | 36 | 8% | 410 | 91% |
| p63* | 446 | 100% | 14 | 3% | 432 | 97% | 8 | 2% | 34 | 8% | 404 | 91% |
| TTF-1 8G7G3/1 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| TTF-1 SPT24** | 27 | 6% | 19 | 70% | 8 | 30% | 17 | 63% | 7 | 26% | 3 | 11% |
| Napsin A** | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Chromogranin A | 45 | 10% | 41 | 91% | 4 | 9% | 40 | 89% | 4 | 9% | 1 | 2% |
| Synaptophysin** | 17 | 4% | 16 | 94% | 1 | 6% | 11 | 65% | 5 | 29% | 1 | 6% |
| CD56 | 70 | 16% | 36 | 51% | 34 | 49% | 59 | 84% | 7 | 10% | 4 | 6% |
No tissue microarray core in 3 cases.
No tissue microarray core in 1 case.
With regard to neuroendocrine markers, Chromogranin A was positive in 45 (10%) cases, Synaptophysin was positive in 17 (4%) cases, and CD56 was positive in 70 (16%) cases. However, most of them were focal or weak expression, and none of them showed typical neuroendocrine morphology. In addition, expression of none of these neuroendocrine makers showed any prognostic impact for overall survival using the Kaplan–Meier method (P-value > 0.5 for all markers).
Table 2 summarizes immunohistochemical profiles of squamous cell carcinomas according to histologic subtypes and tumor differentiation. Almost all of keratinizing and nonkeratinizing tumors were diffusely positive for p40 (99% and 98%, respectively) and p63 (99% and 98%, respectively) while basaloid tumors less frequently showed diffuse expression for p40 (82%) and p63 (85%). TTF-1 (SPT24) was positive in 7% of keratinizing tumors and 6% of nonkeratinizing tumors while it was negative in all basaloid tumors. Diffuse TTF-1 (SPT24) expression was more frequently identified in nonkeratinizing tumors (50%) than keratinizing tumors (18%).
Table 2.
Immunohistochemical profile according to histologic subtypes and tumor differentiation in lung squamous cell carcinomas confirmed with immunohistochemistry (n = 449)
| Marker/Histologic subtypes & differentiation | Positive | Distribution
|
Intensity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Focal | Diffuse | Weak | Moderate | Strong | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| p40 | ||||||||||||
| keratinizing | 250 | 100% | 3 | 1% | 247 | 99% | 1 | 0% | 14 | 6% | 235 | 94% |
| nonkeratinizing | 165 | 100% | 4 | 2% | 161 | 98% | 2 | 1% | 16 | 10% | 147 | 89% |
| basaloid | 34 | 100% | 6 | 18% | 28 | 82% | 0 | 0% | 6 | 18% | 28 | 82% |
| well | 51 | 100% | 0 | 0% | 51 | 100% | 0 | 0% | 1 | 2% | 50 | 98% |
| moderately | 176 | 100% | 2 | 1% | 174 | 99% | 0 | 0% | 8 | 5% | 168 | 95% |
| poorly | 222 | 100% | 11 | 5% | 211 | 95% | 3 | 1% | 27 | 12% | 192 | 86% |
| p63 | ||||||||||||
| keratinizing* | 249 | 100% | 3 | 1% | 246 | 99% | 2 | 1% | 12 | 5% | 235 | 94% |
| nonkeratinizing** | 163 | 100% | 5 | 3% | 158 | 98% | 4 | 2% | 19 | 12% | 140 | 86% |
| basaloid | 34 | 100% | 6 | 18% | 28 | 85% | 2 | 6% | 3 | 9% | 29 | 88% |
| well | 51 | 100% | 0 | 0% | 51 | 100% | 0 | 0% | 2 | 4% | 49 | 96% |
| moderately* | 175 | 100% | 2 | 1% | 173 | 99% | 1 | 1% | 8 | 5% | 166 | 95% |
| poorly** | 220 | 100% | 12 | 6% | 208 | 95% | 7 | 3% | 24 | 11% | 189 | 87% |
| TTF-1 SPT24 | ||||||||||||
| keratinizing* | 17 | 7% | 14 | 82% | 3 | 18% | 11 | 65% | 5 | 29% | 1 | 6% |
| nonkeratinizing | 10 | 6% | 5 | 50% | 5 | 50% | 6 | 60% | 2 | 20% | 2 | 20% |
| basaloid | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| well | 8 | 16% | 7 | 88% | 1 | 13% | 4 | 50% | 3 | 38% | 1 | 13% |
| moderately* | 13 | 7% | 9 | 69% | 4 | 31% | 9 | 69% | 3 | 23% | 1 | 8% |
| poorly | 6 | 3% | 3 | 50% | 3 | 50% | 4 | 67% | 1 | 17% | 1 | 17% |
No tissue microarray core in 1 case.
No tissue microarray core in 2 cases.
Of all squamous cell carcinomas confirmed via immunohistochemistry, 51 (11%) cases were classified as well-differentiated tumor, 176 (39%) cases as moderately-differentiated tumor, and 222 (49%) cases as poorly-differentiated tumor. Every well-differentiated tumor was diffusely positive for p40 and p63, with strong intensity in the vast majority of cases (98% and 96%, respectively). Conversely, poorly-differentiated tumors exhibited strong intensity less frequently (86% and 86%, respectively). Tumors positive for TTF-1 showed weak to strong intensity, regardless of tumor differentiation.
Immunohistochemical and Morphological Profile of Tumors Reclassified into Other Histology From Squamous Cell Carcinoma
Table 3 summarizes the immunohistochemical profile of tumors reclassified into other histology from squamous cell carcinoma. Of reclassified adenocarcinomas (n = 20), 3 (15%) cases were focally positive for p40. In 8 (40%) cases, p63 was positive and most of those cases showed focal expression (n = 7). There were 13 (65%) cases that were positive for TTF-1 (8G7G3/1) (Figure 4D), approximately half of which showed focal (n = 8) and weak (n = 6) expression. All cases were positive for TTF-1 (SPT24) and most of them showed diffuse (n = 16) and strong (n = 17) expression. There were 13 (65%) cases positive for Napsin A. Most of these showed diffuse expression (n = 10) and half showed strong expression (n = 7). Among tumors positive for both TTF-1 SPT 24 and 8G7G3/1 (n = 13), most cases (n = 12) were also positive for Napsin A. In contrast, among cases positive for TTF-1 SPT 24 but negative for TTF-1 8G7G3/1 (n = 7), only one case was positive for Napsin A. The cases that showed any positivity for neuroendocrine markers (Chromogranin A and Synaptophysin) did not have typical neuroendocrine morphology.
Table 3.
Immunohistochemical profile in tumors reclassified into other histology from squamous cell carcinoma (n=30)
| Histology/Marker | Positive | Distribution
|
Intensity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Focal | Diffuse | Weak | Moderate | Strong | ||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Adenocarcinoma (n=20) | ||||||||||||
| p40 | 3 | 15% | 3 | 100% | 0 | 0% | 1 | 33% | 2 | 67% | 0 | 0% |
| p63 | 8 | 40% | 7 | 88% | 1 | 13% | 4 | 50% | 4 | 50% | 0 | 0% |
| TTF-1 (8G7G3/1) | 13 | 65% | 8 | 62% | 5 | 38% | 6 | 46% | 2 | 15% | 5 | 38% |
| TTF-1 (SPT24) | 20 | 100% | 4 | 20% | 16 | 80% | 0 | 0% | 3 | 15% | 17 | 85% |
| Napsin A | 13 | 65% | 3 | 23% | 10 | 77% | 2 | 15% | 4 | 31% | 7 | 54% |
| Chromogranin A | 3 | 15% | 3 | 100% | 0 | 0% | 3 | 100% | 0 | 0% | 0 | 0% |
| Synaptophysin | 2 | 10% | 2 | 100% | 0 | 0% | 2 | 100% | 0 | 0% | 0 | 0% |
| CD56 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||||||||
| Neuroendocrine carcinoma (n=6) | ||||||||||||
| p40 | 1 | 17% | 0 | 0% | 1 | 100% | 0 | 0% | 0 | 0% | 1 | 100% |
| p63 | 3 | 50% | 2 | 67% | 1 | 33% | 2 | 67% | 0 | 0% | 1 | 33% |
| TTF-1 (8G7G3/1) | 2 | 33% | 2 | 100% | 0 | 0% | 2 | 100% | 0 | 0% | 0 | 0% |
| TTF-1 (SPT24) | 3 | 50% | 1 | 33% | 2 | 67% | 1 | 33% | 0 | 0% | 2 | 67% |
| Napsin A | 1 | 17% | 1 | 100% | 0 | 0% | 1 | 100% | 0 | 0% | 0 | 0% |
| Chromogranin A | 3 | 50% | 3 | 100% | 0 | 0% | 1 | 33% | 2 | 67% | 0 | 0% |
| Synaptophysin | 3 | 50% | 2 | 67% | 1 | 33% | 1 | 33% | 2 | 67% | 0 | 0% |
| CD56 | 5 | 83% | 0 | 0% | 5 | 100% | 2 | 40% | 2 | 40% | 1 | 20% |
|
| ||||||||||||
| Large cell carcinoma (n=4) | ||||||||||||
| p40 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| p63 | 1 | 25% | 1 | 100% | 0 | 0% | 0 | 0% | 1 | 100% | 0 | 0% |
| TTF-1 (8G7G3/1) | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| TTF-1 (SPT24) | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Napsin A | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Chromogranin A | 1 | 25% | 1 | 100% | 0 | 0% | 1 | 100% | 0 | 0% | 0 | 0% |
| Synaptophysin | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| CD56 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
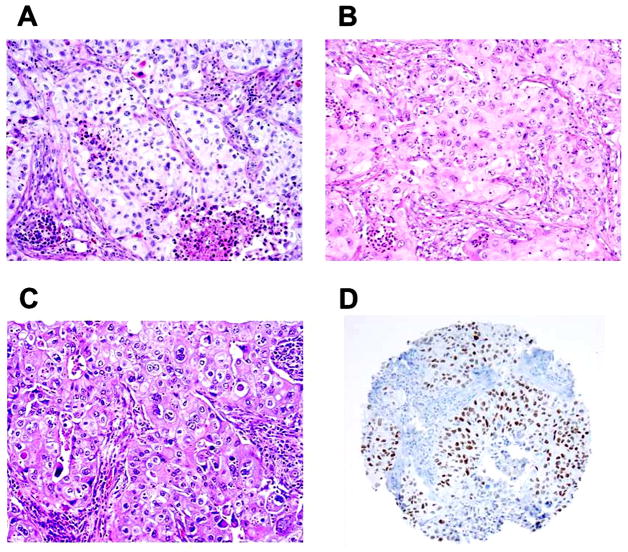
Figure 4. Squamoid morphology of lung adenocarcinoma (original magnification, x100: A–C).
(A) Cytoplasmic keratinization-like feature (eosinophilic cytoplasm with pyknotic nuclei) identified near necrotic area (bottom right). (B) Squamoid tumor cells that exhibited abundant eosinophilic cytoplasm. (C) Squamoid tumor cells the exhibited abundant eosinophilic cytoplasm with sharp cell borders and intercellular bridge-like features. (D) Squamoid adenocarcinoma positive for TTF-1 (8G7G3/1).
Morphologically, all tumors reclassified as adenocarcinomas from squamous cell carcinomas were poorly-differentiated (solid subtype) tumors with no clear glandular structure. They showed focal squamoid morphology, including cytoplasmic findings that were suggestive of keratinization such as abundant eosinophilic cytoplasm with sharp cell borders and pyknotic nuclei, and features that were suggestive of intercellular bridges (Figures 4A, 4B, and 4C). These were occasionally observed near necrotic areas with acute inflammation. Of all squamoid adenocarcinomas reclassified from squamous cell carcinomas in this series, only one case was described in our prior publication.21
Of tumors reclassified as neuroendocrine carcinomas (large cell neuroendocrine carcinoma [n = 4] and small cell carcinomas [n = 2]), p63 was positive in 3 cases. One of those was also positive for p40 with squamous morphology, and was classified as combined large cell neuroendocrine carcinoma and squamous cell carcinoma.
All tumors reclassified as large cell carcinomas (n = 4) were negative for p40, TTF-1 (8G7G3/1 and SPT24), Napsin A, Synaptophysin, and CD56. One case was focally positive for p63 but was not classified as squamous cell carcinoma because it was negative for p40 and did not show keratinization. One case was focally positive for Chromogranin A but was not classified as neuroendocrine carcinoma because it did not show neuroendocrine morphology.
Discussion
One of the significant advances in lung cancer classification in the past decade was the discovery that immunohistochemical analysis was required for precise diagnosis in a significant percentage of tumors.11–17 While this discovery was in the context of small biopsies and cytology samples from patients with advanced lung cancer, data from our study showed that immunostains were also needed for proper histologic classification of resected tumors, particularly poorly-differentiated tumors. In a prior study of 98 resected tumors with original diagnosis of squamous cell carcinoma from our institution, immunohistochemical analysis reclassified 4 cases as solid variant of adenocarcinoma with squamoid morphologic features.21 In support of immunophenotypic re-classification, it was found that some of these tumors harbor mutations typical of adenocarcinoma (EGFR, KRAS) rather than squamous cell carcinoma.21 To expand on these observations, in this study we reviewed 480 tumors that had been originally diagnosed as squamous cell carcinoma, and performed immunohistochemistry for squamous cell carcinoma markers (p40 and p63), adenocarcinoma markers (TTF-1, both 8G7G3/1 and SPT24, antibodies and Napsin A), and neuroendocrine markers (Chromogranin A, Synaptophysin, and CD56) using tissue microarray analysis. After this re-evaluation, 449 (93.5%) cases were confirmed as squamous cell carcinoma. The remainder of the cases were reclassified as adenocarcinoma (n = 20), adenosquamous carcinoma (n = 1), large cell carcinoma (n = 4), and high grade neuroendocrine carcinoma (small cell carcinoma or large cell neuroendocrine carcinoma; n = 6).
In the upcoming revision of the WHO Classification of Tumours of the Lung, squamous cell carcinoma will be classified as either keratinizing, nonkeratinizing, or basaloid subtype.27 The results of our study underscore the importance of immunohistochemistry for tumors that are classified as either nonkeratinizing squamous cell carcinomas or basaloid carcinoma.
Introduction of immunohistochemistry into routine diagnostic work requires awareness of sensitivity and specificity of the various antibodies that are available. Our group and others have previously reported that p40 was equivalent to p63 in sensitivity for squamous cell carcinoma; p40 still had greater specificity than p63.16, 17 In lung cancer, previous reports have found that TTF-1 (clone 8G7G3/1) was more specific (positive in 1% of squamous cell carcinomas), yet less sensitive (65–77%), for lung adenocarcinoma while TTF-1 (clone SPT24) was less specific (positive in 17% of squamous cell carcinomas), yet more sensitive (72–84%), for adenocarcinomas when differentiated from squamous cell carcinomas.17, 19, 20 In order to differentiate adenocarcinoma from tumors originally diagnosed as squamous cell carcinoma, we first classified them by p40 and TTF-1 (8G7G3/1) because of their high specificity for squamous cell carcinoma and adenocarcinoma, respectively. After this evaluation, 449 cases (94%) were confirmed as squamous cell carcinoma (p40 positive/TTF-1 [8G7G3/1] negative).
In all squamous cell carcinoma cases confirmed via immunohistochemistry (p40 positive and TTF-1 [8G7G3/1] negative) in our series, the vast majority of them were diffusely (97%) or strongly (91%) positive for p40. Similarly, all squamous cell carcinoma cases were also positive for p63 and the vast majority of them were diffusely (97%) or strongly (91%) positive for p63. These findings confirmed high sensitivity of p40 and p63 for the detection of squamous cell carcinomas. Among the subset of squamous cell carcinoma cases that were completely negative for TTF-1 (8G7G3/1), 27 (6%) cases were focally or diffusely positive for TTF-1 (SPT24) with weakly to strong intensity; these cases were diffusely positive for p40. In contrast, all squamous cell carcinoma cases were negative for Napsin A. These findings confirmed that TTF-1 (SPT24) had lower specificity than TTF-1 (8G7G3/1) and Napsin A in the classification of poorly-differentiated tumors into adenocarcinomas. All of these findings suggest that the anti-TTF-1 antibody that is more specific for adenocarcinoma (clone 8G7G3/1) is better suited as the primary antibody in routine clinical practice for addressing the differential of lung adenocarcinoma versus squamous cell carcinoma. It also emphasizes the importance of staining for both squamous and adenocarcinoma markers in poorly differentiated solid carcinomas rather than only adenocarcinoma or only squamous markers.
Of all tumors reclassified as adenocarcinoma in our series (n = 20), only 3 cases were positive for p40—yet all of them had only focal positivity for p40—and no case showed clear squamous differentiation by morphology or strong positivity for p40. Focal labeling for p40 in lung adenocarcinomas has been previously described by our group.16 On the other hand, p63 was positive (n = 8) in more cases—although staining was only focal in most cases (n = 7)—and no case showed strong intensity for p63. These findings may support the previous reported results that demonstrated that p40 had higher specificity than p63 for squamous cell carcinoma.16, 17 Among tumors reclassified as adenocarcinomas, 13 cases were positive for TTF-1 (8G7G3/1), which is more specific antibody for lung adenocarcinoma; most of those (n = 12) were also positive for Napsin A. The rest of them (n = 7) were negative for TTF-1 (8G7G3/1) but positive for TTF-1 (SPT 24), which is less specific antibody for lung adenocarcinoma; however, only one of those were positive for Napsin A. Given the lower specificity but higher sensitivity of TTF-1 SPT24 antibody, it cannot be determined in individual cases whether labeling for SPT24 in 8G7G3/1-negative cases reflects true low-level expression - as may be suspected in our single Napsin A-positive case - versus non-specific labeling, as seen in a subset of squamous cell carcinomas. Further studies are warranted to investigate, in poorly differentiated lung carcinomas, whether TTF-1 SPT24 antibody can reliably classify tumors that are negative with highly specific markers of adenocarcinoma and squamous cell carcinoma (TTF-1 8G7G3/1 and p40) into either adenocarcinoma or large cell carcinoma. Regardless, for decisions about molecular testing and management, there is no major clinical impact whether a tumor is considered to be a solid adenocarcinoma or a large cell carcinoma.
All squamous cell carcinomas cases that were reclassified as adenocarcinomas (n = 20) were poorly-differentiated with some amount of squamoid morphology, as originally described by Rekhtman et al.21—abundant eosinophilic cytoplasm, sharp cell borders, and intercellular bridges—and often near necrotic area of the tumors, but they did not have typical layered keratinization. It can be very difficult to classify these cases as either poorly-differentiated adenocarcinoma or squamous cell carcinoma using only histological assessment, via H&E stained slides, and may require further immunohistochemical analysis. It must also be emphasized that immunohistochemistry was not required for the diagnosis of every squamous cell carcinoma. If squamous differentiation in the tumor—layered keratin and cytoplasmic keratinization with intercellular bridge—was clearly distinguishable based on morphological assessment on H&E stained slides and if there was no secondary component that morphologically raised the possibility of the tumor classified as adenocarcinoma, small cell carcinoma, or large cell neuroendocrine carcinoma. The positive staining for small percentages of neuroendocrine markers has been previously recognized but is not known to have any clinical significance in adenocarcinomas or squamous cell carcinomas that lack neuroendocrine morphology or that are a component of a combined neuroendocrine tumor such as large cell neuroendocrine carcinoma or small cell carcinoma.10, 28–30 The lack of prognostic significance for neuroendocrine markers in lung squamous cell carcinoma was confirmed in our data.
In addition to the distinction between lung squamous cell carcinoma and adenocarcinoma, high-grade neuroendocrine carcinoma and large cell carcinoma also need to be differentiated from poorly-differentiated squamous cell carcinoma, especially if the tumors have a basaloid feature. In our series, 4 out of the 6 cases (2 large cell carcinoma and 2 small cell carcinoma) reclassified as high grade neuroendocrine carcinoma had predominant (≥50%) basaloid-like patterns and they were difficult to differentiate from basaloid squamous cell carcinomas based on morphology alone.
In conclusion, we demonstrated the need for immunohistochemistry in poorly-differentiated tumors that were originally considered squamous cell carcinomas, particularly those of the nonkeratinizing or basaloid types. We reclassified 6% (n = 31) of the tumors originally diagnosed as squamous cell carcinoma (n = 480) into other histologic types—adenocarcinoma (n = 20), adenosquamous carcinoma (n = 1), large cell carcinoma (n = 4), and high grade neuroendocrine carcinoma (n = 6). We confirmed 94% (n = 449) as squamous cell carcinoma after re-evaluation with slide review and immunohistochemistry. In the cases where typical adenocarcinoma morphology—lepidic, acinar, papillary, or micropapillary structures—was not identified and clear squamous morphology was not evident, immunohistochemical analysis using p40 and TTF-1 (8G7G3/1), which are markers for squamous cell carcinoma and adenocarcinoma, respectively, may be useful. In tumors that have basaloid morphology, immunohistochemistry for neuroendocrine markers and TTF-1 should be considered, particularly if p40 is negative; this remains true even if the tumor has some squamoid morphology.
Acknowledgments
Our laboratory work is supported in part by the William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; the National Cancer Institute (grants R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705-06, U54 CA137788, P50 CA086438-13, and U54 CA132378); and the U.S. Department of Defense (grants PR101053 and LC110202). We thank Dr. Ronald A. Ghossein, our Head and Neck pathology expert at Memorial Sloan Kettering Cancer Center, for advice on the evaluation of histological features and for reviewing many of the cases in this study, Joe Dycoco for help with the lung carcinoma database in the Thoracic Service, Department of Surgery, and Irina Linkov, Avani Giri and Louie Lopez, for help with tissue microarray construction and immunohistochemistry.
Footnotes
Disclosure/conflict of interest
The authors made no disclosures.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 5.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 8.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis W, Brambilla E, Muller-Hermelink H, et al. Tumours of the Lung, Pleura, Thymus, and Heart. IARC Press; Lyon, France: 2004. World Health Organization Classification of Tumours. Pathology and Genetics. [Google Scholar]
- 11.Savci-Heijink CD, Kosari F, Aubry MC, et al. The role of desmoglein-3 in the diagnosis of squamous cell carcinoma of the lung. Am J Pathol. 2009;174:1629–1637. doi: 10.2353/ajpath.2009.080778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348–1359. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 13.Whithaus K, Fukuoka J, Prihoda TJ, et al. Evaluation of napsin A, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:155–162. doi: 10.5858/arpa.2011-0232-OA. [DOI] [PubMed] [Google Scholar]
- 14.Turner BM, Cagle PT, Sainz IM, et al. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. 2012;136:163–171. doi: 10.5858/arpa.2011-0320-OA. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Shin HC, Shin KC, et al. Best immunohistochemical panel in distinguishing adenocarcinoma from squamous cell carcinoma of lung: tissue microarray assay in resected lung cancer specimens. Ann Diagn Pathol. 2013;17:85–90. doi: 10.1016/j.anndiagpath.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 17.Nonaka D. A study of DeltaNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol. 2012;36:895–899. doi: 10.1097/PAS.0b013e3182498f2b. [DOI] [PubMed] [Google Scholar]
- 18.Rekhtman N, Tafe LJ, Chaft JE, et al. Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod Pathol. 2013;26:511–522. doi: 10.1038/modpathol.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comperat E, Zhang F, Perrotin C, et al. Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod Pathol. 2005;18:1371–1376. doi: 10.1038/modpathol.3800422. [DOI] [PubMed] [Google Scholar]
- 20.Matoso A, Singh K, Jacob R, et al. Comparison of Thyroid Transcription Factor-1 Expression by 2 Monoclonal Antibodies in Pulmonary and Nonpulmonary Primary Tumors. Appl Immunohistochem Mol Morphol. 2010;18:142–149. doi: 10.1097/PAI.0b013e3181bdf4e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res. 2012;18:1167–1176. doi: 10.1158/1078-0432.CCR-11-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadota K, Nitadori JI, Woo KM, et al. Comprehensive Pathological Analyses in Lung Squamous Cell Carcinoma: Single Cell Invasion, Nuclear Diameter, and Tumor Budding Are Independent Prognostic Factors for Worse Outcomes. J Thorac Oncol. 2014;9:1126–1139. doi: 10.1097/JTO.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes L, Eveson J, Reichart P, et al. Tumours of the Head and Neck. IARC Press; Lyon, France: 2005. World Health Organization Classification of Tumours. Pathology and Genetics. [Google Scholar]
- 24.Moro-Sibilot D, Lantuejoul S, Diab S, et al. Lung carcinomas with a basaloid pattern: a study of 90 cases focusing on their poor prognosis. Eur Respir J. 2008;31:854–859. doi: 10.1183/09031936.00058507. [DOI] [PubMed] [Google Scholar]
- 25.Kadota K, Nitadori J, Sarkaria IS, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer. 2013;119:931–938. doi: 10.1002/cncr.27863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: Tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travis W, Brambilla E, Nicholson A, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer (IARC); Lyon, France: 2015. [DOI] [PubMed] [Google Scholar]
- 28.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ionescu DN, Treaba D, Gilks CB, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation--an entity of no clinical or prognostic significance. Am J Surg Pathol. 2007;31:26–32. doi: 10.1097/01.pas.0000213319.04919.97. [DOI] [PubMed] [Google Scholar]
- 30.Sterlacci W, Fiegl M, Hilbe W, et al. Clinical relevance of neuroendocrine differentiation in non-small cell lung cancer assessed by immunohistochemistry: a retrospective study on 405 surgically resected cases. Virchows Arch. 2009;455:125–132. doi: 10.1007/s00428-009-0812-0. [DOI] [PubMed] [Google Scholar]