Abstract
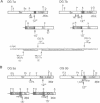
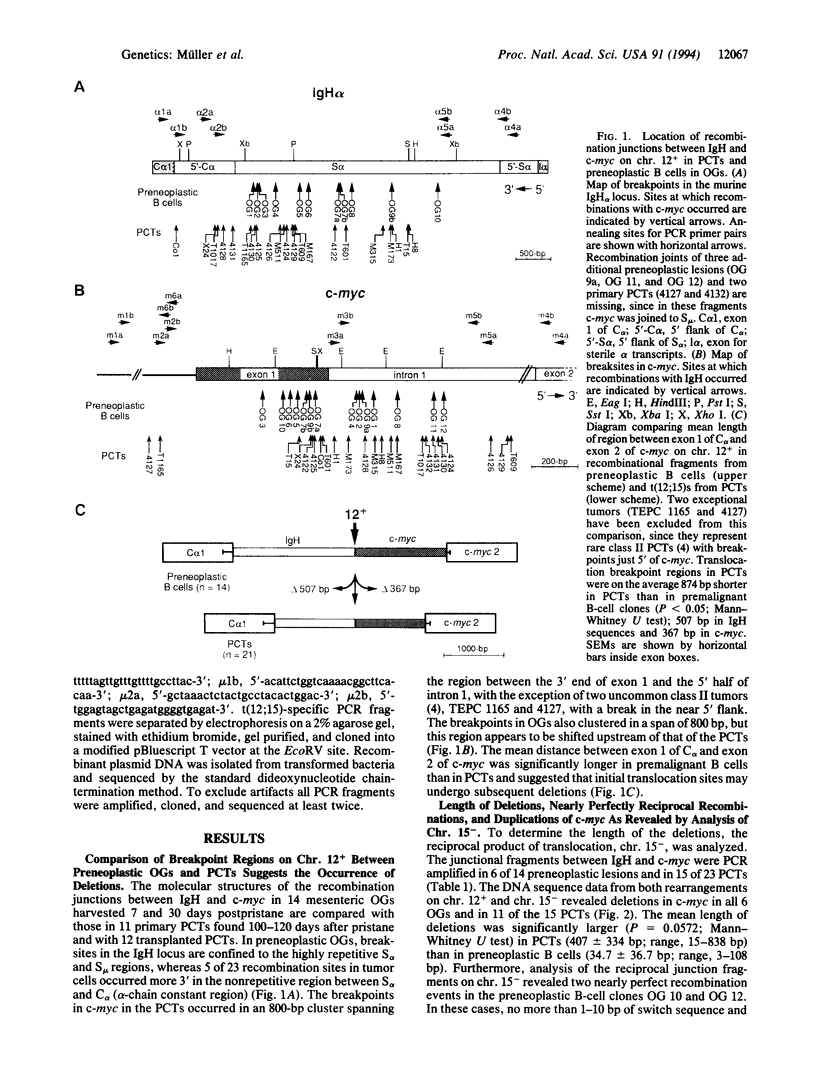
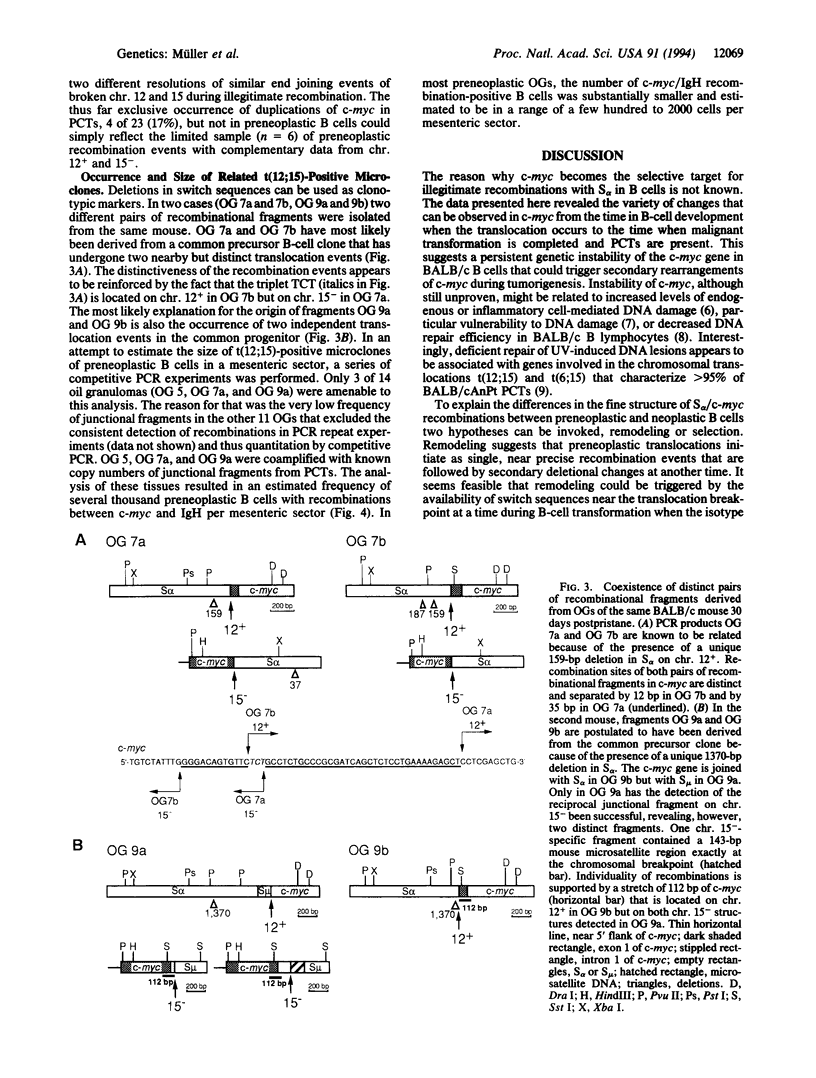
The translocation of c-myc on chromosome (chr.) 15 to an immunoglobulin heavy-chain switch region on chr. 12 is the critical oncogenic step in pristane-induced plasmacytoma (PCT) development in BALB/cAnPt mice. Applying a recently developed PCR method, we have been able to detect the most commonly occurring illegitimate recombinations between alpha-chain switch region (S alpha) and c-myc in preneoplastic B cells residing in mesenteric oil granuloma (OG) tissues 7-30 days postpristane. In this study, we compare the nucleotide sequences at the S alpha/c-myc breaksites on both the c-myc-activating chr. 12+ and the reciprocal chr. 15- from eight transplanted PCTs, seven primary PCTs, and five OGs that contained six B-cell clones. These junction sequences revealed a remarkable diversity of S alpha/c-myc recombinations. In nine cases--four PCTs and five B-cell clones--nearly precise reciprocal exchanges with a loss of only 3-35 bp in c-myc were found. Large deletions in c-myc that removed 369-878 bp were observed in seven PCTs but not in early B cells. Duplications of c-myc ranging from 103 to 229 bp were also restricted to PCTs and noticed in four cases. Clonally related but different reciprocal recombinations, 38 bp apart on chr. 12+ and 15 bp apart on chr. 15-, were isolated from two different specimens of the same OG tissue from a BALB/c mouse 30 days postpristane. A second OG from another 30-day mouse yielded four recombinational fragments--two clonally related chr. 12(+)-specific fragments and two chr. 15(-)-specific fragments--one of which carried a 143-bp insertion of a microsatellite at the breaksite. We suggest that the initial recombinational break-point regions between S alpha and c-myc in plasmacytoma precursor cells at the time of immunoglobulin heavy-chain switching are intrinsically labile and characterized by a persisting instability of c-myc, which can result in large secondary deletions of c-myc.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beecham E. J., Jones G. M., Link C., Huppi K., Potter M., Mushinski J. F., Bohr V. A. DNA repair defects associated with chromosomal translocation breaksite regions. Mol Cell Biol. 1994 Feb;14(2):1204–1212. doi: 10.1128/mcb.14.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Cory S. Activation of cellular oncogenes in hemopoietic cells by chromosome translocation. Adv Cancer Res. 1986;47:189–234. doi: 10.1016/s0065-230x(08)60200-6. [DOI] [PubMed] [Google Scholar]
- Dunnick W., Hertz G. Z., Scappino L., Gritzmacher C. DNA sequences at immunoglobulin switch region recombination sites. Nucleic Acids Res. 1993 Feb 11;21(3):365–372. doi: 10.1093/nar/21.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick W., Wilson M., Stavnezer J. Mutations, duplication, and deletion of recombined switch regions suggest a role for DNA replication in the immunoglobulin heavy-chain switch. Mol Cell Biol. 1989 May;9(5):1850–1856. doi: 10.1128/mcb.9.5.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Littlewood T. D. The role of c-myc in cell growth. Curr Opin Genet Dev. 1993 Feb;3(1):44–49. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Janz S., Müller J., Shaughnessy J., Potter M. Detection of recombinations between c-myc and immunoglobulin switch alpha in murine plasma cell tumors and preneoplastic lesions by polymerase chain reaction. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7361–7365. doi: 10.1073/pnas.90.15.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz S., Shacter E. Activated murine neutrophils induce unscheduled DNA synthesis in B lymphocytes. Mutat Res. 1993 Jan;293(2):173–186. doi: 10.1016/0921-8777(93)90068-r. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., McDowell M., Steinberg C. M., Wabl M. Looping out and deletion mechanism for the immunoglobulin heavy-chain class switch. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1581–1585. doi: 10.1073/pnas.85.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J., Dunnick W. A. Products and implied mechanism of H chain switch recombination. J Immunol. 1989 Apr 15;142(8):2932–2935. [PubMed] [Google Scholar]
- Potter M., Sanford K. K., Parshad R., Tarone R. E., Price F. M., Mock B., Huppi K. Genes on chromosomes 1 and 4 in the mouse are associated with repair of radiation-induced chromatin damage. Genomics. 1988 Apr;2(3):257–262. doi: 10.1016/0888-7543(88)90010-9. [DOI] [PubMed] [Google Scholar]
- Potter M., Wiener F. Plasmacytomagenesis in mice: model of neoplastic development dependent upon chromosomal translocations. Carcinogenesis. 1992 Oct;13(10):1681–1697. doi: 10.1093/carcin/13.10.1681. [DOI] [PubMed] [Google Scholar]
- Shacter E., Lopez R. L., Beecham E. J., Janz S. DNA damage induced by phorbol ester-stimulated neutrophils is augmented by extracellular cofactors. Role of histidine and metals. J Biol Chem. 1990 Apr 25;265(12):6693–6699. [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Shichiri M., Hanson K. D., Sedivy J. M. Effects of c-myc expression on proliferation, quiescence, and the G0 to G1 transition in nontransformed cells. Cell Growth Differ. 1993 Feb;4(2):93–104. [PubMed] [Google Scholar]
- Siebenkotten G., Esser C., Wabl M., Radbruch A. The murine IgG1/IgE class switch program. Eur J Immunol. 1992 Jul;22(7):1827–1834. doi: 10.1002/eji.1830220723. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Sirlin S., Abbott J. Induction of immunoglobulin isotype switching in cultured I.29 B lymphoma cells. Characterization of the accompanying rearrangements of heavy chain genes. J Exp Med. 1985 Mar 1;161(3):577–601. doi: 10.1084/jem.161.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Wallen R., Patel V., DePinho R. A. Role of first exon/intron sequences in the regulation of myc family oncogenic potency. Oncogene. 1993 Sep;8(9):2547–2553. [PubMed] [Google Scholar]