Abstract
BACKGROUND
Voiding disorders in humans, particularly in children are associated with increased incidence of behavioral issues as well as past history of childhood abuse. We hypothesized that creating stress in mice, utilizing either a chronic social defeat model (SD) or restraint stress in shallow water model (RSSW), would engender changes in bladder function, morphology, and behavior, thereby enabling us to study the resultant voiding dysfunction.
METHODS
For SD stress (14 days), C57BL/6 male mice were exposed daily to a larger aggressive CD-1 male for 10 minutes, followed by sensory exposure in a barrier cage for 24 hours. Control mice were similarly housed with no exposure. For RSSW (21 days), C57BL/6 mice were put in a perforated conical tube with feet immersed in water daily for 4 hours, then returned to single housing cages. Control mice were also in single housing. After the stress period, voiding patterns were obtained on filter paper, followed by behavioral tests. At necropsy, blood was taken for corticosterone analysis, and bladder and body weights measured. Bladder cryosections were stained with hematoxylin and eosin (H&E) for morphological assessment. Sequential sections were immunostained with antibodies to Ki-67 as a proliferation marker, CD31 (endothelial cell marker), and Uroplakin-II. Image J software was used to measure bladder wall thickness on blinded H&E photomicrographs as well as quantitate CD31 staining. Both Ki-67 -positive and -negative nuclei were counted with Imaris software to obtain a proliferation index.
RESULTS
Only SD mice had a single large void pattern. Bladder -to -body weight ratios increased in SD mice (p≤ 0.02) but not in RSSW mice. Plasma corticosterone levels were elevated in all stressed mice. SD mice exhibited lower levels of locomotor activity compared with controls; RSSW mice were hyperactive. In SD mice, bladder wall thickness was increased (p ≤ 0.003) but no change was seen in Ki-67 proliferation index, consistent with hypertrophy. No difference with control mice was seen in vascularity as visualized by CD31 staining. Uniform Uroplakin-II staining lined the urothelium of both SD and control mice.
CONCLUSIONS
Mice exposed to repeated SD (14 days) respond with altered voiding indicative of urine retention, and exhibit bladder wall changes consistent with hypertrophy while the urothelial barrier is maintained. These changes were not observed with repeated RSSW. SD, in contrast to RSSW, provides a model of psychological stress to further study the interplay of behavior and bladder dysfunction, enabling an improved understanding of voiding dysfunction, and the ability to create innovative and more effective management pathways for children who present with voiding dysfunction.
Keywords: social defeat stress, restraint stress, bladder, lower urinary tract symptoms, urinary voiding pattern
1. Introduction
Disturbances of urinary bladder filling and voiding cycles are reflected in a number of diverse symptoms including urgency, incontinence, and changes in frequency of voiding, that are collectively termed LUTS (lower urinary tract symptoms). The prevalence of LUTS in adults, obtained from large-scale population studies, is high for both men and women (Coyne et al., 2009; Irwin et al., 2006) with symptoms such as urgency self-reported in 22.4% of men and 35.7% of women. In a cross-sectional study of school-age children LUTS were detected in 21.8%, with urgency reported in 13.7% (Vaz et al., 2012); a recent review estimated the prevalence of incontinence in children at 17% (Deshpande, Craig, Smith, & Caldwell, 2012).
LUTS may arise from an underlying neurological abnormality (termed neurogenic LUTS) or from non-neurogenic abnormalities that may be due to anatomic malformation or be of unknown etiology. Behavioral and psychological disorders have been documented to co-exist in children with LUTS more often than for children with normal voiding habits (Logan, Correia, McCarthy, & Slattery, 2014; Oliver, Campigotto, Coplen, Traxel, & Austin, 2013; Schast, Zderic, Richter, Berry, & Carr, 2008; von Gontard, Baeyens, Van Hoecke, Warzak, & Bachmann, 2011). Findings from a number of studies agree that 20–25% of children with LUTS also have mental or behavioral health problems including anxiety, depression, attention deficit/hyperactivity disorder, or oppositional-defiant disorder. These findings have therapeutic implications for optimal treatment strategies for these children. Furthermore, it has been speculated that this close association may indicate an underlying role of psychosocial disorders in the etiology of LUTS in certain individuals (von Gontard, 2012). While it is established that the central nervous system controls certain basic aspects of lower urinary tract function, the influence of psychosocial disorders and chronic stress on neural circuits controlling micturition has yet to be fully characterized and understood. This gap in knowledge is a significant barrier to improving clinical outcomes for those affected with LUTS.
For rodents, the subordination of one male by a more aggressive male (social defeat) provides a paradigm for socially-induced psychological stress and leads to alterations in behavior indicative of high anxiety and/or depression (Golden, Covington, Berton, & Russo, 2011; Kinsey, Bailey, Sheridan, Padgett, & Avitsur, 2007; Krishnan et al., 2007). Similar study designs have also illustrated changes in immune function and visceral tissues, including the appearance of spontaneous colitis (Reber et al., 2007) and evidence of cardiac remodeling (Costoli et al., 2004). Splenomegaly occurs, accompanied by decreased sensitivity of immune cells to glucocorticoids, perhaps as an adaptive response to social defeat and the increased likelihood of sustaining a bite wound (Avitsur, Stark, & Sheridan, 2001). Of interest, it has been demonstrated that even age-matched mice of the same inbred strain and genetic background studied under identical conditions will vary in their responses to social defeat (Avitsur, Powell, Padgett, & Sheridan, 2009; Krishnan, et al., 2007).
During an episode of social defeat the dominant mouse exhibits aggressive behavior, such as chasing, grappling and biting, while the subordinate displays avoidance behaviors including defensive upright posturing and immobility. Urinary voiding is affected by social rank in male mice so that the subordinate male withholds from voiding (Desjardins, Maruniak, & Bronson, 1973). Previous work with rodent models of social defeat have shown that chronic social stress in the subordinate animal leads to changes in voiding patterns, and eventual physiological and molecular alterations in the bladder (Chang et al., 2009; Wood, Baez, Bhatnagar, & Valentino, 2009). In this paper we sought to replicate the voiding dysfunction in mice caused by social defeat stress as well as examine the effects of another chronic psychogenic stress modality. For this we employed repeated daily periods of restraint during which the mice were also placed in shallow water.
2 Material and Methods
2.1 Mice and housing
Mice were housed in a pathogen-free vivarium that uses the Modular Animal Caging System (Alternative Design, Siloam Spring, AR) with HEPA filtered air supplied via the Flex-Air System (Alternative Design, Siloam Spring, AR) at 30 air changes/h. Water was provided ad libitum by an automated system (SE Lab Group, Napa, CA). Each cage had ad libitum food, contained corncob bedding, and a nestlet to provide partial enrichment. Mice were maintained on a 14 h light:10 h dark (lights on at 600 h) schedule. Protocols were approved by the Institutional Animal Care and Use Committee. The vivarium is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.2 Social defeat model (SD)
Male C57BL/6N (B6) mice (46–63 days old) and CD-1 mice (4–6 months old, retired breeders) were obtained from Charles River Breeding Laboratories (Wilmington, MA). All mice were single housed in polysulfone plastic cages on stainless steel grid platforms over corncob bedding for 1 week prior to experimentation. Housing on a grid platform was required in order to minimize chewing and tearing of the filter papers used in obtaining urinary void patterns (see below). Daily social defeat encounters were carried out during the light period from 3–5 pm each day for 14 days, based on the protocol of Golden and colleagues (Golden, et al., 2011) with the following modifications. Retired breeder CD-1 male mice were used as the resident (aggressor) mice. To test the aggressive behavior of the CD-1 mice, we used a separate cohort of B6 mice. Briefly, a naïve B6 mouse was added to the home cage of the CD-1 mouse and the time to attack was measured. Only CD-1 mice that attacked within 3 min were used in subsequent social defeat experiments. One week prior to the start of the experiment each CD-1 mouse was singly housed in a Thoren Weaning Cage (31×31×14 cm, Thoren Caging System, Inc., Hazleton, PA) with a stainless steel platform grid over corncob bedding and a nestlet. Each cage had a stainless steel double feeder with 2 water bottle holders, a top filter cover, and a perforated Plexiglas divider (crafted in house) that can be inserted in the middle to provide separate housing.
On Day 1, a naïve B6 male (n =20) was added to a Thoren cage containing a resident CD-1 mouse and a timer was started. The time to first attack was noted and the mice were kept in physical contact for a total of 10 min under constant observation. Then a perforated Plexiglas divider was inserted into the cage, separating the 2 mice physically but allowing sensory contact. Fresh nestlets were added to each compartment. If wounding occurred prior to 10 min, the mice were separated immediately. 24 h later the B6 mice were rotated to the home Thoren cage of a different CD-1 male, and the procedure repeated, for a total of 14 consecutive days. Platform grids were cleaned between each encounter. If no attack occurred within 3 min, the B6 mouse was removed and placed with another CD-1 mouse. Control B6 mice (n = 16) were singly housed on one side of the Plexiglas divider in grid-floored Thoren cages with no CD-1 mouse present. Each day, the grid platforms of the control cages were cleaned and the mice given new nestlets.
2.3 Restraint stress in shallow water model (RSSW)
Adult male C57BL/6J mice (~56 days of age) were obtained from Jackson Laboratories (Bar Harbor, ME) and singly housed in polysulfonate cages for 7 days prior to experimentation. Mice were randomly selected as controls (10 mice) or restraint condition (10 mice). Water-restraint stress was performed as described by Tomita and colleagues (Tomita et al., 2011) with the following modifications. Mice were restrained in 50 ml conical tubes that had 10 holes to allow the mice to breathe. The tubes were then placed horizontally in a 12 × 8 cm plastic box and water (22 ± 1°C) added to a depth of 0.9 cm to cover their ventral surface. Restraint occurred daily for 21 days for 4 h, beginning between the hours of 9 am and 1 pm each day. Control mice were left undisturbed in their individual home cages during this time.
2.4 Void pattern determination
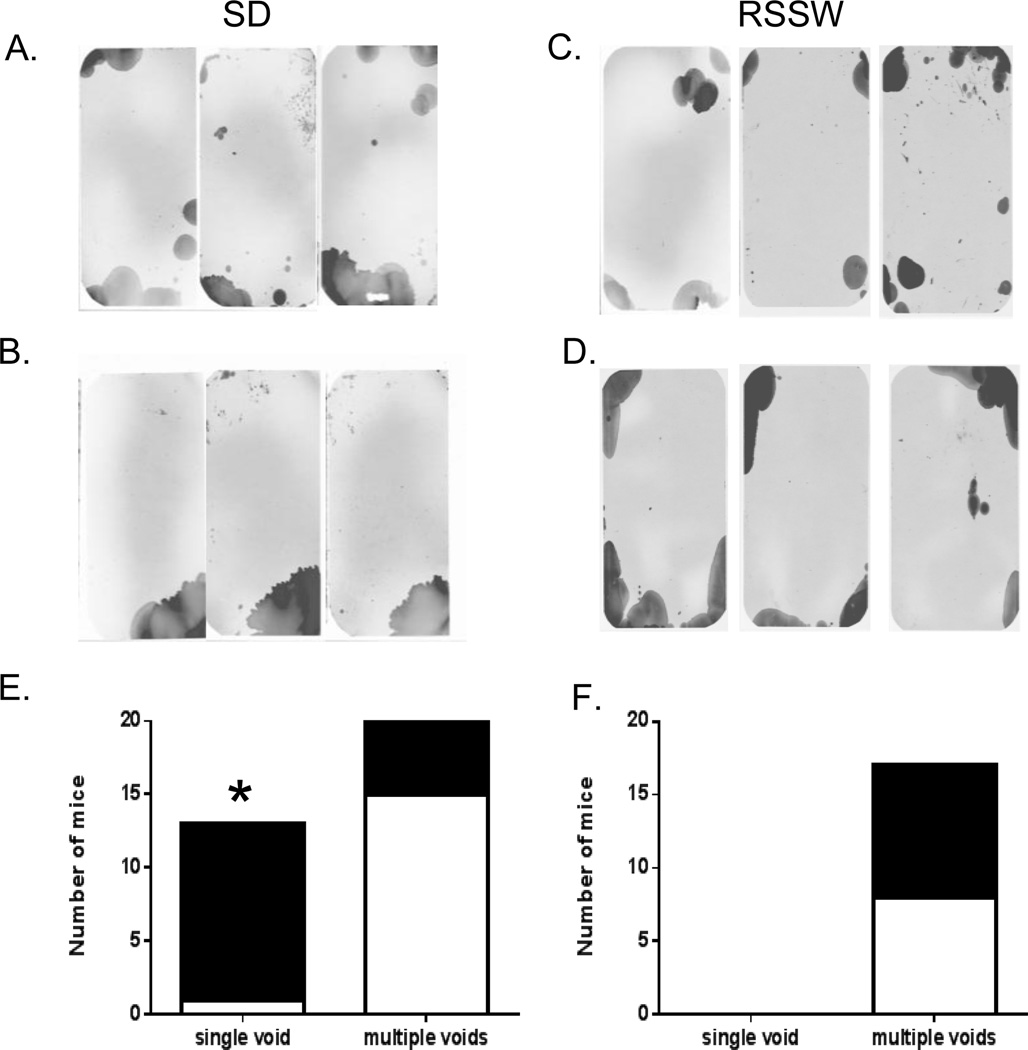
Bladder voiding patterns for control and stressed mice were obtained on Whatman 3 MM chromatography paper (Fisher Scientific, Pittsburgh, PA). For each mouse, two filter papers cut to a size corresponding to the bottom of the cage were placed beneath the stainless steel grid immediately after social defeat or at 5 pm for control mice and restraint stress mice. The following morning, the filters were removed and air-dried. Urine spots on the top filter were visualized by fluorescence at 457 nm, using the Typhoon FLA 9500 laser scanner (General Electric Healthcare, Pittsburgh, PA) and digitally recorded. Visual inspection of the number of spots was performed in a blinded fashion. Further analysis of the filters was performed to measure void areas using the “analyze particles” function in ImageJ (http://imagej.nih.gov/ij) based on the macro described by Yu and coworkers (Yu et al., 2014). We first applied drops of different volumes (1–150 µl) of mouse urine onto Whatman 3 MM paper. A linear relationship was found between volume and area (R2 = 0.996). Spots of 1 µl corresponded to an area of 0.105 cm2 and this was used as the lower limit in the “analyze particles” function in ImageJ, to avoid capturing small marks not due to urine deposition. All spots identified by the macro were curated by comparison with the original filter to rule out artifacts, such as tail drags, or marks due to feces. As noted by Yu and co-workers, this method is semiquantitative due to the presence of multiple overlapping spots during spontaneous voiding, which also occurred for both control and stressed mice in our study (Figure 1). Individual serial voids in the same location cannot be distinguished by this method, and therefore the calculated area is an underestimate of the volume.
Figure 1.
Difference between voiding patterns of SD and RSSW mice. (A – D) Representative voiding patterns of mice are shown with dark areas corresponding to urine spots. (A and C) are from control mice. Control patterns are similar in terms of the number of urine spots as well as the area of individual spots. (B) The voiding patterns of stressed mice of the SD group exhibit a single large void, confined to one corner, while the voiding patterns of RSSW stressed mice (D) are similar to those of control mice. (E) The majority of SD mice (black) showed a single void pattern while most control mice (white) had a multiple voids pattern (*, p ≤ 0.001, Fisher’s exact test). None of the mice in the RSSW group (F) had a single void pattern. Black -stressed mice; white -control mice.
2.5 Behavioral tests
All behavioral assessments were done by personnel blinded to the conditions of the animals.
2.5.1 Locomotor activity
Locomotor activity was performed in a 40 × 40 cm automated locomotor activity chamber (PAS: San Diego Instruments, San Diego, CA) to assess initial exploratory activity levels and activity during habituation to the chamber (Hautman et al., 2014). Mice were placed in the chamber for 1 h with data collected every 5 min. The number of infrared beam breaks (total horizontal activity) was the dependent measure. Locomotor activity was assessed for SD mice one day after the final stress session, and for RSSW mice, 4 days post-stress.
2.5.2 Forced swim test
A modified version of the Porsolt rat Forced Swim Test (FST) was used (Cryan, Valentino, & Lucki, 2005; Porsolt, Bertin, Blavet, Deniel, & Jalfre, 1979). Animals were placed in a transparent glass cylindrical vessel 10 cm in diameter (i.d.) and 25 cm tall that was filled to a depth of 6 cm with 22 ± 1°C water. On day 1 (one day post-stress for SD mice; 6 days post-stress for RSSW mice), mice were placed in the chamber and allowed to swim for 15 min. On day 2, mice were returned to the chamber for 5 min and scored for immobility, latency to immobility, and swimming time every minute. Immobility was defined as minimal motion for the animal to stay afloat.
2.6 Corticosterone Analysis
Trunk blood from SD and RSSW mice was collected at necropsy. Blood was spun down, plasma collected, and stored in aliquots at −20°C. Corticosterone levels were assessed by ELISA (kit for RSSW model mice from Enzo Life Sciences Inc., Farmingdale, NY and for SD model from Immunodiagnostic Systems Inc., Fountain Hills, AZ). Values were normalized to the median of the respective control group, and the data presented as percent of control ± SEM.
2.7 Animal Weight and Organ Weights
The weight of the animals was taken every two to three days to monitor their overall health. At the end of the studies the urinary bladder and spleen were removed at necropsy (SD, 2 days post-stress; RSSW, 18 days post-stress) and the weights of these organs and body weight were recorded.
2.8 Bladder Analysis
Each bladder was divided in half along the longitudinal axis and embedded in OCT (Tissue Tek, Thermo Fisher Scientific, Waltham, MA) or Neg-50 (Richard Allen Scientific, Thermo Fisher Scientific, Waltham, MA). Sagittal sections (10 µm) were stained with hematoxylin and eosin (H&E). Sections from the center of the bladder with intact mucosa, complete muscle wall, and devoid of artifacts were selected for subsequent analyses. One mouse was excluded because it did not meet these criteria. All analyses were performed by an investigator blinded to the experimental condition of the animals.
2.8.1 Immunostaining
Sections were fixed with 4% PFA for 5 min followed by 1XPBS washes. Sections were permeabilized with 1XPBS containing 0.1% Triton-X-100 (PBST) for 20 min at room temperature. After blocking with 10% serum containing 4% BSA for 1 h at room temperature in a humidity chamber, the sections were incubated overnight at 4°C with primary antibody: Rabbit anti - Ki-67 (1:250, Thermo Fisher Scientific, Waltham, MA), Goat polyclonal anti - uroplakin II (UPKII, 1:50, Santa Cruz Biotechnology Inc. Dallas, TX); Rat anti - CD31 (1:50, cat. 550274, BD Biosciences, Franklin Lakes, NJ) or Rabbit anti - α smooth muscle actin (1:50, Abcam, Cambridge MA). Only blocking buffer was added to control sections. Sections were washed the next day with PBST four times and incubated with species matched Alexa Fluor 488 or 555 conjugated secondary antibody for 1 h at room temperature and counterstained with DAPI (Sigma, St. Louis, MO). Sections were again washed with PBST three times and mounted with ProLong Gold antifade reagent (Life Technologies, Carlsbad, CA).
2.8.2 Ki-67 image analysis
Tiled bladder images were generated of a section from each mouse with a Nikon A1Rsi confocal microscope (Nikon Instruments, Inc., Melville, NY) using a 20X objective and “large image” function in NIS Elements ND acquisition. In order to determine a proliferation index, muscle and urothelial areas were defined, and total and Ki-67 -positive cell counts from each area were obtained using the spots function in Imaris software (Bitplane, South Windsor,CT). Ki-67 positive cells were marked as having increased fluorescence over background plus being positive for DAPI.
2.8.3 Measurement of bladder wall thickness
Images of H&E stained bladder sections from stressed and control mice were generated using a Zeiss Axioplan 2 Imaging microscope (Carl Zeiss Microscopy, LLC, Peabody, MA) with 5× objective. Multiple muscle wall thickness measurements were performed on blinded photomicrographs, from the serosa to the innermost part of the muscularis propria, perpendicular to the lumen, using the ImageJ program for Macintosh (http://imagej.nih.gov/ij).
2.8.4 Evaluation of blood vessel density
The vascularity of bladders from stressed and control mice was measured by determining the relative density of endothelial cells using CD31 immunostaining. Images of bladder sections were acquired using a Zeiss ApoTome Axio Imager 2 (Carl Zeiss Microscopy, LLC, Peabody, MA) with a 5× objective. The relative area of CD31 immunostaining was quantified using ImageJ (http://imagej.nih.gov/ij) as described by Kalluri and co-workers (Ayala de la Pena et al., 2011).
2.9 Statistical Analysis
Data were analyzed between stressed and control groups within a model by Fisher’s exact test or t-test using GraphPad Prism version 6.03 for Windows (GraphPad Software, La Jolla, CA www.graphpad.com). Behavioral data were analyzed using mixed linear factorial analysis of variance (SAS v9.3, SAS Institute, Cary, NC). The between subjects factors were Group (stress vs control) and the repeated measure factor was Interval (for locomotor activity). Significant interactions were further analyzed using slice-effect ANOVAs on the repeated measure factor. Degrees of freedom used the Kenward-Roger method. Locomotor test data are presented as least squared means ± SEM, due to the use of repeated measures analysis for this test, all other data are presented as means ± SEM. A p ≤ 0.05 was used as the test of significance.
3 Results
3.1 Lower urinary tract dysfunction occurred only in SD mice
We first compared the physiological effects of the SD and RSSW models. We used bladder void patterns as the primary outcome measure of voiding dysfunction. A single pattern obtained overnight at the end of the stress period in the SD and RSSW models was used to assess alterations in voiding between control and stressed mice (Figure 1). Multiple overlapping voids were present in both control and stressed mice, and precluded an accurate count of individual voidings. Control mice voided in several locations along the sides and corners of the cage in a pattern similar to previous results with C57BL/6 strain mice (Yu, et al., 2014). The majority (12 of 20) of SD mice exhibited a single large void pattern (Figure 1B) while only 1 of 16 control mice (Figure 1A) had a single void pattern at the end of the experiment (Figure 1E), suggesting underlying bladder dysfunction in the form of urinary retention (p ≤ 0.001). To compare the relative volume of urine voided by each group, we measured the area of the urine spots based on a method by Yu and co-workers (Yu, et al., 2014). While the total area was not significantly different (p ≤ 0.09, control, 39.9 ± 3.9 cm2, n = 16; SD, 51.0 ± 4.7 cm2, n = 20, mean ± SEM), the primary void area, defined as the area of the largest urine spot per filter, was increased (p ≤ 0.02) in SD mice (40.5 ± 2.8 cm2) compared to controls (29.6 ± 3.7 cm2).
The void patterns of control and post-RSSW mice were similar and also exhibited overlapping void spots (Figure 1C & D, respectively). In contrast to the SD group none of the mice exposed to RSSW had a single void pattern (Figure 1F). There were no significant differences in either the total area of urine spots (p ≤ 0.2, control, 49.8 ± 6.8 cm2, n = 8; RSSW, 39.4 ± 3.8 cm2, n = 8, mean ± SEM) or in the primary void area of control versus RSSW mice (p ≤ 0.4, control, 27.0 ± 7.4 cm2; RSSW, 20.9 ± 2.1 cm2, mean ± SEM).
3.2 Changes in visceral organ weights
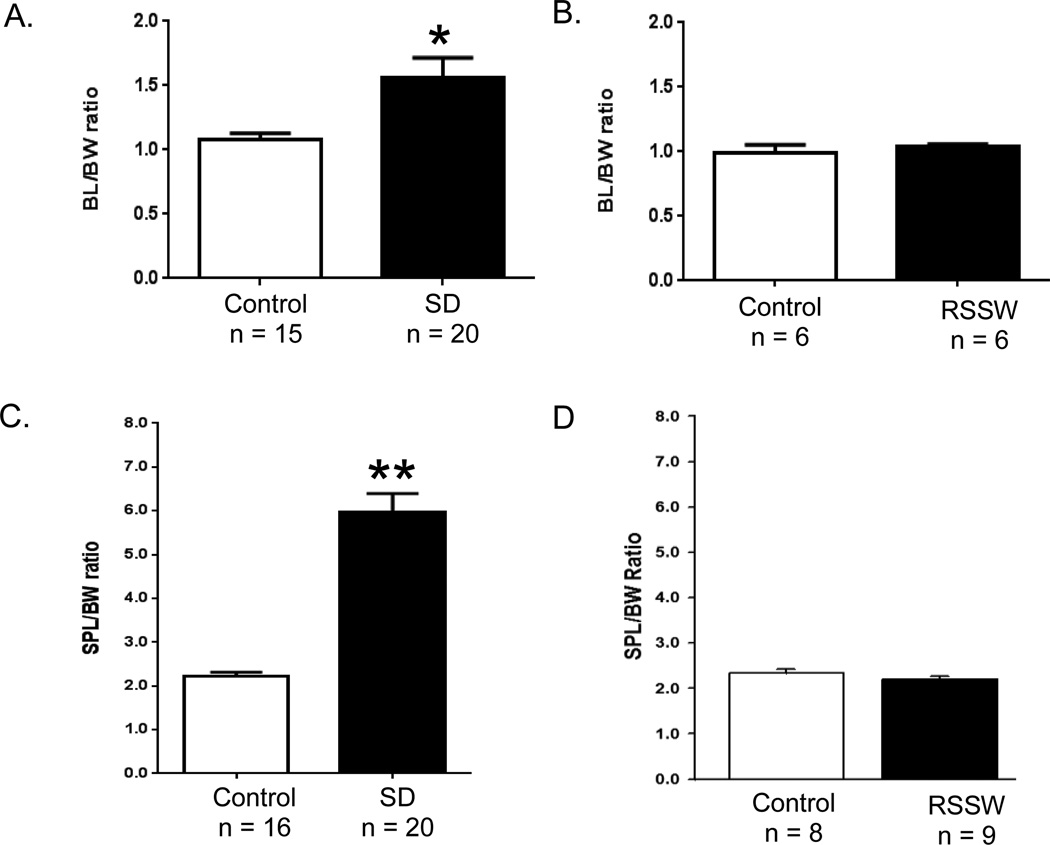
In a surgical model of murine bladder outlet obstruction, chronic retention of urine induced detrusor hypertrophy, reflected in an increase in bladder mass (Austin, Chacko, DiSanto, Canning, & Zderic, 2004). This was also noted in two previously reported 4 week long studies of water avoidance stress (McGonagle et al., 2012) and social defeat stress (Chang, et al., 2009) in mice. In our current 2 week study, we also identified an overall increase (Figure 2A) in the bladder -to -body weight ratio (BL:BW) of SD mice compared with controls (p ≤ 0.02) while no difference was seen in body weight (control, 25.3 ± 0.54 g, n = 16; SD, 25.25 ± 0.32 g, n = 20, mean ± SEM). However, not all mice responded to the same extent. Only 60% of mice (12 of 20) exhibited an increase that was greater than 2 standard deviations (S.D.) from the mean of the controls; these mice will be referred to as responders. There was also no difference in body weight between control and RSSW mice at necropsy (control, 26.1 ± 0.88 g, n =6; RSSW, 25.57 ± 0.57 g, n = 6, mean ± SEM). In contrast to SD, there was no change in bladder -to -body weight ratio seen in the RSSW model (Figure 2B). Splenomegaly has been observed in a social disruption stress model in mice (Avitsur, et al., 2009; Avitsur, et al., 2001) so we also examined spleen -to -body weight ratios in our models (Figure 2C, D). This ratio was increased in all mice in the SD experiment (p ≤ 0.0001) but was not altered in the RSSW model.
Figure 2.
Bladder and spleen weights were increased in SD mice. (A) The mean of the bladder (mg) -to -body weight (g) ratio (BL:BW) in stressed mice of the SD group was increased 1.5 times the mean of the control group, and the spleen -to -body weight ratio (SPL:BW) was 3 times higher (C), while these measures were unchanged in the RSSW group (B, D). *, p ≤ 0.02; **, p ≤ 0.0001, t-test. Data are presented as the mean ± SEM.
3.3 Locomotor activity
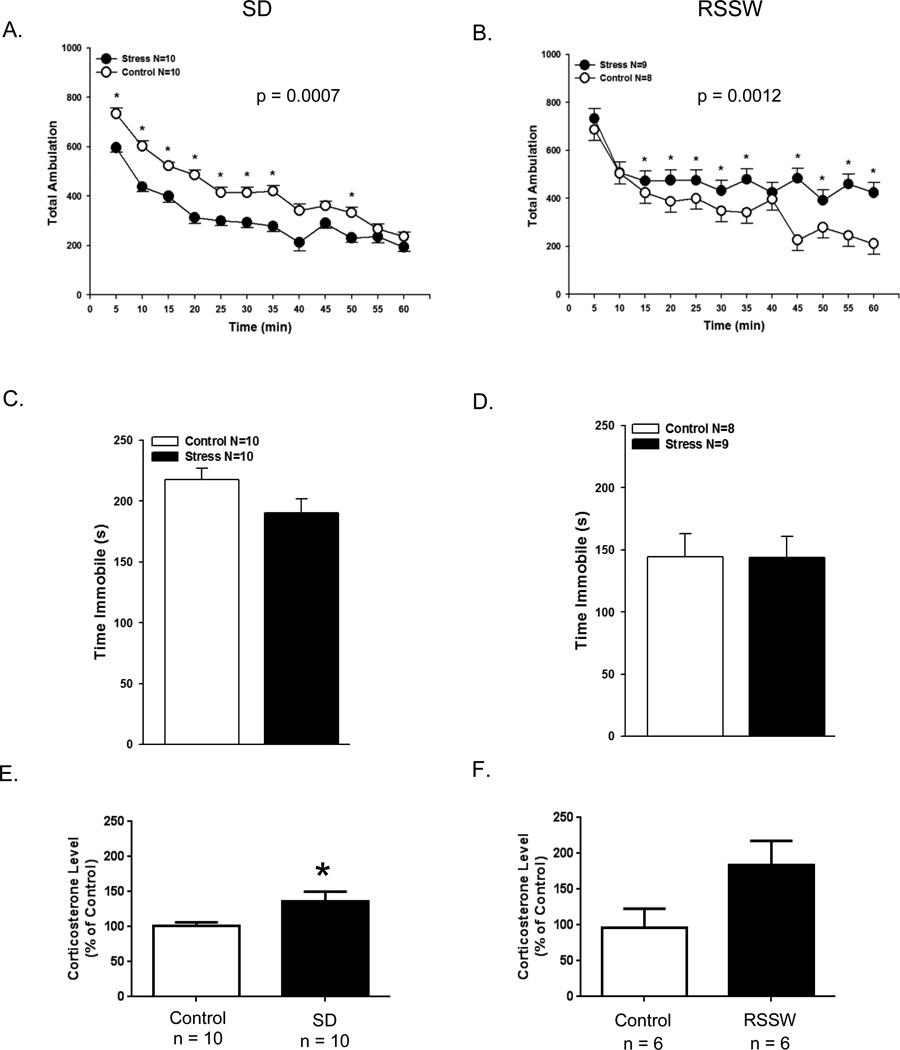
We next measured behavioral alterations in stressed and control mice at the completion of the 2 stress protocols. The measure analyzed in the automated locomotor activity test was the total number of horizontal beam breaks collected every 5 min over a 60 minute period (Figure 3A, B). Compared with control mice, SD mice exhibited hypolocomotion throughout the first 50 min of the test (p = 0.0007). In contrast, mice that had undergone RSSW stress acted in a similar fashion to controls at the beginning of the test but were significantly more active from the 15 min mark onwards (p = 0.0012).
Figure 3.
Locomotor activity differs in SD and RSSW mice. (A, B) Mice from both the SD and the RSSW groups exhibit different locomotor behavior compared with their respective controls. Data presented as least squared mean ± SEM. (C, D) Neither stress elicited depressive-type behavior in the forced swim test (data presented as mean ± SEM). (E, F) At necropsy, SD mice exhibited a significant elevation of plasma corticosterone while RSSW mice exhibited a trend towards increased corticosterone compared with controls (p ≤ 0.07). Data are presented as the mean ± SEM. *p ≤ 0.03
3.4 Forced swim test
The forced swim test evaluates swimming immobility as a measure of despair. There was no difference between controls and stressed mice in the duration of immobility with either the SD or the RSSW model. (Figure 3C,D).
3.5 Plasma corticosterone level
Activation of the stress response is accompanied by a rise in systemic corticosterone. Immediately after the forced swim test, the level of corticosterone present in trunk blood from SD mice was analyzed (Figure 3E). There was a significant elevation in plasma corticosterone from social defeat stressed mice compared with control mice. Although there was a trend towards increased levels in RSSW stressed mice at necropsy (Figure 3F), the difference was not statistically significant (p ≤ 0.07). Assessment of plasma corticosterone levels from submandibular bleeds at Day 14 of the RSSW protocol did detect significant elevation in stressed mice compared with controls (p ≤ 0.01, Control, 19.7 ± 5.1 ng/ml, n = 6; RSSW, 63.3 ± 14.1 ng/ml, n = 5, mean± SEM).
3.6 Detrusor muscle width is increased in bladders from SD mice
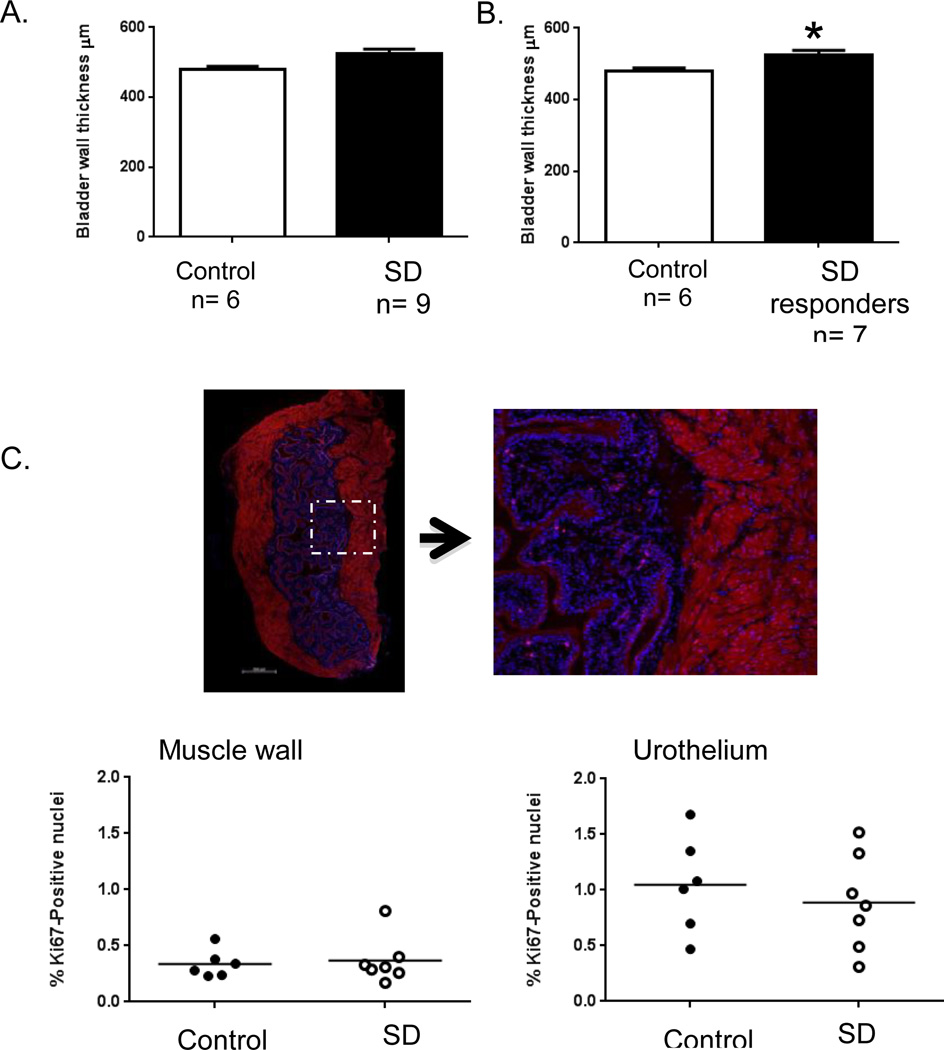
To further address the increased bladder -to -body weight ratio seen in the SD mice, we performed wall measurements on H&E stained bladder sections from control and stressed mice (Figure 4A, B). While there was no difference in the mean muscle wall thickness when all SD mice were compared with controls, there was a significant increase in width when only measurements from responder mice (who had increased bladder -to -body weight ratios) were compared with control mice (control, 479 ± 9.5 µm vs. responder, 525.8 ± 11.9 µm, mean ± SEM, p ≤ 0.003).
Figure 4.
Bladder wall thickness is increased in SD mice while the proliferation index is unchanged. (A, B) Measurements of the detrusor wall perpendicular to the lumen were made in bladder sections from control and SD mice (data presented as mean ± SEM). A statistical difference was seen only when comparing sections from control versus responder mice (those mice with a bladder -to -body weight ratio greater than 2 S.D. from the mean of the control). (C) Representative bladder section immunostained with Ki-67 (magenta) and counterstained with DAPI (blue). Background fluorescence from the bladder muscle is seen in red. Both Ki-67 and total nuclei were counted and a proliferation index computed using Imaris software. No significant difference is seen between SD responders and control mice, either in the muscle or the urothelium. Line is drawn at the mean value of each group. *, p ≤ 0.003
The increase in thickness may result from increased muscle cell number so we examined proliferation in bladder sections using Ki-67 immunostaining as a marker (Figure 4C). There was no significant difference in the proliferation index (the percent of Ki-67-positive nuclei) calculated for the detrusor between control and responder stressed mice. We analyzed the urothelial layer, including the lamina propria, as well and likewise found no difference in the proliferation index.
3.7 SD bladders have intact urothelial barrier and normal vascularity
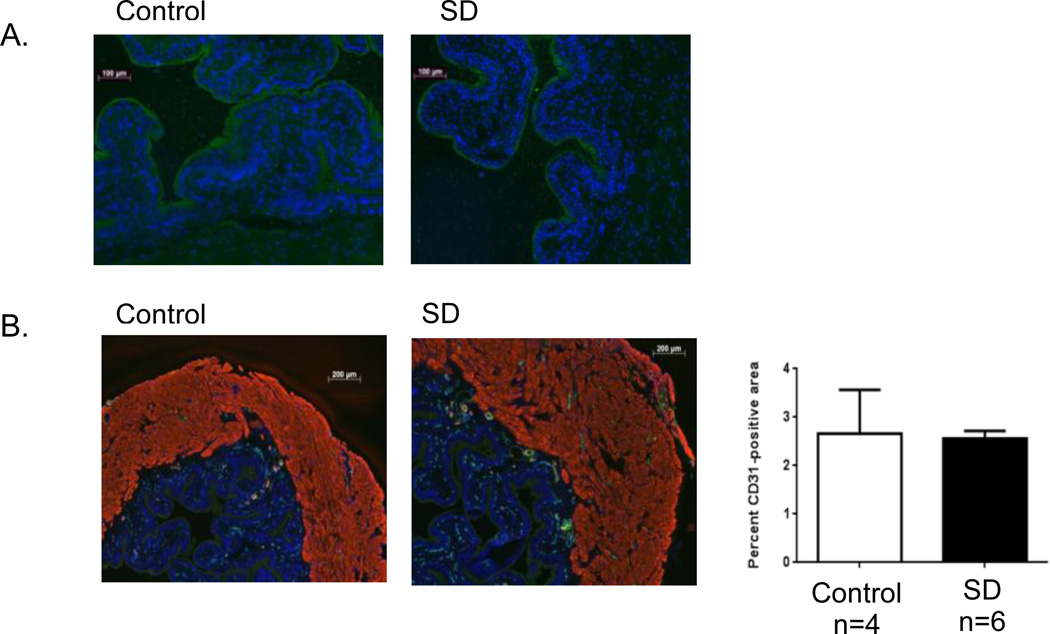
We examined sequential bladder sections for the presence of additional morphological changes. To rule out urothelial damage in our model of SD, we performed uroplakin immunostaining (Figure 5A). Uniform, continuous staining of the umbrella cells of the urothelium was detected in sections from both stressed and control mice. CD31 is present on endothelial cells and can be quantified by immunofluorescent techniques as a measure of vascularity. There was no difference in the relative area of CD31 positive cells between sections of SD stressed and control bladders (Figure 5B).
Figure 5.
Urothelial integrity and bladder vascularity are preserved in SD. Immunostaining of bladder sections from control and SD stressed mice for: (A) the urothelium-specific transmembrane protein uroplakin II (green), counterstained with DAPI (blue), at 100×, and (B) the endothelial marker CD31 (green), α smooth muscle actin (red) and DAPI (blue), at 50×. Following quantification with ImageJ no significant difference in the relative area of endothelial cell staining (CD31 positive) between groups was detected. Data are presented as the mean ± SEM.
4. Discussion
The mechanisms underlying non-neurogenic LUTS are likely multifactorial and may include a psychological component. In the current studies we have addressed this in animal models by comparing the effects of two paradigms of psychological stress (i.e., social defeat and restraint stress in shallow water), on bladder voiding function, physiology, and behavioral changes in C57BL/6 male mice. Changes in voiding frequency and urine retention have long been known to accompany the formation of social hierarchies in male mice (Desjardins, et al., 1973; Lumley, Sipos, Charles, Charles, & Meyerhoff, 1999), and have been interpreted as a natural stress adaptation on the part of the subordinate male. Other studies have suggested that repeated social defeat in rats and mice can also result in pathologic changes to the bladder, including hypertrophy and altered urodynamic parameters, similar to those seen due to bladder outlet obstruction (Chang, et al., 2009; Mingin, Peterson, Erickson, Nelson, & Vizzard, 2014; Wood, et al., 2009). While we have not performed urodynamic studies, the majority of SD mice in this study exhibited bladder hypertrophy, as evidenced by increased bladder mass and increased thickness of the bladder wall in the absence of increased proliferation.
In our current studies, voiding patterns of mice subjected to 2 weeks of repeated social defeat showed single large voids compared with multiple smaller voids exhibited by control mice. These mice were also less likely to explore a novel environment, and had on average, a 50% increase in bladder mass upon necropsy. This significant alteration in bladder mass that we observed is consistent with a hypertrophic early response to chronic urine retention. In contrast, mice exposed to 3 weeks of daily restraint stress in shallow water had voiding patterns similar to those of control mice, no increase in bladder mass, and were similar in activity to control mice during the initial exploration period. These findings underscore the concept that different types of chronic stress may elicit different neuroendocrine responses with correspondingly different physiological outcomes (Ulrich-Lai & Herman, 2009). Future studies, including cystometry in mice to monitor bladder pressure and voiding volume, and identification of alterations in molecular pathways regulating micturition, are necessary to determine the full effect on bladder function and the dynamics of voiding in chronically defeated mice.
The timing of behavioral tests and necropsy were done at different intervals post-stress for the 2 models (see Materials and Methods). Such differences may constitute a confounding factor in our analysis. However, our primary measure for changes in bladder voiding, the void pattern determination, was performed immediately after the last stress session in both models. The temporal relationship between cessation of stress and ongoing behavioral alterations and/or physiological bladder changes has not been determined by us, and would be of interest in future studies.
Persistent urine retention in mice driven by long-term social defeat resulted in functional and physiological changes in the bladder of FVB male mice (Chang, et al., 2009). After 4 weeks of daily defeat by resident C57BL/6 mice, voiding pattern analyses and in vivo cystometry revealed changes indicative of decreased frequency and increased fluid volume at micturition compared with control mice. Urinary volumes doubled with no increase in bladder threshold pressure. At necropsy, bladder weight was found to be double that of control mice, and there was evidence of bladder wall remodeling due to both hypertrophy and increased proliferation. This increase was determined following continuous BrdU administration, and the proliferating cells were found primarily in the urothelial and lamina propria components of the stressed bladder. We used a shorter time course of 2 weeks and found similar results for C57BL/6 mice: a single large void micturition pattern and a mean 50% increase in bladder weight. In contrast, we did not detect changes in cellular proliferation; however, we used a different technique, Ki-67 immunostaining, which provides a one-time identification at necropsy of bladder cells in the G1, S, G2, or M stages of the cell cycle. Differences between the two studies may be due as well to the duration and/or intensity of the stress, or the strain of mouse used since inbred mouse strains have been shown to have different behavioral and metabolic outcomes to repeated social defeat stress (Razzoli, Carboni, Andreoli, Ballottari, & Arban, 2011; Razzoli et al., 2011; Savignac et al., 2011).
We extended our study to include a behavioral analysis of the mice after cessation of the stressor. Corticosterone levels were elevated in both stress paradigms (Figure 4). Social defeat and chronic restraint stress have been associated in previous studies with both anxiety-like behavior and depressive behavior (Buynitsky & Mostofsky, 2009; Chaouloff, 2013; Liu et al., 2013). To examine depressive type behavior, mice were observed in the forced swim test. There was no difference in duration of immobility between stressed and control mice in either model in the current study. The locomotor activity test measures exploration in response to a novel environment as well as habituation rate as the environment becomes familiar with time. We observed that SD and RSSW each produced specific effects on locomotor behavior. Chronic RSSW mice remained more active with increasing time in the chamber than control mice, showing reduced habituation. SD stressed mice were less active than control mice from the start, exhibiting less exploration, which may suggest increased anxiety. Additional tests are needed to firmly establish the behavioral response. However, this behavior did not correlate with bladder weight change which only occurred in a subset of the SD mice. There was no obvious correlation of bladder weight with any parameter measured including corticosterone response, splenomegaly, or weight change (not shown).
Recent work has begun to investigate the nature of the mediators involved in the effects of stress on micturition. Efforts have focused on Barrington’s nucleus in the pons region of the brainstem as the control center for voiding. Corticotrophin releasing factor (CRF) is an important neurohormone that is increased in Barrington’s nucleus during stress and is known to have an inhibitory effect on micturition (Valentino, Wood, Wein, & Zderic, 2011). In a rat model of social stress, treatment with a CRF-antagonist prevented urodynamic changes, while not altering the increased expression of CRF in Barrington’s nucleus (Wood et al., 2013). A recent study has demonstrated the importance of the calcineurin-nuclear factor of activated T-cells (NFAT) transcription factor pathway (Long et al., 2014). This pathway is active both in bladder, where it has been implicated in the hypertrophic response of the detrusor, and in the brain, where it has been linked to behavior modification. Mice with a total knockout of the NFATc4 isoform were resistant to voiding dysfunction after social defeat and did not exhibit increased CRF expression in Barrington’s nucleus. Susceptibility and or resistance to the effects of chronic social defeat on the bladder as seen in our study may depend on the timing and level of expression of CRF (or of other neurotransmitters) in Barrington’s nucleus, and it will be important in future studies to examine neural circuits in our models.
Chronic social defeat stress, based on an innate response during rodent male-male interaction, provides a non-surgical model to study both the genesis and sequelae of voiding dysfunction. In the presence of a dominant male, subordinate mice withhold voiding (while the dominant male increases urine marking behavior). These behavioral responses are plastic in nature, yet in the laboratory repeated defeat over time leads to molecular and structural changes to the bladder and/or regulatory changes to micturition (Chang, et al., 2009; Wood, et al., 2009). This time line parallels what is generally thought of as the evolution of bladder disease in some children, from an initial event (often a urinary tract infection or stress) to voiding dysfunction, that may still resolve or progress to pathological bladder change in the presence of persistent stressful stimuli. Molecular analysis of genetic and epigenetic pathways active in bladder muscle and urothelium as well as adaptive changes in the central nervous system and parasympathetic neurons, will lead to increased understanding of the mechanisms involved, including those responsible for long-term changes sustained after the initiating event(s). This systemic approach, in turn, may provide candidate biomarkers of human disease as we begin to understand the causal mechanistic pathways as well as provide the basis for research on new therapies for LUTS.
Highlights.
Two chronic stressors on mouse behavior and bladder function were studied
The stressors generated different effects on locomotor behavior
Susceptibility to bladder dysfunction occurred only in response to social defeat
Acknowledgements
We thank Matt Kofron, Ph.D. and Michael Muntifering, Confocal Imaging Core, Cincinnati Children’s Hospital Medical Center, for technical support with imaging and Imaris analysis. The helpful suggestions and comments of Joo-Seop Park, Ph.D. and Eunah Chung, Ph.D., Cincinnati Children’s Hospital Medical Center, are greatly appreciated. This work was partially supported by National Institutes of Health Grant T32 ES07051 (J.R.H.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth A. Mann, Email: Elizabeth.Mann@cchmc.org.
Zaheer Alam, Email: Zaheer.Alam@cchmc.org.
Jillian R. Hufgard, Email: Jillian.Hufgard@cchmc.org.
Melissa Mogle, Email: Melissa.Mogle@cchmc.org.
Michael T. Williams, Email: Michael.Williams@cchmc.org.
Charles V. Vorhees, Email: Charles.Vorhees@cchmc.org.
Pramod Reddy, Email: Pramod.Reddy@cchmc.org.
References
- Austin JC, Chacko SK, DiSanto M, Canning DA, Zderic SA. A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology, contractility and Myosin isoform expression. J Urol. 2004;172(4 Pt 1):1524–1528. doi: 10.1097/01.ju.0000138045.61378.96. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Powell N, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Immunol Allergy Clin North Am. 2009;29(2):285–293. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39(4):247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Ayala de la Pena F, Kanasaki K, Kanasaki M, Tangirala N, Maeda G, Kalluri R. Loss of p53 and acquisition of angiogenic microRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinoma. J Biol Chem. 2011;286(23):20778–20787. doi: 10.1074/jbc.M110.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33(7):1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol. 2009;297(4):F1101–F1108. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Social stress models in depression research: what do they tell us? Cell Tissue Res. 2013;354(1):179–190. doi: 10.1007/s00441-013-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoli T, Bartolomucci A, Graiani G, Stilli D, Laviola G, Sgoifo A. Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. Am J Physiol Heart Circ Physiol. 2004;286(6):H2133–H2140. doi: 10.1152/ajpheart.00869.2003. [DOI] [PubMed] [Google Scholar]
- Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, Wein AJ. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104(3):352–360. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29(4–5):547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Deshpande AV, Craig JC, Smith GH, Caldwell PH. Management of daytime urinary incontinence and lower urinary tract symptoms in children. J Paediatr Child Health. 2012;48(2):E44–E52. doi: 10.1111/j.1440-1754.2011.02216.x. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182(4115):939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautman ER, Kokenge AN, Udobi KC, Williams MT, Vorhees CV, Skelton MR. Female mice heterozygous for creatine transporter deficiency show moderate cognitive deficits. J Inherit Metab Dis. 2014;37(1):63–68. doi: 10.1007/s10545-013-9619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, Abrams P. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1314. doi: 10.1016/j.eururo.2006.09.019. discussion 1314-1305. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21(4):458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu R, Tai F, Ma L, Wei B, Yang X, Jia R. Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res. 2013;1502:71–80. doi: 10.1016/j.brainres.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Logan BA, Correia K, McCarthy J, Slattery MJ. Voiding dysfunction related to adverse childhood experiences and neuropsychiatric disorders. J Pediatr Urol. 2014 doi: 10.1016/j.jpurol.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Butler S, Fesi J, Frank C, Canning DA, Zderic SA. Genetic or pharmacologic disruption of the calcineurin-nuclear factor of activated T-cells axis prevents social stress-induced voiding dysfunction in a murine model. J Pediatr Urol. 2014 doi: 10.1016/j.jpurol.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav. 1999;67(5):769–775. doi: 10.1016/s0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- McGonagle E, Smith A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic SA. Water avoidance stress results in an altered voiding phenotype in male mice. Neurourol Urodyn. 2012;31(7):1185–1189. doi: 10.1002/nau.22207. [DOI] [PubMed] [Google Scholar]
- Mingin GC, Peterson A, Erickson CS, Nelson MT, Vizzard MA. Social stress induces changes in urinary bladder function, bladder NGF content, and generalized bladder inflammation in mice. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R893–R900. doi: 10.1152/ajpregu.00500.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JL, Campigotto MJ, Coplen DE, Traxel EJ, Austin PF. Psychosocial comorbidities and obesity are associated with lower urinary tract symptoms in children with voiding dysfunction. J Urol. 2013;190(4) Suppl:1511–1515. doi: 10.1016/j.juro.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57(2–3):201–210. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Carboni L, Andreoli M, Ballottari A, Arban R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav Brain Res. 2011;216(1):100–108. doi: 10.1016/j.bbr.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Carboni L, Andreoli M, Michielin F, Ballottari A, Arban R. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol Biochem Behav. 2011;97(3):566–576. doi: 10.1016/j.pbb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148(2):670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Finger BC, Pizzo RC, O'Leary OF, Dinan TG, Cryan JF. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–536. doi: 10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Schast AP, Zderic SA, Richter M, Berry A, Carr MC. Quantifying demographic, urological and behavioral characteristics of children with lower urinary tract symptoms. J Pediatr Urol. 2008;4(2):127–133. doi: 10.1016/j.jpurol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Tomita M, Katsuyama H, Watanabe Y, Hidaka K, Yoshitome K, Miyaishi S, Nata M. Water-restraint stress enhances methamphetamine-induced cardiotoxicity. Chem Biol Interact. 2011;190(1):54–61. doi: 10.1016/j.cbi.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol. 2011;8(1):19–28. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz GT, Vasconcelos MM, Oliveira EA, Ferreira AL, Magalhaes PG, Silva FM, Lima EM. Prevalence of lower urinary tract symptoms in school-age children. Pediatr Nephrol. 2012;27(4):597–603. doi: 10.1007/s00467-011-2028-1. [DOI] [PubMed] [Google Scholar]
- von Gontard A. Does psychological stress affect LUT function in children? ICI-RS 2011. Neurourol Urodyn. 2012;31(3):344–348. doi: 10.1002/nau.22216. [DOI] [PubMed] [Google Scholar]
- von Gontard A, Baeyens D, Van Hoecke E, Warzak WJ, Bachmann C. Psychological and psychiatric issues in urinary and fecal incontinence. J Urol. 2011;185(4):1432–1436. doi: 10.1016/j.juro.2010.11.051. [DOI] [PubMed] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1671–R1678. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden K, Griffin T, Wolfe JH, Zderic S, Valentino RJ. A corticotropin-releasing factor receptor antagonist improves urodynamic dysfunction produced by social stress or partial bladder outlet obstruction in male rats. Am J Physiol Regul Integr Comp Physiol. 2013;304(11):R940–R950. doi: 10.1152/ajpregu.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol. 2014;306(11):F1296–F1307. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]