Abstract
Critical limb ischemia (CLI) represents the most advanced stage of peripheral arterial obstructive disease (PAOD) with a severe obstruction of the arteries which markedly reduces blood flow to the extremities and has progressed to the point of severe rest pain and/or even tissue loss. Recent therapeutic strategies have focused on restoring this balance in favor of tissue survival using exogenous molecular and cellular agents to promote regeneration of the vasculature. These are based on stimulation of angiogenesis by extracellular and cellular components. This review article carries out a systematic analysis of the most recent scientific literature on the application of stem cells in patients with CLI. The results obtained from the detailed analysis of the recent literature data have confirmed the beneficial role of cell therapy in reducing the rate of major amputations in patients with CLI and improving their quality of life.
1. Introduction
Critical limb ischemia (CLI) is an important condition in the general population with a strong social impact [1]; the prevalence of CLI in the population aged 60–90 years is estimated as 1% (0.5–1.2%) [1, 2] with male to female ratio around 3 : 1 and 5–10% of patients with asymptomatic peripheral arterial obstructive disease (PAOD) or claudication will progress to CLI at 5 years from the first diagnosis. Several studies have shown that over 50% of CLI patients do not have any PAOD symptoms 6 months prior to the onset of CLI [3]. The major risk factors for PAOD include smoking, hyperlipidemia, hypertension, and—for development of CLI—diabetes. Diabetic patients are, at least, fivefold more likely to develop CLI than nondiabetic patients.
CLI is the end stage of PAOD and the macrovascular lesions induce a reduction of distal perfusion. Nutrient blood flow to the tissues and microcirculation exchange are severely altered [4].
Strategies to treat CLI and its related symptoms include both pharmacologic therapy and invasive procedures [5]; however, about 25% of patients still progress each year to limb amputations [6]. Pathophysiologically, chronic ischemia exceeds tissue capacity for oxygen diffusion and nutrients from peri-ischemic territories, as well as for endogenous remodeling. Recent therapeutic strategies have focused on restoring this balance in favor of tissue survival using exogenous molecular and cellular agents to promote regeneration of the vasculature: these are based on stimulation of angiogenesis by extracellular and cellular components [7–9]. Several studies have shown that bone marrow-derived endothelial and hematopoietic progenitors may restore tissue vascularization after ischemic events in limbs, retina, and myocardium [10–14]. Dysfunction in the vascular bed in ischemic conditions, attrition of the microvasculature, and the difficulty or impossibility to adapt to the need for increased blood flow are the critical points through which we investigate cellular mediators and tissue-specific chemokines, which facilitate selective recruitment of bone marrow-derived stem and progenitor cells to specific organs and the factors that promote differentiation of the progenitor cells [15, 16]. The different families of chemokines are determined by the numbers and spacing of cysteine residues adjacent to the amino terminus: CC, CXC, CX3C, and XC. The CC chemokines primarily attract mononuclear cells, including monocytes, eosinophils, basophils, dendritic cells, and T lymphocytes. CXC chemokines primarily attract neutrophils (CXCL1–3 and CXCL5–8) or lymphocytes (CXCL4 and CXCL9–16). Peripheral blood monocytes express CCR1, CCR2, CCR3, CCR5, and CXCR4. Evidences show that a large cohort of chemokines affects monocytes/macrophage recruitment and consequently influences arteriogenesis and response of tissues to ischemia.
The principle that characterizes the therapeutic application of stem cells is the restoration of vascular cellularity, the control and the support of the newly formed vessels which must ensure an adequate supply of oxygen in critical ischemic areas. Thus, oxygen tension plays several roles in the expression of different genes such as the vascular endothelial growth factor (VEGF) family and proangiogenic growth factor.
The aim of this study is to perform a systematic analysis of the most recent scientific literature on the application of stem cells in patients with CLI of different etiologies.
2. Material and Methods
PubMed, Scopus, and ScienceDirect databases were searched for articles using the terms: Peripheral Arterial Obstructive Disease, Critical Limb Ischemia, Stem Cells Therapy, Angiogenesis and Limb Loss.
Only publications in English were included. Titles and abstracts were screened by 1 author (B. L.) to identify potentially relevant studies. All potentially eligible studies were subsequently evaluated in detail by 1 reviewer (B. L.) through consideration of the full text. Reference lists of retrieved articles were also searched for relevant publications.
Inclusion required clinical trials in which therapy with stem cells in CLI patients was performed. Studies were excluded if not performed in English language, if performed in animals or in vitro, if the cohort was defined by the presence of CLI and an additional confounding disease process (e.g., chronic renal failure or cerebrovascular diseases), or if CLI specific results could not be distinguished from those of a larger population consisting of individuals without CLI. Studies were excluded when the primary focus was carotid artery disease, aortic aneurysmal disease, intracranial vascular disease, inflammatory diseases, cancer, nonvascular diseases, and treatment with chemotherapy.
3. Results
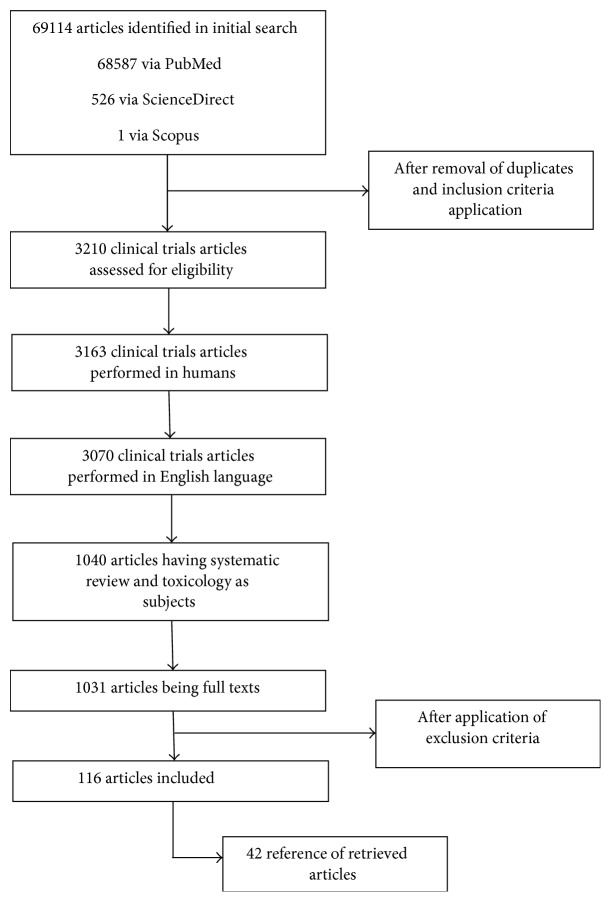
Study Selection. The initial database searches yielded 68587 studies from PubMed, 526 from ScienceDirect, and 1 from Scopus in the last 5 years. We evaluated 1031 eligible full text articles (Figure 1).
Figure 1.
Flow of papers identified from search strategy.
The biology and physiology of stem cells and their differentiation in vascular cells, the current methods of sampling of stem cells found in literature, the relationship with clinical and adverse effects in treated patients, and the description of the indications to the stem cell therapy in patients with CLI are given below.
3.1. Biology of Vascular Stem Cells
Embryonic stem cells (ESCs) have the competency to self-renew indefinitely while maintaining the potential to give rise to all cell types in the human body; the first human cell line was generated in 1998 by Thompson et al. [17]. Many studies were made to clarify the physiology of stem cells, the stage specific embryonic antigens, and the several factors which maintain “stemness” [18–25]. During embryogenesis, the inner cell mass (ICM), the internal cell component of the blastocyst, gives rise to the primitive endoderm and epiblast, which consists of three primary germ layers: ectoderm, mesoderm, and endoderm. Vascular cells including endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) are predominantly descendants of mesodermal cells; however, an ectoderm origin for VSMCs was detected [26, 27]. The differentiation of mesoderm in vascular cells is regulated by important factors in a complex process with a fine regulation: Brachyury, a transcription factor required for posterior mesoderm formation and differentiation and then downregulated when cells undergo specific development into mesoderm-derived tissues, including cardiac muscle, endothelium, and blood cells [28, 29], bone morphogenetic protein (BMP), a member of the transforming growth factor- (TGF-) β superfamily [30, 31], MIXL1, a homeobox gene involved in hematopoietic specification [32, 33], Nodal [34, 35], CD31 [34], [36], CD34 [37, 38], Sca-1 [39, 40], N-cadherin [41, 42], platelet-derived growth factor receptor- (PDGFR-) α [43, 44], and vascular endothelial growth factor receptor- (VEGFR-) 2 [45, 46].
Blood vessels arise from endothelial precursors through a process known as developmental vasculogenesis [47, 48]: resulting capillaries are small and cannot sufficiently compensate for a large occluded transport artery due to Hagen-Poiseuille law [49, 50]. Arteriogenesis, also called collateral growth, is the transformation of preexistent collateral arterioles into functional collateral arteries: evidences have shown that human bone marrow-derived stromal cells promote arteriogenesis through paracrine mechanisms [51, 52].
Studies showed that ischemia induces plasma elevation of stem and progenitor cell-active cytokines, including soluble kit-ligand (sKitL) and thrombopoietin, progenitor-active cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin. Thrombopoietin and sKitL may release stromal-derived factor-1 (SDF-1) from platelets accelerating revascularization of the ischemic limbs through mobilization of hemangiocytes [50, 53, 54]. Hemangiocytes induce neovascularization by releasing angiogenic factors and by physically supporting the assembly of endothelial cells. The risk factors due to insufficient collateralization (diabetes, smoking, hyperlipidemia, and advanced age) are the same for a lower number of circulating, monocytic progenitor cells (MPCs) [55–57].
Also immature VSMCs play a central role in blood vessel morphogenesis; they proliferate and migrate and produce extracellular matrix (ECM) components of the blood vessel wall such as collagen, elastin, and proteoglycans. Vascular growth stimuli, such as ischemic injury, in large and small vessels can trigger a process of differentiation of VSMC in which the matrix proteases, known as matrix metalloproteinases (MMPs), may play several roles: MMPs, thus, are not only involved in many vascular [58–72] and nonvascular diseases [73].
3.2. Mobilization of Stem Cells
The hematopoietic stem cell (HSC) resides in the bone marrow (BM) but several chemokines and cytokines have been shown to enhance trafficking of HSC into the peripheral blood. This process, known as stem cell mobilization, results in HSC microenvironmental interactions with the critical ligands, receptors and cellular proteases. Peripheral blood progenitor cells (PBPCs) advantages are avoidance of general anesthesia and pain and other adverse effects related to BM collection. Studies have shown that peripheral blood after cytokines stimulation contained a major number of CD34+ cells and T and NK cells than those in BM collection [74].
Granulocyte colony stimulating factor (G-CSF) and GM-CSF represent the two major cytokines used to mobilize the stem cells. These two factors can stimulate the stem cells that are released from the BM niches into peripheral blood. The BM niche is a structured microenvironment composed of supporting cells that anchor, through cell interaction, stem cells and regulates the self-regeneration, proliferation, and release into the circulation. Supporting cells also provide stem cells survivor with chemical signals: neurotransmitters induce membrane type-1 metalloproteinase (MT1-MMP) expression and MMP-2 activity [58–72, 75], which mediate the cleavage of ties (CXCR4, VLA4, VCAM-1, and SCF) holding the stem cells in the BM niche and supporting their blood release.
Several studies have shown that administration of G-CSF and GM-CSF leads to a dose-dependent increase of endothelial progenitor cells (EPCs) in peripheral blood [76–78]. G-CSF promotes not only granulocyte expansion but also reduction of adhesion molecules and disruption of the SDF-1/CXCR4 axis: proteolytic enzymes, neutrophil elastase (NE), and cathepsin G (CG) cleave adhesion molecules as VCAM-1, SDF-1 and CXCR4, and c-kit [79, 80]. CXC chemokine receptor-4 antagonists can mobilize EPCs increasing MMP-9 signaling in the BM [58–72, 81, 82].
GM-CSF, instead, is rarely used because it mobilizes a reduced number of cells compared to G-CSF [83]. Generally, VEGF, fibroblastic growth factors, and stromal cell-derived factors have the ability to recruit EPCs; parathyroid hormone, statins,and other ligands may be used to mobilize stem cells, alone or in combination with G-CSF [84, 85].
Stem cell treatments with BM-derived cells (BMDCs) show safety outcomes but also adverse events related to cell collection and mobilization. Porat et al. postulated that alternatively activated dendritic cells (DCs) can promote the generation of EPC-enriched stem cells within a one-day culture [86].
Another source of stem cells can be satellite cells of skeletal muscle: these cells are one of the well-studied adult tissue-specific stem cells and have served as an excellent model for investigating adult stem cells. Myogenic precursor cells of postnatal muscle are responsible for the repair and regeneration of muscle fibers in adult tissue, either by fusing together and forming new fibers or incorporating themselves into damaged muscle cells and their myonuclei [87]. Satellite cells are mitotically quiescent or slow-cycling, committed to myogenesis, but undifferentiated. Satellite cells are the only source of new myoblasts in the adult tissue but they decrease with the age. In ischemic conditions these cells can be activated and their behavior is similar to those of bone marrow stem cells [88]. Satellite cells are activated by Myf-5 [89], a transcription factor, and CD34 is required for maintaining the quiescent state of myogenic stem cells [88]. After 6 hours from injury, satellite cells are activated and migrate, after disruption of basal lamina, from adjacent myofibers by projecting across tissue bridges initiated from an outpouching process of the satellite cell itself [90, 91]. Currently, the limit of this method is represented by the low number of in vivo studies which show the effectiveness of neoangiogenesis in patients with CLI.
Mesenchymal stem cells (MSCs) are multipotent cells showing adaptability and secretory capacity: thus, they can mediate reparative processes from the through release of soluble molecules, MSC-derived growth factors, and extracellular matrix components with paracrine mechanisms. It is likely that lower secretion of these important factors is the cause of failed tissue reparation. Studies have shown that cells obtained from older patients with multiple risk factors have impaired functions [92, 93]: for this reason, therapeutic success in CLI patients could be increased by using MSCs from young donors.
Therapeutic administration of stem cells does not have to be derived only from bone marrow but also from adipose tissue [93–95] and umbilical cord [96, 97] and other sources and released cytokines are main driving molecules in reparatory processes in CLI patients [98–100] with different results (Table 1).
Table 1.
Comparison of advantages and limitations of different types of stem cells.
| Stem cell type | Limitations | Advantages |
|---|---|---|
| Embryonic stem cells | Ethical dilemmas, possible immune rejection after implantation, a small number of differentiated cardiomyocytes being generated, leading to teratocarcinomas; genetic instability | Differentiating into cells of all three germ layers |
| Pluripotent stem cells | Genetic instability, more research needed before using for cardiovascular repair/regeneration | Avoiding ethical concerns |
| Adult stem cells | Natural regeneration capacity of CSCs being too limited, acquisition and isolation difficulties, more research needed | Avoiding ethical concerns, lower risk of immune rejection |
| Mesenchymal stem cells | More research needed | Allowing for allogeneic grafting without the use of immunosuppressive agents, self-renewal, proliferating, and differentiating, promoting growth of adjacent cells, less susceptible to mutations, easy to collect |
| Hematopoietic stem cells | High maintenance, low frequencies, unknown signaling pathways | Proliferating and migrating to injury site in response to physiological/pathological stimuli, capable of myogenesis and angiogenesis |
| Endothelial progenitor cells | Extremely low numbers in peripheral blood and bone marrow making ex vivo expansion difficult | Increasing its numbers in response to ischemia/cytokine stimuli and migrating to injury site and differentiating into new myocytes |
3.3. Intramuscular versus Intra-Arterial Administration of Stem Cells
Intramuscular and intra-arterial injection or a combination of both may be proposed in the treatment of human PAD. The principle of intramuscular injection is the creation of a cell depot with paracrine activity in the ischemic area. Experimental animal studies indicate that BM-derived cells contribute to vascular and muscle regeneration by physically integrating into the tissue and/or by secreting growth factors [101, 102]. The principle of intramuscular injection is the creation of a cell depot with paracrine activity in the ischemic area. Injection of bone marrow mononuclear cells has been reported to promote neovascularization of ischemic tissues effectively. This angiogenic effect may be related to their ability to induce vascular and muscle regeneration by direct de novo vascular and muscle differentiation or paracrine mechanisms through vascular endothelial growth factor secretion. Bone marrow mononuclear cells (BM-MNCs) contained the cell fractions that include EPCs and released various angiogenic factors: incorporation of EPCs in newly formed vessels as well as angiogenesis/arteriogenesis by angiogenic factors released from injected cells likely contributes to the increase in blood. Studies indicate that BMDCs contribute to vascular and muscle regeneration by physically integrating into the tissue and/or by secreting growth factors [103–105].
Intramuscular injection was performed into the gastrocnemius muscle; furthermore, injections were also placed along the occluded native arteries, because the density of preformed collaterals is highest in parallel orientation to the axial arteries: this is the preferred location for collateral growth [106, 107].
The effects of intra-arterial or intra-arterial plus intramuscular cell administration were compared to the effects of intramuscular cell administration. Ankle-brachial index (ABI) and transcutaneous partial pressure of oxygen (TcPO2) were found to be significantly improved only after intramuscular or combined therapy and not after intra-arterial cell therapy only [50, 108, 109]. On the other hand, significantly improved pain and pain-free walking distance were detected and there was no difference between the two. Intramuscular cell therapy significantly improved ulcer healing, while this could not be assessed in detail in trials of intra-arterial cell therapy. Pilot studies by the small number of patients reported the improvement of clinical signs and symptoms of intractable patients with CLI by injection of G-CSF [110, 111] or intramuscular injection of G-CSF-mobilized peripheral blood mononuclear cells [112–114]. Tateno et al. [115] postulated that the implanted peripheral blood mononuclear cells stimulate ischemic skeletal muscle cells to produce muscle-derived angiogenic factors, thereby promoting neovascularization. In all studies, there were no significant differences in the clinical characteristics between patients treated with intra-arterial or intra-arterial plus intramuscular cells [50, 116, 117].
3.4. Adverse Effects in Stem Cells Therapy
Injection of BM-MNCs significantly improved pain-free walking time, rest pain, and tissue oxygen pressure on average 6 months after treatment, whereas injection of peripheral blood mononuclear cells did not exert significant effects [118, 119]. Several studies reported very low mortality rate (<15%) in patients treated with autologous stem cells implantation for CLI [108, 120–122]. These findings suggest that the angiogenic cell therapy using intramuscular implantation of BM-MNCs is valid therapeutic choice and not inferior to the conventional revascularization therapies in patients with CLI. Because of the increased risk and the reduced potential of the treatment, peripheral blood stem cell treatment is less appropriate in the older age [123]. In some studies, deaths have been reported: these were mostly due to acute myocardial infarction, congestive heart failure, and stroke while perforation peritonitis and sepsis have been reported as exceptional [124, 125]. In 2012, Jonsson et al. reported a high incidence of serious adverse events in patients treated with peripheral blood mononuclear cells, causing the investigators to terminate the study [126]. Out of 9 patients, 2 had a myocardial infarction that was believed to be related to the bone marrow stimulation and 1 of the 2 patients died. Another patient had a minor stroke 1 week after stem-cell implantation.
As previously showed, hemodialysis [127], diabetes mellitus [128, 129], and complication with coronary artery disease (CAD) [130–132] are factors that negatively impaired angiogenesis or limb salvage in animal experiments and clinical settings.
3.5. Patients in Whom Endovascular and Surgical Revascularisation Are Not Believed to Be Possible
Patients with poor outflow vessels and extensive comorbidities resulting in unacceptable risk of a revascularization procedure, as well as patients who had previously failed revascularization attempts, are not candidate to surgical and/or endovascular procedures [133–136]. This subgroup of patients without revascularization options, known as NO-CLI, is a population with a high rate of limb loss and death.
One primary endpoint for evaluating the outcomes of NO-CLI therapy is major amputation (AMP), which is usually combined with mortality for AMP-free survival (AFS) [137–140]. AFS captures two hard endpoints, mortality and amputation, that are obviously important to patients and clinicians. Despite the fact that the AFS and mortality are the focal points used in the majority of RCTs in the literature for NO-CLI, they necessarily require periodic review and updating.
In the absence of arterial reconstruction options, novel approaches, such as pharmacologic, gene, or stem cell therapy, are proposed; in particular, regenerative medicine has recently emerged as a new speciality that has created great expectations in the scientific community [141]. Improvement of neovascularization is a therapeutic option to rescue tissue from critical ischemia.
4. Discussion
The gold-standard treatment of severe PAOD and CLI is surgical or endovascular revascularization. However, up to 30% of patients are not candidate for such interventions, due to excessive operative risk or unfavorable vascular involvement. Despite the progress in medical and surgical therapy of patients with CLI, the prognosis of patients with no option for revascularization remains poor: the amputation rate is high as well as mortality rate (20%) within six months.
During the last two decades, a novel therapeutic strategy has been proposed: the stem cells therapy. BMDCs include autologous BMCs, BMMNCs, and EPCs; peripheral blood-derived cells include PBMNCs, PMNCs, ECFCs, CPCs, and EPC, while other cells mainly include MSCs and ADSCs. In 1997, Asahara et al. discovered that bone marrow-derived circulating cells are able to differentiate into endothelium and promote new vessel growth. These cells, known as EPCs, were able to improve tissue perfusion in myocardial and peripheral ischemia through the stimulation of vasculogenesis [142]. As showed in our review, the studies which have applied the stem cell therapy in NO-CLI patients are very numerous. Despite some failures associated with factors that invalidated the functionality of the different stem cells (i.e., diabetes), the results obtained from the detailed analysis of the recent literature data have confirmed the beneficial role of cell therapy in reducing the rate of major amputations, improving distal perfusion, increasing walking distance, reducing pain, improving ABI and TcPO2, and improving overall ischemic symptoms in patients with CLI and their quality of life (Table 2).
Table 2.
Clinical trials using stem cells for treatment of critical limb ischemia.
| Authors | Type of cells | Clinical outcomes |
|---|---|---|
| Nizankowski et al. [143] | BMCs | Improvement of symptoms (pain, cold sensation), increase in ABI and TcPO2, new collateral vessels |
| Napoli et al. [144] | BMCs | Increase in ABI and walking distance, ulcer healing, reduction of amputation rates |
| Procházka et al. [145] | BMCs | Improvement in toe pressure, TBI, LDI, and TcPO2 |
| Matoba et al. [146] | BMMNCs | Long-term improvement in pain scale, ulcer size, and walking distance, reduced amputation rates |
| Amann et al. [8] | BMMNCs | Limb salvage, increase in ABI and TcPO2 |
| Kawamura et al. [147] | PBMNCs with GcSF | Reduced amputations, mostly in nondiabetic nondialysis patients |
| Huang et al. [148] | PBMNCs versus BMMNCs | PBMNC administration: higher overall efficacy, improvement in ABI, skin temperature, rest pain, walking distance, TcPO2, ulcers, and amputation rates (both treatments) |
| Tateishi-Yuyama et al. [149] | Bone marrow MNCs | Increased ABPI and TcPO2 pressure, decreased rest pain |
| Motukuru et al. [150] | Bone marrow MNCs | Increased ABPI and improvement in ulcer healing |
| Lara-Hernandez et al. [108] | Peripheral blood CD34+ CD133+ cells after G-CSF mobilization | Increased ABPI and improvement in ulcer healing |
| Benetti et al. [151] | Human fetal-derived stem cells | |
| Burt et al. [152] | EPCs (CD34/CD133) | Improvement in amputation-free survival, exercise capacity, pain relief, collateral formation, perfusion, and QoL |
| MESENDO (II) Clinicaltrials.gov # NCT00721006 [153] | Stem cell mixture | Completed; pending publication |
| Lasala et al. [154] | BM-MNC EPC + BM-MSC |
↑ABI, ↑angiogenesis (MRA), ↑AFS, ↑TcPO2, ↑WH, ↑WT, ↓pain |
| Dash et al. [155] | BM-MSC | ↑angiogenesis (biopsy), ↑WH, ↑WD, ↓pain |
| NCT01257776 [156] | Adipose-MSC | ABI, AFS, DSA improved |
| NCT01216865 [156] | Cord-MSC | ABI, AFS, pain, WT, WH improved |
Bone marrow aspiration was well tolerated, the most frequent adverse reaction being local pain, responsive to nonsteroidal anti-inflammatory drugs; common adverse event was mild anemia. G-CSF stimulation was generally well tolerated, with prevalently minor side effects, including flu-like symptoms, myalgia, fever, and bone pain. Intramuscular or intra-arterial delivery associated with intramuscular injections of BMMNCs cells had positive results in the majority of clinical studies: the procedure appeared to be generally safe and well tolerated and most adverse reactions were expected given the severe underlying disease and could not be directly attributed to cell therapy. The intramuscular administration seems preferable maybe because cells could hardly reach the target tissue when infused intra-arterially in severely compromised arterial beds.
Thus, bone marrow cells or peripheral blood cells administration? The latter seems to be easier to perform and it might be repeated but it does not appear to be inferior in efficacy: BMMCs seemed to be more effective than mobilized peripheral blood cells in inducing reparative processes because these cells are transiently dysfunctional due to cleavage of the chemokine receptor CXCR4, which is directly involved in stem cell homing. PB-MNCs show comparable or even superior efficacy in comparison to BM-MNCs. In conclusion, BMCs, BM-MNCs, and PB-MNCs are the main cell types used and there is no clear superiority of one cell type over the others. Current literature supports that intramuscular BM cell administration is a relatively safe, feasible, and possibly effective therapy for patients with CLI not susceptible to conventional revascularization.
Based on the recent literature data, treatment-induced improvements are sustainable at 2-3 years: if long-term efficacy becomes definitively established, the stem cell therapy for severe inoperable PAOD will be strongly enhanced. For this reason, multicenter, large-scale and randomized controlled clinical trials may be fundamental and mandatory to prove the safety and efficacy of promoting angiogenesis by the administration of stem cells and for this therapy to become a standard treatment strategy for the patients suffering with CLI.
Another key aspect is that stem cell therapy is an expensive treatment and its cost-effectiveness has not been determined. Thus, a detailed cost-benefit analysis is desirable.
Abbreviations
- ABI:
Ankle-brachial index
- ADSCs:
Adipose tissue-derived stem cells
- AFS:
AMP-free survival
- AMP:
Amputation
- BM:
Bone marrow
- BM-MNCs:
Bone marrow mononuclear cells
- BMDCs:
BM-derived cells
- BMP:
Bone morphogenetic protein
- CAD:
Coronary artery disease
- CG:
Cathepsin G
- CLI:
Critical limb ischemia
- CPCs:
Circulating progenitor cells
- DCs:
Dendritic cells
- ECM:
Extracellular matrix
- ECs:
Endothelial cells
- ECFCs:
Endothelial colony forming cells
- EPCs:
Endothelial progenitor cells
- ESCs:
Embryonic stem cells
- G-CSF:
Granulocyte colony stimulating factor
- GM-CSF:
Granulocyte-macrophage colony-stimulating factor
- HSC:
Hematopoietic stem cell
- ICM:
Inner cell mass
- MMPs:
Matrix metalloproteinases
- MPCs:
Monocytic progenitor cells
- MSCs:
Mesenchymal stem cells
- MT1-MMP:
Membrane type 1 matrix metalloproteinase
- NE:
Neutrophil elastase (NE)
- NO-CLI:
Patients with CLI and without revascularization options
- PAOD:
Peripheral arterial obstructive disease
- PBMNCs:
Peripheral blood mononuclear cells
- PBPCs:
Peripheral blood progenitor cells
- PDGFR-α:
Platelet-derived growth factor receptor-α
- PMNCs:
Peripheral mononuclear Cells
- SDF-1:
Stromal-derived factor-1
- sKitL:
Soluble kit-ligand
- TcPO2:
Partial pressure of oxygen
- TGF-β:
Transforming growth factor-β
- VCAM-1:
Vascular cell adhesion protein-1
- VEGF:
Vascular endothelial growth factor
- VEGFR-2:
Vascular endothelial growth factor receptor-2
- VSMCs:
Vascular smooth muscle cells.
Conflict of Interests
The authors declare no conflict of interests.
Authors' Contribution
Rita Compagna and Bruno Amato participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data and also participated substantially in the drafting and editing of the paper. Salvatore Massa and Maurizio Amato participated substantially in data collection and in the analysis and interpretation of data. Raffaele Grande and Lucia Butrico participated substantially in data collection and execution of the study and in the analysis and interpretation of data and also participated substantially in the drafting and editing of the paper. Stefano de Franciscis and Raffaele Serra participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data and also participated substantially in the drafting, editing, and critical revision of the paper. Rita Compagna and Bruno Amato contributed equally to this work and share the first authorship. Stefano de Franciscis and Raffaele Serra contributed equally to this work and share the senior authorship.
References
- 1.Novo S., Coppola G., Milio G. Critical limb ischemia: definition and natural history. Current Drug Targets—Cardiovascular and Haematological Disorders. 2004;4(3):219–225. doi: 10.2174/1568006043335989. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L., Hiatt W. R., Bell K., et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) European Journal of Vascular and Endovascular Surgery. 2007;33(1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Simons J. P., Goodney P. P., Nolan B. W., Cronenwett J. L., Messina L. M., Schanzer A. Failure to achieve clinical improvement despite graft patency in patients undergoing infrainguinal lower extremity bypass for critical limb ischemia. Journal of Vascular Surgery. 2010;51(6):1419–1424. doi: 10.1016/j.jvs.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2045–2051. doi: 10.1161/atvbaha.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hioki H., Miyashita Y., Miura T., et al. Prognostic improvement by multidisciplinary therapy in patients with critical limb ischemia. Angiology. 2014 doi: 10.1177/0003319714523113. [DOI] [PubMed] [Google Scholar]
- 6.Henry A. J., Hevelone N. D., Belkin M., Nguyen L. L. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. Journal of Vascular Surgery. 2011;53(2):330.e1–339.e1. doi: 10.1016/j.jvs.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matoba S., Tatsumi T., Murohara T., et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. The American Heart Journal. 2008;156(5):1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Amann B., Luedemann C., Ratei R., Schmidt-Lucke J. A. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplantation. 2009;18(3):371–380. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 9.Rafii S., Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nature Medicine. 2003;9(6):702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg-Cohen N., Avraham-Lubin B.-C. R., Sadikov T., Askenasy N. Effect of coadministration of neuronal growth factors on neuroglial differentiation of bone marrow-derived stem cells in the ischemic retina. Investigative Ophthalmology & Visual Science. 2013;55(1):502–512. doi: 10.1167/iovs.13-12223. [DOI] [PubMed] [Google Scholar]
- 11.Strauer B. E., Brehm M., Zeus T., et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 12.Amato B., Compagna R., della Corte G. A., et al. Peripheral blood mono-nuclear cells implantation in patients with peripheral arterial disease: a pilot study for clinical and biochemical outcome of neoangiogenesis. BMC Surgery. 2012;12(supplement 1, article S1) doi: 10.1186/1471-2482-12-s1-s1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Franz R. W., Parks A., Shah K. J., Hankins T., Hartman J. F., Wright M. L. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Journal of Vascular Surgery. 2009;50(6):1378–1390. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 14.Franz R. W., Shah K. J., Johnson J. D., et al. Short- to mid-term results using autologous bone-marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Vascular and Endovascular Surgery. 2011;45(5):398–406. doi: 10.1177/1538574411405545. [DOI] [PubMed] [Google Scholar]
- 15.Rafii S., Meeus S., Dias S., et al. Contribution of marrow-derived progenitors to vascular and cardiac regeneration. Seminars in Cell and Developmental Biology. 2002;13(1):61–67. doi: 10.1006/scdb.2001.0285. [DOI] [PubMed] [Google Scholar]
- 16.Rafii S., Heissig B., Hattori K. Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Therapy. 2002;9(10):631–641. doi: 10.1038/sj/gt/3301723. [DOI] [PubMed] [Google Scholar]
- 17.Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 18.Kiskinis E., Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. The Journal of Clinical Investigation. 2010;120(1):51–59. doi: 10.1172/jci40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rustad K. C., Wong V. W., Sorkin M., et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33(1):80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel G. “Stemness” genes still elusive. Science. 2003;302(5644, article 371) doi: 10.1126/science.302.5644.371a. [DOI] [PubMed] [Google Scholar]
- 21.Evsikov A. V., Solter D. Comment on ““Stemness”: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science. 2003;302(5644):393–393. doi: 10.1126/science.1082380. [DOI] [PubMed] [Google Scholar]
- 22.Gerrits A., Dykstra B., Otten M., Bystrykh L., De Haan G. Combining transcriptional profiling and genetic linkage analysis to uncover gene networks operating in hematopoietic stem cells and their progeny. Immunogenetics. 2008;60(8):411–422. doi: 10.1007/s00251-008-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya B., Puri S., Puri R. K. A review of gene expression profiling of human embryonic stem cell lines and their differentiated progeny. Current Stem Cell Research and Therapy. 2009;4(2):98–106. doi: 10.2174/157488809788167409. [DOI] [PubMed] [Google Scholar]
- 24.Zhu D., Wan X., Huang H., et al. Knockdown of Bmi1 inhibits the stemness properties and tumorigenicity of human bladder cancer stem cell-like side population cells. Oncology Reports. 2014;31(2):727–736. doi: 10.3892/or.2013.2919. [DOI] [PubMed] [Google Scholar]
- 25.de Felici M., Farini D., Dolci S. In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells. Current Stem Cell Research and Therapy. 2009;4(2):87–97. doi: 10.2174/157488809788167391. [DOI] [PubMed] [Google Scholar]
- 26.Topouzis S., Majesky M. W. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-β . Developmental Biology. 1996;178(2):430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 27.Descamps B., Emanueli C. Vascular differentiation from embryonic stem cells: novel technologies and therapeutic promises. Vascular Pharmacology. 2012;56(5-6):267–279. doi: 10.1016/j.vph.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Barresi V., Ieni A., Branca G., Tuccari G. Brachyury: a diagnostic marker for the differential diagnosis of chordoma and hemangioblastoma versus neoplastic histological mimickers. Disease Markers. 2014;2014:7. doi: 10.1155/2014/514753.514753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aramaki S., Hayashi K., Kurimoto K., et al. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Developmental Cell. 2013;27(5):516–529. doi: 10.1016/j.devcel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Leung A. W., Kent Morest D., Li J. Y. H. Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells. Developmental Biology. 2013;379(2):208–220. doi: 10.1016/j.ydbio.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurpinski K., Lam H., Chu J., et al. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28(4):734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe A. D., Downs K. M. Mixl1 localizes to putative axial stem cell reservoirs and their posterior descendants in the mouse embryo. Gene Expression Patterns. 2014;15(1):8–20. doi: 10.1016/j.gep.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis R. P., Ng E. S., Costa M., et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood. 2008;111(4):1876–1884. doi: 10.1182/blood-2007-06-093609. [DOI] [PubMed] [Google Scholar]
- 34.Fuerer C., Nostro M. C., Constam D. B. Nodal·Gdf1 heterodimers with bound prodomains enable serum-independent nodal signaling and endoderm differentiation. The Journal of Biological Chemistry. 2014;289(25):17854–17871. doi: 10.1074/jbc.M114.550301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall V., Hyttel P. Breaking down pluripotency in the porcine embryo reveals both a premature and reticent stem cell state in the inner cell mass and unique expression profiles of the naïve and primed stem cell states. Stem Cells and Development. 2014;23(17):2030–2045. doi: 10.1089/scd.2013.0502. [DOI] [PubMed] [Google Scholar]
- 36.Hall V., Hyttel P. Breaking down pluripotency in the porcine embryo reveals both a premature and reticent stem cell state in the inner cell mass and unique expression profiles of the naïve and primed stem cell states. Stem Cells and Development. 2014;23(17):2030–2045. doi: 10.1089/scd.2013.0502. [DOI] [PubMed] [Google Scholar]
- 37.Reményi P., Gopcsa L., Marton I., et al. Peripheral blood stem cell mobilization and engraftment after autologous stem cell transplantation with biosimilar rhG-CSF. Advances in Therapy. 2014;31(4):451–460. doi: 10.1007/s12325-014-0114-z. [DOI] [PubMed] [Google Scholar]
- 38.Shalaby M. N., Saad M., Akar S., Reda M. A., Shalgham A. The role of aerobic and anaerobic training programs on CD(34+) stem cells and chosen physiological variables. Journal of Human Kinetics. 2012;35(1):69–79. doi: 10.2478/v10078-012-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saravanakumar M., Devaraj H. Distribution and homing pattern of c-kit+ Sca-1+ CXCR4+ resident cardiac stem cells in neonatal, postnatal, and adult mouse heart. Cardiovascular Pathology. 2013;22(4):257–263. doi: 10.1016/j.carpath.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Houlihan D. D., Mabuchi Y., Morikawa S., et al. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α . Nature Protocols. 2012;7(12):2103–2111. doi: 10.1038/nprot2012125. [DOI] [PubMed] [Google Scholar]
- 41.Ishimine H., Yamakawa N., Sasao M., et al. N-Cadherin is a prospective cell surface marker of human mesenchymal stem cells that have high ability for cardiomyocyte differentiation. Biochemical and Biophysical Research Communications. 2013;438(4):753–759. doi: 10.1016/j.bbrc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K., Pang K., Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells and Development. 2014;23(12):1340–1354. doi: 10.1089/scd.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Hong W. X., Lan B., et al. PDGF mediates derivation of human embryonic germ cells. Differentiation. 2014;86(4-5):141–148. doi: 10.1016/j.diff.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Valente S., Alviano F., Ciavarella C., et al. Human cadaver multipotent stromal/stem cells isolated from arteries stored in liquid nitrogen for 5 years. Stem Cell Research and Therapy. 2014;5(1, article 8) doi: 10.1186/scrt397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao X., Ping Y., Liu Y., et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0057188.e57188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano M., Fukuda K. The selective VEGFR inhibitor PTK787/ZK 222584 represses the activities of VEGFR-negative bone marrow-derived mesenchymal stem cells. Cancer Biology and Therapy. 2009;8(13):1249–1251. doi: 10.4161/cbt.8.13.8996. [DOI] [PubMed] [Google Scholar]
- 47.Voskuil M., van Royen N., Hoefer I., Buschmann I., Schaper W., Piek J. J. Angiogenesis and arteriogenesis: the long journey from concept to clinical application. Nederlands Tijdschrift voor Geneeskunde. 2001;145(14):670–675. [PubMed] [Google Scholar]
- 48.van Royen N., Piek J. J., Buschmann I., Hoefer I., Voskuil M., Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovascular Research. 2001;49(3):543–553. doi: 10.1016/s0008-63630000206-6. [DOI] [PubMed] [Google Scholar]
- 49.Raval Z., Losordo D. W. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circulation Research. 2013;112(9):1288–1302. doi: 10.1161/circresaha.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawall H., Bramlage P., Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. Journal of Vascular Surgery. 2011;53(2):445–453. doi: 10.1016/j.jvs.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 51.Eitenmüller I., Volger O., Kluge A., et al. The range of adaptation by collateral vessels after femoral artery occlusion. Circulation Research. 2006;99(6):656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 52.Heil M., Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (Arteriogenesis) Circulation Research. 2004;95(5):449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 53.Park B., Hoffman A., Yang Y., et al. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. Journal of Vascular Surgery. 2010;51(1):165–173. doi: 10.1016/j.jvs.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Y., Meng H., Li C., et al. Umbilical cord-derived mesenchymal stem cells isolated by a novel explantation technique can differentiate into functional endothelial cells and promote revascularization. Stem Cells and Development. 2010;19(10):1511–1522. doi: 10.1089/scd.2009.0321. [DOI] [PubMed] [Google Scholar]
- 55.Kim W., Myung H. J., Suk H. C., et al. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circulation Journal. 2006;70(8):1052–1057. doi: 10.1253/circj.70.1052. [DOI] [PubMed] [Google Scholar]
- 56.Gómez-Cerezo J. F., Pagán-Muñoz B., López-Rodríguez M., Estébanez-Muñoz M., Barbado-Hernández F. J. The role of endothelial progenitor cells and statins in endothelial function: a review. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2007;5(4):265–272. doi: 10.2174/187152507782109836. [DOI] [PubMed] [Google Scholar]
- 57.Adams V., Linke A., Breuckmann F., et al. Circulating progenitor cells decrease immediately after marathon race in advanced-age marathon runners. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(5):602–607. doi: 10.1097/hjr.0b013e328309c756. [DOI] [PubMed] [Google Scholar]
- 58.Serra R., Buffone G., Costanzo G., et al. Altered metalloproteinase-9 expression as least common denominator between varicocele, inguinal hernia, and chronic venous disorders. Annals of Vascular Surgery. 2014;28(3):705–709. doi: 10.1016/j.avsg.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 59.Amato B., Coretti G., Compagna R., et al. Role of matrix metalloproteinases in non-healing venous ulcers. International Wound Journal. 2013 doi: 10.1111/iwj.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serra R., Buffone G., Falcone D., et al. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair and Regeneration. 2013;21(3):395–401. doi: 10.1111/wrr.12035. [DOI] [PubMed] [Google Scholar]
- 61.Serra R., Grande R., Buffone G., Gallelli L., de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Acta Phlebologica. 2013;14(3):99–107. [Google Scholar]
- 62.Serra R., Grande R., Butrico L., et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. International Wound Journal. 2014 doi: 10.1111/iwj.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serra R., Gallelli L., Buffone G., et al. Doxycycline speeds up healing of chronic venous ulcers. International Wound Journal. 2013 doi: 10.1111/iwj.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serra R., Gallelli L., Conti A., et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Design, Development and Therapy. 2014;8:519–527. doi: 10.2147/dddt.s61770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serra R., Grande R., Buffone G., et al. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. International Wound Journal. 2014 doi: 10.1111/iwj.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Busceti M. T., Grande R., Amato B., et al. Pulmonary embolism, metalloproteinases and neutrophil gelatinase associated lipocalin. Acta Phlebologica. 2013;14(3):115–121. [Google Scholar]
- 67.de Franciscis S., Mastroroberto P., Gallelli L., Buffone G., Montemurro R., Serra R. Increased plasma levels of metalloproteinase-9 and neutrophil gelatinaseeassociated lipocalin in a rare case of multiple artery aneurysm. Annals of Vascular Surgery. 2013;27(8):1185.e5–1185.e7. doi: 10.1016/j.avsg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 68.de Franciscis S., Gallelli L., Battaglia L., et al. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. International Wound Journal. 2013 doi: 10.1111/iwj.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serra R., Grande R., Gallelli L., et al. Carotid body paragangliomas and Matrix metalloproteinases. Annals of Vascular Surgery. 2014 doi: 10.1016/j.avsg.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 70.de Franciscis S., Grande R., Butrico L., et al. Resection of Carotid Body Tumors reduces arterial blood pressure. An underestimated neuroendocrine syndrome. International Journal of Surgery. 2014;12(supplement 2):S63–S67. doi: 10.1016/j.ijsu.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 71.Serra R., Grande R., Buffone G., et al. Effects of glucocorticoids and tumor necrosis factor-alpha inhibitors on both clinical and molecular parameters in patients with Takayasu arteritis. Journal of Pharmacology & Pharmacotherapeutics. 2014;5(3):193–196. doi: 10.4103/0976-500X.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serra R., Volpentesta G., Gallelli L., et al. Metalloproteinase-9 and neutrophil gelatinase-associated lipocalin plasma and tissue levels evaluation in middle cerebral artery aneurysms. British Journal of Neurosurgery. 2014 doi: 10.3109/02688697.2014.913777. [DOI] [PubMed] [Google Scholar]
- 73.Lombard C., Saulnier J., Wallach J. Assays of matrix metalloproteinases (MMPs) activities: a review. Biochimie. 2005;87(3-4):265–272. doi: 10.1016/j.biochi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Siddiq S., Pamphilon D., Brunskill S., Doree C., Hyde C., Stanworth S. Bone marrow harvest versus peripheral stem cell collection for haemopoietic stem cell donation in healthy donors. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD006406.pub2.CD006406 [DOI] [PubMed] [Google Scholar]
- 75.Vagima Y., Avigdor A., Goichberg P., et al. MT1-MMP and RECK are involved in human CD34+ progenitor cell retention, egress, and mobilization. Journal of Clinical Investigation. 2009;119(3):492–503. doi: 10.1172/JCI36541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takahashi T., Kalka C., Masuda H., et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Medicine. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 77.Haas R., Murea S. The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells. Cytokines and Molecular Therapy. 1995;1(4):249–270. [PubMed] [Google Scholar]
- 78.Hölig K., Kramer M., Kroschinsky F., et al. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood. 2009;114(18):3757–3763. doi: 10.1182/blood-2009-04-218651. [DOI] [PubMed] [Google Scholar]
- 79.Dar A., Kollet O., Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Experimental Hematology. 2006;34(8):967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Link D. C. Mechanisms of granulocyte colony-stimulating factor induced hematopoietic progenitor-cell mobilization. Seminars in Hematology. 2000;37(1, supplement 2):25–32. doi: 10.1016/s0037-19630090086-6. [DOI] [PubMed] [Google Scholar]
- 81.Devine S. M., Flomenberg N., Vesole D. H., et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2004;22(6):1095–1102. doi: 10.1200/jco.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 82.Pusic I., Dipersio J. F. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Current Opinion in Hematology. 2010;17(4):319–326. doi: 10.1097/moh.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 83.Poole J., Mavromatis K., Binongo J. N., et al. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: a randomized clinical trial. The Journal of the American Medical Association. 2013;310(24):2631–2639. doi: 10.1001/jama.2013.282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ballen K. Targeting the stem cell niche: squeezing blood from bones. Bone Marrow Transplantation. 2007;39(11):655–660. doi: 10.1038/sj.bmt.1705651. [DOI] [PubMed] [Google Scholar]
- 85.Rashidi N., Adams G. B. The influence of parathyroid hormone on the adult hematopoietic stem cell niche. Current Osteoporosis Reports. 2009;7(2):53–57. doi: 10.1007/s11914-009-0010-7. [DOI] [PubMed] [Google Scholar]
- 86.Porat Y., Assa-Kunik E., Belkin M., et al. A novel potential therapy for vascular diseases: blood-derived stem/progenitor cells specifically activated by dendritic cells. Diabetes/Metabolism Research and Reviews. 2014;30(7):623–634. doi: 10.1002/dmrr.2543. [DOI] [PubMed] [Google Scholar]
- 87.Gussoni E., Soneoka Y., Strickland C. D., et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–394. doi: 10.1038/43922. [DOI] [PubMed] [Google Scholar]
- 88.Hart C. A., Tsui J., Khanna A., Abraham D. J., Baker D. M. Stem cells of the lower limb: their role and potential in management of critical limb ischemia. Experimental Biology and Medicine. 2013;238(10):1118–1126. doi: 10.1177/1535370213503275. [DOI] [PubMed] [Google Scholar]
- 89.Kassar-Duchossoy L., Gayraud-Morel B., Gomès D., et al. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature. 2004;431(7007):466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- 90.White T. P., Esser K. A. Satellite cell and growth factor involvement in skeletal muscle growth. Medicine and Science in Sports and Exercise. 1989;21(5):S158–S163. [PubMed] [Google Scholar]
- 91.Kawiak J., Brzóska E., Grabowska I., et al. Contribution of stem cells to skeletal muscle regeneration. Folia Histochemica et Cytobiologica. 2006;44(2):75–79. [PubMed] [Google Scholar]
- 92.Epstein S. E., Fuchs S., Zhou Y. F., Baffour R., Kornowski R. Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovascular Research. 2001;49(3):532–542. doi: 10.1016/s0008-63630000217-0. [DOI] [PubMed] [Google Scholar]
- 93.Kinnaird T., Stabile E., Burnett M. S., Epstein S. E. Bone marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circulation Research. 2004;95(4):354–363. doi: 10.1161/01.res.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 94.Bura A., Planat-Benard V., Bourin P., et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 95.Zhi K., Gao Z., Bai J., et al. Application of adipose-derived stem cells in critical limb ischemia. Frontiers in Bioscience. 2014;19:768–776. doi: 10.2741/4243. [DOI] [PubMed] [Google Scholar]
- 96.Prather W. R., Toren A., Meiron M., Ofir R., Tschope C., Horwitz E. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemi. Cytotherapy. 2009;11(4):427–434. doi: 10.1080/14653240902849762. [DOI] [PubMed] [Google Scholar]
- 97.Bilic G., Zeisberger S. M., Mallik A. S., Zimmermann R., Zisch A. H. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplantation. 2008;17(8):955–968. doi: 10.3727/096368908786576507. [DOI] [PubMed] [Google Scholar]
- 98.Pratama G., Vaghjiani V., Tee J. Y., et al. Changes in culture expanded human amniotic epithelial cells: implications for potential therapeutic applications. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0026136.e26136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzuki H., Iso Y. Clinical application of vascular regenerative therapy for peripheral artery disease. BioMed Research International. 2013;2013:6. doi: 10.1155/2013/179730.179730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song G., Li X., Shen Y., et al. Transplantation of iPSc restores cardiac function by promoting angiogenesis and ameliorating Cardiac remodeling in a post-infarcted swine model. Cell Biochemistry and Biophysics. 2014 doi: 10.1007/s12013-014-0369-7. [DOI] [PubMed] [Google Scholar]
- 101.Sasaki S., Inoguchi T., Muta K., et al. Therapeutic angiogenesis by ex vivo expanded erythroid progenitor cells. The American Journal of Physiology—Heart and Circulatory Physiology. 2007;292(1):H657–H665. doi: 10.1152/ajpheart.00343.2006. [DOI] [PubMed] [Google Scholar]
- 102.Sasaki K.-I., Heeschen C., Aicher A., et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(39):14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kinnaird T., Burnett E. S., Shou M., et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.cir.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 104.Kondoh K., Koyama H., Miyata T., Takato T., Hamada H., Shigematsu H. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovascular Research. 2004;61(1):132–142. doi: 10.1016/j.cardiores.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Versari D., Lerman L. O., Lerman A. The importance of reendothelialization after arterial injury. Current Pharmaceutical Design. 2007;13(17):1811–1824. doi: 10.2174/138161207780831239. [DOI] [PubMed] [Google Scholar]
- 106.Boda Z., Udvardy M., Farkas K., et al. Autologous bone marrow-derived stem cell therapy in patients with severe peripheral arterial disorder. Orvosi Hetilap. 2008;149(12):531–540. doi: 10.1556/OH.2008.28125. [DOI] [PubMed] [Google Scholar]
- 107.van Tongeren R. B., Hamming J. F., Fibbe W. E., et al. Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemia. Journal of Cardiovascular Surgery. 2008;49(1):51–58. [PubMed] [Google Scholar]
- 108.Lara-Hernandez R., Lozano-Vilardell P., Blanes P., Torreguitart-Mirada N., Galmés A., Besalduch J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Annals of Vascular Surgery. 2010;24(2):287–294. doi: 10.1016/j.avsg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 109.Dong Z., Chen B., Fu W., et al. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia. Journal of Vascular Surgery. 2013;58(2):404.e3–411.e3. doi: 10.1016/j.jvs.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 110.Subramaniyam V., Waller E. K., Murrow J. R., et al. Bone marrow mobilization with granulocyte macrophage colony-stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. American Heart Journal. 2009;158(1):53.e1–60.e1. doi: 10.1016/j.ahj.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 111.Winter J. N., Lazarus H. M., Rademaker A., et al. Phase I/II study of combined granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor administration for the mobilization of hematopoietic progenitor cells. Journal of Clinical Oncology. 1996;14(1):277–286. doi: 10.1200/JCO.1996.14.1.277. [DOI] [PubMed] [Google Scholar]
- 112.Moazzami K., Majdzadeh R., Nedjat S. Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. The Cochrane Database of Systematic Reviews. 2011;(12) doi: 10.1002/14651858.CD008347.pub2.CD008347 [DOI] [PubMed] [Google Scholar]
- 113.Shimamura M., Nakagami H., Koriyama H., Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. BioMed Research International. 2013;2013:8. doi: 10.1155/2013/186215.186215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mangiafico R. A., Mangiafico M. Medical treatment of critical limb ischemia: current state and future directions. Current Vascular Pharmacology. 2011;9(6):658–676. doi: 10.2174/157016111797484107. [DOI] [PubMed] [Google Scholar]
- 115.Tateno K., Minamino T., Toko H., et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circulation Research. 2006;98(9):1194–1202. doi: 10.1161/01.res.0000219901.13974.15. [DOI] [PubMed] [Google Scholar]
- 116.Fadini G. P., Agostini C., Avogaro A. Autologous stem cell therapy for peripheral arterial disease. Meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209(1):10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 117.Gu Y.-Q., Zhang J., Guo L.-R., et al. Transplantation of autologous bone marrow mononuclear cells for patients with lower limb ischemia. Chinese Medical Journal. 2008;121(11):963–967. [PubMed] [Google Scholar]
- 118.Tateishi-Yuyama E., Matsubara H., Murohara T., et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. The Lancet. 2002;360(9331):427–435. doi: 10.1016/s0140-67360209670-8. [DOI] [PubMed] [Google Scholar]
- 119.Boda Z., Veréb Z., Rajnavolgji É. Autologous bone marrow stem cell or peripheral blood endothelial progenitor cell therapy in patients with peripheral limb ischaemia. Orvosi Hetilap. 2006;147(25):1155–1160. [PubMed] [Google Scholar]
- 120.Hirsch A. T. Critical limb ischemia and stem cell research: anchoring hope with informed adverse event reporting. Circulation. 2006;114(24):2581–2583. doi: 10.1161/circulationaha.106.666719. [DOI] [PubMed] [Google Scholar]
- 121.Moriya J., Minamino T., Tateno K., et al. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circulation: Cardiovascular Interventions. 2009;2(3):245–254. doi: 10.1161/CIRCINTERVENTIONS.108.799361. [DOI] [PubMed] [Google Scholar]
- 122.Losordo D. W., Kibbe M. R., Mendelsohn F., et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circulation: Cardiovascular Interventions. 2012;5(6):821–830. doi: 10.1161/circinterventions.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sugihara S., Yamamoto Y., Matsuura T., et al. Age-related BM-MNC dysfunction hampers neovascularization. Mechanisms of Ageing and Development. 2007;128(9):511–516. doi: 10.1016/j.mad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 124.Powell R. J., Comerota A. J., Berceli S. A., et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. Journal of Vascular Surgery. 2011;54(4):1032–1041. doi: 10.1016/j.jvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 125.Powell R. J. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. Journal of Vascular Surgery. 2012;56(1):264–266. doi: 10.1016/j.jvs.2012.03.255. [DOI] [PubMed] [Google Scholar]
- 126.Jonsson T. B., Larzon T., Arfvidsson B., et al. Adverse events during treatment of critical limb ischemia with autologous peripheral blood mononuclear cell implant. International Angiology. 2012;31(1):77–84. [PubMed] [Google Scholar]
- 127.Vlahu C. A., Lemkes B. A., Struijk D. G., Koopman M. G., Krediet R. T., Vink H. Damage of the endothelial glycocalyx in dialysis patients. Journal of the American Society of Nephrology. 2012;23(11):1900–1908. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shin L., Peterson D. A. Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes. Stem Cells Translational Medicine. 2012;1(2):125–135. doi: 10.5966/sctm.2012-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kishore R., Verma S. K., Mackie A. R., et al. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060161.e60161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Honold J., Lehmann R., Heeschen C., et al. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(10):2238–2243. doi: 10.1161/01.atv.0000240248.55172.dd. [DOI] [PubMed] [Google Scholar]
- 131.Finney M. R., Greco N. J., Haynesworth S. E., et al. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biology of Blood and Marrow Transplantation. 2006;12(5):585–593. doi: 10.1016/j.bbmt.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 132.Liao Y.-F., Feng Y., Chen L.-L., Zeng T.-S., Yu F., Hu L.-J. Coronary heart disease risk equivalence in diabetes and arterial diseases characterized by endothelial function and endothelial progenitor cell. Journal of Diabetes and Its Complications. 2014;28(2):214–218. doi: 10.1016/j.jdiacomp.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 133.Slovut D. P., Sullivan T. M. Critical limb ischemia: medical and surgical management. Vascular Medicine. 2008;13(3):281–291. doi: 10.1177/1358863x08091485. [DOI] [PubMed] [Google Scholar]
- 134.Altaner C., Altanerova V., Cihova M., et al. Characterization of mesenchymal stem cells of “no-options” patients with critical limb ischemia treated by autologous bone marrow mononuclear cells. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0073722.e73722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marfella R., Luongo C., Coppola A., et al. Use of a non-specific immunomodulation therapy as a therapeutic vasculogenesis strategy in no-option critical limb ischemia patients. Atherosclerosis. 2010;208(2):473–479. doi: 10.1016/j.atherosclerosis.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Strauss B. H. Diabetic patients receiving bare-metal stents: no option patients? Journal of the American College of Cardiology. 2013;61(16):1686–1687. doi: 10.1016/j.jacc.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 137.Norgren L., Hiatt W. R., Dormandy J. A., Nehler M. R., Harris K. A., Fowkes F. G. R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Journal of Vascular Surgery. 2007;45(1) supplement:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 138.Benoit E., O'Donnell T. F., Jr., Kitsios G. D., Iafrati M. D. Improved amputation-free survival in unreconstructable critical limb ischemia and its implications for clinical trial design and quality measurement. Journal of Vascular Surgery. 2012;55(3):781–789. doi: 10.1016/j.jvs.2011.10.089. [DOI] [PubMed] [Google Scholar]
- 139.González-Fajardo J. A., Brizuela-Sanz J. A., Aguirre-Gervás B., et al. Prognostic significance of an elevated neutrophil-lymphocyte ratio in the amputation-free survival of patients with chronic critical limb ischemia. Annals of Vascular Surgery. 2014;28(4):999–1004. doi: 10.1016/j.avsg.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 140.Saarinen E., Sugano N., Biancari F., et al. Therapeutic approach to CLI with tissue loss—a comparative prospective cohort study in Finland and Japan. Annals of Vascular Surgery. 2014;28(6):1426–1431. doi: 10.1016/j.avsg.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 141.Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 142.Asahara T., Murohara T., Sullivan A., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 143.Nizankowski R., Petriczek T., Skotnicki A., Szczeklik A. The treatment of advanced chronic lower limb ischaemia with marrow stem cell autotransplantation. Kardiologia Polska. 2005;63(4):351–360. [PubMed] [Google Scholar]
- 144.Napoli C., Farzati B., Sica V., et al. Beneficial effects of autologous bone marrow cell infusion and antioxidants/L-arginine in patients with chronic critical limb ischemia. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(6):709–718. doi: 10.1097/hjr.0b013e3283193a0f. [DOI] [PubMed] [Google Scholar]
- 145.Procházka V., Gumulec J., Chmelová J., et al. Autologous bone marrow stem cell transplantation in patients with end-stage chronical critical limb ischemia and diabetic foot. Vnitrni Lekarstvi. 2009;55(3):173–178. [PubMed] [Google Scholar]
- 146.Matoba S., Tatsumi T., Murohara T., et al. TACT Follow-up Study Investigators. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. The American Heart Journal. 2008;156(5):1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 147.Kawamura A., Horie T., Tsuda I., et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. Journal of Artificial Organs. 2006;9(4):226–233. doi: 10.1007/s10047-006-0351-2. [DOI] [PubMed] [Google Scholar]
- 148.Huang P. P., Yang X. F., Li S. Z., Wen J. C., Zhang Y., Han Z. C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thrombosis and Haemostasis. 2007;98(6):1335–1342. doi: 10.1160/th07-02-0137. [DOI] [PubMed] [Google Scholar]
- 149.Tateishi-Yuyama E., Matsubara H., Murohara T., et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. The Lancet. 2002;360(9331):427–435. doi: 10.1016/s0140-67360209670-8. [DOI] [PubMed] [Google Scholar]
- 150.Motukuru V., Suresh K. R., Vivekanand V., Raj S., Girija K. R. Therapeutic angiogenesis in Buerger's disease (thromboangiitis obliterans) patients with critical limb ischemia by autologous transplantation of bone marrow mononuclear cells. Journal of Vascular Surgery. 2008;48(6):53S–60S. doi: 10.1016/j.jvs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 151.Benetti F., Peñaherrera E., Maldonado T., Vera Y. D., Subramanian V., Geffner L. Direct myocardial implantation of human fetal stem cells in heart failure patients: long-term results. Heart Surgery Forum. 2010;13(1):E31–E35. doi: 10.1532/hsf98.20091130. [DOI] [PubMed] [Google Scholar]
- 152.Burt R. K., Testori A., Oyama Y., et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplantation. 2010;45(1):111–116. doi: 10.1038/bmt.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ouma G. O., Jonas R. A., Usman M. H. U., Mohler E. R. Targets and delivery methods for therapeutic angiogenesis in peripheral artery disease. Vascular Medicine. 2012;17(3):174–192. doi: 10.1177/1358863x12438270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lasala G. P., Silva J. A., Gardner P. A., Minguell J. J. Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology. 2010;61(6):551–556. doi: 10.1177/0003319710364213. [DOI] [PubMed] [Google Scholar]
- 155.Dash N. R., Dash S. N., Routray P., Mohapatra S., Mohapatra P. C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Research. 2009;12(5):359–366. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 156.Yan J., Tie G., Xu T. Y., Cecchini K., Messina L. M. Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Reviews and Reports. 2013;9(3):360–372. doi: 10.1007/s12015-013-9433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]