Abstract
Herein, we review evidence that systemic insulin resistance diseases linked to obesity, type 2 diabetes, and non-alcoholic steatohepatitis promote neurodegeneration. Insulin resistance dysregulates lipid metabolism, which promotes ceramide accumulation with attendant inflammation and ER stress. Mechanistically, we propose that toxic ceramides generated in extra-CNS tissues, e.g. liver, get released into peripheral blood, and subsequently transit across the blood-brain barrier into the brain where they induce brain insulin resistance, inflammation, and cell death (extrinsic pathway). These abnormalities establish or help propagate a cascade of neurodegeneration associated with increased ER stress and ceramide generation, which exacerbate brain insulin resistance, cell death, myelin degeneration, and neuro-inflammation. The data suggest that a mal-signaling network mediated by toxic ceramides, ER stress, and insulin resistance should be targeted to disrupt positive feedback loops that drive the AD neurodegeneration cascade.
Key Phrases: Diabetes mellitus, insulin resistance, neurodegeneration, non-alcoholic steatohepatitis, ceramide, central nervous system
Crisis of insulin resistance
Insulin resistance diseases, including Alzheimer's disease (AD), obesity, type 2 diabetes mellitus (T2DM), non-alcoholic steatohepatitis (NASH), and metabolic syndrome are prevalent in modern high tech societies, and they are costly because they consume large percentages of healthcare budgets, lead to disability, and cause premature death . The unrelenting appetite for highly processed, high starch, high fat, and high caloric content foods is literally eroding health status across all age groups in the United States. Thanks to the robust domestic and international research efforts over the past decade, it is now clear that insulin resistance can afflict any organ and tissue in the body. The consequences include deficits in energy metabolism, increased inflammation and oxidative stress, and proneness to cellular degeneration and death. No thanks to commercial luring of the uninformed who seek the comfortable lifestyles of the West, insulin resistance diseases are quickly spreading throughout the world and beginning to bear their tolls on global health.
Insulin resistance disease states
Insulin stimulates lipogenesis and increases triglyceride storage in liver and adipose tissue [Capeau 2008; Leonard et al. 2005]. This process helps to maintain energy balance. Chronic high caloric intake disrupts homeostatic mechanisms and causes insulin resistance [Capeau 2008; de la Monte and Wands 2008; Kraegen and Cooney 2008; Lyn-Cook et al. 2009]. In liver, insulin resistance is associated with conversion of simple hepatic steatosis (lipid storage) to steatohepatitis (fatty liver with inflammation and cell injury). The accompanying inflammation, pro-inflammatory cytokine activation, oxidative stress, and increased cell death via mitochondrial or apoptotic mechanisms promote liver degeneration. Insulin resistance mediated lipolysis is yet another factor contributing to progression of liver disease in NASH [Kao et al. 1999]. Lipolysis leads to increased production of toxic lipids, including ceramides (see below), that further impair insulin signaling, mitochondrial function, and cell viability [Holland and Summers 2008; Kraegen and Cooney 2008; Langeveld and Aerts 2009]. Endoplasmic reticulum (ER) stress and mitochondrial dysfunction can worsen insulin resistance, lipolysis, and ceramide accumulation [Anderson and Borlak 2008; Kaplowitz et al. 2007; Malhi and Gores 2008; Sundar Rajan et al. 2007].
ER functions such as protein synthesis, modification, and folding, calcium signaling, and lipid biosynthesis are driven by glucose utilization. Impaired glucose uptake and metabolism in insulin resistance diseases such as obesity, T2DM, and NASH activates ER stress pathways [Kaplowitz, Than, Shinohara and Ji 2007; Malhi and Gores 2008; Sharma et al. 2008; Sundar Rajan, Srinivasan, Balasubramanyam and Tatu 2007]. ER stress contributes to lipid dyshomeostasis by activating pro-inflammatory, pro-ceramide, and pro-death pathways that lead to increased generation of toxic lipids, e.g. ceramides [Banerjee et al. 2008; de la Monte et al. 2009; Kaplowitz and Ji 2006; Ronis et al. 2008]. Correspondingly, ceramide levels and pro-ceramide gene expression are increased in livers with chronic steatohepatitis [Longato et al. 2012; Setshedi et al. 2011].
The consequences of insulin resistance, particularly the stress responses, themselves promote insulin resistance. Unchecked, the rates of injury eventually exceed those of repair. Organ-system degeneration is mediated by the combined effects of impaired energy balance, lipid dyshomeostasis, loss of membrane integrity, and ER stress, all of which contribute to increased ceramide generation [DeFronzo 2010; Eckardt et al. 2011; Holland et al. 2007; Kaplowitz, Than, Shinohara and Ji 2007; Lipina and Hundal 2011; Malhi and Gores 2008; Summers 2010], which itself causes insulin resistance (see below). Therefore, chronic insulin resistance initiates a harmful positive feedback loop that results in propagation of chronic diseases and tissue degeneration. Underlying pathophysiological mechanisms include increased ceramide generation, inflammation, tissue injury, ER stress, and mitochondrial dysfunction, all of which worsen insulin resistance.
Ceramides-the back story
Ceramides comprise a family of sphingolipids [Reynolds et al. 2004; Summers 2010] that regulates diverse functions including growth, motility, adhesion, differentiation, senescence, and apoptosis. Ceramides also contribute to cell membrane structure by participating in lipid microdomains, i.e. rafts [Sonnino and Prinetti 2010]. Ceramides differ in length of their fatty acid chains (up to C24), and are formed via complex biosynthetic [Reynolds, Maurer and Kolesnick 2004; Stiban et al. 2010], catabolic [Clarke et al. 2011; Reynolds, Maurer and Kolesnick 2004], or salvage [Gault et al. 2010; Mullen and Obeid 2011; Reynolds, Maurer and Kolesnick 2004] mechanisms. The rapid turnover and short half-life of ceramides facilitate their role as second messengers for intracellular signaling.
Ceramides generated de novo regulate physiological functions, whereas those produced via catabolic pathways are generated in response to drugs, physical agents, chemotherapeutic agents, pro-inflammatory cytokines, trophic factor withdrawal, and ionizing radiation [Adibhatla and Hatcher 2008; Farooqui et al. 2010; Holland and Summers 2008; Liu et al. 1997; Nikolova-Karakashian and Reid 2011; Reynolds, Maurer and Kolesnick 2004; Summers 2006], indicating a link to stress and disease states. The salvage pathway accounts for 50% to 90% of sphingolipid biosynthesis in cells, and accomplishes this by recycling sphingoid bases released by acid ceramidases for use by ceramide synthases. Ceramide profiles in different organelles and cell types can shift as an adaptive or pathophysiological response. Accumulation of ceramides in lipid rafts [Sonnino and Prinetti 2010] causes small rafts to merge into larger units to modify membrane structure and protein function, including receptor responsiveness, signal transduction, and stress responses [Corre et al. 2010; Hajduch et al. 2001; Li et al. 2010; Lingwood et al. 2010; Lingwood et al. 2010].
Ceramides cause insulin resistance and insulin resistance increases ceramides
Ceramides are lipid signaling molecules that can be cytotoxic, cause insulin resistance [Arboleda et al. 2007; Chalfant et al. 1999; Chavez et al. 2005; Chavez et al. 2003; Delarue and Magnan 2007; Holland and Summers 2008; Kraegen and Cooney 2008; Liu, Obeid and Hannun 1997], and activate pro-inflammatory cytokines. Ceramides cause insulin resistance [DeFronzo 2010; Eckardt, Taube and Eckel 2011; Holland, Knotts, Chavez, Wang, Hoehn and Summers 2007; Kaplowitz, Than, Shinohara and Ji 2007; Lipina and Hundal 2011; Malhi and Gores 2008; Summers 2010] in obesity, T2DM, NASH [Alessenko et al. 2004; Han et al. 2008; Katsel et al. 2007; Summers 2010], and probably AD [de la Monte et al. 2012]. Ceramides cause insulin resistance by activating pro-inflammatory cytokines [Bryan et al. 2008; Summers 2006; Van Brocklyn 2007] and inhibiting insulin signaling at various levels in the pathway. For example, ceramides: 1) inhibit signaling through PI3 kinase-Akt [Bourbon et al. 2002; Hajduch, Balendran, Batty, Litherland, Blair, Downes and Hundal 2001; Nogueira et al. 2008; Powell et al. 2003]; 2) alter the phosphorylation states of proteins that regulate insulin signaling [Silveira et al. 2008]; 3) inhibit Akt [Arboleda, Huang, Waters, Verkhratsky, Fernyhough and Gibson 2007] by activating protein phosphatase 2A [Chalfant, Kishikawa, Mumby, Kamibayashi, Bielawska and Hannun 1999] and glycogen synthase kinase 3β (GSK-3β) [Arboleda et al. 2010; Stoica et al. 2003], and recruiting phosphatase and tensin homologue deleted on chromosome 10 (PTEN) [Hajduch et al. 2008]; and 4) stimulate pro-apoptotic mechanisms such as interleukin-1β converting enzyme (ICE)-like proteases [Liu, Obeid and Hannun 1997]. Therefore, ceramide homeostasis is needed to maintain insulin responsiveness and minimize cell injury. Correspondingly, inhibition of ceramide synthesis and accumulation was shown to prevent obesity-associated insulin resistance [Chavez, Holland, Bar, Sandhoff and Summers 2005; Holland, Knotts, Chavez, Wang, Hoehn and Summers 2007].
In chronic obesity, T2DM and NASH, lipid dyshomeostasis results in increased generation of ceramide in adipose tissue and/or liver [Alessenko, Bugrova and Dudnik 2004; Han, Park, Shinzawa, Kim, Chung, Lee, Kwon, Lee, Park, Chung, Hwang, Yan, Song, Tsujimoto and Lee 2008; Katsel, Li and Haroutunian 2007; Summers 2010]. Pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [Summers 2006], are activated in obesity, T2DM, NASH, and AD [Lieber et al. 2004; Rosenberg 2005; Sahai et al. 2004; Sastre et al. 2006; Satapathy et al. 2004; Tuppo and Arias 2005; Yalniz et al. 2006]. Inflammation and insulin resistance increase ceramide production, and ceramides promote oxidative and ER stress. Consequently, these interconnecting pathophysiological processes induce a positive feedback mal-signaling loops that establishes a cascade of progressive organ-system degeneration.
Brain metabolic derangements and insulin resistance in Alzheimer's disease
AD shares several features in common with systemic insulin resistance diseases including, reduced insulin-stimulated growth and survival signaling, increased oxidative stress, pro-inflammatory cytokine activation, mitochondrial dysfunction, and impaired energy metabolism [de la Monte 2012; de la Monte et al. 2009]. The concept that AD represents a metabolic disease stems from the findings that cerebral glucose utilization is impaired in the early stages of AD [Adolfsson et al. 1980; Caselli et al. 2008; Fujisawa et al. 1991; Langbaum et al. 2010; Mosconi et al. 2009; Mosconi et al. 2008], and that brain metabolic derangements worsen with AD progression [Hoyer and Nitsch 1989; Hoyer et al. 1991].
Human postmortem studies established that brain insulin resistance mediated by reduced insulin receptor expression and insulin receptor binding were consistent and fundamental abnormalities in AD [Rivera et al. 2005; Steen et al. 2005]. In AD, the deficits in brain insulin and IGF signaling involves pathways needed to maintain neuronal survival, energy production, gene expression, and plasticity [Frolich et al. 1998]. Correspondingly, nearly all of the critical features of AD, including increased: 1) activation of kinases that aberrantly phosphorylate tau and lead to the accumulation of neurofibrillary tangles, dystrophic neuritic plaques and neuorpil threads; 2) expression of amyloid-beta precursor protein (AβPP) and accumulation of AβPP-Aβ peptides that are neurotoxic and result in senile plaque formation; 3) oxidative and ER stress that propagate cell death cascades; 4) mitochondrial dysfunction which causes energy deficits; and 5) disruption of cholinergic homeostasis needed for neuronal plasticity, memory, and cognition, could represent consequences of brain insulin/IGF resistance.
In the central nervous system (CNS), insulin and insulin-like growth factor (IGF) signaling networks regulate a broad array of functions including cell growth and survival, metabolism, gene expression, protein synthesis, cytoskeletal assembly, synapse formation, neurotransmitter function, and plasticity [D'Ercole and Ye 2008; de la Monte and Wands 2005; Hoyer 2004]. Impairments in insulin and IGF signaling could be mediated by reduced ligand availability, reduced receptor rexponsiveness, or inhibition of downstream signaling. Chronic insulin/IGF-1 resistance has dire consequences on the functional integrity of the CNS [de la Monte and Wands 2005; Schubert et al. 2003; Schubert et al. 2004; Xu et al. 2003] due to impairments in neuronal survival, energy production, gene expression, and plasticity [Frolich, Blum-Degen, Bernstein, Engelsberger, Humrich, Laufer, Muschner, Thalheimer, Turk, Hoyer, Zochling, Boissl, Jellinger and Riederer 1998]. Moreover, inhibition of insulin/IGF signaling disrupts cholinergic homeostasis, thereby compromising one of the most important neurotransmitter systems utilized for neuronal plasticity, memory, and cognition.
Another major adverse effect of insulin/IGF resistance in the brain is chronically increased stress caused by oxidative and endoplasmic reticulum (ER) stress, generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that damage proteins, RNA, DNA, and lipids [Reddy et al. 2009], mitochondrial dysfunction; and activation of pro-inflammatory and pro-death cascades [de la Monte, Longato, Tong and Wands 2009; de la Monte and Wands 2005; de la Monte et al. 2008; Haan 2006; Lester-Coll et al. 2006; Rivera, Goldin, Fulmer, Tavares, Wands and de la Monte 2005; Steen, Terry, Rivera, Cannon, Neely, Tavares, Xu, Wands and de la Monte 2005; Tilg and Moschen 2008]. Oxidation of amino acid residues leads to formation of advanced glycation end products (AGEs) or advanced oxidation protein products. Oxidation of proteins causes them to become unfolded, inactivated, and more susceptible to cleavage. Moreover, oxidation of aliphatic side-chains leads to the formation of peroxides and carbonyls (aldehydes and ketone). Peroxides attack other molecules and produce radicals. Carbonyls are toxic and cause stress-induced AGE accumulation, which contributes to progressive impairment of cellular functions in aging, diabetes, AD, experimental models of AD, and degenerative diseases [Greilberger et al. 2010; Greilberger et al. 2008; Gu et al. 2008; Stadtman 2001]. In AD, elevated levels of AGE in amyloid plaques and neurofibrillary tangles [Gella and Durany 2009; Krautwald and Munch 2010; Rahmadi et al. 2011] quite likely contribute to the ongoing cell death and neurodegeneration [Gella and Durany 2009; Krautwald and Munch 2010; Reddy, Zhu, Perry and Smith 2009].
Peripheral insulin resistance states are linked to cognitive impairment and Alzheimer's disease
The molecular and biochemical abnormalities in brains with AD closely mimic those in T2DM and NASH, and until recently, the vast majority of sporadic AD had no association with diabetes. In fact, prior to 1980, the epidemiological trends for AD (increasing prevalence) were opposite those for diabetes mellitus (declining as a cause of death) [de la Monte, Neusner, Chu and Lawton 2009]. Within the past 2-3 decades, morbidity and mortality rates have trended upward for diabetes and other insulin resistance diseases, including metabolic syndrome (dyslipidemic states), non-alcoholic steatohepatitis (NASH), and obesity, despite improvements in medical care [de la Monte, Neusner, Chu and Lawton 2009]. At the same time, the increasing overlap between cognitive impairment or AD and peripheral insulin resistance diseases has raised concerns about the potential contributions or even causal roles of obesity and diabetes mellitus in neurodegeneration and dementia [de la Monte, Neusner, Chu and Lawton 2009; Qiu et al. 2007].
Epidemiologic studies showed that people with glucose intolerance, deficits in insulin secretion, T2DM, obesity/dyslipidemic disorders, and NASH had significantly higher risks for developing MCI or AD-type dementia [Craft 2005; Craft 2006; Craft 2007; de la Monte, Longato, Tong and Wands 2009; Hoyer 2004; Luchsinger et al. 2007; Martins et al. 2006; Pasquier et al. 2006]. For example, obese individuals were found to have higher rates of MCI [Lokken et al. 2009] and impaired performance on executive function tests [Gunstad et al. 2007; Lokken, Boeka, Austin, Gunstad and Harmon 2009]. In adition, their risk for developing dementia or AD was at least two-fold higher than for the general population [Yaffe 2007]. These results were corroborated by experimental data showing that chronic high fat feeding and diet induced obesity with T2DM impair spatial learning and memory [Winocur and Greenwood 2005; Winocur et al. 2005] and cause atrophy, insulin resistance, inflammation, oxidative stress, and cholinergic dysfunction in the brain [Lyn-Cook, Lawton, Tong, Silbermann, Longato, Jiao, Mark, Wands, Xu and de la Monte 2009; Moroz et al. 2008]. Moreover, in NASH, which is associated with hepatic insulin resistance, the rates of neuropsychiatric diseases such as depression and anxiety [Elwing et al. 2006], and risks for developing cognitive impairment [Felipo et al. 2011] are significantly increased. On the other hand, weight loss leading to reduced peripheral insulin resistance improves cognitive performance [Baker et al. 2010; Baker et al. 2010] and enhances neuropsychiatric function including mood and behavior [Bryan and Tiggemann 2001].
Several studies have shown that cognitive impairment and neuropsychiatric dysfunction occur with liver disease caused by various agents, including alcohol abuse, obesity, chronic Hepatitis C virus infection, Reyes syndrome, and nitrosamine exposure [Elwing, Lustman, Wang and Clouse 2006; Karaivazoglou et al. 2007; Kopelman et al. 2009; Loftis et al. 2008; Perry et al. 2008; Schmidt et al. 2005; Weiss and Gorman 2006]. These diseases are linked because they are all associated with hepatic steatosis or steatohepatitis and hepatic insulin resistance, endoplasmic reticulum (ER) stress, and increased generation of cytotoxic sphingolipids, including ceramides [de la Monte et al. 2006; Lester-Coll, Rivera, Soscia, Doiron, Wands and de la Monte 2006; Lyn-Cook, Lawton, Tong, Silbermann, Longato, Jiao, Mark, Wands, Xu and de la Monte 2009; Moroz, Tong, Longato, Xu and de la Monte 2008; Tong et al. 2010; Tong et al. 2009]. Mechanistically, inflammation, superimposed on disease states that promote lipid storage in hepatocytes promotes ER stress, oxidative damage, mitochondrial dysfunction, and lipid peroxidation, which together drive hepatic insulin resistance [Capeau 2008; Kraegen and Cooney 2008].
Hepatic insulin resistance stimulates lipolysis [Kao, Youson, Holmes, Al-Mahrouki and Sheridan 1999], and lipolysis leads to increased generation of toxic lipids e.g. ceramides, which further impair insulin signaling, mitochondrial function, and cell viability [Holland and Summers 2008; Kraegen and Cooney 2008; Langeveld and Aerts 2009]. Moreover, with steatohepatitis, hepatic and peripheral insulin resistance are accompanied by increased local and peripheral levels of ceramides [de la Monte, Tong, Lester-Coll, Plater and Wands 2006; Lester-Coll, Rivera, Soscia, Doiron, Wands and de la Monte 2006; Moroz, Tong, Longato, Xu and de la Monte 2008; Tong, Longato and de la Monte 2010; Tong, Neusner, Longato, Lawton, Wands and de la Monte 2009], suggesting that distant target organs may be susceptible to their toxic effects. Experimentally, molecular and biochemical abnormalities associataed with AD can be produced by in vitro exposure to short-chain cytotoxic ceramides [Adibhatla and Hatcher 2008; Alessenko, Bugrova and Dudnik 2004]. In addition, in vivo administration (i.p.) of toxic ceramides causes cognitive-motor deficits, brain insulin resistance, oxidative stress, metabolic abnormalities, and neurodegeneration, similar to AD-type neurodegeneration [Tong and de la Monte 2009]. Further investigations showed that toxic ceramides delivered into peripheral blood by i.p. injection localize in brain membranes and therefore cross the blood brain barrier [de la Monte 2012].
Toxic lipids produced in peripheral insulin resistance states contribute to brain insulin-resistance and neurodegeneration
In obesity, adipose tissue, skeletal muscle, and liver have abnormal sphingolipid metabolism results in increased ceramide production, inflammation, and activation of pro-inflammatory cytokines, with impairments in glucose homeostasis and insulin responsiveness [Delarue and Magnan 2007; Shah et al. 2008; Summers 2006]. In human [Kolak et al. 2007] and experimental models of NASH [Cong et al. 2008], ceramide levels in adipose tissue are elevated due to increased activation of serine palmitoyl transferase, and acidic and neutral sphingomyelinases [Liu, Obeid and Hannun 1997]. In addition, liver ceramide synthase and serine palmitoyl transferase mRNA levels are increased in the early stages of hepatic steatosis, but with the development of NASH and neurodegeneration, ceramide synthase mRNA transcripts decline while sphingomyelinase gene expression increases [Lyn-Cook, Lawton, Tong, Silbermann, Longato, Jiao, Mark, Wands, Xu and de la Monte 2009]. Since neurodegeneration in models of obesity and diabetes have not been associated with increased CNS expression of pro-ceramide genes, we suspect that the AD-type neurodegeneration with brain insulin/IGF resistance is mediated by secondary effects of peripheral insulin resistance, i.e. dysregulated lipid metabolism, increased production of cytotoxic ceramides, and increased trafficking of cytotoxic ceramides from peripheral blood to brain.
Corresponding with the above concept, mass spectrometry-based lipidomics analysis of plasma detected elevated levels of saturated sphingolipids (N16:0 and N21:0) in AD relative to control subjects, and linked severity of cognitive impairment with altered levels of specific very long chain ceramides [Han 2010]. In addition, elevated plasma levels of very long-chain saturated ceramides (C22:0 and C24:0) were found to be predictive of memory loss and hippocampal atrophy in patients with MCI [Mielke et al. 2010], whereas increased ratios of dihydrosphingomyelin to dihydroceramide and sphingomyelin to ceramide were shown to be correlated with slower progression of AD [Mielke et al. 2011]. Although these studies did not interrogate the sources of plasma sphingolipids and ceramides or the presence of underlying peripheral insulin resistance diseases, they provide evidence that shifts in plasma spingolipid profiles and levels could be used as peripheral biomarkers for individuals at risk for progression from MCI to dementia. Ideally, it would be beneficial to determine the degree to which peripheral blood very long chain ceramide profiles shift with treatment of AD with insulin, insulin sensitizers, or measures to support neurotransmitter function and metabolic homeostasis in the CNS.
Hypothesis: Peripheral insulin resistance diseases can cause MCI and contribute to progressive AD-type neurodegeneration
The aggregate results from several studies suggest that peripheral, including hepatic insulin resistance with associated chronic injury, inflammation, and metabolic dysfunction leads to dysregulated lipid metabolism with increased ceramide production. Intra-hepatic (or visceral fat) accumulation of cytotoxic ceramides promotes ER stress, which exacerbates insulin resistance, inflammation, and oxidative stress. Consequences include increased DNA damage, mitochondrial dysfunction, energy depletion, ROS production, and eventually the formation lipid, protein, and DNA adducts, which further impair cellular functions. Finally, a reverberating cascade of mal-signaling and insulin resistance with impaired cell survival gets established, resulting in leakage of toxic ceramides from liver (visceral fat) to peripheral blood [de la Monte, Longato, Tong and Wands 2009]. Toxic lipids, including ceramides can cross the blood-brain barrier and cause insulin resistance by interfering with critical phosphorylation events [Arboleda, Huang, Waters, Verkhratsky, Fernyhough and Gibson 2007; Chalfant, Kishikawa, Mumby, Kamibayashi, Bielawska and Hannun 1999; de la Monte et al. 2010; Liu, Obeid and Hannun 1997; Tong and de la Monte 2009] and activating pro-inflammatory cytokines [Bryan, Kordula, Spiegel and Milstien 2008; Summers 2006; Van Brocklyn 2007], CNS Therefore, brain insulin resistance, which is an early and important feature of AD, may be mediated by chronic exposure to cytotoxic ceramides generated in extra-CNS sources [de la Monte, Tong, Nguyen, Setshedi, Longato and Wands 2010] and capable of penetrating the blood brain barrier. cause CNS insulin resistance, oxidative stress, and pro-inflammatory cytokine activation, which ultimately result in dysregulated lipid metabolism, myelin breakdown, increased endogenous ceramide generation, and ER stress.
The epidemic of peripheral insulin resistance diseases which includes obesity, T2DM, and NAFLD/NASH, is likely responsible for the staggering increases in morbidity and mortality rates from AD across all age groups, 50-years and older [de la Monte, Neusner, Chu and Lawton 2009]. Mechanistically, we propose that this extrinsic pathway of brain insulin/IGF resistance with attendant ER stress is initiated through insults arising from toxic ceramides generated in peripheral tissues, e.g. liver, and traffic through peripheral blood to the CNS to exert their neurotoxic and degenerative effects. Future therapeutic strategies for restoring cognitive function and preventing progression to AD should consider the inclusion of agents that block toxic ceramide and other toxic lipid production in both peripheral tissues and the brain.
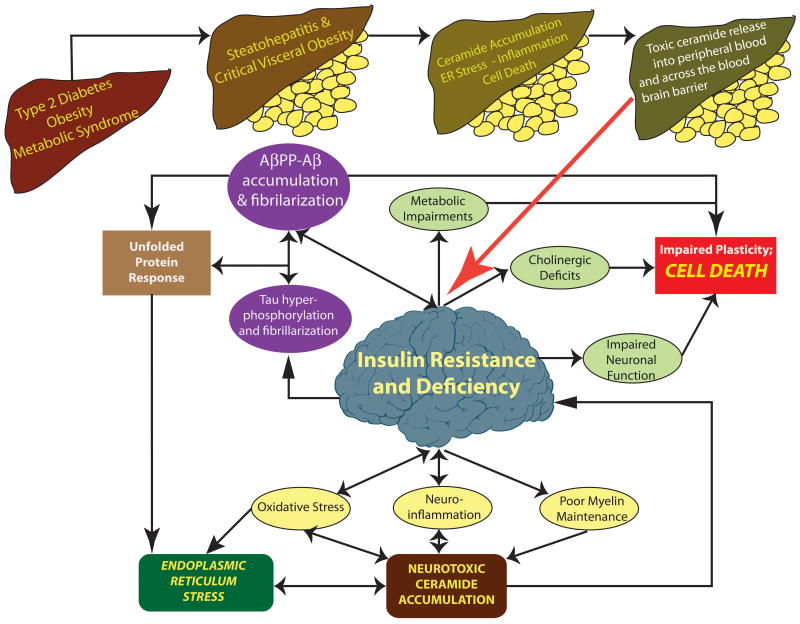
Figure 1.
Extrinsic mechanisms of brain insulin/IGF resistance and neurodegeneration. In Type 2 diabetes, non-alcoholic steatohepatitis, and visceral obesity (visceral obesity shown as yellow discs below livers), excess lipid accumulation leads to insulin resistance, which promotes inflammation, ER stress, and oxidative injury. This process establishes a positive feedback cycle of mal-signaling and insulin resistance with impaired cell survival that results in leakage of toxic ceramides from liver (or visceral fat) to peripheral blood. Toxic ceramides capable of penetrating the blood brain barrier, cause CNS insulin resistance, oxidative stress, and pro-inflammatory cytokine activation, which ultimately result in dysregulated lipid metabolism, myelin breakdown, increased endogenous ceramide generation, and ER stress.
Acknowledgments
Supported by AA-11431 and AA-12908 from the National Institutes of Health
References
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfsson R, Bucht G, Lithner F, Winblad B. Hypoglycemia in Alzheimer's disease. Acta Med Scand. 1980;208(5):387–388. doi: 10.1111/j.0954-6820.1980.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer's disease. Biochem Soc Trans. 2004 Feb;32(Pt 1):144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008 Sep;60(3):311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- Arboleda G, Cardenas Y, Rodriguez Y, Morales LC, Matheus L, Arboleda H. Differential regulation of AKT, MAPK and GSK3beta during C2-ceramide-induced neuronal death. Neurotoxicology. 2010 Dec;31(6):687–693. doi: 10.1016/j.neuro.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Arboleda G, Huang TJ, Waters C, Verkhratsky A, Fernyhough P, Gibson RM. Insulin-like growth factor-1-dependent maintenance of neuronal metabolism through the phosphatidylinositol 3-kinase-Akt pathway is inhibited by C2-ceramide in CAD cells. Eur J Neurosci. 2007 May;25(10):3030–3038. doi: 10.1111/j.1460-9568.2007.05557.x. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis. 2010;22(2):569–579. doi: 10.3233/JAD-2010-100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010 Jan;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Russell WK, Jayaraman A, Ramaiah SK. Identification of proteins to predict the molecular basis for the observed gender susceptibility in a rat model of alcoholic steatohepatitis by 2-D gel proteomics. Proteomics. 2008 Oct;8(20):4327–4337. doi: 10.1002/pmic.200700368. [DOI] [PubMed] [Google Scholar]
- Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002 Feb 1;277(5):3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- Bryan J, Tiggemann M. The effect of weight-loss dieting on cognitive performance and psychological well-being in overweight women. Appetite. 2001 Apr;36(2):147–156. doi: 10.1006/appe.2000.0389. [DOI] [PubMed] [Google Scholar]
- Bryan L, Kordula T, Spiegel S, Milstien S. Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta. 2008 Sep;1781(9):459–466. doi: 10.1016/j.bbalip.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeau J. Insulin resistance and steatosis in humans. Diabetes Metab. 2008 Dec;34(6 Pt 2):649–657. doi: 10.1016/S1262-3636(08)74600-7. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008 Sep;65(9):1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999 Jul 16;274(29):20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005 May 20;280(20):20148–20153. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003 Mar 21;278(12):10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. American journal of physiology. Gastrointestinal and liver physiology. 2011 May;300(5):G697–702. doi: 10.1152/ajpgi.00426.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Wu BX, Hannun YA. The neutral sphingomyelinase family: identifying biochemical connections. Adv Enzyme Regul. 2011;51(1):51–58. doi: 10.1016/j.advenzreg.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008 May 7;82(19-20):983–990. doi: 10.1016/j.lfs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Corre I, Niaudet C, Paris F. Plasma membrane signaling induced by ionizing radiation. Mutat Res. 2010 Apr-Jun;704(1-3):61–67. doi: 10.1016/j.mrrev.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and cognitive impairment: a view through the prism of epidemiology. Arch Neurol. 2005 Jul;62(7):1043–1044. doi: 10.1001/archneur.62.7.1043-a. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4):298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007 Apr;4(2):147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008 Dec;149(12):5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Therapeutic Targets of Brain Insulin Resistance In Sporadic Alzheimer's Disease. Frontiers in bioscience : a journal and virtual library. 2012;E4:1582–1605. doi: 10.2741/482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Triangulated Mal-Signaling in Alzheimer's Disease: Roles of Neurotoxic Ceramides, ER Stress, and Insulin Resistance Reviewed. J Alzheimers Dis. 2012 Feb 15; doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR. The liver-brain axis of alcohol-mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health. 2009 Jul;6(7):2055–2075. doi: 10.3390/ijerph6072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009 Oct;10(10):1049–1060. [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Neusner A, Chu J, Lawton M. Epidemilogical Trends Strongly Suggest Exposures as Etiologic Agents in the Pathogenesis of Sporadic Alzheimer's Disease, Diabetes Mellitus, and Non-Alcoholic Steatohepatitis. J Alzheimers Dis. 2009 Apr 10; doi: 10.3233/JAD-2009-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Re E, Longato L, Tong M. Dysfunctional Pro-Ceramide ER Stress,and Insulin/IGF Signaling Networks with Progression of Alzheimer's Disease. J Alzheimers Dis. 2012 Feb 1; doi: 10.3233/JAD-2012-111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer's disease. J Alzheimers Dis. 2006 Sep;10(1):89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. J Alzheimers Dis. 2010;21(3):967–984. doi: 10.3233/JAD-2010-091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis. 2005 Feb;7(1):45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Alzheimer 's disease is type 3 diabetes: Evidence reviewed. J Diabetes Sci Tech. 2008;2(6):1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, Duan K, Wands JR. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008 Aug;23(8 Pt 2):e477–486. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010 Jul;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007 Mar;10(2):142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011 Sep;12(3):163–172. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006 Jul-Aug;68(4):563–569. doi: 10.1097/01.psy.0000221276.17823.df. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Ong WY, Farooqui T. Lipid mediators in the nucleus: Their potential contribution to Alzheimer's disease. Biochimica et biophysica acta. 2010 Aug;1801(8):906–916. doi: 10.1016/j.bbalip.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Felipo V, Urios A, Montesinos E, Molina I, Garcia-Torres ML, Civera M, del Olmo JA, Ortega J, Martinez-Valls J, Serra MA, Cassinello N, Wassel A, Jorda E, Montoliu C. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metabolic brain disease. 2011 Nov 10; doi: 10.1007/s11011-011-9269-3. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm. 1998;105(4-5):423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Sasaki K, Akiyama K. Increased insulin levels after OGTT load in peripheral blood and cerebrospinal fluid of patients with dementia of Alzheimer type. Biol Psychiatry. 1991 Dec 15;30(12):1219–1228. doi: 10.1016/0006-3223(91)90158-i. [DOI] [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell adhesion & migration. 2009 Jan-Mar;3(1):88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilberger J, Fuchs D, Leblhuber F, Greilberger M, Wintersteiger R, Tafeit E. Carbonyl proteins as a clinical marker in Alzheimer's disease and its relation to tryptophan degradation and immune activation. Clinical laboratory. 2010;56(9-10):441–448. [PubMed] [Google Scholar]
- Greilberger J, Koidl C, Greilberger M, Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D, Oettl K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free radical research. 2008 Jul;42(7):633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- Gu F, Zhu M, Shi J, Hu Y, Zhao Z. Enhanced oxidative stress is an early event during development of Alzheimer-like pathologies in presenilin conditional knock-out mice. Neuroscience letters. 2008 Jul 25;440(1):44–48. doi: 10.1016/j.neulet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive psychiatry. 2007 Jan-Feb;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol. 2006 Mar;2(3):159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001 Feb;44(2):173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J. 2008 Mar 1;410(2):369–379. doi: 10.1042/BJ20070936. [DOI] [PubMed] [Google Scholar]
- Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, Kwon CH, Lee KW, Park CK, Chung WJ, Hwang JS, Yan JJ, Song DK, Tsujimoto Y, Lee MS. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008 Jan;49(1):84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- Han X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer's disease. Biochimica et biophysica acta. 2010 Aug;1801(8):774–783. doi: 10.1016/j.bbalip.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007 Jun;65(6 Pt 2):S39–46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008 Jun;29(4):381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004 Apr 19;490(1-3):115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Nitsch R. Cerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer type. J Neural Transm. 1989;75(3):227–232. doi: 10.1007/BF01258634. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3(1):1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- Kao Y, Youson JH, Holmes JA, Al-Mahrouki A, Sheridan MA. Effects of insulin on lipid metabolism of larvae and metamorphosing landlocked sea lamprey, Petromyzon marinus. Gen Comp Endocrinol. 1999 Jun;114(3):405–414. doi: 10.1006/gcen.1999.7265. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006 Oct;21(Suppl 3):S7–9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007 Nov;27(4):367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- Karaivazoglou K, Assimakopoulos K, Thomopoulos K, Theocharis G, Messinis L, Sakellaropoulos G, Labropoulou-Karatza C. Neuropsychological function in Greek patients with chronic hepatitis C. Liver Int. 2007 Aug;27(6):798–805. doi: 10.1111/j.1478-3231.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochemical research. 2007 Apr-May;32(4-5):845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007 Aug;56(8):1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009 Mar-Apr;44(2):148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008 Jun;19(3):235–241. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- Krautwald M, Munch G. Advanced glycation end products as biomarkers and gerontotoxins - A basis to explore methylglyoxal-lowering agents for Alzheimer's disease? Experimental gerontology. 2010 Oct;45(10):744–751. doi: 10.1016/j.exger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Caselli RJ, Lee W, Reschke C, Bandy D, Alexander GE, Burns CM, Kaszniak AW, Reeder SA, Corneveaux JJ, Allen AN, Pruzin J, Huentelman MJ, Fleisher AS, Reiman EM. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010 Apr;67(4):462–468. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld M, Aerts JM. Glycosphingolipids and insulin resistance. Prog Lipid Res. 2009 Mar 20; doi: 10.1016/j.plipres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Critical reviews in biochemistry and molecular biology. 2011 Jun;46(3):200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- Leonard BL, Watson RN, Loomes KM, Phillips AR, Cooper GJ. Insulin resistance in the Zucker diabetic fatty rat: a metabolic characterisation of obese and lean phenotypes. Acta Diabetol. 2005 Dec;42(4):162–170. doi: 10.1007/s00592-005-0197-8. [DOI] [PubMed] [Google Scholar]
- Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006 Mar;9(1):13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- Li X, Becker KA, Zhang Y. Ceramide in redox signaling and cardiovascular diseases. Cell Physiol Biochem. 2010;26(1):41–48. doi: 10.1159/000315104. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004 Mar;79(3):502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function - where membrane structure and pathology intersect. FEBS Lett. 2010 May 3;584(9):1879–1886. doi: 10.1016/j.febslet.2009.11.089. [DOI] [PubMed] [Google Scholar]
- Lingwood CA, Manis A, Mahfoud R, Khan F, Binnington B, Mylvaganam M. New aspects of the regulation of glycosphingolipid receptor function. Chem Phys Lipids. 2010 Jan;163(1):27–35. doi: 10.1016/j.chemphyslip.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011 Jul;54(7):1596–1607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- Liu B, Obeid LM, Hannun YA. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1997 Jun;8(3):311–322. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2008 Jan 17;430(3):264–268. doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2009 Sep-Oct;5(5):547–552. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Longato L, Ripp K, Setshedi M, Wands JR, de la Monte SM. Advanced human alcoholic liver disease is associated with increased pro-ceramide gene expression, ceramide accumulation, endoplasmic reticulum stress, and insulin/IGF resistance. Oxid Med Cell Longev. 2012 doi: 10.1155/2012/479348. p. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007 Apr;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009 Apr;16(4):715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008 Nov;28(4):360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry. 2006 Aug;11(8):721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Christopher MJ. Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Curr Opin Lipidol. 2011 Jun;22(3):210–215. doi: 10.1097/MOL.0b013e3283453dbe. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Bandaru VV, Weinberg DD, Darby E, Zaidi N, Pavlik V, Doody RS, Lyketsos CG. Plasma Sphingomyelins are Associated with Cognitive Progression in Alzheimer's Disease. J Alzheimers Dis. 2011 Jan 1;27(2):259–269. doi: 10.3233/JAD-2011-110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, Mori S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2010 Sep;6(5):378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and Type 2 diabetes mellitus. J Alzheimers Dis. 2008;15(1):29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, de Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's Disease. Eur J Nucl Med Mol Imaging. 2009 May;36(5):811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Pupi A, de Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann N Y Acad Sci. 2008 Dec;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen TD, Obeid LM. Ceramide and Apoptosis : Exploring the Enigmatic Connections Between Sphingolipid Metabolism and Programmed Cell Death. Anticancer Agents Med Chem. 2011 Jun 27; doi: 10.2174/187152012800228661. [DOI] [PubMed] [Google Scholar]
- Nikolova-Karakashian MN, Reid MB. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. Antioxid Redox Signal. 2011 Nov 1;15(9):2501–2517. doi: 10.1089/ars.2011.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira TC, Anhe GF, Carvalho CR, Curi R, Bordin S, Carpinelli AR. Involvement of phosphatidylinositol-3 kinase/AKT/PKCzeta/lambda pathway in the effect of palmitate on glucose-induced insulin secretion. Pancreas. 2008 Oct;37(3):309–315. doi: 10.1097/mpa.0b013e318168dac3. [DOI] [PubMed] [Google Scholar]
- Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006 Nov;32(5 Pt 1):403–414. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Dig Dis Sci. 2008 Feb;53(2):307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003 Nov;23(21):7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, de Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry. 2007 Jul;20(4):380–385. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- Rahmadi A, Steiner N, Munch G. Advanced glycation endproducts as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer's disease. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2011 Mar;49(3):385–391. doi: 10.1515/CCLM.2011.079. [DOI] [PubMed] [Google Scholar]
- Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer's disease. J Alzheimers Dis. 2009;16(4):763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004 Apr 8;206(2):169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005 Dec;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Butura A, Korourian S, Shankar K, Simpson P, Badeaux J, Albano E, Ingelman-Sundberg M, Badger TM. Cytokine and chemokine expression associated with steatohepatitis and hepatocyte proliferation in rats fed ethanol via total enteral nutrition. Exp Biol Med (Maywood) 2008 Mar;233(3):344–355. doi: 10.3181/0707-RM-203. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB. Clinical aspects of inflammation in Alzheimer's disease. Int Rev Psychiatry. 2005 Dec;17(6):503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004 Nov;287(5):G1035–1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci. 2006 Apr-May;24(2-3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004 Oct;99(10):1946–1952. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, Schmidt PS. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord. 2005;20(5):286–291. doi: 10.1159/000088306. [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003 Aug 6;23(18):7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited Therapeutic Effect of N-Acetylcysteine on Hepatic Insulin Resistance in an Experimental Model of Alcohol-Induced Steatohepatitis. Alcoholism, clinical and experimental research. 2011 Jul 25; doi: 10.1111/j.1530-0277.2011.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem. 2008 May 16;283(20):13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. The Journal of clinical endocrinology and metabolism. 2008 Nov;93(11):4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008 Oct;217(1):1–12. doi: 10.1002/jcp.21514. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Prinetti A. Lipids and membrane lateral organization. Front Physiol. 2010;1:153. doi: 10.3389/fphys.2010.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation in aging and age-related diseases. Annals of the New York Academy of Sciences. 2001 Apr;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005 Feb;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Movsesyan VA, Lea PMT, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003 Mar;22(3):365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006 Jan;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010 Apr;21(2):128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007 Mar;125(3):411–424. [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008 Dec;19(10):371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Tong M, de la Monte SM. Mechanisms of ceramide-mediated neurodegeneration. J Alzheimers Dis. 2009 Apr;16(4):705–714. doi: 10.3233/JAD-2009-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Longato L, de la Monte SM. Early limited nitrosamine exposures exacerbate high fat diet-mediated type2 diabetes and neurodegeneration. BMC Endocr Disord. 2010 Mar 19;10(1):4. doi: 10.1186/1472-6823-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine Exposure Causes Insulin Resistance Diseases: Relevance to Type 2 Diabetes Mellitus, Non-Alcoholic Steatohepatitis, and Alzheimer's Disease. J Alzheimers Dis. 2009 Jun 19; [PMC free article] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005 Feb;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR. Sphingolipid signaling pathways as potential therapeutic targets in gliomas. Mini Rev Med Chem. 2007 Oct;7(10):984–990. doi: 10.2174/138955707782110123. [DOI] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011 Aug;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Weiss JJ, Gorman JM. Psychiatric behavioral aspects of comanagement of hepatitis C virus and HIV. Curr HIV/AIDS Rep. 2006 Nov;3(4):176–181. doi: 10.1007/s11904-006-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005 Dec;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, Mcewen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005 Oct;119(5):1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Xu J, Yeon JE, Chang H, Tison G, Chen GJ, Wands J, de la Monte S. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 2003 Jul 18;278(29):26929–26937. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res. 2007 Apr;4(2):123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- Yalniz M, Bahcecioglu IH, Ataseven H, Ustundag B, Ilhan F, Poyrazoglu OK, Erensoy A. Serum adipokine and ghrelin levels in nonalcoholic steatohepatitis. Mediators Inflamm. 2006;2006(6):34295. doi: 10.1155/MI/2006/34295. [DOI] [PMC free article] [PubMed] [Google Scholar]