Abstract
Hypertonic saline (HS) resuscitation has been studied as a possible strategy to reduce PMN activation and tissue damage in trauma patients. HS blocks PMNs by ATP release and stimulation of A2a adenosine receptors. Here we studied the underlying mechanisms in search of possible reasons for the inconsistent results of recent clinical trials with HS resuscitation. Purified human PMNs or PMNs in whole blood were treated with HS to simulate hypertonicity levels found after HS resuscitation (40 mM beyond isotonic levels). ATP release was measured with a luciferase assay. PMN activation was assessed by measuring oxidative burst. The pannexin-1 (panx1) inhibitor 10panx1 and the gap junction inhibitor carbenoxolone (CBX) blocked ATP release from PMNs in purified and whole blood preparations, indicating that HS releases ATP via panx1 and gap junction channels. HS blocked fMLP-induced PMN activation by 40% in purified PMN preparations and by 60% in whole blood. These inhibitory effects were abolished by 10panx1 but only partially reduced by CBX, which indicates that panx1 has a central role in the immunomodulatory effects of HS. Inhibition of the ectonucleotidases CD39 and CD73 abolished the suppressive effect of HS on purified PMN cultures but only partially reduced the effect of HS in whole blood. These findings suggest redundant mechanisms in whole blood that may strengthen the immunomodulatory effect of HS in vivo. We conclude that HS resuscitation exerts anti-inflammatory effects that involve panx1, CD39, CD73, and other ectonucleotidases, which produce the adenosine that blocks PMNs by stimulating their A2a receptors. Our findings shed new light on the immunomodulatory mechanisms of HS and suggest possible new strategies to improve the clinical efficacy of hypertonic resuscitation.
Keywords: Keywords: Hypertonic saline, ATP release, immunomodulation, neutrophil activation, purinergic signaling
Introduction
Polymorphonuclear neutrophils (PMNs) are the most abundant immune cell population in human blood. PMNs defend the host against invading bacteria using a powerful arsenal of cytotoxic mediators. However, in critical care and trauma patients, excessive PMN activation can cause collateral tissue damage and multi-organ failure, which are considered major causes of morbidity and mortality in these patients (1). Hypertonic saline (HS) resuscitation has been studied as a potential strategy to reduce these complications in trauma patients (2). An early clinical trial comparing HS resuscitation (250 ml of 7.5% NaCl + 6% dextran-70) with normal saline showed that hypertonic resuscitation was safe despite the fact that it elevated plasma Na+ concentrations to levels that were about 20 mM higher than in control patients (2-3). While HS resuscitation did not improve overall survival in this trial, the authors of the study reported fewer complications due to organ failure in the HS group when compared to the normal saline control group (3).
The reasons for these beneficial effects remain unclear; however, the results of the study mentioned above are consistent with the concept that HS reduces PMN activation and host tissue damage (2). This notion is supported by the finding that exposure of purified PMNs to HS at hypertonicity levels equivalent to those found in trauma patients can significantly suppress PMN activation in vitro (4-5). In search of the underlying mechanisms, we found that HS and other compounds that increase the osmolality in the culture medium trigger rapid osmotic cell shrinkage, membrane deformation, and the release of cellular ATP into the extracellular environment (6). We also found that extracellular ATP and adenosine can stimulate various purinergic receptor subtypes on the cell surface of PMNs and that these purinergic receptors alter cell singling processes involved in PMN activation and functional PMN responses such as oxidative burst, degranulation, and chemotaxis (6).
The mechanisms by which intact cells release a portion of their cellular ATP are still not well defined. However, strong evidence implicates vesicular exocytosis, gap junction hemichannels, and pannexin-1 (panx1) channels as key ATP release mechanisms in immune cells (7-8). In our previous work, we identified panx1 as an important channel that facilitates HS-induced ATP release from T cells (9).
Released ATP can activate a number of different purinergic receptors. The mammalian purinergic receptor family comprises nineteen known members that can be divided into three separate subgroups: P1, P2X, and P2Y receptors (7-8). Adenosine is the natural ligand of the P1 receptor family that consists four members: A1, A2a, A2b, and A3. The P2X receptor family has seven known members (P2X1-7) that recognize extracellular ATP and act as ATP-gated ion channels. The eight known P2Y receptor subtypes (P2Y1, 2, 4, 6, 11, 12, 13, 14) that have been cloned and characterized can be stimulated by ATP, ADP, or similar nucleotides. P2Y and P1 receptors belong to the G protein-coupled receptor (GPCR) superfamily. The extracellular concentrations of the ligands of these different purinergic receptor subtypes are regulated by various ectonucleotidase families, including the ecto-nucleoside triphosphate diphosphohydrolases (ENTPDases; e.g., CD39), ecto-nucleotide pyrophosphatase/phosphodiesterases (ENPP), alkaline phosphatases, and ecto-5′-nucleotidase (CD73), which can convert ATP to ADP, AMP, and adenosine (7, 10). Of these different ecto-nucleotidase isoforms, CD39 and CD73 have special roles as they are abundantly expressed in many different tissues and synergize to convert released ATP to adenosine, whereby CD39 converts ATP and ADP to AMP and CD73 converts AMP to adenosine (10).
Thus, CD39 and CD73 define the extracellular concentration of adenosine, an ATP breakdown product that has a central function in the regulation of PMNs (11). Adenosine is recognized by A2a adenosine receptors, the most abundant purinergic receptor subtype in human PMNs (11). A2a receptors are linked to cAMP signaling and are responsible for the inhibition of PMNs by adenosine (5-6). We have previously demonstrated that HS blocks PMNs by stimulation of A2a receptors (6). In the current study, we investigated the upstream mechanisms by which PMNs release ATP and by which adenosine is formed, and how these mechanisms contribute to the suppressive effects of HS on PMNs in purified cultures and in whole blood samples. Our findings reveal a redundancy in these mechanisms that results in a powerful suppressive effect of HS on PMNs in whole blood. These findings corroborate our previous clinical finding that HS resuscitation of trauma patients has significant anti-inflammatory effects on PMNs.
Materials and Methods
Materials
Carbenoxolone (CBX), N-formyl-Met-Leu-Phe (fMLP), dextran, and all other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise stated. Percoll was from Pharmacia (Piscataway, NJ). 10Panx 1, ARL67156 (ARL), and α,β-methylene adenosine 5′-diphosphate (APCP) were purchased from Tocris Bioscience (Ellisville, MI).
PMN isolation
The Institutional Review Board of the Beth Israel Deaconess Medical Center approved all studies involving human subjects. PMNs were isolated from the peripheral blood of healthy volunteers as described previously using dextran sedimentation followed by Percoll gradient centrifugation (12-13). Cell preparations were kept pyrogen-free and osmotic or mechanical stimulation was carefully avoided to limit unintentional ATP release and cell stimulation. In some cases, PMNs were pretreated with different concentrations of CBX, 10panx1, ARL67156, or APCP. These treatments did not change cell viability as determined by trypan blue dye exclusion.
Measurement of ATP in cell supernatants
Freshly isolated human PMNs (5×105/ml) or freshly drawn heparinized whole blood samples were allowed to rest for 30 min at 37°C in a water bath that was placed on a vibration isolation table (TMC, Peabody, MA). Whole blood was diluted with four equal volumes of pre-warmed Hanks' Balanced Salt Solution (HBSS; Gibco, Life Sciences, Grand Island, NY). Then cells were treated with the inhibitors and stimuli as described in the individual experiments below. In order to study ATP release in response to HS, appropriate volumes of HBSS containing an additional 1 M NaCl were added to cell preparations or whole blood samples in order to increase the final NaCl concentration by 40 mM beyond isotonic levels. This level of hypertonicity is similar to that found in the plasma after HS resuscitation (3, 14). The cell preparations were chilled in an ice bath to stop ATP release and centrifuged to remove all cells. After gently centrifuging samples in an Eppendorf centrifuge for 10 min at 2,000 rpm and 0°C, cells were discarded and supernatants centrifuged again to remove platelets and remaining cells (5,000 rpm, 5 min, 0°C). The supernatants were collected and ATP concentrations were determined with a luciferin/luciferase-based ATP Bioluminescence Assay HS II Kit (Roche; Palo Alto, CA) and a Luminoskan plate reader (Labsystems; Helsinki, Finland) as previously described (15).
PMN activation
PMN activation was assessed by determining oxidative burst in response to fMLP stimulation using dihydrorhodamine-123 (DHR123; Invitrogen, Carlsbad, CA) and the flow cytometer method previously described (15-16). Briefly, isolated PMNs or whole blood samples (diluted 1:5 with HBSS) were incubated at 37°C, subjected to the treatments described in the individual experiments below, and DHR123 (2 μM for purified PMNs and 100 μM for whole blood) was added along with fMLP at a final concentration of 100 nM. After 20 min at 37°C, cells were placed on ice for 10 min to stop reactions and rhodamine123 fluorescence resulting from the conversion of DHR123 was assessed immediately using a BD FACSCalibur flow cytometer (Becton Dickinson; Lincoln Park, NJ). PMNs were gated based on their characteristic forward and side scatter properties (FSC and SSC) and rhodamine123 fluorescence was assessed as a measure of oxidative burst in individual PMNs.
Statistical analyses
Data are shown as means ± SD unless otherwise stated. Statistical analyses were done using one-way ANOVA followed by Newman-Keuls test, and differences were considered significant at p<0.05.
Results
Pannexin-1 (panx1) channels contribute to HS-induced ATP release from PMNs
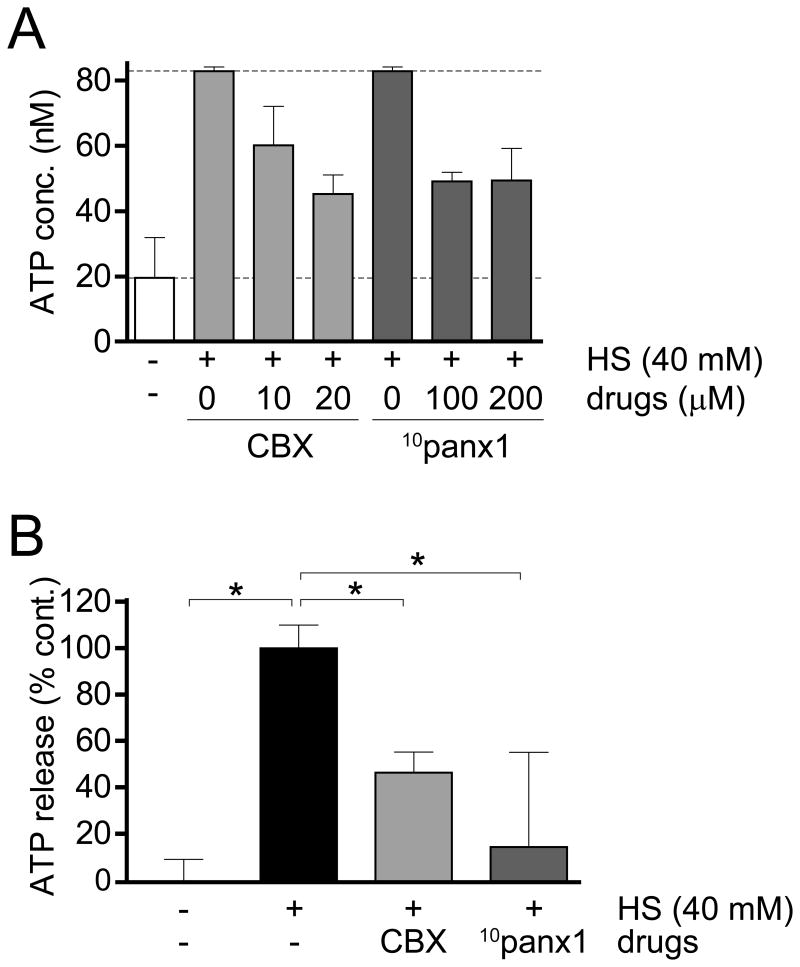
While autocrine purinergic signaling is recognized for its important role in immune cell regulation, the mechanisms by which immune cells release ATP are still poorly defined (7). Pannexin-1 (panx1) is one of three pannexin isoforms that are related to connexin gap junction proteins and have been shown to facilitate ATP release from various cell types (17). We have previously demonstrated that panx1 facilitates ATP release from T cells following HS treatment (9). Therefore, we tested whether panx1 plays a similar role in the response of PMNs to HS. Primary human PMNs were pretreated with the gap junction inhibitor CBX or the mimetic peptide 10panx1 that specifically blocks panx1 channels (13, 17-18). Depending on the donor from whom cells were isolated, baseline ATP levels in purified PMN cultures could range from 20-60 nM (Fig. 1A). On average, we found that pretreatment with CBX or 10panx1 inhibited HS-induced ATP release by 50-80% in purified PMN cultures (Fig. 1B). These results suggest that panx1 and CBX-sensitive gap junction channels contribute to HS-induced ATP release from human PMNs.
Figure 1. Pannexin-1 contributes to HS-induced ATP release from human PMNs.
Purified human PMNs were pretreated for 10 min with the indicated concentrations of the gap junction inhibitor CBX or the panx1 channel inhibitor 10panx1. Then HS was added to increase isotonic levels by an additional 40 mM and 3 min after addition of HS, ATP concentrations in the extracellular space were analyzed with a luciferin/luciferase-based ATP assay. Panel A shows representative results of experiments performed at least three times with cells from different donors. Individual measurements were performed at least in duplicate. Panel B shows cumulative results with cells from three different donors. Results are shown as means ± SD of three separate experiments and statistical analysis was performed with one-way ANOVA, *p<0.05.
Panx1 channels facilitate the suppressive effect of HS on PMNs
Next we investigated how ATP released from panx1 and gap junction channels contributes to the inhibitory effect of HS on purified PMNs. PMNs were pretreated for 10 min with different concentrations of CBX or 10panx1 and then exposed to HS for 3 min to increase the extracellular Na+ concentration by 40 mM above isotonic levels. Then, cells were stimulated with 100 nM fMLP and PMN activation was assessed by measuring oxidative burst using DHR123 and flow cytometry as described in the methods section. HS significantly suppressed PMN activation, reducing oxidative burst to 60% of the response of control cells at isotonic conditions (Fig. 2A-B). Pretreatment of PMNs with CBX or 10panx1 diminished this inhibitory effect of HS by 35 or 80%, respectively (Fig. 2B). These results demonstrate that panx1 channels and, to a lesser extent, CBX-sensitive gap junction channels are involved in the suppression of purified PMNs by HS.
Figure 2. Blocking ATP release reduces the suppressive effect of HS on PMN activation.
Purified human PMNs were pretreated for 10 min with the indicated concentrations of the gap junction inhibitor CBX or the panx1 inhibitor 10panx1. Then HS was added to increase isotonic sodium levels by an additional 40 mM. Cells were stimulated with 100 nM fMLP and oxidative burst was measured using DHR123 and flow cytometry. Representative flow cytometry data are shown in panel A. Results were expressed as the percentage of the average mean florescence intensity (MFI) values of treated cells versus untreated controls (B). Data shown are means ± SD and representative of three experiments with cell preparations from different donors and all measurements were performed at least in duplicate. Statistical analyses were done with one-way ANOVA, *p<0.05 compared to isotonic control, #p<0.05 compared to HS control.
Blocking CD39 and CD73 abolishes the inhibitory effect of HS on PMNs
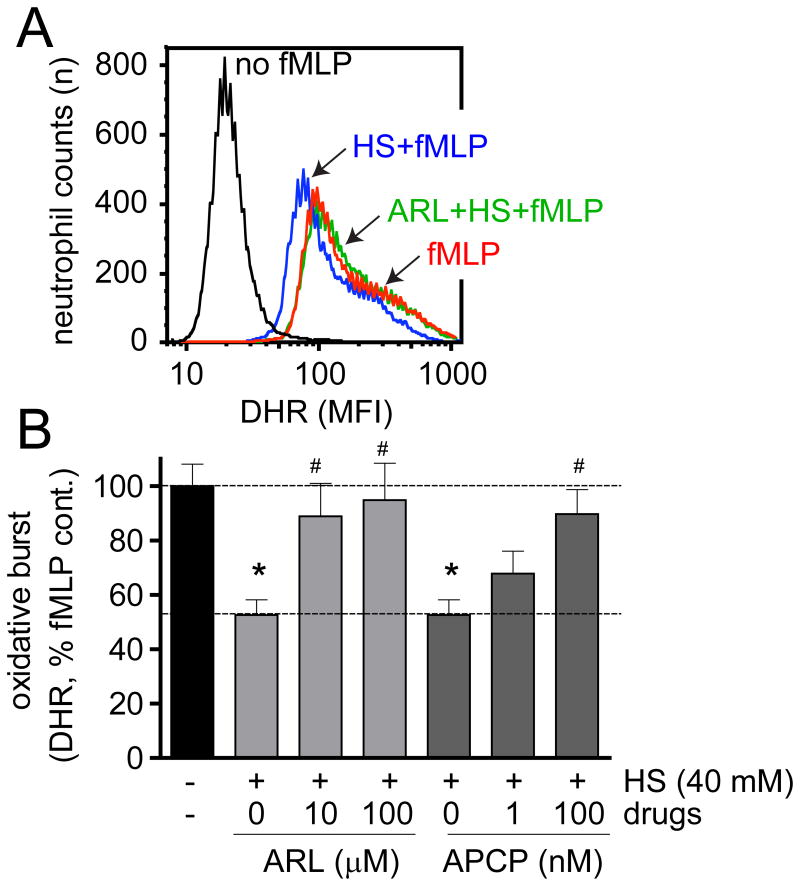
We have previously demonstrated that A2a adenosine receptors contribute to the suppressive effects of HS on PMNs (6). Here we examined the upstream mechanisms involved in the formation of the adenosine that stimulates these A2a receptors. Ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1; EC 3.6.1.5), also known as CD39, is a prominent member of the family of ectonucleotidases that are expressed on the cell surfaces of many mammalian cells (10). CD39 catalyzes the breakdown of extracellular ATP and ADP to AMP. However, the formation of adenosine from released ATP requires an additional enzymatic step that converts AMP to adenosine. Ecto-5′-nucleotidase (5′-NT; EC 3.1.3.5), also known as CD73, is capable of performing this enzymatic breakdown process (7, 10). In order to study whether these two enzymes are involved in the suppressive effect of HS, we used the CD39 inhibitor ARL67156 (ARL) and the CD73 inhibitor α,β-methylene adenosine 5′-diphosphate (APCP). Pretreatment of purified PMNs for 10 min with ARL or APCP reduced the suppressive effect of HS (Fig. 3A-B). These findings indicate that the breakdown of released ATP to AMP and the subsequent conversion of AMP to adenosine depend on CD39 and CD73 and that both ectonucleotidases are required for HS to exert its suppressive effect on purified PMNs.
Figure 3. ATP hydrolysis by CD39 and CD73 is required for the suppressive effect of HS on PMNs.
Purified human PMNs were pretreated for 10 min with the indicated concentrations of the CD39 inhibitor ARL67156 (ARL) or the CD73 inhibitor APCP. Then cells were exposed to HS (additional 40 mM above isotonic level), stimulated with 100 nM fMLP, and oxidative burst was measured with DHR123 and flow cytometry. Panel A shows representative data. The results obtained on different days were expressed as the percentage of the average mean florescence intensity (MFI) values of treated cells versus untreated controls (B). Data shown are means ± SD and representative of three experiments done with different cell preparations. All measurements were carried out at least in duplicate. Statistical analyses were done with one-way ANOVA, *p<0.05 compared to isotonic control, #p<0.05 compared to HS control.
The suppressive effect of HS in whole blood involves panx1, CD39, CD73, and other ectonucleotidases
The studies outlined above revealed that the effect of HS on isolated PMNs involves panx1-induced ATP release and the ectonucleotidases CD39 and CD73 to form the adenosine that blocks PMN activation via their A2a receptors. Our data suggest that all these components must be present in purified PMN cultures. However, when HS is administered in vivo, many other cell types can also be affected by HS and serve as additional sources of ATP. In addition, these other cell types possess ectonucleotidases that influence adenosine formation and the effect of HS on PMNs. Therefore, we studied the mechanisms by which HS alters PMN function in heparinized human whole blood samples. Blood samples were diluted 1:5 with isotonic HBSS. In unstimulated whole blood samples, we found basal ATP concentrations as high as 150 nM. These basal ATP levels varied among the donors who provided blood for our studies. After the addition of HS at a dose that increased the Na+ concentration by 40 mM above the levels under isotonic conditions, extracellular ATP concentrations could reach levels as high as 210 nM (Fig. 4A). Pretreatment with CBX completely abolished ATP release in response to HS, while 10panx1 reduced HS-induced ATP release by ∼80% (Fig. 4B). These findings suggest that HS treatment of whole blood causes ATP release through panx1 channels but also through other, CBX-sensitive gap junction channels. Inhibition of these channels blocked HS-induced ATP release more profoundly in whole blood than in purified PMN preparations (Fig. 1). Taken together, these findings suggest that the cells in whole blood release ATP primarily via panx1 and gap junction channels, while PMNs also utilize additional mechanisms such as vesicular transport.
Figure 4. Panx1 and other gap junction channels facilitate HS-induced ATP release from human whole blood.
Diluted human whole blood samples were pretreated with the indicated concentrations of CBX or 10panx1 for 10 min. Then HS was added to increase isotonic sodium levels by an additional 40 mM and after 3 min, ATP concentrations in the supernatants were analyzed with a luciferin/luciferase-based ATP assay. Panel A shows representative results of three individual experiments using blood from different donors. All measurements were performed at least in duplicate. Panel B shows cumulative results of all experiments done with blood from three donors. Results are shown as means ± SD and statistical analysis was performed with one-way ANOVA, *p<0.05.
Next we tested how panx1 and CBX-sensitive gap junction channels contribute to the suppressive effect of HS in whole blood. Compared to purified PMNs, HS had a more profound suppressive effect on PMNs in whole blood, reducing fMLP-induced oxidative burst by over 60% compared to the 40% observed in purified PMN cultures (Fig. 5A-B). However, while pretreatment with CBX and 10panx1 reduced the suppressive effect of HS in purified PMN preparations (Fig. 2), only 10panx1 was able to reduce HS-induced PMN suppression in whole blood (Fig. 5A). These results suggest that the ATP that elicits the suppressive effect of HS in whole blood originates primarily from panx1 channels, while CBX-sensitive gap junction channels deliver additional ATP that is not directly involved in the suppression of PMNs. A possible explanation for these dual roles of the released ATP may be differences in the subcellular localization of the ATP release channels relative to the location of the ectonucleotidases that generate adenosine and/or the A2a adenosine receptors that ultimately suppress PMNs. However, it should also be noted that ATP release itself contributes to PMN activation by stimulation of P2Y2 receptors and that stimulatory effect of ATP can also be affected by CBX (11-12).
Figure 5. Panx1, CD39, CD73, and other ectonucleotidases contribute to the suppression of PMNs by HS in whole blood.
Diluted human whole blood samples were diluted 1:5 with HBSS and pretreated for 10 min with the indicated concentrations of CBX, 10panx1 (panel A), ARL67156 (ARL), or APCP (panel B). Then HS was added to increase extracellular tonicity by 40 mM, stimulated with 100 nM fMLP, and oxidative burst was measured with DHR123 and flow cytometry. All data shown are cumulative results of three individual experiments performed with blood from different donors. All measurements were performed at least in duplicates. Data are shown as mean ± SD and statistical analysis was done with one-way ANOVA, *p<0.05 compared to isotonic control, #p<0.05 compared to HS control.
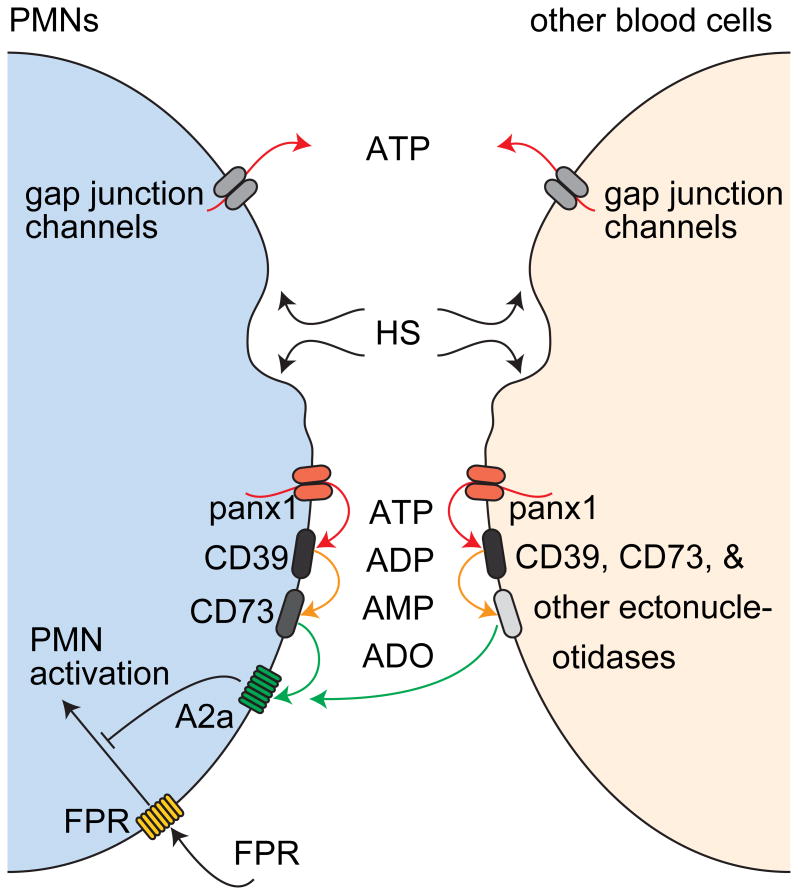
Inhibitors of CD39 and CD73 reduced the suppressive effect of HS in whole blood (Fig. 5B). However, this reduction was less profound compared to the effects of these inhibitors on purified PMN preparations (Fig. 3). These differences suggest that, contrary to our results with purified PMNs, adenosine formation in whole blood also includes mechanisms other than those driven by CD39 and CD73 (Fig. 6).
Figure 6. Proposed mechanism by which HS suppresses PMN activation in whole blood.
HS causes ATP release from PMNs and other cell types in whole blood. While different mechanisms are involved in ATP release, only panx1 delivers the ATP that facilitates the suppressive effect of HS on PMNs. CD39, CD73, and other ectonucleotidases on the surfaces of PMNs and other cell types contribute to the formation of adenosine, which blocks PMN activation by stimulation of A2a receptors. The diversity of ATP sources and the redundancy of ectonucleotidases that convert released ATP to adenosine strengthen the immunomodulatory effect of HS in whole blood.
Overall, our findings indicate that the suppressive effect of HS on PMNs is more robust in whole blood than in purified PMN preparations, because cell types other than PMNs in whole blood can serve as sources of ATP and because adenosine formation is accomplished by a more diverse set of ectonucleotidases that is available on the different cell types in whole blood. Taken together, our findings suggest that whole blood amplifies the inhibitory effect of HS on PMNs and that this amplification may increase the anti-inflammatory potential of HS resuscitation in patients.
Discussion
Several considerations have fueled the interest in hypertonic fluids for the resuscitation of trauma and critical care patients (19-23). Compared to conventional isotonic resuscitation fluids, significantly smaller volumes of hypertonic fluids are sufficient to restore circulation in hypotensive patients (21-22). The ease of transport of the small volumes of hypertonic fluids (250 ml per patient) has particular relevance in military applications. The infusion of such small fluid volumes is considerably easier and faster compared to bulkier isotonic fluids, allowing rapid restoration of blood circulation, which may reduce cell damage and protect host organs from hypoxia and reperfusion injury (21, 23-24).
In addition to these obvious advantages, HS also possesses immunomodulatory properties that can be harnessed to modulate the inflammatory response to shock and trauma (25-27). Of particular interest is the fact that HS can block PMN activation and reduce host organ damage in animal models of shock (25, 27-31). Based on compelling preclinical evidence, the efficacy and immunomodulatory properties of HS resuscitation were recently tested in a large randomized clinical trial (32-33). In this multi-center study, which was carried out in major trauma centers across North America, a 250-ml bolus of 7.5% hypertonic saline (HS) with or without 6% dextran-70 (HSD) was administered to patients in a pre-hospital setting. The trial was stopped prematurely when it became evident that the mortality during the first day after admission was higher in the HS group than in the HSD group and in the control group that received an equal volume of normal saline (31). Nevertheless, the long-term mortality rates and clinical outcome measures did not significantly differ among the three treatment groups. These clinical findings diverge from the results of most preclinical studies with HS that have shown beneficial effects on survival and outcome parameters.
The discrepancy between clinical and preclinical findings is difficult to explain. Numerous in vitro studies demonstrated that pretreatment of PMNs with HS at levels similar to those found in trauma patients can markedly block PMN activation (35). However, these laboratory studies were often carried out with purified PMNs. Therefore, we wondered whether differences in the immunomodulatory mechanisms of HS in purified PMN preparations versus whole blood could be related to the lack of the efficacy of HS resuscitation reported in clinical trials. Our current study was designed to investigate this issue. Interestingly, we found that HS exerts a more profound suppressive effect on PMNs in whole blood than in purified cultures (Figs. 2&5). Panx1 channels are responsible for these effects, apparently by supplying the ATP that contributes to the suppressive effect of HS on PMNs (Figs. 3&5). Moreover, we found that CD39 and CD73 have key roles in the formation of adenosine in purified PMN cultures, while additional ectonucleotidase isoforms are involved in eliciting the suppressive effects of HS in whole blood (Fig. 6). The redundancy of these mechanisms in whole blood increases the probability that the immunomodulatory efficacy of HS is maintained in trauma patients, even when the enzymatic activity of some of the ectonucleotidase isoforms that are involved in adenosine formation may be lost. Overall, our findings suggest that the suppressive effect of HS is more robust in whole blood than in isolated PMN preparations. This concept is supported by a recent clinical study that has shown that HS resuscitation profoundly suppresses PMNs in trauma patients (33).
However, this anti-inflammatory effect of HS resuscitation in trauma patients did not translate into actual survival benefits for these patients (21). These disappointing results may be due to the dual roles of PMNs in host defense and collateral host tissue damage. Because PMNs form the first line of defense against invading bacteria, blocking their activation with HS increases the risk of sepsis in those patients who acquire infections from their injuries or from nosocomial sources. Thus, like other anti-inflammatory treatment strategies, HS resuscitation can prevent host tissue damage in some patients and increase the risk of sepsis in others. Novel diagnostic tools will be needed to rapidly identify those patients who can benefit from HS resuscitation. Until such new tools are available, it will be difficult to anticipate whether HS resuscitation or other anti-inflammatory treatment strategies ameliorate organ failure or increase the risk of sepsis (36).
Acknowledgments
We acknowledge financial support through the National Institute of Health grants GM-51477, GM-60475, AI-072287, AI-080582, and T32 GM-103702, a grant from CDMRP (PR043034; W.G.J.), a Shock Society/Nova Nordisk research grant for Early Career Investigators (Y.C.), and a grant from the German Research Foundation (LE-3209/1-1; C.L.).
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA. Neutrophils--a key component of ischemia-reperfusion injury. Shock. 2013;40(6):463–470. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 2.Bulger EM, Hoyt DB. Hypertonic resuscitation after severe injury: is it of benefit? Adv Surg. 2012;46:73–85. doi: 10.1016/j.yasu.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Mattox KL, Maningas PA, Moore EE, Mateer JR, Marx JA, Aprahamian C, Burch JM, Pepe PE. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The U S A Multicenter Trial Ann Surg. 1991;213(5):482–491. doi: 10.1097/00000658-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junger WG, Hoyt DB, Davis RE, Herdon-Remelius C, Namiki S, Junger H, Loomis W, Altman A. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101(12):2768–2779. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlic T, Loomis WH, Shreve A, Namiki S, Junger WG. Hypertonicity increases cAMP in PMN and blocks oxidative burst by PKA-dependent and –independent mechanisms. Am J Physiol Cell Physiol. 2002;282(6):C1261–C1269. doi: 10.1152/ajpcell.00479.2001. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76(1):245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 7.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99(1):16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, Junger WG. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88(6):1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Yao Y, Sumi Y, Li A, To UK, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3(125):ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao Y, Chen Y, Ledderose C, Li L, Junger WG. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem. 2013;288(31):22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murao Y, Loomis W, Wolf P, Hoyt DB, Junger WG. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock. 2003;20(1):29–34. doi: 10.1097/01.shk.0000071060.78689.f1. [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, Ledderose C, Seier T, Graf AF, Brix B, Chong E, Junger WG. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem. 2014;289(39):26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Junger WG. Measurement of oxidative burst in neutrophils. Methods Mol Biol. 2012;844:115–124. doi: 10.1007/978-1-61779-527-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588(8):1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116(18):3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velasco IT, Pontieri V, Rocha e Silva M, Jr, Lopes OU. Hyperosmotic NaCl and severe hemorrhagic shock. Am J Physiol. 1980;239(5):H664–H673. doi: 10.1152/ajpheart.1980.239.5.H664. [DOI] [PubMed] [Google Scholar]
- 20.Smith GJ, Kramer GC, Perron P, Nakayama S, Gunther RA, Holcroft JW. A comparison of several hypertonic solutions for resuscitation of bled sheep. J Surg Res. 1985;39(6):517–528. doi: 10.1016/0022-4804(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 21.Bulger EM, Hoyt DB. Hypertonic resuscitation after severe injury: is it of benefit? Adv Surg. 2012;46:73–85. doi: 10.1016/j.yasu.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Dubick MA, Shek P, Wade CE. ROC trials update on prehospital hypertonic saline resuscitation in the aftermath of the US-Canadian trials. Clinics (Sao Paulo) 2013;68(6):883–886. doi: 10.6061/clinics/2013(06)25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wade CE, Grady JJ, Kramer GC, Younes RN, Gehlsen K, Holcroft JW. Individual patient cohort analysis of the efficacy of hypertonic saline/dextran in patients with traumatic brain injury and hypotension. J Trauma. 1997;42(5 Suppl):S61–S65. doi: 10.1097/00005373-199705001-00011. [DOI] [PubMed] [Google Scholar]
- 24.Baker AJ, Rhind SG, Morrison LJ, Black S, Crnko NT, Shek PN, Rizoli SB. Resuscitation with hypertonic saline-dextran reduces serum biomarker levels and correlates with outcome in severe traumatic brain injury patients. J Neurotrauma. 2009;26(8):1227–1240. doi: 10.1089/neu.2008.0868. [DOI] [PubMed] [Google Scholar]
- 25.Hampton MB, Chambers ST, Vissers MC, Winterbourn CC. Bacterial killing by neutrophils in hypertonic environments. J Infect Dis. 1994;169(4):839–846. doi: 10.1093/infdis/169.4.839. [DOI] [PubMed] [Google Scholar]
- 26.Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42(4):190–196. [PubMed] [Google Scholar]
- 27.Junger WG, Hoyt DB, Hamreus M, Liu FC, Herdon-Remelius C, Junger W, Altman A. Hypertonic saline activates protein tyrosine kinases and mitogen-activated protein kinase p38 in T-cells. J Trauma. 1997;42(3):437–443. doi: 10.1097/00005373-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Ciesla DJ, Moore EE, Zallen G, Biffl WL, Silliman CC. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: timing is everything. J Trauma. 2000;48(3):388–395. doi: 10.1097/00005373-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Murao Y, Hoyt DB, Loomis W, Namiki S, Patel N, Wolf P, Junger WG. Does the timing of hypertonic saline resuscitation affect its potential to prevent lung damage? Shock. 2000;14(1):18–23. doi: 10.1097/00024382-200014010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Hashiguchi N, Lum L, Romeril E, Chen Y, Yip L, Hoyt DB, Junger WG. Hypertonic saline resuscitation: efficacy may require early treatment in severely injured patients. J Trauma. 2007;62(2):299–306. doi: 10.1097/01.ta.0000222956.88760.33. [DOI] [PubMed] [Google Scholar]
- 31.Inoue Y, Chen Y, Pauzenberger R, Hirsh MI, Junger WG. Hypertonic saline up-regulates A3 adenosine receptor expression of activated neutrophils and increases acute lung injury after sepsis. Crit Care Med. 2008;36(9):2569–2575. doi: 10.1097/CCM.0b013e3181841a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, Brasel KJ, Tisherman SA, Coimbra R, Rizoli S, Minei JP, Hata JS, Sopko G, Evans DC, Hoyt DB. ROC investigators: Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junger WG, Rhind SG, Rizoli SB, Cuschieri J, Shiu MY, Baker AJ, Li L, Shek PN, Hoyt DB, Bulger EM. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline - without dextran - inhibits neutrophil and endothelial cell activation. Shock. 2012;38(4):341–350. doi: 10.1097/SHK.0b013e3182635aca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junger WG, Rhind SG, Rizoli SB, Cuschieri J, Baker AJ, Shek PN, Hoyt DB, Bulger EM. Prehospital hypertonic saline resuscitation attenuates the activation and promotes apoptosis of neutrophils in patients with severe traumatic brain injury. Shock. 2013;40(5):366–374. doi: 10.1097/SHK.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kølsen-Petersen JA. Immune effect of hypertonic saline: fact or fiction? Acta Anaesthesiol Scand. 2004;48(6):667–678. doi: 10.1111/j.1399-6576.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 36.Lansink KWW, Gunning AC, Leenen LPH. Cause of death and time of death distribution of trauma patients in a Level I trauma centre in the Netherlands. Eur J Trauma Emerg Surg. 2013;39(4):375–383. doi: 10.1007/s00068-013-0278-2. [DOI] [PubMed] [Google Scholar]