Abstract
Objectives
Little is known regarding complementary and alternative medicine (CAM) use during pregnancy and the preconception period. Since half of all U.S. pregnancies are unintended, understanding the patterns of CAM use among women of childbearing age has implications for fetal and maternal health.
Methods
Descriptive statistics were generated from the 2012 National Health Interview Survey (NHIS) to estimate weighted prevalence and patterns of CAM use by women of childbearing age. Comparisons were made between pregnant and non-pregnant respondents.
Results
In this sample of 10,002 women, 7% (n=727) were recently pregnant. Over one third of all the women used CAM during the previous year (34%/38%, pregnant/non-pregnant, respectively) and only half disclosed CAM use to conventional providers (50%/49%). In the adjusted model, taking multivitamins (OR=2.52 [2.22–2.86]) and moderate to heavy alcohol use (1.92 [1.53–2.41] were more likely associated with CAM use. The two most commonly used modalities were herbs (14%/17%) and yoga (13%/16%). The top reasons for CAM use were to improve general wellness or to prevent disease (33%/35%) and to treat back pain (16%/18%). When examining all pregnancy-related symptoms treated with CAM, no difference was found in the rates of CAM use between pregnant and non-pregnant users.
Conclusions
CAM use by women of childbearing age in the U.S is common, with over a third of the population using one or more therapies. However, only half disclosed their use to conventional providers despite limited evidence on safety and effectiveness. This study highlights the important need for further research in this area.
Keywords: preconception health, NHIS, CAM, pregnancy
INTRODUCTION
The health status of a woman during pregnancy as well before conception significantly influences maternal and fetal outcomes.(1, 2) In the U.S. half of all pregnancies are unintended,(3, 4) and the infant mortality rate is high compared to other developed countries.(5) In response to this problem, public health initiatives have emerged, which focus on factors that impact maternal health not only during pregnancy but also during the unpredictable time before conception.(6) These efforts emphasize the importance of preconception health counseling to a broadened audience of women who may have planned, as well as unplanned or unintended pregnancies. (7–10) These initiatives include the management of medical conditions and mental health issues that would impact potential pregnancies such as diabetes, hypertension or depression. Preconception health initiatives also emphasize counseling for modifiable health behaviors such as smoking and nutrition.
Complementary and alternative medicine (CAM) is a diverse collection of health care approaches and therapies developed outside of conventional allopathic medicine, which often emphasize health behavior change. Women of childbearing age represent one of the largest groups of CAM users.(11–13) Analysis of the 2007 National Health Interview Study (NHIS) found that almost half of all women aged between 18–49 years old used some type of CAM in the previous year.(14) In other NHIS studies, white, highly educated women and those who live in a western or northeastern part of the United States and were more likely to use CAM.(13, 15, 16) Incidentally, this same socio-demographic profile of women is associated with improved pregnancy outcomes, including lower infant mortality and preterm birth rates,(17–19) although the association of improved pregnancy outcomes with CAM use has not been studied.
Prior analyses of the 2007 NHIS reported no significant difference in CAM use between pregnant or post-partum (36%) and non-pregnant (41%) women.(14) However, the previous analysis didn’t include other important health behaviors, such as smoking, and did not analyze data separately for specific CAM modalities and reasons for use. International studies and convenience samples within the US have reported 20–70% use of CAM by pregnant women for general conditions including pregnancy-related symptoms such as back pain, fatigue and dysuria.(20–24)
While some therapies such as yoga and massage may be beneficial for stress, wellness, and pain reduction,(25, 26) other modalities may be harmful.(27, 28) Clinicians who provide preconception counseling or obstetrical care should be aware of the patterns of CAM use among women of childbearing age so that they may direct care towards therapies known to be safe and effective.
The purpose of this study is to report the prevalence of CAM use and its modalities in the U.S. by women of childbearing age and its subgroup of pregnant women with respect to demographic, behavioral and health factors that are influential to preconception and pregnancy health.
METHODS
We used data from the 2012 NHIS a nationally representative study of the civilian, non-institutionalized U.S. adult population conducted by the National Center for Health Statistics and the U.S. Department of Health and Human Services. The survey was conducted in English or Spanish and oversampled minority populations. Data files from NHIS’s Adult Core, Family Core and CAM questionnaires were analyzed. IRB exemption was obtained from the Institutional Review Board of Beth Israel Deaconess Medical Center in Boston, Massachusetts.
Women of childbearing age are defined to be all adult female respondents between 18 and 49 years old. Those younger than 18 were not included because pregnancy-related questions were only asked of women 18 years or older.(29) Women who were either currently pregnant or pregnant within 1 year of data collection were categorized as pregnant/recently pregnant.
Socio-demographic factors, health behaviors and other health factors that impact preconception health were selected from the Family and Adult Cores. Sub-groups with small sample sizes (n<30), were grouped together to provide sufficient numbers for reporting.
Included socio-demographic factors were: age (continuous), dichotomized race (white or non-white), dichotomized marital status, education (high school graduate or less, some college with no degree, undergraduate degree or more), dichotomized employment, yearly income (<$35K, $35–$75K, $75–$100K or greater than $100K), region of residence (Northeast, Midwest, South and West) and insurance status (none/other/unknown, Medicare/Medicaid, or private).
The health behaviors included were: physical activity (low = no vigorous activity or moderate activity once a week; medium = vigorous activity < 3 times/week or moderate activity which is ≥ 2 times/week and ≤ 5 times/week; high = vigorous activity > 3 times/week or moderate activity ≥ 6 times/week), smoking status (never, former or sometimes/daily), alcohol consumption (not current, light/infrequent or moderate/heavy) and dichotomous multivitamin/mineral use.
The other health factors included: perceived health status (fair/poor/don’t know, good, very good, excellent), BMI (<18.5, 18.5–25, 25–30, >30 kg/m2) and symptoms or diseases that may impact birth or maternal outcomes (menstrual problems, headaches, nausea/vomiting, low back pain, muscle/bone pain, depression, anxiety, frequent stress, insomnia, and fatigue/lack of energy).(1, 30–32)
The NHIS Adult CAM Supplement collected data on the use of 18 different CAM therapies within the past year. These modalities were collapsed into five categories defined by the National Center for Complementary and Alternative Medicine: (33)
Biologic Therapies: Included non-vitamin/non-mineral dietary supplements such as herbs and fish oil, special diets and chelation therapy. Vitamins and minerals were excluded from this group of modalities because 1) they were already accounted for in the health behaviors and 2) it was not possible to distinguish prenatal vitamin use from other vitamin use.
Body Based/Manipulative Therapies: Included osteopathic, chiropractic, massage, Craniosacral, Alexander technique, Feldenkrais, and Trager Psychophysical Integration.
Mind-body Therapies: Included biofeedback, hypnosis, tai chi, qi gong, yoga, and mindful practice/meditation.
Alternative Medical Systems: Included acupuncture, Ayurveda, homeopathy, naturopathy, native and traditional healers.
Energy healing therapies: Included Reiki, Therapeutic Touch and others.
CAM use was defined as use of any of these modalities during the previous year. Univariate, weighted chi-squared analyses were conducted between all the women of childbearing age who did and did not use CAM for each of the demographics, health behaviors and other health factors.
A multivariable logistic regression model was built to identify independent variables associated with CAM use in all woman of childbearing age. All of the listed socio-demographic characteristics, health behaviors and health conditions were included.
Using the previously defined groups of CAM modalities and any individual modality that had n>30, a weighted chi-squared analyses was done to determine the differences between use of any CAM or its subgroups among pregnant and non-pregnant women.
We calculated frequencies and percentages for reasons of use and health conditions for the pregnant and non-pregnant groups of CAM users. From a predefined list of 88 health conditions, participants were asked to select which condition they were treating with their top 3 CAM therapies. Responses were grouped together by physiologic systems including musculoskeletal, psychiatric, gastrointestinal, coronary vascular disease, dermatologic, otolaryngic, gynecologic/genitourinary, infectious disease/rheumatologic, neurologic, respiratory and other. Since the variables available through NHIS that measured conditions directly associated with pregnancy were not linked to CAM treatment, we defined a sub-list of pregnancy-associated symptoms from the 88 health conditions treated with CAM. The specific conditions for each category are listed in Appendix A. Data are reported for cell sizes >30. Weighted chi-squared analyses were done on the pregnant and non-pregnant CAM users for each group of physiologic systems.
Additionally, the percentage of CAM users, pregnant and not, who disclosed their use of CAM to their conventional health care professionals was also calculated.
We used the survey analysis procedures in SAS v9.3 with appropriate population weights to obtain accurate estimates and standard errors for the US population.
RESULTS
Our sample included 10,002 women of childbearing age, representing 67.2 million women. An estimated 38% of all U.S. women of childbearing age (about 25 million women) were CAM users, regardless of pregnancy status. The mean age of the CAM users was 34.2 years (+/−0.23). The mean age of the non-CAM users was 33.1 years (+/−0.16)(Table 1).
TABLE 1.
Characteristics of CAM using women aged 18–49, National Health Interview Survey, 2012
| Any CAM use | No CAM use | |
|---|---|---|
| n=3632 | n=6370 | |
| Demographics | ||
| Estimated US Population Size | 25,206,626 | 41,983,237 |
| Mean Age +/− SD | 34.02 ±0.05 | 33.2 ±0.03 |
| (%) | (%) | |
| White Race | 83 | 74 |
| Married/Living with partner | 59 | 55 |
| Education | ||
| ≤ High school grad | 20 | 44 |
| Some College, no degree | 25 | 23 |
| ≥Undergraduate degree | 55 | 33 |
| Employed | 73 | 62 |
| Yearly Income ($) | ||
| 0–35K | 28 | 45 |
| 35–75K | 29 | 30 |
| 75–100K | 14 | 10 |
| >100K | 28 | 15 |
| Region of Residence | ||
| Northeast | 18 | 18 |
| Midwest | 25 | 21 |
| South | 29 | 41 |
| West | 28 | 20 |
| Insurance | ||
| None/Other/Unknown | 18 | 29 |
| Medicare/Medicaid | 7 | 18 |
| Private | 74 | 54 |
| Behaviors | ||
| Physical Activity | ||
| Low | 18 | 40 |
| Med (Vig<3×/wk or 2<=Mod<=5×/wk) | 27 | 24 |
| High (Vig>=3×/wk or mod >=6×/wk) | 55 | 35 |
| Smoking Status | ||
| Never | 67 | 72 |
| Former | 16 | 9 |
| Current: Sometimes/Daily | 16 | 18 |
| Alcohol Consumption | ||
| Not Currently | 22 | 42 |
| Current: Light/Infrequent | 57 | 48 |
| Current: Moderate/Heavy | 21 | 10 |
| MultiVit/MineralSupplement in past year | 72 | 42 |
| Health Factors | ||
| Perceived Health Status | ||
| Fair/Poor/Don't know | 7 | 11 |
| Good | 21 | 26 |
| Very Good | 35 | 32 |
| Excellent | 37 | 32 |
| Pregnant (Current or within past year) | 6* | 8* |
| BMI (kg/m^2) | ||
| Underweight (<18.5) | 3 | 3 |
| Normal (18.5 – 25) | 51 | 39 |
| Overweight (25–30) | 23 | 24 |
| Obese (>=30) | 24 | 35 |
| Symptoms / Diseases | ||
| Menstrual Problems | 28 | 15 |
| Headaches | 28 | 14 |
| Nausea/Vomiting | 38 | 24 |
| Low Back Pain within past 3 months | 35 | 23 |
| Muscle/Bone Pain | 22 | 16 |
| Depression | 22 | 13 |
| Anxiety | 31 | 17 |
| Frequent Stress | 51 | 29 |
| Insomnia | 27 | 14 |
| Fatigue/Lack of Energy | 23 | 14 |
The only non-significant difference in prevalence (p>.05)
Of the women of childbearing age, 7% were pregnant/recently pregnant (n=727). Among these, we found that 34% (n=239) used at least one type of CAM during the previous year. Univariate analysis found no significant difference between this group and the non-pregnant CAM using women (p=0.06). There was no significant difference in rates of CAM use disclosure to primary care providers between pregnant/recently pregnant (50%) and non-pregnant (49%) CAM users.
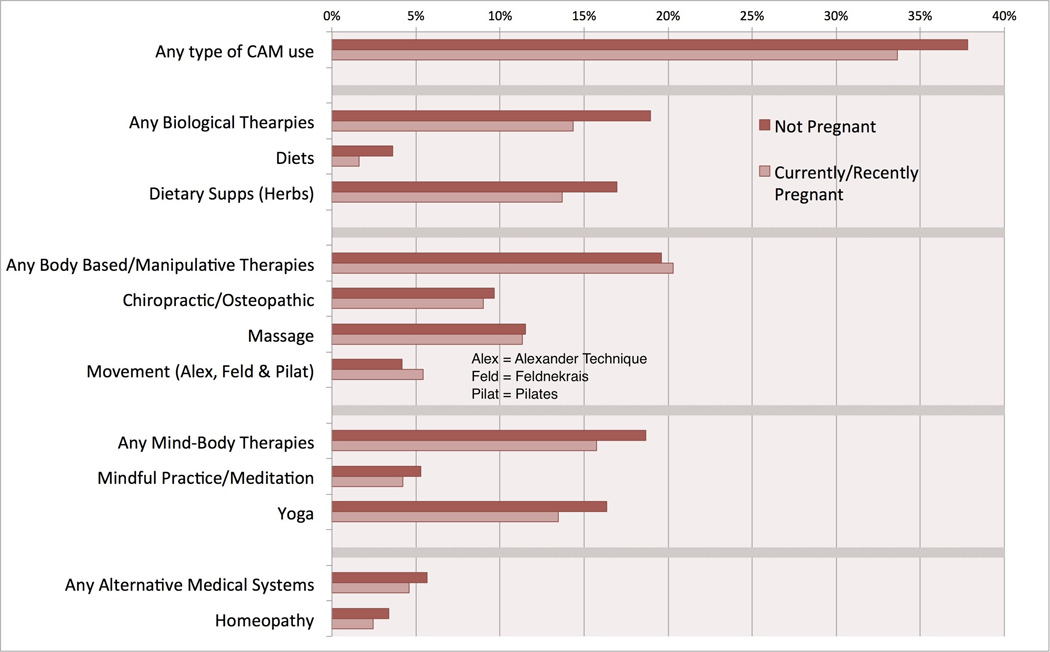
When looking at the most commonly used modalities (Figure 1), non-pregnant women used biologically-based therapies significantly more than the pregnant/recently pregnant group (diets p=0.01, non-vitamin/non-mineral supplements p=0.03, any biologics p<0.01). The number of women using energy-based therapies was not large enough to be accurately reported.
Figure 1.
After controlling for the socio-demographic factors, health behaviors and health conditions (listed in Table 1), we found that pregnant or recently pregnant women were less likely to use CAM during the previous year (p=0.02). Using multivitamins, having an undergraduate degree, moderate to heavy alcohol use, living in the west, and muscle or bone pain were the strongest predictors of CAM use for women of childbearing age. Being obese, engaging in a low amount of physical activity, and having a fair or poor perceived health status predicted the lack of CAM use.
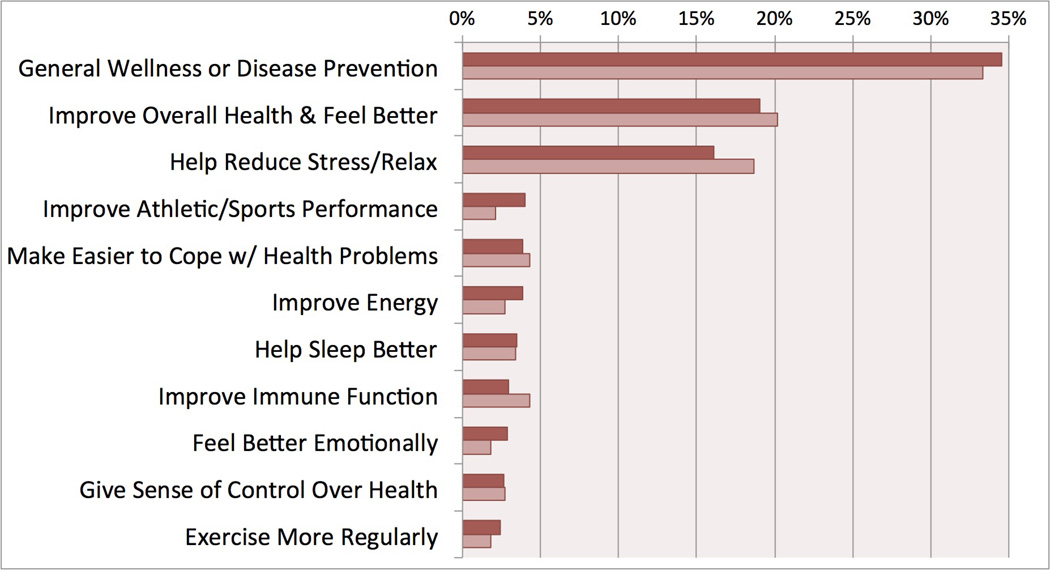
The distribution of reasons why women of childbearing age are using CAM is similar between non-pregnant CAM users (n=3,084) pregnant/recently pregnant CAM users (n=214)(Figure 2). In the non-pregnant group general wellness/disease prevention (35%), improving health/feeling better (19%), and reducing stress/relaxing (16%) were the top three reasons for using CAM. There was no statistical difference between the recently pregnant and non-pregnant groups for any of the reasons of use.
Figure 2.
Twenty-five percent of pregnant/recently pregnant CAM users were treating a musculoskeletal problem with CAM, compared to 28% of non-pregnant women. Back pain was the most prevalent health condition being treated (16% of non-pregnant /18% of pregnant/recently pregnant). When examining the category of pregnancy-associated symptoms (Appendix A), we found that 28% of pregnant/recently pregnant CAM users were treating one of these symptoms in the category with CAM, compared to 29% of non-pregnant CAM users. There was no statistical difference between any of these comparisons.
DISCUSSION
Regardless of pregnancy status, we found that over one third of women of childbearing age in the U.S. used some type of CAM during the previous year. This represents an estimated 25 million women. We estimate that half a million of these women using CAM were pregnant during the previous year. Health behaviors such as using multivitamins and moderate to heavy alcohol use predicted CAM use. The most common modalities were body-based therapies as well as any biological therapy. The top reasons for CAM use were to improve general wellness or prevent disease. Back pain was the most prevalent symptom being treated with CAM. When examining all pregnancy-related health conditions and symptoms treated with CAM, no difference was found in the rates of CAM use between pregnant/recently pregnant and non-pregnant users.
Our finding that only about half of CAM users, pregnant or not, disclosed their CAM use to their primary care providers is similar to prior estimates from convenience samples.(24) This is especially concerning for pregnant women, given the possible risks associated with the use of some herbs (27) and the lack of information regarding the effects of many other herbs during pregnancy. Ideally, as evidence becomes available regarding effectiveness of specific CAM modalities to treat particular health conditions, such as massage for pregnancy-related back pain, providers can guide their patients to appropriate modalities that are not only safe but effective as well.
Similar to previous studies, we found that female CAM users were more likely to be white, educated, well-off and not living in the southern region of the U.S.(13, 15, 16) We also found that having private insurance and taking multivitamins were positive predictors of CAM use. Some of these same characteristics are associated with better birth outcomes.(2, 34) However, we also found that alcohol consumption and health conditions such as low back pain and frequent stress(1, 31, 35) were predictors for CAM use. This suggests a more complex and nuanced relationship between CAM use and its potential influence on maternal health. Further research into CAM’s effectiveness for treating these conditions is encouraged.
Our 34–38% estimated rates of CAM use by women of childbearing age, pregnant or not, mirror current CAM use by all U.S. adults.(14, 36) However, this is less than the national estimates from Australia, which report that 48% of pregnant women consulted a CAM practitioner and 52% used CAM products.(37, 38) These discrepancies may be due, in part, to the birth culture in Australia, which emphasizes midwifery.
In our estimate, the body-based category of modalities, which included therapies such as massage, osteopathic and chiropractic care, was used the most often by 20% of women. In contrast, 17% of women from the 2007 NHIS used body-based modalities, which suggests a possible increased trend of use. The most common modalities reported from 2007 were mind-body practices (24%),(14) which is higher than our 19% estimate for the use of any mind-body therapy. This difference may be due in part to the removal of some modalities from the updated 2012 mind-body category (ie. deep breathing exercises, support groups and stress management classes).
The largest individual modality in the body-based group, massage, was used only by 12% of respondents. This is similar to the 14% of British, postnatal massage users reported by Hall et al in 2014.(21) The Australian national survey found that 34% of women consulted massage therapists during their pregnancies.(38) A bivariate analysis of the Austalian cohort found that these women were less likely to have cesarean sections before labor, but more likely to have one once labor began.(28) Osteopathic and chiropractic manipulation also had a low rate of use in our sample (10%) despite its relative safety in pregnancy(39) and evidence supporting its effectiveness for pregnancy-related back pain.(26)
The use of herbs or non-vitamin/non-mineral dietary supplements was 19% for non-pregnant women and 14% for pregnant/recently pregnant women. This was the only modality of CAM that was significantly different between the two groups of women (p=0.02). This reduced use of herbs and supplements during pregnancy is similar to what the U.S. National Birth Defects Prevention Study reported.(40) Its unclear if this is due to an increased risk perception from patients or providers regarding herb and supplement use during pregnancy.
However, we suspect our estimations to be low since the 2012 NHIS explicitly excluded the use of herbal teas in their questions even though “pregnancy teas” are readily available at grocery stores. A large study from the United Kingdom found that more of their pregnant respondents reported using an herbal tea (17%) compared to those who reported using herbal medicines (5%).(41) An earlier survey from Australia in 2002 found that 51% of their sample used herbal teas during pregnancy.(42) We believe the true U.S. estimate of herbal medicine use during pregnancy to be above our calculated 14% and less than the 73% estimated by a systematic review from 2014.(43)
The suspected underestimate of herb and supplement use is concerning. While its been understudied in the pregnant population, a variety of herbs interact with medications in the general population.(44) The use of herbs and supplements for weight loss by women, who don’t know they are pregnant, is associated with birth defects.(45) For those who are pregnant, some case reports present infants who had seizures or myocardial infarctions after mothers used Blue Cohosh for labor induction.(43, 46)
General wellness and disease prevention were the primary reasons why women of childbearing age used CAM, regardless of pregnancy status. This use of CAM for generalized health reasons shows an overarching goal for wellbeing, a common tenant of the holistic philosophies that many CAM modalities support. The influence that maternal wellbeing during the preconception period can have on maternal and fetal health is seen with mothers who experience stressful life events prior to conception. Chronically stressed pregnant women are more likely to have preterm births.(1) Additionally, they are more likely to give birth to very low birth weight infants.(32) The impact of CAM modalities on wellbeing among pregnant women should be analyzed in future studies.
We found no difference between recently pregnant and non-pregnant women in rates of CAM use to treat pregnancy-related health conditions. In contrast, the Australian national study reported that pregnant women preferentially consulted CAM practitioners over conventional providers to treat specific conditions such as sciatica, yet they preferentially consulted conventional providers for headaches.(37)
There are several limitations to this study. NHIS doesn’t include women who can’t respond in English or Spanish and could possibly be using their native remedies more frequently. NHIS did not ask women specifically about use of CAM for symptoms and conditions directly related to their pregnancy. Also, there are other CAM modalities used in the U.S., which aren’t captured by the CAM supplement such as Theta Healing. Due to its self-reported nature, the data are subject to recall bias. Most importantly NHIS wasn’t specifically designed to study pregnant women. For example, survey participants who were under 18 years old were not asked about pregnancy status. We are also unable to verify that CAM modalities were used precisely when respondents were pregnant. Due to the small sample size of pregnant CAM users who used specific modalities, we are also unable to say if those therapies were used for a certain condition such as back pain. While our sample size is too small to generate a national estimate of pregnant women’s use of specific herbs, future analysis of the 2012 NHIS could look at which particular herbs all women of childbearing age are using. Finally, unlike the Australian study, we are unable to report on birth outcomes.
Despite these limitations, our study is the most accurate U.S. national estimate of CAM use during pregnancy and in the broader population of women of childbearing age, which includes detailed patterns of use. At this time, the sample size from the 2012 NHIS is larger than previous surveys, which asked only if women were currently pregnant. Our analysis also is the first to list the top modalities and associated health conditions used nationally by women of childbearing age and pregnant women. These estimates provide a direction to guide future studies.
Women with known pregnancies are in a unique time in life where they can be more receptive to health messages and be motivated to make healthy lifestyle changes.(48) Healthier lifestyles that include reduced smoking and alcohol consumption or improved nutrition,(2) impact women’s health during pregnancy and that of their future child. While CAM offers a variety of modalities that may enable lifestyle change, these therapies may inappropriately be perceived as natural and therefore safe for the mother and fetus.(22, 24, 49) Care providers of women who are planning a pregnancy or at high risk of having an unintended pregnancy should ask what CAM practices they use in the hopes of harnessing the potential benefits of CAM while minimizing the harms.
Supplementary Material
TABLE 2.
Adjusted Odd Ratios for CAM use among women aged 18–49, National Health Interview Survey 2012
| Demographics | Adjusted OR* (95% CI) |
|---|---|
| White Race | 1.19 (1.03, 1.37) |
| Education | |
| ≤ High school grad | Reference |
| Some College, no degree | 1.60 (1.34, 1.91) |
| ≥Undergraduate degree | 1.96 (1.66, 2.32) |
| Yearly Income ($) | |
| 0–35K | Reference |
| 35–75K | 1.16 (0.98, 1.37) |
| 75–100K | 1.46 (1.17, 1.82) |
| >100K | 1.58 (1.28, 1.95) |
| Region of Residence | |
| Northeast | 1.39 (1.15, 1.68) |
| Midwest | 1.57 (1.31, 1.89) |
| South | Reference |
| West | 1.81 (1.52, 2.16) |
| Insurance | |
| None/Other/Unknown | Reference |
| Medicare/Medicaid | 0.72 (0.56, 0.91) |
| Private | 1.22 (1.05, 1.42) |
| Behaviors | |
| Physical Activity | |
| Low | 0.44 (0.37, 0.53) |
| Med (Vig<3×/wk or 2<=Mod<=5×/wk) | 0.74 (0.63, 0.86) |
| High (Vig>=3×/wk or mod >=6×/wk) | Reference |
| Smoking Status | |
| Never | Reference |
| Former | 1.48 (1.22, 1.79) |
| Current: Sometimes/Daily | 0.95 (0.80, 1.13) |
| Alcohol Use | |
| Not Currently | Reference |
| Current: Light/Infrequent | 1.37 (1.16, 1.62) |
| Current: Moderate/Heavy | 1.92 (1.53, 2.41) |
| MultiVit/MineralSupplement in past year | 2.52 (2.22, 2.86) |
| Health Factors | |
| Perceived Health Status | |
| Fair/Poor/Don't know | 0.62 (0.47, 0.80) |
| Good | 0.80 (0.67, 0.96) |
| Very Good | 0.89 (0.77, 1.03) |
| Excellent | Reference |
| Pregnant (Current or within past year) | 0.75 (0.59, 0.96) |
| BMI (kg/m^2) | |
| Underweight (<18.5) | 1.08 (0.72, 1.60) |
| Normal (18.5 – 25) | Reference |
| Overweight (25–30) | 0.76 (0.65, 0.89) |
| Obese (>=30) | 0.65 (0.55, 0.76) |
| Symptoms / Diseases | |
| Nausea/Vomiting | 1.42 (1.22, 1.64) |
| Low Back Pain | 1.56 (1.33, 1.83) |
| Muscle/Bone Pain | 1.64 (1.36, 1.97) |
| Depression | 1.32 (1.08, 1.60) |
| Frequent Stress | 1.38 (1.17, 1.63) |
| Fatigue / Lack of Energy | 1.29 (1.07, 1.56) |
for all characteristics listed in Table 1
Acknowledgments
Source of Funding:
Dr. Holden is a trainee of Dr. Gloria Yeh’s Grant Number T32AT000051 from the National Center for Complementary & Alternative Medicine.
Dr. Paula Gardiner is the recipient of Grant Number K07 AT005463-01A1 from the National Center for Complementary & Alternative Medicine.
Dr. Gurjeet Birdee is the recipient of Grant Number K23 AT00696 from the National Center for Complementary & Alternative Medicine.
Dr. Davis’s work is supported by Harvard Catalyst (NIH award 1UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers).
Footnotes
Disclosure: The authors report no conflict of interest.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Complementary & Alternative Medicine or the National Institutes of Health.
References
- 1.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual review of psychology. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 2.D'Angelo D, Williams L, Morrow B, Cox S, Harris N, Harrison L, et al. Preconception and Interconception Health Status of Women Who Recently Gave Birth to a Live-Born Infant — Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 Reporting Areas, 2004. Morbitidy and Morltaity Weekly Report. 2007;56(SS-10):1–35. [PubMed] [Google Scholar]
- 3.Pickle S, Wu J, Burbank-Schmitt E. Prevention of Unintended Pregnancy: A Focus on Long-Acting Reversible Contraception. Primary care. 2014;41(2):239–260. doi: 10.1016/j.pop.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Custer M, Waller K, Vernon S, O'Rourke K. Unintended pregnancy rates among a US military population. Paediatric and perinatal epidemiology. 2008;22(2):195–200. doi: 10.1111/j.1365-3016.2007.00896.x. [DOI] [PubMed] [Google Scholar]
- 5.OECDiLibrary. [[cited 2014 June 24, 2014]];Infant Mortality France: OECD Health Data. 2013 Available from: http://www.oecd-ilibrary.org/social-issues-migration-health/infant-mortality_20758480-table9. [Google Scholar]
- 6.CDC. Recommendations to improve preconception health and health care—United States. MMWR Recommendations and Reports. 2006;55(RR-06):1–23. [PubMed] [Google Scholar]
- 7.Cheng TL, Kotelchuck M, Guyer B. Preconception women's health and pediatrics: an opportunity to address infant mortality and family health. Academic pediatrics. 2012;12(5):357–359. doi: 10.1016/j.acap.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack BW, Atrash H, Coonrod DV, Moos MK, O'Donnell J, Johnson K. The clinical content of preconception care: an overview and preparation of this supplement. Am J Obstet Gynecol. 2008;199(6) Suppl 2:S266–S279. doi: 10.1016/j.ajog.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 9.Farahi N, Zolotor A. Recommendations for Preconception Counseling and Care. American Family Physician. 2013;88(8):499–506. [PubMed] [Google Scholar]
- 10.The Role of the Certified Nurse-Midwife/Certified Midwife in Preconception Health and Health Care. American College of Nurse Midwives. 2013:1. [Google Scholar]
- 11.Barnes P, Powell-Griner E, McFann K, Nahin R. Complementary and Alternative Medicine Use Among Adults: United States, 2002. Advance Data. 2004;343:1–20. [PubMed] [Google Scholar]
- 12.Upchurch DM, Chyu L, Greendale GA, Utts J, Bair YA, Zhang G, et al. Complementary and alternative medicine use among American women: findings from The National Health Interview Survey, 2002. Journal of women's health. 2007;16(1):102–113. doi: 10.1089/jwh.2006.M074. [DOI] [PubMed] [Google Scholar]
- 13.Bishop FL, Lewith GT. Who Uses CAM? A Narrative Review of Demographic Characteristics and Health Factors Associated with CAM Use. Evidence-based complementary and alternative medicine : eCAM. 2010;7(1):11–28. doi: 10.1093/ecam/nen023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birdee GS, Kemper K, Rothman R, Gardiner P. Use of Complementary and Alternative Medicine During Pregnancy and the PostPartum Period: An Analysis of the National Health Interview Survey. Journal of women's health. 2014 doi: 10.1089/jwh.2013.4568. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MA, West AN, Weeks WB, Sirovich BE. Health behaviors and utilization among users of complementary and alternative medicine for treatment versus health promotion. Health services research. 2011;46(5):1402–1416. doi: 10.1111/j.1475-6773.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes P, Bloom B, Nahin R. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007. National Health Statistics Report. 2008;12:1–24. [PubMed] [Google Scholar]
- 17.Mendez DD, Hogan VK, Culhane JF. Institutional racism, neighborhood factors, stress, and preterm birth. Ethnicity & health. 2013 doi: 10.1080/13557858.2013.846300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews TJ, MacDorman M. In: Infant Mortality Statistics from the 2010 Period Linked Birth/Infant Death Data Set. Services USDoHaH, editor. Hyattsvilee, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 19.Barfield W, D'Angelo D, Moon R, Lu M, Wong B, Iskander J. In: CDC Grand Rounds: Public Health Approaches to Reducing U.S. Infant Mortality. Prevention CfDCa, editor. Washington, DC: Morbility and Mortality Weekly Report; 2013. p. 6250628. [PMC free article] [PubMed] [Google Scholar]
- 20.Kalder M, Knoblauch K, Hrgovic I, Munstedt K. Use of complementary and alternative medicine during pregnancy and delivery. Archives of gynecology and obstetrics. 2011;283(3):475–482. doi: 10.1007/s00404-010-1388-2. [DOI] [PubMed] [Google Scholar]
- 21.Hall HR, Jolly K. Women's use of complementary and alternative medicines during pregnancy: a cross-sectional study. Midwifery. 2014;30(5):499–505. doi: 10.1016/j.midw.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Adams J, Chi-Wai L, Sibbritt D, Broom A, Wardle J, Homer C, et al. Women's Use of Complementary and Alternative Medicine During Pregnancy: A Critical Review of the Literature. Birth. 2009;36(3):237–245. doi: 10.1111/j.1523-536X.2009.00328.x. [DOI] [PubMed] [Google Scholar]
- 23.Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. American Journal of Obstetrics and Gynecology. 2003;188(4):1039–1045. doi: 10.1067/mob.2003.223. [DOI] [PubMed] [Google Scholar]
- 24.Strouss L, Mackley A, Paul D, Locke R. Complementary and Alternative Medicine use in women during pregnancy: do their healthcare providers know? BMC Complementary and Alterntaive Medicine. 2014;14(85):1–9. doi: 10.1186/1472-6882-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalsen A, Grossman P, Acil A, Langhorst J, Liudtke R, Esch T, et al. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive yoga program. Med Sci Monitor. 2005;11(12):CR556–CR561. [PubMed] [Google Scholar]
- 26.Close C, Sinclair M, Liddle SD, Madden E, McCullough JE, Hughes C. A systematic review investigating the effectiveness of Complementary and Alternative Medicine (CAM) for the management of low back and/or pelvic pain (LBPP) in pregnancy. Journal of advanced nursing. 2014 doi: 10.1111/jan.12360. [DOI] [PubMed] [Google Scholar]
- 27.Dugoua J, Perri D, Mills E, Koren G. Safety and efficacy of Blue Cohosh (Caulophyllum Thalictroides) during pregnancy and lactation. Journal of Population Therapeutics and Clinical Pharamcology. 2008;15(1):66–73. [PubMed] [Google Scholar]
- 28.Steel A, Adams J, Sibbritt D, Broom A, Frawley J, Gallois C. Relationship between complementary and alternative medicine use and incidence of adverse birth outcomes: An examination of a nationally representative sample of 1835 Australian women. Midwifery. 2014 doi: 10.1016/j.midw.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 29.CDC. [updated June 27, 2014];2014 2012:[National Health Interview Survey]. Available from: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm - 2012_NHIS.
- 30.ACOG. The Importance of Preconception Care in the Continuum of Women's Health Care. Danvers, MA: ACOG; 2005. [Google Scholar]
- 31.Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell-Andrews AA, Kain ZN. Low back pain during pregnancy: prevalence, risk factors, and outcomes. Obstetrics and gynecology. 2004;104(1):65–70. doi: 10.1097/01.AOG.0000129403.54061.0e. [DOI] [PubMed] [Google Scholar]
- 32.Witt W, Cheng E, Wisk L, Lizelman K, Chatterjee D, Mandell K, et al. Maternal Stressful Life Events Prior to Conception and the Impact on Infant Birth Weight in the United States. American journal of public health. 2014;104(S1):S81–S89. doi: 10.2105/AJPH.2013.301544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medicine USNLo. Complementary & Alternative Medicine 2003. [cited 2014 July 27]; Available from: http://www.nlm.nih.gov/tsd/acquisitions/cdm/subjects24.html.
- 34.Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC public health. 2011;11(Suppl 3):S19. doi: 10.1186/1471-2458-11-S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergstrom C, Persson M, Mogren I. Pregnancy-related low back pain and pelvic girdle pain approximately 14 months after pregnancy - pain status, self-rated health and family situation. BMC pregnancy and childbirth. 2014;14:48. doi: 10.1186/1471-2393-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tindle H, Davis R, Phillips RS, Eisenberg DM. Trends in Use of COmplementary and Alternative Medicine by US Adults: 1997–2002. Alternative Therapies. 2005;11(1):42–49. [PubMed] [Google Scholar]
- 37.Steel A, Adams J, Sibbritt D, Broom A, Gallois C, Frawley J. Utilisation of complementary and alternative medicine (CAM) practitioners within maternity care provision: results from a nationally representative cohort study of 1,835 pregnant women. BMC Pregnancy & Childbirth. 2012;12(146):1–8. doi: 10.1186/1471-2393-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frawley J, Adams J, Sibbritt D, Steel A, Broom A, Gallois C. Prevalence and determinants of complementary and alternative medicine use during pregnancy: results from a nationally representative sample of Australian pregnant women. The Australian & New Zealand journal of obstetrics & gynaecology. 2013;53(4):347–352. doi: 10.1111/ajo.12056. [DOI] [PubMed] [Google Scholar]
- 39.Stuber KJ, Wynd S, Weis CA. Adverse events from spinal manipulation in the pregnant and postpartum periods: a critical review of the literature. Chiropractic & manual therapies. 2012;20:8. doi: 10.1186/2045-709X-20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broussard CS, Louik C, Honein MA, Mitchell AA National Birth Defects Prevention S. Herbal use before and during pregnancy. Am J Obstet Gynecol. 2010;202(5):443, e1–e16. doi: 10.1016/j.ajog.2009.10.865. [DOI] [PubMed] [Google Scholar]
- 41.Bishop JL, Northstone K, Green JR, Thompson EA. The use of Complementary and Alternative Medicine in pregnancy: data from the Avon Longitudinal Study of Parents and Children (ALSPAC) Complementary therapies in medicine. 2011;19(6):303–310. doi: 10.1016/j.ctim.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Maats F, Crowther C. Patterns of vitamin, mineral and herbal supplement use prior to and during pregnancy. The Australian & New Zealand journal of obstetrics & gynaecology. 2002;42(5):494–496. doi: 10.1111/j.0004-8666.2002.00494.x. [DOI] [PubMed] [Google Scholar]
- 43.Dante G, Bellei G, Neri I, Facchinetti F. Herbal therapies in pregnancy: what works? Current opinion in obstetrics & gynecology. 2014;26(2):83–91. doi: 10.1097/GCO.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 44.Tsai HH, Lin HW, Simon Pickard A, Tsai HY, Mahady GB. Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: a systematic literature review. International journal of clinical practice. 2012;66(11):1056–1078. doi: 10.1111/j.1742-1241.2012.03008.x. [DOI] [PubMed] [Google Scholar]
- 45.Bitsko RH, Reefhuis J, Louik C, Werler M, Feldkamp ML, Waller DK, et al. Periconceptional use of weight loss products including ephedra and the association with birth defects. Birth defects research Part A, Clinical and molecular teratology. 2008;82(8):553–562. doi: 10.1002/bdra.20472. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy D, Lupattelli A, Koren G, Nordeng H. Herbal medicine use in pregnancy: results of a multinational study. BMC complementary and alternative medicine. 2013;13(355) doi: 10.1186/1472-6882-13-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin J, Harmilton B, Sutton P, Ventura S, Matthews MS, Osterman M. Births: Final Data 2008. National Vital Statistics Reports. 2010;59(1):1–72. [PubMed] [Google Scholar]
- 48.Wilkinson S, McIntyre D. Evaluation of the 'healthy start to pregnancy' early antenatal health promotion workshop: a randomized controlled trial. BMC Pregnancy & Childbirth. 2012;12(131):1–12. doi: 10.1186/1471-2393-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warriner S, Bryan K, Brown AM. Women's attitude towards the use of complementary and alternative medicines (CAM) in pregnancy. Midwifery. 2014;30(1):138–143. doi: 10.1016/j.midw.2013.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.