Abstract
Aims
Altered interoception, how the brain processes afferents from the body, may contribute to the urge to take drugs, and subsequently, the development of addiction. Although chronic stimulant dependent individuals exhibit attenuated brain responses to pleasant interoceptive stimuli, it is unclear whether this deficit exists early-on in the process of transition to stimulant addiction.
Methods
To this end, we compared problem stimulant users (PSU; n=18), desisted stimulant users (DSU; n=15), and stimulant naïve comparison subjects (CTL; n=15) during functional magnetic resonance imaging (fMRI) while they anticipated and experienced pleasant soft touch (slow brushstroke to the palm and forearm).
Results
Groups did not differ in behavioral performance or visual analog scale ratings of soft touch stimuli. fMRI results indicated that PSU exhibited greater right anterior insula, left inferior frontal gyrus, and right superior frontal gyrus activation than DSU and CTL during the anticipation and experience of soft touch. Moreover, during the experience of soft touch, PSU demonstrated higher bilateral precentral gyrus/middle insula and right posterior temporal gyrus activation than DSU and CTL.
Conclusions
In contrast to chronic stimulant dependence, individuals who have recently developed stimulant use disorders show exaggerated neural processing of pleasant interoceptive stimuli. Thus, increased processing of body-relevant information signaling pleasant touch in those individuals who develop problem use may be a predictive interoceptive biomarker. However, future investigations will need to determine whether the combination of probing pleasant interoception using neuroimaging is sufficiently sensitive and specific to help identify individuals at high risk for future problem use.
Keywords: amphetamine, cocaine, reward, interoception, functional magnetic resonance imaging
1. INTRODUCTION
Of the one million people who use cocaine and amphetamine recreationally (SAMHSA, 2012), about 20% progress to stimulant dependence (Lopez-Quintero et al., 2011). Although researchers have identified neural substrates linked to chronic addiction (Goldstein and Volkow, 2011; Koob and Le Moal, 2005), identifying brain mechanisms altered during the transition to stimulant addiction is also important for the development of early intervention strategies.
Interoception, the processing and integration of afferent signals from inside the body in response to both internal and external stimuli, has been implicated in addiction (Craig, 2002; Naqvi and Bechara, 2010; Paulus et al., 2009; Verdejo-Garcia and Bechara, 2009). Chronic users may experience attenuated bodily signals to external natural rewards and aversive/stressful events (Paulus and Stewart, 2014; Verdejo-Garcia et al., 2012), reflecting reduced insular cortex function (Craig, 2002). While middle/posterior insula (MI/PI) receives somatosensory activity from thalamocortical pathways, anterior insula (AI) integrates this information with emotionally salient activity to produce a bodily prediction error, motivating fronto-cingulate mechanisms to eliminate homeostatic imbalances (Craig, 2002, 2009; Paulus et al., 2009). An individual’s degree to approach or avoid a stimulus, including drugs of abuse, may result from this error (Paulus et al., 2009), the difference between an experienced versus expected internal state. When considering drug consumption, individuals with substance use disorders may not appropriately engage insular cortex to signal potential aversive outcomes. Instead, these individuals might derive incentive motivation from anticipation of pleasant states. In comparison, others have proposed that decreased striatal responses to natural rewards (Everitt and Robbins, 2005; Kelley and Berridge, 2002; Volkow et al., 2010) in conjunction with attenuated insular processing may result in drug-seeking to maintain perceived bodily homeostasis.
To explore compromised neural mechanisms of addiction, our lab has employed a Soft Touch paradigm, wherein participants anticipate and receive soft brush strokes to the forearm and palm during functional magnetic resonance imaging (fMRI; May et al., 2013; Migliorini et al., 2013). Gentle slow brushstrokes along forearm/palm skin, rated as subjectively pleasant, are detected by afferent fibers projecting to insular cortex (Bjornsdotter et al., 2010; Gordon et al., 2013; Löken et al., 2009, 2011). Chronic methamphetamine dependent adults exhibit lower AI and dorsal striatum activation than controls while anticipating and experiencing Soft Touch (May et al., 2013), results consistent with the notion that chronic addiction is associated with attenuated neural processing of non-drug related pleasant interoceptive feeling states. In contrast, during the experience of Soft Touch, adolescents with current alcohol/marijuana use disorders display lower PI activation in conjunction with greater AI and ventral striatum activation than controls, indicating reduced somatovisceral processing, but heightened emotional feeling states and reward sensitivity, respectively (Migliorini et al., 2013). These studies suggest that interoceptive processing dysfunctions may not be stable during the clinical course of addiction. Instead, drug-using individuals may transition from a primary positive reinforcement mechanism (seeking pleasant experiences) to a negative reinforcement mechanism (avoidance of aversive experiences; Koob and Le Moal, 2005) together with compulsive use patterns (Everitt and Robbins, 2005).
Although a substantial literature has described the nature of striatal changes as a function of addiction development (Chambers et al., 2003; Everitt and Robbins, 2013; Volkow et al., 2012), less is known about how/why insular regions change as a function of substance use. Moreover, research is warranted to clarify whether altered insular/striatal activations to pleasant interoceptive stimuli are a function of the type of drug used, indicators of a general predisposition to experiment with drugs, or markers of problem use. The present study examined these issues by recruiting an initial cohort of young adult recreational stimulant users who, three years later, either progressed to problem stimulant use (PSU) or desisted stimulant use (DSU). Groups were matched with stimulant-naïve healthy comparison subjects (CTL). Participants completed the Soft Touch paradigm during fMRI. We hypothesized that PSU would exhibit greater AI and striatum activation than DSU and CTL during the experience of Soft Touch, consistent with prior work in adolescents with recent-onset alcohol/marijuana substance use disorders (Migliorini et al., 2013), as opposed to insular/striatal attenuation evident in chronic stimulant users (May et al., 2013).
2. METHODS
2.1 Participants
The study protocol was approved by the University of California, San Diego Human Research Protections Program and was carried out in accordance with the Declaration of Helsinki. All subjects provided written informed consent. Recreational, non-dependent stimulant users were recruited and defined by methods described in previous studies (Reske et al., 2011; Stewart et al., 2013). Among this original cohort (n=184), users were contacted three years after their initial lab visit (93% follow-up rate: 171 followed up; 10 unreachable; 3 refused to participate). Each user underwent a standardized three-year follow-up interview to examine extent of interim drug use, allowing us to identify participants who developed problems associated with stimulant use and others who had desisted using. Thus, two stimulant user groups were formed for the present study, termed problem stimulant users (PSU) and desisted stimulant users (DSU). Of the 171 participants who completed the three-year follow-up protocol, 38 met criteria for the PSU group, whereas 83 met criteria for the DSU group. Table S11 demonstrates that PSU who consented to participate in the present study (n=18) possessed greater years of education at the initial visit than PSU who did not participate (n=20). Table S2 indicates that DSU who consented to participate (n=15) reported a greater number of cocaine uses at the initial visit than DSU who were not enrolled in this study (n=68). Otherwise, stimulant users who were enrolled in the present study did not differ in demographic or initial visit drug use variables from those who were not enrolled.
PSU were a priori defined by (1) continued stimulant (dextroamphetamine, cocaine, methylphenidate) use since the initial visit and (2) endorsement of 2+ DSM-IV (American Psychiatric Association, 2000) criteria of stimulant abuse or dependence as determined by the Semi Structured Assessment for the Genetics of Alcoholism II (SSAGA II; Bucholz et al., 1994) occurring together > 6 contiguous months since the initial visit (M=4.83 criteria; SD=1.98; range: 2–8). Within PSU, 56% met criteria for cocaine abuse, 50% met criteria for amphetamine abuse, 28% met criteria of cocaine dependence, and 33% met criteria for amphetamine dependence. DSU were defined as having (1) no 6− month periods of 6+ stimulant uses and (2) no stimulant abuse/dependence or other substance dependence in the interim three-year period. Stimulant-naïve CTL were recruited from the general population and endorsed no lifetime substance dependence (see Figure S1 for schematic overview). No participants were current regular nicotine smokers.
The final cohort consisted of 18 PSU (9 female), 15 DSU (6 female), and 15 CTL (7 female), all right handed (Edinburgh Handedness Inventory; Oldfield, 1971). At the time of the three-year follow-up interview, participants were in their mid-twenties (M=24.47 years, SD=1.64) with three years of college education (M=15.72 years, SD=1.17). Groups were matched on age, education, gender, and ethnicity (see Table S3). Groups did not differ on percentage of participants meeting criteria for alcohol abuse (PSU=61%, DSU=47%, CTL=27%; χ2(2)=3.92, p=.14), alcohol dependence (PSU=17%, DSU=7%, CTL=0%; χ2(2)=3.06, p=.21), or marijuana dependence (PSU=17%, DSU=0%, CTL=0%; χ2(2)=5.33, p=.07). However, a higher percentage of PSU and DSU met criteria for marijuana abuse than CTL (PSU=56%, DSU=67%, CTL=7%; χ2(2)=12.60, p=.002), although PSU and DSU did not differ from each other. Participants completed two more sessions: (1) clinical interview; and (2) fMRI Soft Touch paradigm.
2.2 Clinical Interview Session
Interviewers administered the SSAGA II and diagnoses were based on consensus meetings (clinicians and trained study personnel). Exclusion criteria for all groups were: (1) metal/other factors precluding fMRI; (2) head injuries or loss of consciousness > 5 min; (3) medication for any psychiatric disorder (past 3 years); (4) diagnosed neurological disorder; (5) lifetime psychosis or antisocial personality disorder; (6) current and/or past six month episodes of anxiety disorders or unipolar depression; and (7) positive urine toxicology screen for substances other than marijuana (given that marijuana is present in urine up to six weeks after use).
The Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) was administered to obtain verbal intelligence quotient (IQ). Personality constructs known to correlate with substance use disorders were administered, including the Sensation Seeking Scale (SSS; Zuckerman, 2007), Barratt Impulsiveness Scale (Barratt and Patton, 1983), State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983), and Beck Depression Inventory II (BDI-II; Beck et al., 1996). Baseline visit/interim stimulant and marijuana uses were calculated for PSU and DSU based on the number of sessions each substance was used. Baseline visit uses consisted of cumulative drug sessions up until that visit, whereas interim uses consisted of only drug sessions completed during the time of the baseline visit until the time of the three-year follow-up interview.
2.3 fMRI Session
2.3.1 Urine testing
Subjects were asked to abstain from drugs for 72 hours. Twelve subjects tested positive for marijuana (n=7 PSU; n=5 DSU; PSU and DSU did not differ in number of subjects testing positive: χ2(1) =.11, p=.74). No participants tested positive for any other substance.
2.3.2 Soft Touch stimulus
Trained research assistants used a hand held soft boar bristle brush (OXO International Ltd., NY) on pre-measured and marked 4 cm long regions of skin on the ventral surface of the left forearm and palm (Löken et al., 2009; Olausson et al., 2000; Vallbo et al., 1993). Each brush stroke was performed at a velocity of 2 cm/s in a proximal to distal direction with a force equal to the weight of the brush.
2.3.3 Soft Touch paradigm
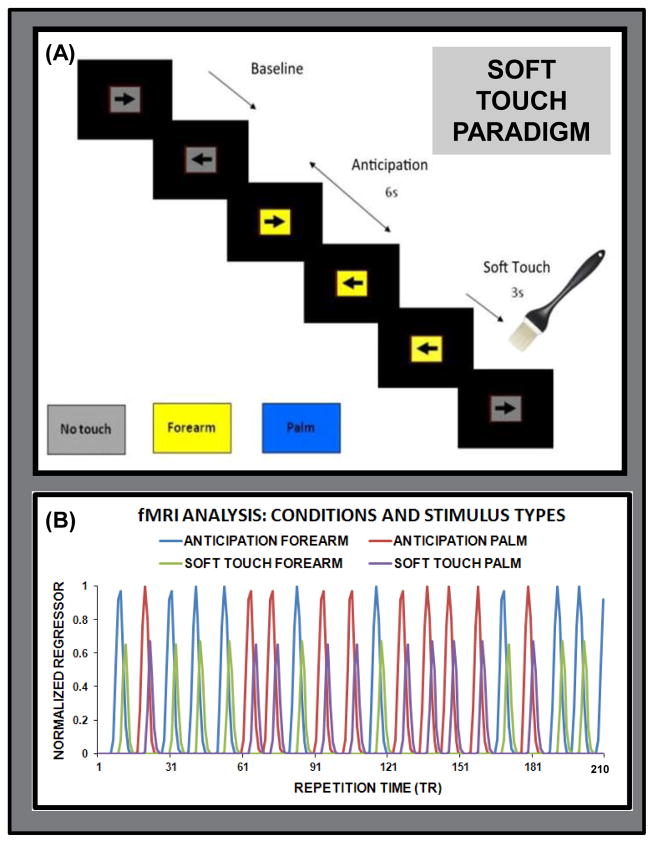
Participants engaged in a continuous performance task (CPT) with cued stimulus presentation designed to focus attention on visual stimuli while maintaining a stable cognitive load. This task was chosen to keep participants engaged while not being too complex that it would distract from external stimuli. A screen presented a left or right black arrow surrounded by a colored rectangle in successive 3 s intervals (see Figure 1A). Subjects responded to the arrow orientation by pressing a left or right button. The arrow remained on the screen for the entire 3 s during which a button could be pressed at any time. Colored rectangle backgrounds signified one of three conditions: (1) baseline (gray) during which no tactile stimulus was expected or administered (variable duration averaging 9 s: three consecutive arrow trials); (2) anticipation (lasting 6 s: two consecutive arrow trials) during which the background color of the presentation indicated an impending soft touch on the left palm (blue) or left forearm (yellow); (3) soft touch (lasting 3 s: 1 arrow trial) during which a soft touch was administered to either the palm or the forearm. Anticipation and soft touch conditions were each presented 20 times (120 s for anticipation, 60 s for soft touch) for each stimulus type (palm, forearm). Response accuracy and reaction time (RT) were obtained. Participants received instruction on task structure and background color meanings prior to the task and completed post-fMRI visual analog scale (VAS) questionnaires. VAS instructions required that participants provide a rating from ‘0 – not at all’ to ‘10 – extremely’ about their Soft Touch experience for pleasant, unpleasant, intensity, tickle, warm, cold, and soft dimensions.
Figure 1.
(A) Illustration of the Soft Touch paradigm, wherein participants anticipated or experienced a soft touch brushstroke on their forearm (signaled by yellow background) or palm (signaled by a blue background); (B) Illustration of normalized condition (anticipation, soft touch) and stimulus type (forearm, palm) regressors included in the fMRI deconvolution as a function of repetition time (TR) during one run of the task.
2.3.4 fMRI image acquisition
Two event-related runs sensitive to blood oxygenation level-dependent contrast were collected using a Signa EXCITE (GE Healthcare, USA) 3.0 Tesla scanner (T2*-weighted echo planar imaging scans, TR=2000 ms, TE=30 ms, FOV=24 cm2, 64 x 64 x 40 matrix, 40 3.0mm axial slices, in-plane resolution of 3.75 x 3.75 x 3mm, flip angle=90 degrees, 210 TRs for each run; each trial = 1.5 TR). A high-resolution T1-weighted anatomical image (spoiled gradient recalled, TR=8 ms, TE=3 ms, slices=172, FOV=25 cm, ~1 mm3 voxels) was also obtained.
2.4 fMRI Data Analysis
2.4.1 Single subjects
Functional data were realigned, slice-time corrected, normalized, and artifact/outlier corrected prior to subject-level deconvolution analyses and spatial smoothing, resulting in percent signal change (PSC) from baseline for each condition per subject to be used as dependent variables in group analysis. Full details are provided in Supplementary Material2.
2.4.2 Group analysis
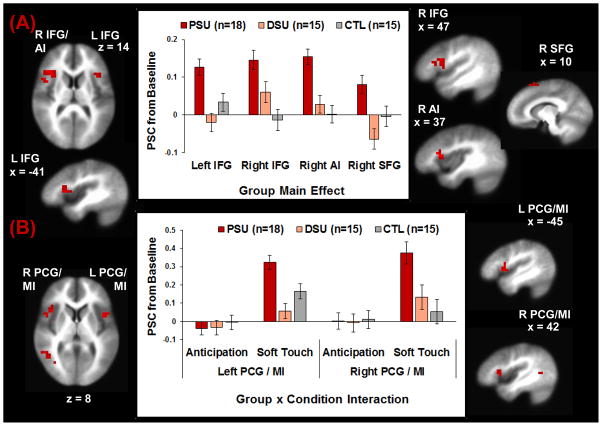
For each voxel within the brain, a linear mixed effects (LME) analysis (Pinheiro et al., 2013) was performed. Subjects were random effects while group (PSU, DSU, and CTL), condition (anticipation, soft touch), and stimulus type (palm, forearm) were fixed effects. PSC was the dependent variable. The main effect of group was examined to determine whether PSU, DSU, and CTL differed across conditions and stimulus types. The group by condition interaction was the primary effect of interest in order to test hypotheses involving anticipation versus experience of pleasant touch in PSU, DSU, and CTL. A threshold adjustment method (AFNI AlphaSim) based on Monte-Carlo simulations was employed to guard against identifying false positive areas of activation; given a per voxel p < 0.0001 threshold, whole-brain volume threshold was 512 μL (8 contiguous voxels) for a clusterwise p < .01 (two-tailed) threshold corrected for multiple comparisons. Voxelwise threshold was based on the following LME degrees of freedom and F values: (1) group main effect: F(2,45)=4.27; (2) group by condition interaction: F(2,135)=4.03; and (3) group by condition by stimulus type interaction: F(2,135)=4.03. Significant LME clusters were followed up by univariate analysis of variance (ANOVA) tests, using pairwise comparisons p<.05: Bonferroni corrections were employed when the homogeneity of variance assumption was met as per Levine’s test, and Games-Howell corrections were used when group variances were heterogeneous). Brain regions emerging as significantly different between PSU and the other two groups are presented in Table 2 along with Cohen’s d, reflecting between-group effect sizes.
Table 2.
Functional Magnetic Resonance Imaging Results
| Vol (μL)/#Voxels | x | y | z | Hem | Regions Within Cluster | BA | Effect Size PSU vs. DSU | Effect Size PSU vs. CTL |
|---|---|---|---|---|---|---|---|---|
| Group Main Effect: PSU ↑ DSU and/or CTL across trials | ||||||||
| 1536/24 | 37 | 19 | 14 | R | Anterior Insula1 | 13 | 1.39 | 1.57 |
| 832/13 | −41 | 16 | 12 | L | IFG1 | 44 | 1.47 | 0.97 |
| 768/12 | 47 | 10 | 16 | R | IFG1 | 44 | ns | 1.35 |
| 640/10 | 46 | −44 | 30 | R | Supramarginal Gyrus1 | 40 | ns | 1.49 |
| 640/10 | 10 | 16 | 61 | R | SFG2 | 6 | 1.19 | 0.97 |
| Group by Condition Interaction: PSU ↑ DSU and/or CTL during Soft Touch condition | ||||||||
| 1344/21 | −45 | 8 | 8 | L | PCG/Middle Insula1 | 13 | 1.48 | 0.96‡ |
| 1152/18 | 51 | −51 | 13 | R | STG1 | 22 | 1.09 | 0.85 |
| 960/15 | 42 | 15 | 8 | R | PCG/Middle Insula1 | 13 | 0.88† | 1.16 |
Note:
Bonferroni corrected post-hoc comparisons p < .05.
Games-Howell corrected post-hoc comparisons p < .05.
Did not achieve significance (.05 < p < .10) when n=3 PSU and n=1 DSU with alcohol dependence were removed from analyses.
Did not achieve significance (.05 < p < .10) when n=3 PSU with marijuana dependence were removed from analyses.
ns = non-significant. PSU = Problem Stimulant Users. DSU = Desisted Stimulant Users. CTL = Healthy Comparison Subjects. Vol = Volume. Hem = Hemisphere. L = Left Hemisphere. R = Right Hemisphere. BA = Brodmann Area. Effect Size = Cohen’s d. Coordinates reflect center of mass. SFG = superior frontal gyrus. IFG = inferior frontal gyrus. PCG = precentral gyrus. STG = superior temporal gyrus. No significant differences emerged between PSU and the other two groups for the anticipation condition.
2.5 Non-fMRI Analysis
Non-imaging statistical analyses were performed in SPSS (PASW Version 18 Statistics for Windows, Chicago, IL). Univariate ANOVAs (using pairwise comparisons p<.05: Bonferroni corrections used when the homogeneity of variance assumption was met, and Games-Howell corrections utilized for heterogeneous group variances) were performed for questionnaires, with group as the between-subjects variable. Due to issues with non-normality, BDI-II distributions were compared between groups using the Kruskal-Wallis test. Similarly, lifetime drug use variables were compared pairwise between PSU and DSU with non-parametric Mann-Whitney U tests due to non-normal distributions. To determine whether total drug use in a particular period (cumulative drug use reported at the baseline visit, as opposed to additional interim drug use reported at the three-year follow up interview) was greater for stimulants as opposed to marijuana, a percentage of drug use due to stimulants score was created consisting of the following formula: (amphetamine uses + cocaine uses)/(amphetamine uses + cocaine uses + marijuana uses). Repeated measures ANOVAs were performed in SPSS to investigate differences in RT and accuracy, wherein group was the between-subject variable and condition and stimulus type were within-subjects variables.
3. RESULTS
3.1 Non-fMRI Data
Table 1 illustrates that PSU and DSU had slightly lower verbal IQ and, as expected, higher interim stimulant and marijuana uses than CTL. PSU also endorsed higher depression than DSU and CTL as well as greater trait anxiety than DSU. Although PSU and DSU did not differ in rates of baseline cocaine or baseline/interim marijuana uses, PSU reported greater baseline visit/interim amphetamine and interim cocaine uses than DSU. Moreover, PSU endorsed higher % drug uses due to stimulants as opposed to marijuana in the interim than DSU, but PSU and DSU did not differ in % drug uses due to stimulants at baseline. By definition, only PSU subjects met criteria for current stimulant use disorder. Table S3 shows that groups did not differ in impulsivity, sensation seeking, or state anxiety.
Table 1.
Group Characteristics.
| PSU (n=18) | DSU (n=15) | CTL (n=15) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| IQ and Anxiety | M | SD | M | SD | M | SD | df | F | p | Description |
| WTAR Verbal IQ | 108.06 | 5.95 | 110.33 | 5.70 | 116.92 | 7.82 | 2, 41 | 6.91 | 0.003 | CTL > PSU/DSU1 |
| STAI Trait | 35.67 | 8.69 | 29.20 | 4.68 | 33.27 | 6.86 | 2, 45 | 3.45 | 0.04 | PSU > DSU2 |
|
| ||||||||||
| Depression | M | SD | M | SD | M | SD | Kruskal-Wallis p | Description | ||
|
| ||||||||||
| BDI-II | 5.28 | 6.27 | 0.47 | 0.52 | 1.73 | 1.87 | .002 | PSU > DSU/CTL | ||
|
| ||||||||||
| Initial Visit Drug Use | M | SD | M | SD | M | SD | M-W-U p PSU vs DSU | Description | ||
|
| ||||||||||
| Amphetamine | 36.11 | 49.49 | 6.53 | 6.69 | N/A | N/A | .01 | PSU > DSU | ||
| Cocaine | 37.22 | 85.64 | 32.40 | 53.18 | N/A | N/A | .61 | ns | ||
| Marijuana | 837.61 | 1349.05 | 1085.27 | 1626.89 | N/A | N/A | .63 | ns | ||
| % Use Due to Stimulants | 32.69 | 36.28 | 15.66 | 18.72 | N/A | N/A | .16 | ns | ||
|
| ||||||||||
| Interim Drug Use | M | SD | M | SD | M | SD | M-W-U p PSU vs DSU | Description | ||
|
| ||||||||||
| Amphetamine | 170.00 | 290.16 | 1.87 | 3.20 | 0 | 0 | .001 | PSU > DSU/CTL | ||
| Cocaine | 546.22 | 1204.76 | 5.80 | 16.29 | 0 | 0 | .01 | PSU > DSU/CTL | ||
| Marijuana | 621.89 | 1424.27 | 466.53 | 575.36 | 25.53 | 43.87 | .23 | PSU/DSU > CTL | ||
| % Use Due to Stimulants | 72.20 | 33.15 | 6.98 | 14.08 | 0 | 0 | .001 | PSU > DSU/CTL | ||
Note:
Bonferroni post-hoc corrected p<.05.
Games-Howell post-hoc corrected p<.05.
PSU = problem stimulant users. DSU = desisted stimulant users. CTL = healthy comparison subjects. WTAR = Wechsler Test of Adult Reading. IQ = intelligence quotient. BDI-II = Beck Depression Inventory II. STAI = State Trait Anxiety Inventory. Due to BDI-II non-normal distributions the Kruskal-Wallis test was used to determine whether group distributions differed from one another. Drug use was quantified as the number of distinct sessions each substance was used for a particular period; given non-normal distributions for these measures, Mann-Whitney U (M-W-U) tests were employed to test differences in drug use between groups. ns = non-significant. Data were not available for WTAR (n=1 PSU; n=3 CTL).
Groups did not differ in VAS ratings (all ps>.23). Across participants, soft touch stimuli were rated as moderately pleasant (M=5.58, SE=0.41) and soft (M=5.58, SE=0.39) as well as and mildly ticklish (M=2.85, SE=0.36), but relatively low in unpleasantness (M=1.47, SE=0.33), warmth (M=1.97, SE=0.30), coldness (M=1.77, SE=0.31), and intensity (M=1.71, SE=0.28).
Behavioral data were not available for four participants (n=1 PSU; n=2 DSU; n=1 CTL). A condition main effect emerged (F(1,41)=8.57, p=.01), wherein soft touch was associated with longer RT (M=668.25, SE=25.92) than anticipation (M=631.70, SE=20.05). No other RT effects emerged (all p>.25). No effects emerged for accuracy (all p>.20), which averaged 99.3% across groups, conditions, and stimulus types.
3.2 fMRI Data
Table 2 demonstrates differences between PSU and the other two groups and Figure 2A illustrates the group main effect, wherein PSU exhibited greater activation in left inferior frontal gyrus (IFG), right AI, and right superior frontal gyrus (SFG) than DSU and CTL. Although PSU also showed greater right IFG and right supramarginal gyrus activation than CTL, PSU did not differ from DSU in these regions after post-hoc corrections for multiple comparisons. Figure 2B shows that for the group by condition interaction, during soft touch PSU exhibited greater bilateral precentral gyrus (PCG)/MI and right superior temporal gyrus (STG) activation than DSU and CTL. No clusters emerged for the group by condition by stimulus type interaction. Follow-up univariate ANOVAs with post-hoc corrections for multiple comparisons indicated that group effects remained significant when participants with alcohol dependence (n=3 PSU; n=1 DSU) and marijuana dependence (n=3 PSU) were removed from analyses except for bilateral PCG/MI, which fell short of significance (.05<p<.10; see Table 2).
Figure 2.
Neuroimaging results for: (A) the group main effect, wherein problem stimulant users (PSU) exhibited greater left inferior frontal gyrus (IFG), right anterior insula (AI), and right superior frontal gyrus (SFG) activation than desisted stimulant users (DSU) and healthy comparison subjects (CTL) across anticipation and soft touch trials. PSU also displayed greater activation than CTL in right IFG but did not differ from DSU according to Bonferroni corrections; and (B) the group x condition interaction, wherein PSU displayed higher bilateral precentral gyrus (PCG)/middle insula (MI) activation than DSU and CTL during the soft touch condition but not the anticipation condition. Error bars reflect + 1 standard error of the mean.
3.3 Follow-Up Analyses: PSU brain-behavior correlations
Since PSU endorsed higher depression scores and greater number of baseline/interim amphetamine and interim cocaine uses than the other two groups, it is inappropriate to use these variables as covariates in group analysis of covariance using brain activations as the dependent measure (Miller and Chapman, 2001). Therefore, these measures were correlated with brain activations (using SPSS Pearson correlations) to further explain right AI, averaged bilateral IFG, and averaged bilateral PCG/MI results. Left and right ROI values were averaged together prior to statistical comparisons. Stimulant uses and BDI-II scores were natural log-transformed due to non-normality. No effects were significant when corrected for multiple comparisons (4 measures x 3 brain regions: p = .05/12 = .004 threshold).
4. DISCUSSION
This study examined processing differences between PSU, DSU, and CTL in response to pleasant interoceptive stimuli. We predicted that PSU would demonstrate greater AI and striatum activation during soft touch than DSU and CTL, indicative of heightened interoceptive awareness (Craig, 2009) and reward responsivity (Delgado et al., 2000), consistent with prior work in adolescents with recent-onset alcohol/marijuana substance use disorders (Migliorini et al., 2013). This hypothesis was partially supported. PSU exhibited greater right AI activation across anticipation and soft touch trials than DSU and CTL, suggesting that heightened interoceptive awareness is present in recent-onset stimulant use disorders not only during somatosensory stimulation, as hypothesized, but also when preparing for stimulation to occur. Therefore, heightened AI activation to pleasant stimuli may be a neural marker for recent transition to substance use disorders independent of the substance used (stimulants vs. alcohol/marijuana: Migliorini et al., 2013). Contrary to prediction, PSU did not exhibit greater striatum activation than DSU or CTL in response to soft touch and small sample sizes reduced statistical power to find small/medium effects. It may be the case that young adults transitioning to stimulant use disorder do not show excessive responsivity to natural rewards as much as drug-relevant rewards. Additional research is warranted to test this prediction.
Across anticipation and soft touch trials, PSU also exhibited greater bilateral IFG than CTL and DSU (although differences in right IFG between PSU and DSU did not survive Bonferroni correction). In addition, PSU exhibited greater PCG/MI activation than DSU and CTL specifically during the experience of Soft Touch. A recent meta-analysis indicates that MI is most often activated during sensorimotor tasks, particularly those involving touch to the hand (Kurth et al., 2010). IFG/PCG activations are linked to goal-motivated hand actions (Johnson-Frey et al., 2003), soft hand stimulation (Ebisch et al., 2011), and inhibitory motor control (Swann et al., 2009). Furthermore, IFG/PCG activations are reduced when attention to movement is paired with a secondary attention task within the context of a dual task paradigm, presumably because both tasks are competing for processing resources (Johansen-Berg and Matthews, 2002). The present study might also be considered a dual task paradigm, since participants experience soft touch to their left palm/forearm while they are pressing buttons with their right hand, a process that may involve divided attention to motor and visual signals. Therefore, heightened IFG/PCG in PSU may reflect greater recruitment of resources needed to override attention directed to pleasant interoceptive stimuli in order to perform motor responses. Lastly, in response to soft touch, PSU exhibited greater right STG activation than DSU and CTL. Right STG is linked to processing of slow as opposed to fast affective touch to the forearm, thought to reflect enhanced somatosensory integration (Gordon et al., 2013; Voos et al., 2013). In this case, PSU may be more sensitive to pleasant tactile signals than young adults not actively using stimulants.
Whereas in the present study PSU show higher AI/IFG activation than DSU and CTL within the context of pleasant touch, prior work indicates that PSU exhibit lower AI/IFG activation than DSU and CTL during an unpleasant inspiratory breathing load manipulation (Stewart et al., 2015). These studies contribute to an emerging narrative about individuals at high risk for addiction. Afferent bodily information has profound effects on incentive motivation, what a person approaches or avoids. An approach-avoidance imbalance may impact whether one engages in risky behaviors such as drug taking. PSU expend more neural processing resources as evidenced by the degree of fMRI activation to positively versus negatively valenced interoceptive afferents. Problem use might emerge because the brain fails to appropriately process negative consequences of drug use and –instead – has a more salient trace of the positive bodily effects of drug consumption. Neural differences are not paralleled by subjective ratings, suggesting that these processes occur outside of awareness. Paralleling the proposed transition from impulsive to compulsive use (Everitt and Robbins, 2005) or from positive to negative reinforcement driven behavior (Koob, 2013), there is a change from processing positively valenced interoceptive afferents to negatively valenced afferents. Chronic stimulant use may deplete cognitive control mechanisms that drive goal-oriented behavior (Baler and Volkow, 2006; Salo et al., 2009) while blunting the sensory experience of non-drug related stimuli, resulting in neural attenuation in brain regions involved in interoceptive processing (May et al., 2013; Paulus and Stewart, 2014).
Amplified AI, IFG, and PCG/MI activations were not correlated with self-report measures that were higher in PSU than the other two groups (depression, baseline/interim amphetamine use, and interim cocaine use), suggesting that neural findings differentiating PSU cannot simply be attributed to comorbid psychopathology or one type of substance examined in isolation. Neural findings between groups emerged in the absence of heightened impulsivity and sensation seeking in PSU, suggesting that perceived pursuit of rewarding and/or risky sensations may not mirror neural measures of heightened processing of bodily sensations.
There are several limitations to this study. First, sample sizes were small, precluding detection of small to medium effects, and post-hoc pairwise comparisons were employed to test between-group differences while avoiding type I error inflation. Second, groups did not differ in behavioral performance or subjective VAS ratings, consistent with prior reports (May et al., 2013; Migliorini et al., 2013). In future iterations of the Soft Touch paradigm, we hope to: (1) incorporate VAS ratings into the task itself to obtain ephemeral changes in visceral responses to pleasant interoceptive stimuli; and (2) merge the Soft Touch manipulation with a more cognitively demanding task to determine if somatosensory processing disrupts behavioral responses to attentional challenge as a function of addiction. Third, PSU and DSU reported multiple baseline/interim sessions of marijuana use and over half met criteria for current marijuana abuse (some of whom tested positive for marijuana), indicating that participants in this sample were not pure stimulant users. However, given that PSU and DSU did not differ in baseline/interim marijuana uses, brain activation differences between PSU and DSU cannot be attributed to differences in marijuana consumption. Fourth, although no PSU subjects tested positive for stimulants prior to the fMRI session, indicating no recent stimulant use, it could instead be the case that subtle withdrawal effects due contributed to group differences. Fifth, given that fMRI was only recorded at one time point after PSU had already begun the transition to addiction, this study cannot reliably track the change in neural processing of interoceptive stimuli from occasional use to problem use. Future studies could employ a longitudinal design, wherein fMRI is measured once during occasional active use and again 1–3 years later after onset of problem use.
This study adds to a growing literature showing a complex alteration of interoceptive function to positively valenced stimuli. Individuals early-on in their stimulant addiction show exaggerated neural processing of these stimuli, which may explain positive reinforcement mechanisms hypothesized to be important for addiction development. However, attenuated neural processing of interoceptive stimuli during later stages of drug addiction may reflect an adaptation of the interoceptive system, possibly as a consequence of years of drug exposure with profound stimulation of the interoceptive system, and a change toward a negative reinforcement mechanism of addiction.
Supplementary Material
Highlights.
Problem and desisted stimulant users (PSU and DSU) compared to controls on pleasant interoception
PSU show heightened anterior insula/prefrontal activation to soft touch anticipation/experience
PSU users show heightened precentral gyrus and middle insula activation to soft touch
Early stage of stimulant addiction linked to brain hyperactivity
Acknowledgments
Role of Funding Source
Financial support for the conduct of research in the present study was provided by the National Institute on Drug Abuse [DA016663-01A1 to M. P. Paulus]. NIDA had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The present study was supported by the National Institute on Drug Abuse [DA016663-01A1 to M. P. Paulus].
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
J. L. Stewart assisted in collecting and processing the data, primarily analyzed and interpreted the data, and wrote the original draft of the manuscript. A. C. May assisted in collecting and processing the data, assisted in data interpretation, and edited drafts of the manuscript. S. F. Tapert assisted in study design and data interpretation, and edited drafts of the manuscript. M. P. Paulus designed the study, assisted in data interpretation, and edited drafts of the manuscript. All authors approved the final version of the paper for submission. The article is the authors’ original work, has not received prior publication and is not under consideration for publication elsewhere.
Conflict of Interest Disclosure Statement
J. L. Stewart: Conflict of interest: none.
A. C. May: Conflict of interest: none.
S. F. Tapert: Conflict of interest: none.
M. P. Paulus: Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer L. Stewart, Email: jennifer.stewart@qc.cuny.edu.
April C. May, Email: acmay@ucsd.edu.
Susan F. Tapert, Email: stapert@ucsd.edu.
Martin P. Paulus, Email: mpaulus@ucsd.edu.
References
- American Psychiatric Association. Diagnostic Criteria From DSM-IV-TR. American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Patton JH. Impulsivity: cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M, editor. Biological Bases of Sensation Seeking, Impulsivity, and Anxiety. Erlbaum; Hillsdale, NJ: 1983. pp. 77–116. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bjornsdotter M, Morrison I, Olausson H. Feeling good: on the role of C fiber mediated touch in interoception. Exp Brain Res. 2010;207:149–155. doi: 10.1007/s00221-010-2408-y. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Ferri F, Salone A, Perrucci MG, D’Amico L, Ferro FM, Romani GL, Gallese V. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cog Neurosci. 2011;23:1808–1822. doi: 10.1162/jocn.2010.21551. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD. Brain mechanisms for processing affective touch. Hum Brain Mapp. 2013;34:914–922. doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensori-motor areas, including primary motor cortex. Exp Brain Res. 2002;142:13–24. doi: 10.1007/s00221-001-0905-8. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39:1053–1058. doi: 10.1016/S0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press; London: 2005. [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Löken LS, Minde J, Wessberg J, Perini I, Nennesmo I, Olausson H. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134:1116–1126. doi: 10.1093/brain/awr011. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, Blanco C. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–669. doi: 10.1111/j.1360-0443.2010.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Depend. 2013;131:238–246. doi: 10.1016/j.drugalcdep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini R, Stewart JL, May AC, Tapert SF, Paulus MP. What do you feel? Adolescent drug and alcohol users show altered brain response to pleasant interoceptive stimuli. Drug Alcohol Depend. 2013;133:661–668. doi: 10.1016/j.drugalcdep.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Kakuda N. Tactile directional sensibility: peripheral neural mechanisms in man. Brain Res. 2000;866:178–187. doi: 10.1016/S0006-8993(00)02278-2. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacol. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, Saikat D, Deepayan S R Development Core Team. nlme: Linear and nonlinear mixed effects models. R package Version 3.1–111 2013 [Google Scholar]
- Reske M, Delis DC, Paulus MP. Evidence for subtle verbal fluency deficits in occasional stimulant users: quick to play loose with verbal rules. J Psychiatr Res. 2011;45:361–368. doi: 10.1016/j.jpsychires.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2011 national survey on drug use and health: summary of national findings. 2012 http://www.samhsa.gov/data/NSDUH/2011SummNatFindDetTables/Index.aspx.
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR. State-Trait Anxiety Inventory (STAI) BiB. 1983;2010:180. [Google Scholar]
- Stewart JL, Flagan TM, May AC, Reske M, Simmons AN, Paulus MP. Young adults at risk for stimulant dependence show reward dysfunction during reinforcement-based decision making. Biol Psychiatry. 2013;73:235–241. doi: 10.1016/j.biopsych.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Juavinett AL, May AC, Davenport PW, Paulus MP. Do you feel alright? Attenuated neural processing of aversive interoceptive stimuli in current stimulant users. Psychophysiology. 2015;52:249–262. doi: 10.1111/psyp.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-S. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacol. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos AC, Pelphrey KA, Kaiser MD. Autistic traits are associated with diminished neural response to affective touch. Soc Cogn Affect Neurosci. 2013;8:378–386. doi: 10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; Antonio, TX: 2001. [Google Scholar]
- Zuckerman M. The sensation seeking scale V (SSS-V): Still reliable and valid. Pers Individ Dif. 2007;43:1303–1305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.