Abstract
Exposure to stimuli and environments associated with drug use is considered one of the most important contributors to relapse among substance abusers. Neuroimaging studies have identified neural circuits underlying these responses in cocaine-dependent subjects. But these studies are often difficult to interpret because of the heterogeneity of the participants, substances abused, and differences in drug histories and social variables. Therefore, the goal of this study was to assess the functional effects of exposure to cocaine-associated stimuli in a nonhuman primate model of cocaine self-administration, providing precise control over these variables, with the 2-[14C]deoxyglucose method. Rhesus monkeys self-administered 0.3 mg/kg/injection cocaine (n=4) under a fixed-interval 3-min (FI 3-min) schedule of reinforcement (30 injections/session) for 100 sessions. Control animals (n = 4) underwent identical schedules of food reinforcement. Sessions were then discontinued for 30 days, after which time monkeys were exposed to cocaine- or food-paired cues, and the 2DG experiment was conducted. The presentation of the cocaine-paired cues resulted in significant increases in functional activity within highly restricted circuits that included portions of the pre-commissural striatum, medial prefrontal cortex, rostral temporal cortex, and limbic thalamus when compared to control animals presented with the food-paired cues. The presentation of cocaine-associated cues increased brain functional activity in contrast to the decreases observed after cocaine consumption. Furthermore, the topography of brain circuits engaged by the expectation of cocaine is similar to the distribution of effects during the earliest phases of cocaine self-administration, prior to the onset of neuroadaptations that accompany chronic cocaine exposure.
Keywords: Cocaine, Craving, Cue reactivity, Glucose utilization, Rhesus monkey, Self-administration
Introduction
The effects of drugs of abuse are considered to be the product of multiple processes that include the pharmacological effects attributed to the specific actions of the drug at the cellular and receptor level, as well as the conditioned or learned effects that are produced by the repeated pairing of the drug with the internal and external environment. These learned associations mediate the anticipation or expectation of drug effects that precede drug administration and induce craving and drug seeking (O’Brien et al., 1998). In the case of drugs of abuse such as cocaine, these learned associations can intensify the pleasurable feelings engendered by cocaine (Volkow et al., 2006; Volkow et al., 2003) as well as result in profound desires for the drug when cues associated with the drug are encountered. These cravings can be elicited by exposure to drug paraphernalia, places where the cocaine is used or obtained, or the people with whom drug use is associated, and are often accompanied by signs and symptoms that are similar to the effects of the cocaine itself (Childress et al., 1999). Thus, cue-induced craving is one of the most insidious characteristics of addiction and is thought to be one of the main factors responsible for relapse, even after months or years of successful abstinence (Dackis and O’Brien, 2001).
Neuroimaging studies have been central to our understanding of the networks of brain structures underlying this phenomenon, particularly in human addicts. The presentation of cues associated with the availability of cocaine can alter the pattern of functional activity in such areas as the amygdala, orbitofrontal and dorsolateral prefrontal cortex, anterior cingulate gyrus, ventral striatum, insula, cerebellum, dorsal striatum, and portions of the parietal cortex (Chase et al., 2011; Kuhn and Gallinat, 2011; see recent reviews: Wilson et al., 2004). In addition, the degree of activation has been shown to correlate with the severity of craving in regions including the anterior cingulate, amygdala, and insula (Bonson et al., 2002; Risinger et al., 2005; Sinha et al., 2005; Wang et al., 1999). Generally, studies of cue-induced craving rely on the measurement of the brain response that results from exposure to drug paraphernalia, videos depicting drug use and associated stimuli, or autobiographical scripts of drug experiences. Responses associated with exposure to the drug are then compared with those elicited by exposure to neutral stimuli, all within the framework of the laboratory.
Although there is evidence that some laboratory measures may be valid predictors of “real-world” treatment outcomes (Ghitza et al., 2010; Kosten et al., 2006; Sinha et al., 2006) among substance abusers, cognitive and neurobiological laboratory studies of phenomena like craving in this population can be challenging to interpret. There is often large variability in the duration, frequency, and pattern of cocaine use, differences in routes of cocaine administration, the presence of co-morbid neuropsychiatric and neurological symptoms, and differences in social, vocational, and educational experiences that can influence the results of these studies. Such differences are present within studies, but also across studies, making it difficult to compare findings among reports. Furthermore, although substance abusers may report craving and intense feelings for drug, these are laboratory settings distinct from the environments in which drugs are used.
Animal models offer ways to obviate many of these problems. With animal models, precise control over drug experience in terms of duration of exposure, total intake, and use of other drugs can be carefully controlled. Systematic manipulation of these and other variables can ensure that the results are attributable to the variables in question. Importantly with studies in animal models, the experimental manipulations take place in the environments in which the drug is routinely self-administered. The goal of the current study, therefore, was to use metabolic mapping with the 2-[14C]deoxyglucose (2DG) method (Sokoloff et al., 1977) to investigate the effects of the presentation of cocaine-associated cues to nonhuman primates with identical histories of cocaine self-administration, that produce neurobiological adaptations consistent with those found in human cocaine addicts (Beveridge et al., 2008; Letchworth et al., 2001; Nader et al., 2002; Porrino et al., 2004).
There is considerable overlap in the circuits activated by cues associated with various drugs including cocaine, nicotine, heroin and alcohol (for review see Chase et al., 2011). There is further overlap with the patterns of activation accompanying the presentation of cues that have been paired with natural reinforcers such as food (Tang et al., 2012; van der Laan et al., 2011) and other types of reinforcers including money (Kim et al., 2011; Knutson et al., 2001; Rademacher et al., 2010). Despite the strong similarities often observed among these stimuli, it is important to identify unique brain networks sensitive to cocaine cues that might aid in the development of therapeutic interventions for addiction treatment. Thus, a second goal of this study was to identify the functional response to cues specifically associated with cocaine. To this end we compared the response to cocaine cues to functional activity in a group of animals with a history of exposure to food rather than cocaine under an identical schedule of reinforcement.
Materials and methods
Subjects
Seven experimentally-naïve adult male rhesus monkeys (Macaca mulatta) weighing between 9.0 – 13.0 kg (mean ± SD; 10.6 ± 1.2) at the start of the study served as subjects. Monkeys were individually housed in stainless steel cages with water ad libitum; animals had physical and visual contact with each other. Body weights were maintained at approximately 95% of free-feeding weights by banana-flavored pellets earned during the experimental sessions and by feeding of Lab Diet Monkey Chow, provided no sooner than 30 minutes post-session supplemented by fresh fruits and vegetables. All procedures were performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. In addition, all procedures were reviewed and approved by the Animal Care and Use Committee of Wake Forest University.
Behavioral Apparatus
Cocaine self-administration and food-reinforced responding occurred in ventilated and sound-attenuated operant chambers (1.5 x 0.74 x 0.76m, Med Associates, East Fairfield, VT) designed to accommodate a primate chair (Model R001, Primate Products, Redwood City, CA). Chambers contained an intelligence panel (48 x 69 cm), which consisted of two retractable levers (5 cm wide) and three stimulus lights. The levers were positioned within easy reach of the monkey sitting in the primate chair. One-gram food pellets were delivered from a feeder located on the top of the chamber. A peristaltic infusion pump (7531-10, Cole-Parmer Co., Chicago, IL) was used to deliver drug injections at a rate of approximately 1.5 ml/10 sec to those animals self-administering cocaine.
Surgical Procedures
All monkeys, including controls, were surgically prepared, under sterile conditions, with indwelling intravenous catheters and vascular access ports (Model GPV, Access Technologies, Skokie, IL). Monkeys were anesthetized with a combination of ketamine (15 mg/kg, i.m.) and butorphanol (0.03 mg/kg, i.m.) and an incision was made near the femoral vein. After blunt dissection and isolation of the vein, the proximal end of the catheter was inserted into the vein for a distance calculated to terminate at the inferior vena cava. The distal end of the catheter was threaded subcutaneously to an incision on the back. The vascular access port was placed within a pocket formed by blunt dissection near this incision. Monkeys were given 24–48 hours recovery time prior to returning to food-reinforced responding.
Self-administration procedures
Monkeys were initially trained to respond on one of two levers, in the presence of a white light above the lever, by reinforcing each response on the correct lever with a 1g banana-flavored pellet. Pellet delivery was accompanied by extinguishing the white light and illuminating a red light above the lever for 10 sec. Over a period of approximately three weeks the interval between availability of food pellets was gradually increased until a three-minute interval was achieved (i.e., fixed-interval 3-minute schedule; FI 3-min). Under the final schedule conditions, the first response on the lever after three minutes resulted in the delivery of a food pellet; sessions ended after 30 food presentations. All monkeys responded under the FI 3-min schedule of food presentation for at least 20 sessions and until stable performance was obtained (± 20% of the mean for three consecutive sessions, with no trends in response rates). When food-maintained responding was stable, the feeder was unplugged and extinction of responding was examined for five consecutive sessions, after which responding was reestablished and maintained by food presentation.
After baseline performance had been re-established, monkeys were randomly assigned to one of two groups; monkeys assigned to cocaine self-administration groups were surgically prepared with venous catheters, as described above. One group of monkeys served as controls (N=4) and continued to respond under the FI 3-min schedule of food presentation for a total of 100 sessions followed by 30 days during which time monkeys remained in their home cages with no operant sessions conducted. For both groups, reinforcement was always accompanied by extinguishing the white light and illuminating the red light (conditioned stimulus).
A second group of monkeys (N=4) was assigned to chronic exposure to cocaine self-administration, consisting of 100 sessions in which responding was maintained by 0.3 mg/kg/injection cocaine (30 injections per session, for a total of 9.0 mg/kg/session), followed by cessation of operant session for 30 days. Prior to each experimental session, the back of the animal was cleaned with 95% ethanol and betadine scrub and a 22-gauge Huber Point Needle (Model PG20-125) was inserted into the vascular access port leading to the venous catheter, connecting an infusion pump containing the cocaine solution to the catheter. Prior to the start of the session, the pump was operated for approximately three seconds, filling the port with cocaine. At the end of each session, the port was filled with heparinized saline (100 Units/ml) to help prevent clotting.
Daily experimental sessions, conducted at approximately the same time each day, continued for 100 sessions. In an effort to develop a measure of ‘”craving”, monkeys self-administering cocaine and their food-maintained control monkeys were exposed to ‘probe’ sessions approximately every 14 days. A probe session consisted of a 2-h experimental session in which the schedule of reinforcement was FI 2-h. For these probe sessions, responding over the 2-h session occurred in the presence of the discriminative stimulus (white light) signaling cocaine or food, but responding did not lead to the conditioned stimulus (red light) or reinforcement. Probe sessions were conducted following 1, 2, or 3 day periods during which no operant sessions were conducted, each of which was evaluated twice throughout the course of the experiment, with the order of testing occurring in a semi-random fashion, for a total of six probe sessions. Following completion of 100 sessions operant sessions were discontinued for 30 days. Animals remained in their home cages for 30 days. Although no self-administration or food-reinforced sessions were conducted during this time, all monkeys were placed in their restraint chairs, catheters were flushed with heparinized saline (100 Units/ml) and then animals were returned to their home cages three times per week (Mon–Fri).
Thirty days following the cessation of food or cocaine self-administration, the 2DG experiment was conducted. Two days prior to the 2DG study, monkeys were surgically implanted with an arterial catheter in the opposite leg of the venous catheter. Food control monkeys had two catheters implanted, one in the femoral vein and one in the femoral artery of the same leg. The final session involved the 2DG experiment in which monkeys were placed in the experimental chamber and the white lever light, which was the discriminative stimulus light signaling food or cocaine availability was illuminated; the 2DG was administered approximately 2 min after the start of the stimulus presentation. No levers were in the chamber and no drug was available on the day of the procedure. For that reason, we refer to the measures of glucose utilization in the cocaine and control groups during the final session as relating to “expectation” of the availability of reinforcement.
Measurement of local cerebral glucose utilization
Catheters exited through a small opening in the rear of the chamber allowing infusions and sampling to be accomplished remotely with minimal disruption to the animal. The 2DG procedure was initiated at the end of the last session, two minutes into the timeout, by the infusion of an intravenous pulse of 2.76 MBq/kg 2-deoxy-D-[14C]glucose (PerkinElmer, Waltham, MA; specific activity 1850-2035 MBq/mmol) followed by a flush of heparinized saline. Timed arterial blood samples were drawn at a schedule sufficient to define the time course of the arterial 2DG and glucose concentrations. Arterial blood samples were centrifuged immediately. Plasma 14C concentrations were determined by liquid scintillation spectrophotometry (Beckman Instruments, Fullerton, CA), and plasma glucose concentrations were assessed using a glucose analyzer (Analox Instruments, London, UK)). Approximately 45 minutes after tracer injection, the animals were euthanized by an intravenous overdose of sodium pentobarbital (100 mg/kg). Brains were removed rapidly, blocked in three parts, frozen in isopentane (−45 °C), and stored at −80 °C until they were processed for autoradiography. Coronal sections (20 μm) were cut in a cryostat maintained at −22 °C. Sections were thaw-mounted on glass coverslips, dried on a hot plate at 60 °C, and apposed to Kodak MR-1 film (Rochester, NY) for 15–30 days, along with a set of [14C]methylmethacrylate standards (Amersham, Arlington Heights, IL) calibrated for their equivalent 14C concentration in 20 μm brain sections. Autoradiograms were developed in Kodak GBX developer, indicator stop bath, and rapid fix at 68 °C.
Quantitative densitometry of autoradiograms was accomplished with a computer-assisted image-processing system (MCID, Interfocus Imaging Ltd., Linton, UK). Optical density measurements for each structure were made in a minimum of eight brain sections. Measurements were made bilaterally and averaged across hemispheres. Tissue 14C concentrations were determined from the optical densities and a calibration curve obtained by densitometric analysis of the autoradiograms of the calibrated standards. Glucose utilization was then calculated using the operational equation of the method (Sokoloff et al., 1977), local-tissue 14C concentrations, the time course of the plasma 2DG and glucose concentrations, and the appropriate kinetic constants (Kennedy et al., 1978). Because of differences in the baseline levels of glycemia and 2DG concentrations, the lumped constant was adjusted appropriate to the glucose levels according to procedures based on previous work (Kennedy et al., 1978; Porrino et al., 2004; Schuier et al., 1990; Suda et al., 1990). Nomenclature for specific brain regions was based on the atlas of Paxinos et al. (2000).
Statistical analysis
Rates of glucose utilization (adjusted for glycemia levels as described above) were measured in 39 discrete brain regions. Values of rates of local cerebral glucose utilization obtained for individual brain structures were analyzed in four neuroanatomical groups (basal ganglia, cortex, thalamus, and limbic regions) by means of a two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure). This was followed by Bonferroni’s post-hoc test for multiple comparisons where appropriate. In all cases, p<0.05 was considered statistically significant.
Pearson product-moment correlations were used to correlate rates of glucose utilization with the average percentage increase in response rate across probe sessions for each animal, as corrected for multiple tests. Correlations were conducted in control and cocaine groups combined.
Results
Behavior
After 100 cocaine or food self-administration sessions, the mean rate of responding (last 5 sessions prior to cessation of self-administration) for monkeys in the food control group was 0.55 ± .17 (mean ± SEM) responses/s, as compared to 0.02 ± .003 responses/s for the cocaine group. Mean session length for the last 5 sessions prior to cessation of self-administration was 104.2 ± 7.3 min for food controls and 301.9 ± 40.1 min for the cocaine group.
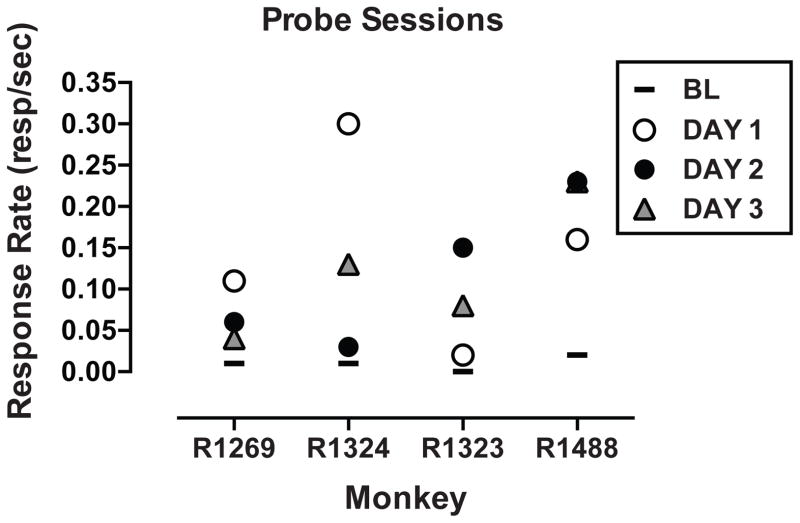
Data from probe sessions (i.e., 2 hr sessions with an FI 2-h schedule of reinforcement) for the cocaine self-administration monkeys are shown in Figure 1. Response rates during probe sessions for the food controls were .39 ± .18 responses/s, an average of 64% of food reinforced rates during regular experimental sessions, whereas response rates for the cocaine self-administering monkeys were 0.14 ± .03 responses/s, a 600% increase over cocaine-reinforced rates. Irrespective of the number of days without access to self-administration days (1–3), response rates during probe sessions were always higher than baseline response rates; there were no statistical differences between probe days (Figure 1).
Figure 1.
Response rates during probe sessions for monkeys self-administering cocaine (0.3 mg/kg per session). Most points are the mean of 2–3 determinations. Different symbols represent different abstinence periods (days in which cocaine self-administration sessions were not conducted). The solid line represents the mean response rate prior to each probe session.
Rates of Local Cerebral Glucose Utilization
Plasma glucose levels measured just prior to the initiation of the 2DG procedure did not differ significantly between or within groups: food group, 1.01 ± 0.5 mg/ml (mean ± SEM); cocaine group, 0.96 ± 0.3 mg/ml. Rates of local cerebral glucose metabolism were measured in 39 brain regions. Data from specific brain regions are described in detail below.
Basal ganglia
Rates of cerebral glucose utilization for regions of the basal ganglia are shown in Table 1. Statistical analysis demonstrated a significant main effect of brain region (F(12,60) = 34.251, P = 0.000), but not treatment (F(1,5) = 1.779, NS), as well as a significant interaction of treatment with brain region (F(12,60) = 1.986, P = 0.048). Further analysis demonstrated that exposure to cocaine-related cues resulted in significantly higher rates of glucose utilization than exposure to food related cues in the medial (+16.3%) and lateral (+20.1%) pre-commissural caudate nucleus, dorsal (+16.3%) and ventral (+18.0%) pre-commissural putamen, and the core (+20.9%) and shell (+14.0%) regions of the nucleus accumbens. There were no other significant differences between groups, most notably an absence of difference in the post-commissural caudate and putamen.
Table 1.
Striatum/ Basal Ganglia
| Brain Region | Food Cues (N=3) | Cocaine Cues (N=4) | Percentage Difference |
|---|---|---|---|
| Pre-commissural Striatum | |||
| Caudate-dorsomedial | 48 ± 2.1 | 56 ± 0.5 ** | 16.3% |
| Caudate-dorsolateral | 50 ± 1.9 | 60 ± 0.6 ** | 20.1% |
| Putamen-dorsal | 54 ± 3.2 | 63 ± 1.0 * | 16.3% |
| Putamen-ventral | 46 ± 2.2 | 56 ± 0.8 * | 18.0% |
| Nucleus accumbens-core | 38 ± 1.0 | 46 ± 0.9 ** | 20.9% |
| Nucleus accumbens-shell | 38 ± 1.9 | 43 ± 0.9 ** | 14.0% |
| Post-commissural Striatum | |||
| Caudate | 50 ± 1.8 | 49 ± 5.7 | −1.7% |
| Putamen | 54 ± 1.4 | 51 ± 5.8 | −6.0% |
| Basal ganglia | |||
| Ventral tegmental area | 21 ± 2.5 | 20 ± 1.9 | −5.7% |
| Globus pallidus | 31 ± 0.9 | 35 ± 2.3 | 12.9% |
| Subthalamus | 55 ± 4.7 | 51 ± 5.1 | −7.4% |
| Substantia nigra pars compacta | 37 ± 2.3 | 38 ± 5.0 | 0.6% |
| Substantia nigra pars reticulata | 34 ± 1.9 | 32 ± 3.4 | −5.6% |
Data represent rates of local cerebral glucose utilization (μmol/100g/min) expressed as means ± S.E.M.
P < 0.05,
P < 0.01, Bonferroni t-tests for multiple comparisons following two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure).
Rates of glucose utilization in the core of the nucleus accumbens were positively correlated (rxy = +. 852) with the average increase in responding during probe sessions as compared to the average rate of the three sessions prior to each probe session. Correlations were significant at the P <.05 level, corrected for multiple correlations. No significant correlations were observed between changes in rates of responding and rates of glucose utilization in any other brain region.
Prefrontal and temporal cortex
Rates of cerebral glucose utilization for regions of the prefrontal and temporal cortex are shown in Table 2. Statistical analysis demonstrated a significant main effect of brain region (F(5,25) = 63.280, P = 0.000) and group (F(1,5) = 15.245, P = .011) but no significant interaction of treatment with brain region (F(5,25) = 0.182, NS). Further analysis demonstrated that exposure to cocaine-related cues resulted in significantly higher rates of glucose utilization than exposure to food related cues in the medial prefrontal cortex including regions 32, 25, 14 and 24 (+18.4%), the temporal pole (+26.4%) and the anterior insula, including both dorsal and ventral subdivisions (+17.7%). There were no significant differences in the dorsal and orbital portions of the prefrontal cortex.
Table 2.
Cortico-thalamic Areas
| Brain Region | Food Cues (N=3) | Cocaine Cues (N=4) | Percentage Difference |
|---|---|---|---|
| Prefrontal Cortex | |||
| Medial (areas 24,25,14,32) | 47 ± 1.3 | 56 ± 2.5 * | 18.4% |
| Orbital (areas 11,12,13) | 53 ± 3.3 | 60 ± 2.6 | 12.6% |
| Dorsolateral (areas 45,46) | 56 ± 0.6 | 63 ± 3.3 | 11.2% |
| Temporal Cortex | |||
| Temporal pole | 31 ± 1.4 | 39 ± 0.5 ** | 26.4% |
| Insula | 44 ± 0.8 | 51 ± 1.7 * | 17.7% |
| Temporal gyrus (areas STG, TE, TF) | 41 ± 2.0 | 48 ± 0.9 | 17.3% |
| Thalamus | |||
| Midline | 46 ± 0.7 | 52 ± 1.3 * | 12.7% |
| Mediodorsal | 46 ± 2.1 | 55 ± 1.5 * | 19.5% |
| Ventral anterior | 36 ± 0.6 | 45 ± 1.6 ** | 24.5% |
| Parafascicular/Centromedian | 39 ± 0.9 | 44 ± 1.6 * | 13.8% |
| Ventrolateral | 40 ± 1.4 | 45 ± 1.4 | 11.4% |
| Habenula | 30 ± 1.1 | 42 ± 0.7** | 38.5% |
Data represent rates of local cerebral glucose utilization (μmol/100g/min) expressed as means ± S.E.M.
P < 0.05,
P < 0.01, Bonferroni t-tests for multiple comparisons following two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure).
Thalamus
Rates of cerebral glucose utilization for regions of thalamus are shown in Table 2. Statistical analysis demonstrated a significant main effect of brain region (F(6,30) = 37.180, P = 0.000) and group (F(1,5) = 30.697, P = .003) and a significant interaction of treatment with brain region (F(6,30) = 2.436, P = .049). Further analysis demonstrated that exposure to cocaine-related cues resulted in significantly higher rates of glucose utilization than exposure to food-related cues within midline thalamic regions (+12.7%), the ventral anterior thalamus (+24.5%), all levels of the mediodorsal thalamus (+19.5%), and the parafasicular-centromedian complex (+13.8%). In addition, there were significant differences in the habenula (+38.5%).
Limbic areas and hypothalamus
Rates of cerebral glucose utilization for regions of the limbic system including amygdala, hippocampus, and extended amygdala and hypothalamus are shown in Table 3. Statistical analysis demonstrated a significant main effect of brain region (F(11.55) = 18.587, P = 0.000), but no significant effects of treatment group (F(1,5) = 1.012, NS) or interaction of treatment with brain region (F(11,55) = 0.058, NS). There were no significant differences between treatment groups in any of these regions.
Table 3.
Limbic and related subcortical areas
| Brain Region | Food Cues (N=3) | Cocaine Cues (N=4) | Percentage Difference |
|---|---|---|---|
| Amygdala | |||
| Lateral | 31 ± 2.4 | 31 ± 3.6 | −0.5% |
| Central | 23 ± 1.3 | 20 ± 2.5 | −12.0% |
| Medial | 26 ± 1.9 | 24 ± 3.1 | −6.7% |
| Basolateral | 30 ± 1.7 | 31 ± 3.0 | 2.1% |
| Hypothalamus | |||
| Preoptic area | 28 ± 0.6 | 30 ± 2.7 | 4.2% |
| Lateral | 29 ± 1.9 | 29 ± 3.7 | 0.6% |
| Medial | 29 ± 2.0 | 29 ± 4.1 | −0.4% |
| Paraventricular | 32 ± 2.2 | 27 ± 2.9 | −14.5% |
| Limbic-Associated | |||
| Bed nucleus of stria terminalis | 24 ± 2.1 | 23 ± 1.8 | −5.4% |
| Lateral septum | 29 ± 2.9 | 31 ± 3.3 | 5.9% |
| Hippocampus (CA1,CA3,dentate) | 32 ± 4.8 | 32 ± 3.2 | 0.5% |
| Subiculum | 39 ± 1.2 | 36 ± 3.3 | 8.4% |
Data represent rates of local cerebral glucose utilization (μmol/100g/min) expressed as means ± S.E.M.
P < 0.05,
P < 0.01, Bonferroni t-tests for multiple comparisons following two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure).
Discussion
These results demonstrate that the presentation of cues previously associated with the availability of cocaine self-administration resulted in significant increases in functional activity, as reflected by rates of glucose utilization, in highly restricted portions of the pre-commissural striatum, medial prefrontal cortex, rostral temporal cortex, and the limbic thalamus when compared to rates of glucose utilization in animals presented with cues associated with food. Furthermore, these findings show that even one month without drug exposure does not dampen the ability of cocaine-discriminative cues to produce substantial brain changes that may be associated with relapse. The cocaine-associated discriminative stimulus can be hypothesized to lead to expectation of cocaine, which elevated functional brain activity over and above that of a discriminative stimulus associated with a natural reward, in this case-food. Therefore, the increased activity in these cortico-striatal-thalamic circuits specifically represents the consequences of the expectation of cocaine rather than the effects of discriminative stimuli associated with reinforcement in general. The neural correlates of the expectation of cocaine identified in the present study are in sharp contrast to those that accompany consummatory processes identified in previous studies. Metabolic mapping in animals with similar histories of cocaine intake (total intake of ~900 mg/kg over 100 sessions) after the completion of self-administration sessions results in alterations in functional activity in a far wider expanse of the striatum, prefrontal cortex and limbic regions (Beveridge et al., 2006; Porrino et al., 2002; Porrino et al., 2004). Although there was considerable overlap in the anatomical topography, particularly in the ventral striatum and medial prefrontal cortex, there was a clear distinction between the neural correlates related to the discriminative stimulus associated with cocaine (i.e., expectation) and the pharmacology of cocaine (i.e., consumption).
In many studies of cocaine self-administration the anticipatory and consummatory phases of reward processing are closely intermingled, making it difficult to distinguish between the substrates of the two processes. In our previous metabolic mapping studies of brain functional activity, we focused on the consummatory effects of cocaine self-administration, mapping drug effects at the end of self-administration sessions. In contrast, in order to separate the two processes, the present study focused solely on the anticipatory phase of cocaine self-administration by mapping brain function in the presence of a cocaine-associated discriminative stimulus. One of the most prominent differences between these phases was that the presentation of the cocaine discriminative stimulus (i.e., anticipation) produced significant increases in functional activation while cocaine self-administration (i.e., consumption) resulted in reductions in brain activation (Beveridge et al., 2006). Consistent with our hypothesis regarding the role of cocaine-associated discriminative stimuli, the elevations in functional brain activity seen here are consistent with studies that have demonstrated activation and arousal during the anticipation of drug (Kufahl et al., 2008; Volkow et al., 2003). A similar separation of increases and decreases in patterns of brain activation associated with anticipation and consumption of alcohol has been reported during the performance of a memory task (Gundersen et al., 2008). These authors found significant correspondence between the topography of the responses in the anterior cingulate and prefrontal cortex, but opposite effects on brain activation.
This anatomical circuitry underlying the effects of the presentation of cocaine-predictive cues more closely coincided with the topography of the functional response to the pharmacological effects of cocaine self-administration in the earliest phases of drug exposure (Porrino et al., 2002), as compared to those seen after more chronic exposure (Beveridge et al., 2006; Porrino et al., 2004). This is illustrated in Figure 2. The initial phases of drug self-administration, as investigated previously, represented a time just following acquisition of the self-administration behavior, but prior to the onset of any significant behavioral or neural adaptations. This early time point was chosen to model initial drug experimentation in humans, when cocaine use is recreational before any transition to more chronic addictive patterns of drug taking. Presentation of the cocaine-associated discriminative stimulus produced activation in these same networks (including the medial and orbital prefrontal cortex, ventral striatum, rostral caudate, and limbic thalamus), but not in those that are additionally affected after more prolonged experience. Expectation, then, activates those brain circuits that underlie the effects of cocaine experienced early in drug use before the development of more habitual and compulsive drug taking and the neuroadaptations that accompany more protracted use of cocaine. One could speculate that the sensations that underlie the anticipatory response to cocaine are more likely to reflect the positive or appetitive effects of the drug than the negative or aversive consequences often associated with long term use. What is “expected” upon presentation of cocaine-associated discriminative stimuli may be the pleasurable aspects of early drug use that lead to repeated use, thus outweighing the more negative sensations that accompany compulsive use and withdrawal.
Figure 2.
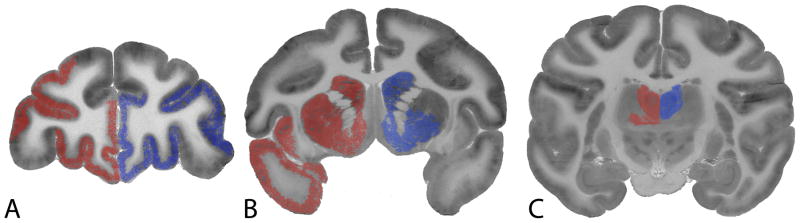
Similarities in the distribution of alterations in functional activity in response to the presentation of cues associated with cocaine self-administration (left side of each brain image) and post-cocaine self-administration (right side of each brain image). Shown are autoradiograms of 2-[14C]deoxyglucose uptake in coronal sections at the level of the A, prefrontal cortex, B, striatum, and C, thalamus of the rhesus monkey. Areas shaded in red (left) depict those regions in which significant increases in rate of glucose utilization were measured following the presentation of cocaine-associated stimuli. Areas shaded in blue (right) depict those areas of significant alterations in rates of glucose utilization in response to cocaine self-administration in its earliest stages (5 days exposure). Note that in both cases, effects were bilateral, however for the purposes of comparison they are depicted on one hemisphere.
In the current study the pattern of functional activation during the presentation of the cocaine discriminative stimulus was compared to the pattern associated with expectation of a natural reinforcer (i.e., the presentation of the discriminative stimulus associated with banana-flavored pellets). This may help to account for the absence of differences in activation in some brain regions such as the amygdala, which have been implicated in reward expectancy in previous studies (Childress et al., 1999; Hampton et al., 2007; Malik et al., 2011; Schneider et al., 2001; Tang et al., 2012). These areas may be part of a more generalized circuit that is responsive to cues regardless of the nature of the reinforcer. In contrast, in areas such as the ventral striatum the cocaine-related cue produced a greater neural response than the cue associated with a natural reinforcer suggesting greater reward magnitude associated with the cocaine discriminative stimulus. Furthermore, in the present study, rates of glucose utilization, specifically in the core portion of the nucleus accumbens were positively correlated with the degree of increase in responding during probe sessions. In these probe sessions, reinforcement (food or cocaine) was not available throughout the session. Thus, responding reflected the intensity of anticipation of reward that in turn was coded in the nucleus accumbens. Similar findings in humans have shown that neural activation, as measured by fMRI BOLD signals, in the nucleus accumbens increases with increasing reward value (Knutson et al., 2001; Knutson et al., 2005; Spreckelmeyer et al., 2009), thus, further substantiating the role of the ventral striatum in reward magnitude. By far one of the best-studied paradigms in human addiction research is that of cue reactivity, in which the response to drug-related stimuli is measured, often accompanied by neuroimaging to investigate the underlying substrates of this behavior. In general, these investigations compare the reactions of drug users to stimuli associated with cocaine or other drugs of abuse to the reactions to neutral or non-drug related stimuli. Despite the substantial differences between the paradigms used in these studies and the current study, there is still considerable overlap among many of the findings. Recent reviews and meta-analyses have identified areas of concurrence among drugs of abuse (largely cocaine, nicotine and alcohol) and among the factors that modulate these cue-evoked responses (cf. Chase et al., 2011; Jasinska et al., 2014; Wilson et al., 2004). For example, portions of the prefrontal cortex, including orbitofrontal and dorsolateral areas, of drug users are activated by drug-related stimuli in non-treatment seeking subjects, but not generally in treatment seekers. Furthermore, greater responses in these cortical areas were seen when there was the expectancy of drug administration as compared to those situations where drug was clearly unavailable. The activation observed here in these regions would parallel these findings. In contrast, activation in the amygdala was frequently seen in treatment seekers when there was no expectation of drug administration, thus supporting the absence of any alterations in functional activity in this brain region here. Again, although the contrasts differ (cocaine vs. natural reward as opposed to drug vs neutral stimuli) substantially, there are many regions of overlap that emphasize the critical importance of this circuitry by which drug-related cues and contexts exert such a powerful influence over behavior.
The present data are consistent with many investigations of specific brain regions in animal models, both rodent and nonhuman primate. Lesion and microinjection studies have identified roles for, among others, the nucleus accumbens, prefrontal cortex, amygdala and hippocampus (Pickens et al., 2011; Weiss, 2005) in stimulus processing. For example, electrophysiological investigations have reported changes in the firing patterns of neurons in the orbitofrontal and anterior cingulate cortices during cue presentation (Baeg et al., 2009) distinguishing between the effects of discriminative and conditioned stimuli in nonhuman primates. Similarly, changes in the firing patterns of rodent nucleus accumbens neurons were reported during discriminative stimulus presentations associated with different reinforcer delays (Day et al., 2011). Studies in rodents using metabolic mapping, (Knapp et al., 2002), c-fos expression (cf. Kufahl et al., 2009; Miller and Marshall, 2005; Nic Dhonnchadha et al., 2012), or fMRI (Tsurugizawa et al., 2012) have also identified the nucleus accumbens, prefrontal cortex, ventral midbrain and amygdala as substrates of cue-induced craving. However because most of these studies have not compared across reinforcers as in the present study, the findings reflect the consequences of discriminative and/or conditioned stimuli in general and are not, therefore, specific to cocaine.
There are some limitations of the current study that should be considered. The absence of a neutral stimulus condition to compare to the effects of the presentation of either food or cocaine-related stimuli and contexts limits more direct comparisons to studies in human drug users, although as noted above there is still considerable overlap across studies. The current study tested a single withdrawal time, so it is not possible to determine the development or the persistence of these effects. The striking correspondence between the topography of patterns of functional activation following discriminative stimulus presentation and the response to cocaine self-administration in its early phases, however, suggests that responses to cocaine-related stimuli develop early in the course of drug exposure. These questions are important directions that should be considered in future studies.
In summary, the anticipation of cocaine availability engendered by the presentation of a cocaine discriminative stimulus was associated with a pattern of increased functional activation, as reflected by increased rates of glucose utilization, in a highly restricted interconnected network of brain regions that included the ventral striatum, medial pre-commissural caudate nucleus, rostral temporal cortex and limbic thalamus, when compared to the patterns in animals anticipating food reward. This network associated with anticipation corresponded closely to the anatomical topography of the response to the effects of long-term cocaine self-administration, but in opposing directions; glucose utilization in the presence of the cocaine discriminative stimulus increased (indicative of arousal), whereas rates of glucose utilization decreased during cocaine consumption (i.e., reinforcement available). The overlap was most striking between the circuits underlying expectation and those sub-serving the effects of cocaine in the earliest stages of cocaine self-administration when cocaine use is controlled more by its positive or rewarding effects, rather than after the transition to more compulsive drug use marked by extensive neuroadaptations and the development of negative affect.
Acknowledgments
The authors wish to acknowledge Susan Nader and Tonya Calhoun for their assistance in conducting the self-administration studies. This work was supported by grants from the National Institute on Drug Abuse DA09085 and DA06634.
Footnotes
Authors’ contributions
LJP and MAN conceptualized and designed the study. MAN was responsible for the behavioral experiments and associated data analysis, and TJRB and HRS conducted the 2DG experiments and collected and analyzed the data. LJP drafted, and MAN, TJRB, and HRS critically reviewed and edited the manuscript for intellectual content. All authors approved the final version for publication.
The authors have no conflicts of interest to disclose.
References
- Baeg EH, Jackson ME, Jedema HP, Bradberry CW. Orbitofrontal and anterior cingulate cortex neurons selectively process cocaine-associated environmental cues in the rhesus monkey. J Neurosci. 2009;29:11619–11627. doi: 10.1523/JNEUROSCI.3206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Carelli RM. Nucleus accumbens neurons encode predicted and ongoing reward costs in rats. Eur J Neurosci. 2011;33:308–321. doi: 10.1111/j.1460-9568.2010.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B, Boyd SJ, Copersino ML, Frost JJ, Gorelick DA. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biol Psychiatry. 2010;68:697–703. doi: 10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Specht K, Gruner R, Ersland L, Hugdahl K. Separating the effects of alcohol and expectancy on brain activation: an fMRI working memory study. Neuroimage. 2008;42:1587–1596. doi: 10.1016/j.neuroimage.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L. Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol. 1978;4:293–301. doi: 10.1002/ana.410040402. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb Cortex. 2011;21:769–776. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Printseva B, Cottam N, Kornetsky C. Effects of cue exposure on brain glucose utilization 8 days after repeated cocaine administration. Brain Res. 2002;950:119–126. doi: 10.1016/s0006-8993(02)03011-1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kufahl P, Li Z, Risinger R, Rainey C, Piacentine L, Wu G, Bloom A, Yang Z, Li SJ. Expectation modulates human brain responses to acute cocaine: a functional magnetic resonance imaging study. Biol Psychiatry. 2008;63:222–230. doi: 10.1016/j.biopsych.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Dagher A. State of expectancy modulates the neural response to visual food stimuli in humans. Appetite. 2011;56:302–309. doi: 10.1016/j.appet.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Lovascio BF, Shrestha N, Lin A, Leite-Morris KA, Man HY, Kaplan GB, Kantak KM. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav Brain Res. 2012;234:100–106. doi: 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2000. [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schuier F, Orzi F, Suda S, Lucignani G, Kennedy C, Sokoloff L. Influence of plasma glucose concentration on lumped constant of the deoxyglucose method: effects of hyperglycemia in the rat. J Cereb Blood Flow Metab. 1990;10:765–773. doi: 10.1038/jcbfm.1990.134. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Grunder G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda S, Shinohara M, Miyaoka M, Lucignani G, Kennedy C, Sokoloff L. The lumped constant of the deoxyglucose method in hypoglycemia: effects of moderate hypoglycemia on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1990;10:499–509. doi: 10.1038/jcbfm.1990.92. [DOI] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Uematsu A, Uneyama H, Torii K. Functional brain mapping of conscious rats during reward anticipation. J Neurosci Methods. 2012;206:132–137. doi: 10.1016/j.jneumeth.2012.02.014. [DOI] [PubMed] [Google Scholar]
- van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage. 2006;32:1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]