Abstract
Tuberculosis disease (TB) may progress at different rates and have different outcomes. Neutrophils have been implicated in TB progression; however, data on their role during TB are controversial. Here we show that in mice, TB progression is associated with the accumulation of cells that express neutrophilic markers Gr-1 and Ly-6G, but do not belong to conventional neutrophils. The cells exhibit unsegmented nuclei, have Gr-1dimLy-6GdimCD11b+ phenotype and express F4/80, CD49d, Ly-6C, CD117, CD135 markers characteristic not of neutrophils, but of immature myeloid cells. The cells accumulate in the lungs, bone marrow, spleen and blood at the advanced (pre-lethal) stage of M. tuberculosis infection and represent a heterogeneous population of myeloid cells at different stages of their differentiation. The accumulation of Gr-1dimCD11b+ cells is accompanied by the disappearance of conventional neutrophils (Gr-1hiLy-6Ghi-expressing cells). The Gr-1dimCD11b+ cells suppress T cell proliferation and IFN-γ production in vitro via NO-dependent mechanisms, i.e. they exhibit characteristics of myeloid-derived suppressor cells (MDSCs). These results document the generation of MDSCs during TB, suggesting their role in TB pathogenesis, and arguing that neutrophils do not contribute to TB pathology at the advanced disease stage.
Introduction
Immune reactions play both protective and pathological roles during TB. Immunological mechanisms mediating TB protection have been studied in details. In contrast, mechanisms driving TB progression remain poorly understood.
One of the mechanisms that have been implicated in TB pathology and progression is neutrophilic inflammation. Indeed, neutrophils are known to induce tissue damage. In humans, high numbers of neutrophils were found in broncho-alveolar lavage fluids of patients with severe TB disease and lung tissue cavitation (1–3). In mice, cells expressing neutrophilic markers Gr-1 and/or Ly-6G accumulated abundantly in the lungs of susceptible animals; in several studies, depletion of Gr-1-expressing cells ameliorated TB outcome (3–6). On the other hand, a large number of observations support the role for neutrophils in TB protection: these cells can phagocytize mycobacteria, produce numerous bactericidal molecules, arm macrophages to kill mycobacteria and promote initiation of T cell immune responses (7–10). An association between low neutrophilic numbers, low plasma levels of neutrophil-derived bactericidal peptides and high risk of TB development has been demonstrated (11, 12), further supporting the impact of these cells in TB protection.
To address the role for neutrophils during TB, we have recently evaluated their response in (A/Sn × I/St)F2 hybrids originating from TB-resistant A/Sn and TB-susceptible I/St mice. Following Mtb challenge, F2 mice exhibited different rates of TB progression (13). F2 mice that succumbed rapidly to Mtb infection had extremely high numbers of Gr-1- and Ly-6G-expressing cells in their lungs. Gr-1/Ly-6G-expressing cells are usually considered as neutrophils. However, neutrophils express Gr-1/Ly-6G markers at high levels (14). The cells accumulating in the lungs of TB-susceptible F2 mice in our study expressed Gr-1dim/Ly-6Gdim phenotype. The nature of these cells and their relationship to neutrophils were unclear.
While almost nothing was known about Gr-1dim/Ly-6Gdim cells during TB, similar cells had been described and characterized in detail during other pathological conditions, in particular, during tumor progression (15, 16). The cells expressed a common myeloid marker CD11b (phenotype Gr-1dimCD11b+), inhibited T cell responses and were therefore referred to as myeloid-derived suppressor cells (MDSCs). MDSCs represent a heterogeneous population of myeloid cells found at different stages of their differentiation. Two main subsets of MDSCs, monocytic and granulocytic, have been described according to their phenotype (Gr-1intCD11b+Ly-6G−/lowF4/80+CD115+CD49d+ or Gr-1hiCD11b+Ly-6GhiF4-80−CD115−CD49d−, respectively) and nuclear morphology (15–17). MDSCs are rare in steady state conditions, but they accumulate abundantly during different pathologies (especially those inducing hyper-inflammatory response) and contribute to their progression (18–23).
We hypothesized that Gr-1dim cells, identified in our previous studies, represented MDSCs (24). In this study, we present evidence supporting this hypothesis. We demonstrate that Gr-1dimCD11b+ cells: (i) accumulate at advanced stage of TB infection in mice of different strains; (ii) exhibit characteristics of immature myeloid cells; (iii) suppress T cell responses; (iv) are rather associated with TB progression and lethality than mature neutrophils. To our knowledge, this is the first description of MDSCs and their role during TB.
Materials and Methods
Mice
Inbred female I/StYCit (I/St), A/JSnYCit (A/Sn) and C57BL/6YCit (B6) mice 2–3 mo of age were used (19–21 g). All mice were bred in the Animal Care Facility at the Central Tuberculosis Research Institute (Moscow, Russia) in accordance with Russian Ministry of Health Guideline no. 755 and the US National Institutes of Health Office of Laboratory Animal Welfare Assurance #A5502-01. Water and food were provided ad libitum. All experimental procedures were approved by the CTRI IACUC.
Bacteria and infection
In most experiments mice were infected intratracheally with 2,3×103 CFUs/mouse of mid-log-phase Mtb strain H37Rv Pasteur as described earlier (25). To monitor disease progression, mice were weighed before the challenge, and then every 3–7 days. If not indicated otherwise, experiments were performed on days 17 and 24 post-infection. Un-infected mice were included in all experiments (“day 0”). In some experiments, mice were infected with 102 CFUs using an Inhalation Exposure System (Glas-Col, Terre Haute, IN) and analyzed on days 22, 80, 160 and 210. Mycobacterial loads were assessed by preparing homogenates of the lungs (upper right lobe) or suspensions of spleen and bone marrow cells, and plating 0.1 ml of their serial 10-fold dilutions onto Dubos Agar; colonies were enumerated after 18–20 days.
Flow cytometry
Suspensions of lung cells were prepared using an enzyme digestion method (26). Lungs were perfused with 0.02% EDTA-PBS to wash blood vessels, incubated in RPMI 1640 containing collagenase/DNase I, and cell suspensions were washed. Spleen cell suspensions were prepared by mild homogenization. To obtain BM cells, tibia and femurs were flushed with 199 medium, containing 1% FCS and 10 mM HEPES. Cells were treated with anti-CD16/CD32 mAbs (eBioscience, San Diego, CA) and stained with different combinations of the following Abs: FITC-anti-F4/80, PE-anti-Gr-1 (clone RB6-8C5), APC-anti-CD11b (all obtained from eBioscience); FITC-anti-Ly-6G (clone 1A8), PerCP-anti-CD4 (BD Pharmigen, San Diego, CA); PerCP-anti-Ly-6C (clone HK1.4), biotin-anti-CD49d followed by PerCP-SA, APC-anti-CD8 (all obtained from Biolegend, San Diego, CA). In preliminary experiments, cells were also stained with LIVE/DEAD Fixable Far Red Dead Cell Stain Kit (Life Technologies, Carlsbad, CA). The experiments confirmed that Gr-1dimCD11b+ cells accumulating in the lungs and bone marrow at the advanced stage of infection were alive. Cells were analyzed using a BD Biosciences FACSort or Canto II with CellQuest Pro, BD FACSDiva 7 (BD Biosciences) and FlowJo (Tree Star) software. In most experiments, at least 40,000 events were analyzed.
Magnetic cell sorting
Suspensions of BM cells were enriched for Gr-1dimCD11b+ and Gr-1hiCD11b+ populations using two-step magnetic cell sorting. Briefly, BM cells were stained with PE-anti-Gr-1 mAbs (eBioscience, San Diego, CA), incubated with anti-PE MultiSortMicroBeads (MiltenyiBiotec, San Diego, CA), washed and separated by sequential passage through LS MACS column (Miltenyi Biotec). The positive fraction was enriched for Gr-1hi cells (purity, 60–75%, all CD11b+, Fig. 2F). The negative fraction was composed of Gr-1dimCD11b+ and Gr-1negCD11b− cells. For the isolation of Gr-1dimCD11b+ cells, the negative fraction was incubated with APC-anti-CD11b mAbs, magnetically labeled with anti-APC MultiSort MicroBeads (Miltenyi Biotec), and sorted on LS MACS column to collect the positive fraction. The later contained mostly Gr-1dimCD11b+ cells (purity, 90–95%, Fig. 3A).
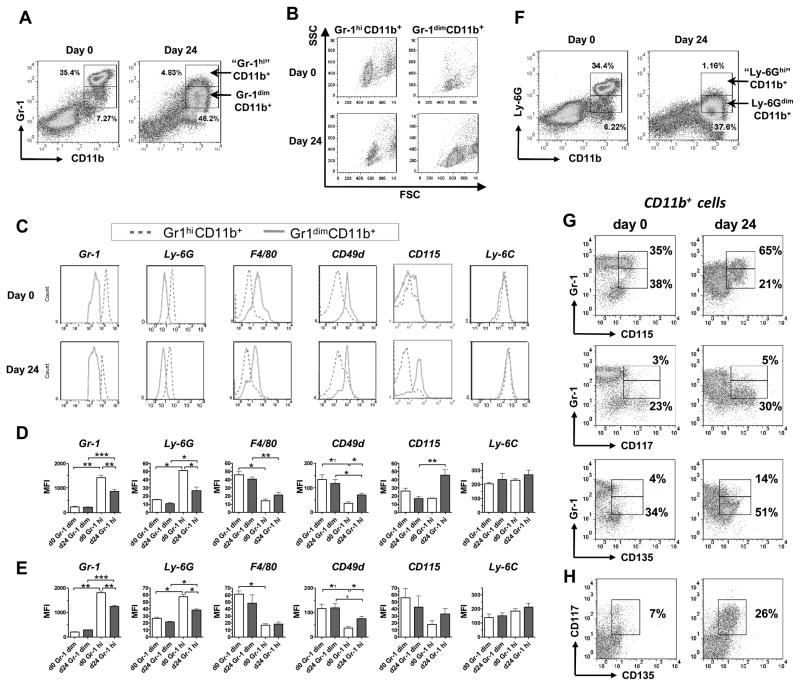
FIGURE 2.
Gr-1dimCD11b+ cells have phenotype of immature myeloid cells. A–C, Flow cytometry analysis of Gr-1dimCD11b+ and Gr-1hiCD11b+ BM cells. Cells were isolated from un-infected (day 0) or infected (day 24) mice. A, Gating strategy. B, Forward and side scatter. C, Expression of indicated markers by Gr-1dimCD11b+ and Gr-1hiCD11b+ cells (representative histograms). D, E, Expression of indicated markers (MFI) by Gr-1dimCD11b+ and Gr-1hiCD11b+ cells isolated from the BM (D) or lungs (E) (summarized data; 3 independent experiments, n=4–12/group/marker). F, Staining of BM cells for CD11b and Ly-6G. G, Expression of CD115, CD117 and CD135 by Gr-1dim and Gr-1hi BM cells (gated on CD11b+ cells). Figures on the dotplots show percent of cells expressing the indicated marker within Gr-1hi (upper line) and Gr-1dim (low line) cells. H, Co-expression of CD117 and CD135 (BM cells, gated on CD11b+ cells).
FIGURE 3. Gr-1dimCD11b+ and Gr-1hiCD11b+ cells differ by nuclear morphology.
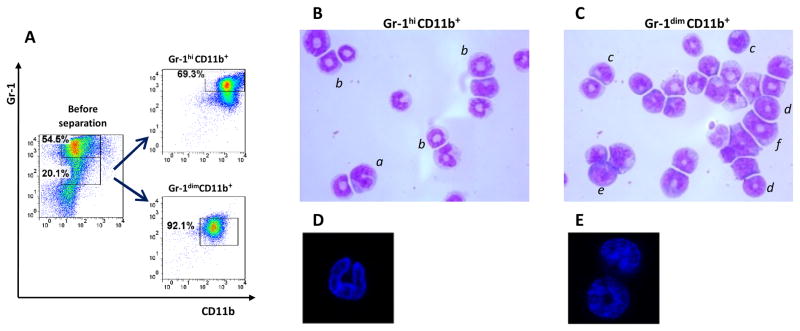
A, Enrichment of BMd24 cells for Gr-1dimCD11b+ and Gr-1hiCD11b+ populations. B, C, Nuclear morphology of Gr-1hiCD11b+ cells (B) and Gr-1dimCD11b+ cells (C) analyzed by light microscopy (cytospin preparations, Giemsa staining). a, PMN cell with segmented nucleus; b, PMN-like ring cell with ring-shaped nucleus and cytoplasmic center that is larger than the width of the ring; c, MNC-like cell with bean-shaped nucleus; d, MNC-like ring cell with a ring-shaped nucleus and a cytoplasmic center that is smaller than the width of the ring; e, MNC with closed nucleus; f, Precursor type of MNC-like ring cell showing a broad nuclear ring of round shape and regular contour and a small ring center. Original magnification ×1600. Cells are classified according to Biermann and coauthors (31). D, E Nuclear morphology of Gr-1hiCD11b+ cells (D) and Gr-1dimCD11b+ (E) cells analyzed by confocal microscopy (DAPI staining).
Suppression assays
SC were isolated from the spleens of un-infected mice, labeled with CFDA-SE (1–10 uM, 10 min, room temperature), washed with PBS containing 10% FCS and cultured in 24- or 96-well plates (9 × 105 cells/ml) covered with immobilized anti-CD3 mAbs (1 ug/ml). Suppressor cells were added to the cultures at 1:1 ratio. Suppressors were BM cells either unseparated or enriched for Gr-1dimCD11b+ or Gr-1hiCD11b+ populations. Four days later cells were harvested, stained with PE-anti-CD4 and PerCP-anti-CD8 mAbs, and proliferation of CD4+ and CD8+ cells was assessed by flow cytometry using CFSE dilution assay. IFN-γ production was measured in culture supernatants using Mouse IFN gamma ELISA Ready-SET-Go! (eBioscience). In some experiments, SC were stimulated with immobilized anti-CD3 mAb and percentages of IFN-γ producing cells were determined using intracellular cytokine staining (27).
To test the ability of BM cells to suppress Mtb-specific responses, I/St mice were immunized with a complete Freund’s adjuvant (100 ug given s.c. into the foodpads). Ten days later, popliteal LN cells were isolated and cultured in vitro in the presence of Mtb sonicate (10 ug/ml) and BM cells, tested for their suppressor activity. Proliferation of LN cells was assessed using [3H]-thymidine incorporation and CFSE dilution assays.
In inhibition experiments, SC and BM cells were co-cultured in the presence of one of the following inhibitors: 500 uM N-hydroxy-L-arginine (NOHA, Merck, Germany), 100 uM NG-monomethyl-L-arginine (L-NMMA, Calbiochem, San Diego, CA), 1000 U/ml catalase (Sigma Aldrich, St Louis, MO). In transwell experiments, SC were stimulated with anti-CD3 at the bottom of transwells, and BM cells were placed in 3 um transwell chambers (Costar-Corning, The Netherlands).
Adoptive cell transfer
To test the ability of Gr-1dimCD11b+ cells to inhibit T cell responses in vivo, adoptive transfer experiments were performed. Donor mice were challenged with Mtb i.t. On day 24 post-challenge, BM cells were isolated and enriched for Gr-1dimCD11b+ population. The cells were transferred into recipient mice (2 × 106/mouse) that had been challenged with Mtb either i.t. (17 days prior the transfer) or aerogenically (74 days prior to the transfer). Three days after the first transfer (i.e., on days 20 or 77, respectively), recipient mice received the second transfer of Gr-1dimCD11b+ cells (the later were freshly isolated from another group of donor mice, challenged 24 days prior to the experiment). Plating of Gr-1dimCD11b+ cells showed that the inoculum contained less than 100 CFUs (i.e., less than 0,1% of Mtb present in the lungs of recipient mice). Injection of 100 CFUs into recipient mice did not add to their Mtb burden, i.e. the contamination of Gr-1dimCD11b+ cells with Mtb did not affect recipient Mtb burden significantly. Three days after the second transfer, recipient mice were sacrificed and the levels of IFN-γ production in the lungs were analyzed using ELISA. The time-points for cell transfers were chosen based on the following: (i) antimycobacterial T cell responses are known to set up after the second week of infection (28); (ii) in i.t. challenge model, recipient mice died by the end of week 4 post-challenge; (iii) in aerosol challenge model, on days 74–80 mice were at the stable stage of infection.
Statistical analysis
Data are shown as mean ± SEM. Differences between the means of experimental groups were analyzed using the non-parametric Mann-Whitney test. Differences were considered significant where p<0.05. The correlation between body weight loss and MFI of Gr-1 was assessed using nonparametric Spearman correlation analysis (GraphPad Software Inc., San Diego, CA).
Results
Gr-1dimCD11b+ cells accumulated in the lungs, spleen, bone marrow and blood of mice at the advanced stage of TB infection
We first sought to characterize the kinetics of Gr-1dim response following Mtb challenge. We therefore challenged TB-susceptible I/St mice with Mtb given i.t. and followed TB progression and the frequencies and the numbers of Gr-1dimCD11b+ cells in different organs. TB progression was evaluated by determining post-infection body weight loss, a vital indicator of TB severity. All mice gained weight during first two weeks post-challenge (“stable stage” of infection), started to undergo wasting after day 17 (“initiation of disease progression”) and lost 15 ± 2% of their initial weight by day 24 (pre-lethal stage of disease; Fig. 1A). CFU numbers in the lungs increased 50 fold since day 17 till day 24 (Fig. 1B). If not sacrificed, mice died by day 26–28 post-challenge.
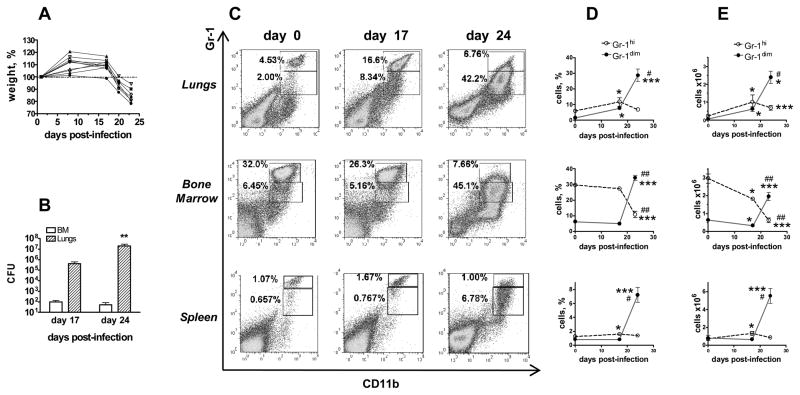
FIGURE 1.
Gr-1dimCD11b+ cells accumulate in different organs of Mtb infected mice at pre-lethal stage of infection. A, Kinetics of post-infection body weight loss. 100% - weight on day 1. The representative results of one (n=10) out of four (n=29) experiments. B, Lung and BM Mtb loads on days 17 and 24 post-infection (2 experiments, n=6 for day 17, n=4 for day 24). C–E, Accumulation of Gr-1dimCD11b+ cells at the advanced stage of Mtb infection. C, Typical flow cytometry data. D, E, Percentages (D) and numbers (E) of Gr-1dimCD11b+ and Gr-1hiCD11b+ cells in indicated organs (2–4 independent experiments, n=6–13 per time-point per organ for days 0 and 24; n=3 for day 17 post-challenge). *,# p< 0.05, **,## p< 0.01, ***,### p< 0.001 compared to day 0 (*) or day 17 (#).
In un-infected mice, Gr-1dimCD11b+ cells were largely absent from the lungs, spleen and blood (<4%) and were also rare in the bone marrow (<8%, Fig. 1C–E and data not shown). The frequencies and the numbers of Gr-1dimCD11b+ cells slightly increased in the lungs by day 17 (2–3-fold, p<0.05) and dramatically increased in all organs by day 24 post-challenge (7–40 fold in the lungs and spleen, 3–10 fold in the BM and blood, p<0.01; Fig. 1D, E and data not shown). As Gr-1dimCD11b+ cells accumulated, the frequencies and the numbers of Gr-1hiCD11b+ cells decreased. It should be noted that cells which in infected mice fall into the “Gr-1hi” gate had a lower expression of Gr-1 compared to Gr-1hi cells from uninfected mice; moreover, in infected mice, “Gr-1hi” cells represented rather an extent of Gr-1dim population than a separate cell population (Fig. 1C, day 24). Thus, the accumulation of Gr-1dimCD11b+ cells and the disappearance of true Gr-1hiCD11b+ cells were associated with TB severity and progression.
Gr-1dimCD11b+cells had phenotype of immature myeloid cells
Gr-1-specific antibodies (clone RB6-8C5) interact with two different molecules, Ly-6G and Ly-6C, that are expressed on neutrophils (Ly-6G and Ly-6C) and monocytes (Ly-6C) (29). To more precisely characterize Gr-1dimCD11b+ cells, we examined the expression of markers that are known to be expressed differentially by neutrophils and monocytes. Special attention was paid to compare Gr-1dimCD11b+ population with typical neutrophils (Gr-1hiCD11b+ cells).
In un-infected mice, Gr-1hiCD11b+ cells had high side scatter, expressed high levels of neutrophil-specific marker Ly-6G and did not express macrophage-specific marker F4/80, i.e. had characteristics of conventional neutrophils (Fig. 2A–E). In contrast, Gr-1dimCD11b+ cells had significantly lower side scatter (Fig. 2B), indicating cell lower granularity. Phenotypically, Gr-1dimCD11b+ cells expressed F4/80, CD49d (α4 integrin, which expression has been associated with monocytic population of MDSCs, (17) and intermediate levels of Ly-6G (Fig. 2C–E). All Gr-1hiCD11b+ and all Gr-1dimCD11b+ cells were positive for Ly-6C. CD115 (M-CSF receptor) was found on some but not all Gr-1hiCD11b+ and Gr-1dimCD11b+ cells (20–65%) (Fig. 2C–E). Overall, Gr-1hiCD11b+ cells expressed markers of neutrophils, while Gr-1dimCD11b+ cells co-expressed neutrophilic and monocytic markers. These differences were seen in all analyzed organs (Fig. 2 and data not shown).
Following the infection, true Gr-1hiCD11b+ cells disappeared: all Gr-1 expressing cells appeared as one large scattered population that represented a continuum of cells with different levels of Gr-1 expression (Fig. 1C and 2A). Previously, several authors have demonstrated that the levels of Gr-1 and Ly-6G expression are indicative of a degree of granulocytic cell differentiation and maturation (14, 30). We, therefore, compared the phenotype of CD11b+ that in infected mice expressed Gr-1 at different levels, i.e., Gr-1dim and “Gr-1hi” cells (Fig. 2A). Compared to “Gr-1hi” cells, Gr-1dim cells had a lower expression of Ly-6G and a higher expression of F4-80 and CD49d, i.e., Gr-1dimCD11b+ cells appeared as less mature and more monocytic. When “Gr-1hi” cells found in infected mice were compared to true Gr-1hi cells present in the BM of un-infected mice, “Gr-1hi” cells had a lower expression of Gr-1 and Ly-6G, and a higher expression of CD49d and CD115 (Fig. 2D, F). Gr-1 expressing cells derived from the lung tissue had similar expression profiles (Fig. 2E).
These data indicated that at the advanced stage of the disease, Gr-1 expressing cells were less differentiated. We, therefore, analyzed the expression of CD117 (c-kit) and CD135 (Flt-3) on Gr-1+CD11b+ cells. In un-infected mice, CD117 and CD135 expressing cells were largely absent. In infected mice, a subset of cells that co-expressed CD117 and CD135 appeared within the Gr-1dimCD11b+ population (Fig. 2G, H). These results confirmed immature state of Gr-1dimCD11b+ cells and emphasized their heterogeneity.
Gr-1dimCD11b+cells had mononuclear morphology
To further characterize Gr-1dimCD11b+ population, we isolated BM cells from Mtb-infected mice, enriched them with Gr-1hiCD11b+ and Gr-1dimCD11b+ populations and analyzed their nuclear morphology using light and confocal microscopy (Fig. 3). The majority of Gr-1hiCD11b+ cells had either segmented nuclei (>20% of cells) or constricted ring-shaped nuclei with a wide cytoplasmic center (larger than the width of the ring, >50% of cells; Fig. 3B, D), i.e. exhibited characteristics of PMN-like cells (31). In contrast, Gr-1dimCD11b+ population was heterogenic and largely composed of MNC-type cells and myelo/monocytic precursors: the cells had bean-shaped nuclei, ring-shaped nuclei with a small cytoplasmic center (the cytoplasmic center of the ring smaller than the width of the ring, a feature characteristic for mononuclear cells (31), or closed nuclei (Fig. 3C, E). The percentage of PMN-like cells in Gr-1dimCD11b+ population was small (<10%).
Thus, in both phenotypic and morphological analyses, Gr-1dimCD11b+ cells appeared as a heterogeneous population of immature myeloid cells, largely of MNC type.
Gr-1dimCD11b+ cells exhibited suppressor activity and inhibited T cell proliferation and IFN-γ production
We then tested whether Gr-1dimCD11b+cells were similar to MDSCs and could suppress T cell responses. As a source of Gr-1dimCD11b+ cells, we used BM cells. The usage of lung cells was difficult, because their magnetic sorting was of low efficiency, and fluorescence-activated sorting of Mtb-infected lung cells was unavailable.
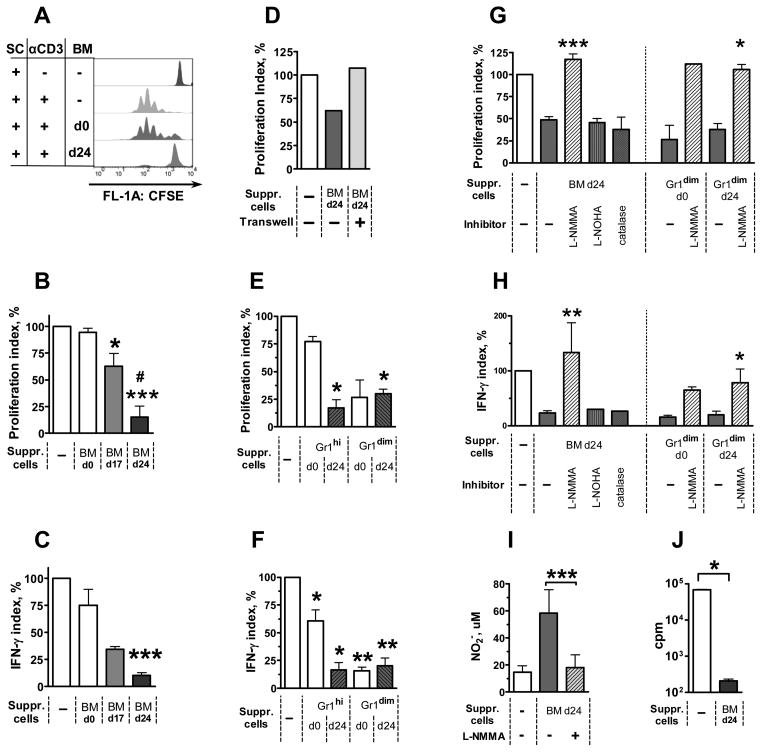
In the first experimental setting, total populations of BM cells were tested for their suppressive activity. BM cells were isolated from un-infected (BMd0) or Mtb-infected mice on day 17 (“BMd17”) or 24 (“BMd24”) post-infection. The cells were added to naive SC stimulated polyclonally with anti-CD3 mAbs, and proliferation of CD4+ and CD8+ lymphocytes was assessed using CFSE dilution assay (Fig. 4A). BMd0 did not inhibit proliferation of CD4+ T cells; BMd17 and BMd24 inhibited proliferation by 38±12% and 85±10%, respectively (Fig. 4A, B). Similar results were obtained when proliferation of CD8+ cells was assessed (data not shown). Besides proliferation, BMd24 also blocked IFN-γ production, as evidenced by a decrease in the concentrations of IFN-γ in culture supernatants (Fig 4C) and in the frequencies of IFN-γ producing cells (data not shown). Separation of SC and BM cells by a Transwell membrane abrogated suppression (Fig. 4D), indicating that direct contacts between suppressor and responding cells or their close co-localization were necessary for successful suppression.
FIGURE 4. Gr-1dimCD11b+ cells suppress T cell proliferation and IFN-γ production.
A–I Naive SC were stimulated with anti-CD3 mAbs in the presence of unseparated BM cells (AD, I) or their subsets (E–H) isolated from un-infected (day 0) or infected (days 17 and 24) mice. A, Example of flow cytometry data showing proliferation of CD4+ SC assessed using CFSE dilution assay. B, Suppression of CD4+ T cell proliferation by BMd17 and BMd24 cells (4 experiments, n=3–13 per group; for CD8+ cells, similar results were obtained (not shown). C, BMd24 cells suppress IFN-γ production (same cultures). D, Culturing in Transwells abrogates BM-mediated suppression. E, F, Suppression of CD4+ cell proliferation (E) and IFN-γ production (F) by Gr-1dimCD11b+ and Gr-1hiCD11b+ cells. G, H, L-NMMA, but not L-NOHA or catalase, abrogates suppression. I, Nitrite concentrations in the cultures of SC and BM cells. J, BM cells suppress proliferation of LN cells induced by Mtb-specific antigens. LN cells were isolated from mice immunized with Complete Freund’s Adjuvant and cultured in the presence of Mtb sonicate and BMd24. Proliferation was assessed as [3H]-thymidine incorporation.
Proliferation index = (MFIexp− MFIneg) / (MFIpos− MFIneg) × 100%, where MFI is MFI of CFSE in: un-stimulated SC (MFIneg); anti-CD3-stimulated SC (MFIpos); anti-CD3-stimulated SC cultured in the presence of BM cells (MFIexp).
IFN-γ index = (IFNexp− IFNneg) / (IFNpos− IFNneg) × 100%, where IFN is concentration of IFN-γ in the same cultures. *, p<0.05; ** p<0.01; ***, p<0.001.
In a separate experiment we tested whether BM cells were able to suppress T-cell response to Mtb-specific antigens. Mice were immunized with CFA; lymph node cells were isolated and stimulated in vitro with Mtb sonicate in the presence or absence of BMd24 cells. In the presence of BMd24 cells, a complete inhibition of cell proliferation was registered ([3H]-thymidine incorporation and CFSE dilution assays, Fig. 4J and data not shown).
We next examined whether suppressor activity of BM cells was mediated by Gr-1dimCD11b+ population (as we supposed). For that, we enriched BM cells with Gr-1dimCD11b+ cells using magnetic cell sorting and tested their ability to suppress SC proliferation and IFN-γ production. Gr-1dimCD11b+ cells isolated either from un-infected or Mtb-infected mice (days 0 and 24, respectively) inhibited T-cell proliferation and IFN-γ production by 70–80% Fig. 4E, F). Simultaneously with Gr-1dimCD11b+ cells, we also isolated Gr-1hiCD11b+ cells. These cells exhibited suppressor activity mainly when were isolated from Mtb-infected mice (day 24 post-challenge, Fig. 4E, F).
To summarize, Gr-1dimCD11b+cells exhibited suppressor activity when were isolated from the BM of either un-infected or Mtb-infected mice. In contrast, total population of BM cells and Gr-1hiCD11b+ cells acquired suppressor activity following infection. Of note, in infected mice (day 24), BM contained 6-fold more Gr-1dimCD11b+ cells and 3-fold less Gr-1hiCD11b+ cells than in control mice. Further, as discussed above, “Gr-1hi” cells found in the BM of infected mice differed from typical Gr-1hi cells found in the BM of control mice, and were less differentiated and more monocytic. Thus, our observations suggested that suppressor activity was the intrinsic property of immature (Gr-1dim) myeloid cells and that these cells, rather than neutrophils, were responsible for BM-mediated suppression of T cell responses.
Gr-1dimCD11b+ cells utilized NO to suppress T cell proliferation and function
It has been reported that suppression of T cell responses by MDSCs may be mediated by different mechanisms, including iNOS-dependent production of NO, arginase I-dependent depletion of arginine from the environment of T cells, and generation of ROI (32). We investigated whether inhibition of these pathways altered immunosuppression mediated by Gr-1dimCD11b+ cells.
The addition of either L-NOHA, an inhibitor of arginase, or catalase, ROI eliminator, did not alter the suppression. In contrast, L-NMMA, an inhibitor of iNOS, almost completely abrogated the suppression and restored T-cell proliferation and IFN-γ production in all suppressed cultures, suggesting that the suppression was NO-mediated (Fig. 4G, H and data not shown).
A role for NO in the suppression of T cell responses was further confirmed by high concentrations of nitrite in all cultures where suppression was detected (i.e., in the cultures of SC and Gr-1dimCD11b+ cells, BMd24, or Gr-1hiCD11b+ cells isolated from BMd24) and by low nitrite concentrations in all the cultures where suppression was absent, either initially (cultures containing SC and BMd0 or Gr-1hiCD11b+ cells isolated from BMd0) or due to its inhibition (cultures containing SC, “suppressive” BM cells and L-NMMA, Fig. 4I and data not shown).
Altogether, in our model, the suppression was mediated mainly by NO, the mechanism of suppression characteristic of monocytic population of MDSCs (33, 34).
Accumulation of Gr-1dimCD11b+ cells is a characteristic feature of severe TB disease in mice of different strains
The studies described above were performed in I/St mice, highly susceptible to Mtb infection (26, 35). We next examined whether in A/Sn and B6 mice genetically more resistant to Mtb infection (26, 35, 36), TB progression was also accompanied by the generation and the accumulation of Gr-1dimCD11b+ cells.
Similarly to I/St mice, in un-infected A/Sn mice Gr-1dimCD11b+ cells were rare. On day 24 post-challenge, the frequencies and the numbers of Gr-1dimCD11b+ cells increased in I/St mice 5–20 fold, while in A/Sn mice only 2–6-fold (Fig. 5A). Of note, I/St mice died by days 26–28 post-challenge, while A/Sn mice survived until days 33–35. On day 31, A/Sn mice exhibited significant wasting and a pronounced increase in Gr-1dimCD11b+ cells in the lungs (33-fold) and BM (8-fold). B6 mice started exhibiting significant wasting by day 26. At this time-point, the frequencies and the numbers of Gr-1dimCD11b+ cells in their lungs, BM and spleen also increased (Fig. 5B–D).
FIGURE 5.
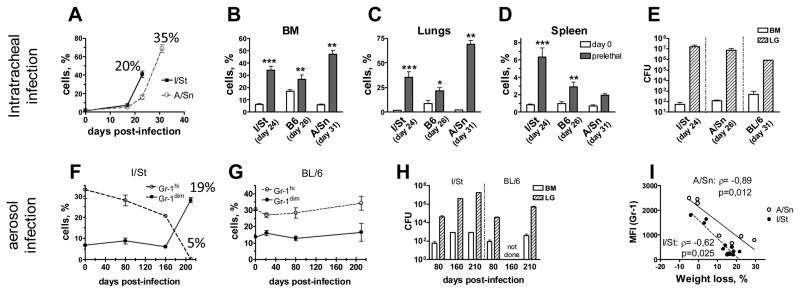
Gr-1dimCD11b+ cells accumulate in TB-susceptible and TB-resistant mice at pre-lethal stage of acute and chronic infection. A–E, acute infection; mice were challenged i.t. with 2,3×103 CFUs. F–H, Chronic infection; mice were challenged aerogenically with 102 CFUs. A, Kinetics of Gr-1dimCD11b+ cells in the lungs of I/St and A/Sn mice. Percentages indicate body weight loss at the corresponding time-points. B–D, Frequencies of Gr-1dimCD11b+ cells in different organs of I/St, A/Sn and B6 mice at indicated time-points (2 experiments; n=6–10 per group). E, H, Mtb loads in the lungs and BM of mice challenged i.t. (E) or aerogenically (H). F, G, Frequencies of Gr-1dimCD11b+ and Gr-1hiCD11b+ cells in the BM of I/St (F) and B6 (G) mice following the chronic infection. I, Correlation between body weight loss and the levels of Gr-1 expression in I/St and A/Sn mice (n=20).
In the experiments described above, the rate of TB progression in susceptible I/St and resistant B6 mice differed only slightly. This was likely due to a relatively high dose of bacteria used for the infection (2,3×103 CFU/mouse given i.t.). We next addressed whether a low-dose challenge would also lead to the accumulation of Gr-1dimCD11b+ cells. For that, I/St and B6 mice were challenged with 102 CFUs of Mtb given aerogenically.
During the stable stage of infection (days 22, 80, 160) Gr-1dimCD11b+ cells were rare, the percentages of Gr-1hiCD11b+ remained stable in all mice (Fig. 5F, G); mice did not exhibit wasting, although Mtb loads progressively increased (Fig. 5H). By day 210, B6 mice did not waste significantly, while I/St mice exhibited significant (>15%) wasting, suggesting they reached the pre-lethal stage of disease. All mice were sacrificed for the analysis. In B6 mice, the frequencies of Gr-1dimCD11b+ and Gr-1hiCD11b+ cells did not change significantly (Fig. 5G). In I/St mice, the frequencies of Gr-1dimCD11b+ cells increased (5-fold); Gr-1hiCD11b+ cells disappeared (Fig. 5F). Mtb burden increased in both B6 and I/St mice (compared to day 80), although to a different degree (B6 mice: 18-fold in the lungs and 3-fold in the BM; I/St mice: 200-fold in the lungs and 12-fold in the BM, Fig. 5H). Phenotypic analysis of Gr-1dimCD11b+ cells accumulating during chronic infection showed that they expressed phenotype similar to Gr-1dimCD11b+ cells found in acutely infected mice. Functional analysis revealed their ability to suppress T-cell proliferation in vitro, i.e. the cells could be ascribed to MDSC (data not shown).
Thus, MDSCs accumulated following both acute and chronic infection when mice reached the advanced stage of disease.
As discussed above, the levels of Ly-6G and Gr-1 expression are indicative of a degree of myeloid cell differentiation and maturation (14, 30). We therefore assessed whether in our model there was an association between TB progression and the levels of Gr-1 expression by the whole population of CD11b+ cells. A significant inverse correlation between body weight loss and the levels of Gr-1 expression was found (r>0.6; p<0.02; Fig. 5I), supporting an association between TB progression and myeloid cell immaturity.
To summarize, hematopoietic shifts resulting in the generation and the accumulation of immature myeloid cells with suppressor activity were a common trait of progressing TB infection in mice.
Adoptive transfer of Gr-1dimCD11b+cells
We next addressed whether Gr-1dimCD11b+ cells could mediate immune suppression in vivo. For that, we transferred Gr-1dimCD11b+ cells adoptively. The cells were isolated from the BM of Mtb-infected donor mice challenged i.t. 24 days before the transfer. In the first experimental setting, we addressed how the transfer of Gr-1dimCD11b+ cells affected host immune response and TB progression during the acute infection. Recipient mice were challenged with Mtb given i.t.; on days 17 and 20 post-challenge (i.e., before endogenous Gr-1dimCD11b+ cells started accumulating and mice progressed to fatal disease), Gr-1dimCD11b+ donor cells were transferred into the recipient mice. Compared to control mice, recipients of Gr-1dimCD11b+ cells had significantly lower content of IFN-γ in the lung tissue (p=0.02, Fig 6A). There was a tendency towards an accelerated wasting of recipient mice (p=0.06, Fig. 6C), but Mtb burden in the lungs, spleen and bone marrow did not change (p>0,6; Fig. 6B and data not shown).
FIGURE 6.
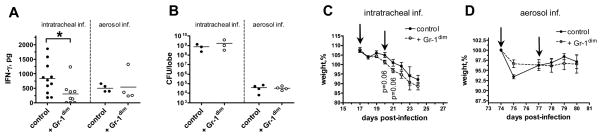
Effect of Gr-1dimCD11b+ cells on IFN-γ production in vivo. Recipient mice were challenged i.t. (2,3×103 CFU/mouse, A–C) or aerogenically (102 CFU/mouse, A, B, D) and transferred with Gr-1dimCD11b+ cells. A, B, IFN-γ production (A) and Mtb loads (B) in the lungs of recipient mice (n=3–11 per group). C, D, Weight loss of recipient mice. Arrows indicate days of cell transfer.
In the second experimental setting, we examined whether Gr-1dimCD11b+ cells could affect immune response during the stable phase of the chronic infection. Recipient mice were infected aerogenically with a low dose of Mtb; Gr-1dimCD11b+ cells were transferred on days 74 and 77 post-challenge, and mice were analyzed on day 80. We saw no significant changes in IFN-γ production in the lungs of recipient mice (p=0.34, Fig. 6A); the transfer did not affect mice weight and Mtb burden (Fig. 6B, D). Thus, in vivo Gr-1dimCD11b+ cells suppressed IFN-γ response at the advanced stage of the acute TB infection, but did not exhibit evident suppressor activity during the stable stage of the chronic infection.
There are several possible explanations for these discrepancies. First, it is possible that the effect of cell transfer depended on the ability of the host to counteract the suppression: such ability could be more efficient during the stable stage of infection, but could go out of control at the advanced stage of disease. Second, it is possible that Gr-1dimCD11b+ cells acquired suppressor activity when transferred into the acutely-infected recipients but failed to do so when transferred into the chronically-infected mice. Indeed, it is known that MDSCs need to be activated to acquire suppressive activity, and IFN-γ is one of their most potent activators (21, 37). As shown in Fig. 6A, in the acutely-infected recipients, the levels of IFN-γ production were higher (Fig. 6A, control groups of mice), which could account for a higher suppressive activity of Gr-1dimCD11b+ cells in these recipients.
Irrespective of whether Gr-1dimCD11b+ cells were able to suppress immune response in vivo or not, our results for the first time demonstrate that Gr-1dimCD11b+ cells accumulating during progressing Mtb infection, do not belong to typical neutrophils but rather represent a population of immature myeloid cells. The accumulation of these cells occurs as a result of hematopoietic shifts, as it is accompanied by the disappearance of neutrophils and is observed in different organs, including the BM. Hematopoietic shifts described in our study represent a common trait of fatal TB in mice, and potentially may represent a new pathogenic factor of TB progression and severity in human.
Discussion
Gr-1dimCD11b+cells and neutrophils during Mtb infection
In this study we have demonstrated that progression of Mtb infection is tightly associated with the generation and the accumulation of Gr-1dimCD11b+ cells in the lungs, bone marrow, spleen and blood of infected mice. Phenotypic, morphological and functional analysis of Gr-1dimCD11b+ cells characterized them as immature myeloid suppressor cells: (i) the cells co-expressed neutrophilic (Gr-1 and Ly-6G) and monocytic (F4/80, CD49d) markers; (ii) the cells had nuclei of immature monocytes/granulocytes and myelomonocytic precursors; (iii) a subset of Gr-1dimCD11b+ cells expressed CD117 and CD135; (iv) the cells were able to suppress T cell responses in vitro. To the best of our knowledge, this is the first description of MDSCs during TB.
During other pathological conditions, two major subsets of MDSCs, monocytic and granulocytic have been described (16). Gr-1dimCD11b cells described in our study likely belonged to the monocytic lineage and contained cells that were at different stages of their differentiation. This is supported by cell phenotype, nuclear morphology and NO-mediated suppression (feature characteristic for monocytic population of MDSCs).
Previously, several studies have described the accumulation of Gr-1-expressing cells during severe TB infection and have associated the accumulation of these cells with TB susceptibility and severity (4–6). However, most of the studies did not take into account the levels of Gr-1 expression and considered all Gr-1-expressing cells as neutrophils. Our study for the first time demonstrates that Gr-1-expressing cells that accumulate abundantly at the late stages of Mtb infection differ from typical neutrophils: in our model, the cells expressed Gr-1 and Ly-6G at low levels and represented a mixture of myeloid cells that were at different stages of their differentiation/maturity. Moreover, the accumulation of Gr-1dimCD11b+ cells was accompanied by the disappearance of conventional neutrophils (Gr-1hiCD11b+ cells), indicating on severe hematopoietic shifts that took place at the advanced stage of TB disease. Of note, the more severe was TB disease, the lower were the levels of Gr-1/Ly6G expression on CD11b+ cells, i.e. the lower was their maturity (Fig. 5I). Thus, hematopoietic shifts resulting in the accumulation of immature myeloid cells and gradual disappearance of neutrophils was a characteristic trait of advanced TB infection in mice.
Our data on immaturity of Gr-1dimCD11b+ cells accumulating during severe TB infection can help explain some of conflicting data on the role for neutrophils in TB pathogenesis. Indeed, on the one hand, neutrophils were shown to phagocyte and kill mycobacteria, produce microbicidal peptides, activate macrophages for Mtb killing, and stimulate initiation of antimycobacterial T-cell responses (8, 10, 38, 39). In high TB-burden settings, low counts of neutrophils and low plasma levels of neutrophil-derived microbicidal peptides were associated with high risk of TB development (11, 12), suggesting a significant role for neutrophils in TB protection. On the other hand, we and others have previously demonstrated that neutrophils (i.e., Gr-1 expressing cells) accumulate abundantly in the lungs of mice susceptible to Mtb infection (5, 6); in some studies, neutralizing anti-Gr-1 antibodies were shown to ameliorate the course of TB infection in mice (4,6). Based on these observations, neutrophils were implicated in TB progression and pathology (40). Results obtained in our study demonstrate that Gr-1 expressing cells accumulating during severe TB do not necessarily represent neutrophils. Thus, conclusions on the pathological role for neutrophils made in experimental studies, in which neutrophils were identified (or depleted) based on their Gr-1/Ly-6G positivity, should be revised to take into account the fact that Gr-1/Ly-6G markers are also expressed by immature myeloid cells. Of note, other authors also noticed atypical features of “neutrophils” accumulating in the mouse lung during severe TB. For example, earlier Keller and coauthors (6) analyzed Gr-1+ cells in the lungs of TB-susceptible and TB-resistant mice. While in TB-resistant mice Gr-1+ cells were granulocytic, Gr-1+ cells accumulating in the lungs of susceptible mice had signs of “aberrant differentiation”. This observation corresponds to the results of our study.
Altogether, hematopoietic shifts resulting in the accumulation of immature myeloid cells and gradual disappearance of neutrophils was a characteristic feature of the advanced stage of TB.
Mechanisms promoting Gr-1dimCD11b+cell generation during TB
Results obtained in our study raise several questions. One of the questions is which factors induce Gr-1dimCD11b+cells during TB.
Studies performed in other pathological conditions (e.g., tumors) suggest that the main factors that induce MDSC generation and activation are growth factors and pro-inflammatory cytokines (i.e., CSF, GM-CSF, G-CSF IL-1β, IL-6, PGE2 et al. (32, 41–43). Of note, these factors are abundantly produced during severe TB infection. For example, we have recently demonstrated high expression of IL-1β, IL-6, MMP8, CXCL2 and several other factors in the lungs of (A/SnxI/St)F2 mice highly susceptible to TB (13). More recently, we found much higher expression of GM-CSF in I/St compared to A/Sn mice (unpublished observations). Other authors reported overproduction of G-CSF, IL-1β, IL-6 and other inflammation-related factors during lethal TB infection in CARD9 and IL-1 receptor knock-out mice (4, 44). Thus, exacerbated inflammation represents a common feature of severe lethal TB infection and likely can serve as a major cause for the accumulation of Gr-1dimCD11b+ cells at the advanced stage of TB.
Among other factors that potentially can switch hematopoiesis to generate Gr-1dimCD11b+ cells are mycobacteria and mycobacteria-derived factors. Indeed, at the pre-lethal stage of infection, i.e. at the time-point when Gr-1dimCD11b+ cells became abundant, Mtb burden increased significantly in different organs. Moreover, Mtb could be found in the BM of infected mice. Next, it has been demonstrated that HSC express TLR, and TLR-mediated signals skew hematopoiesis to myeloid lineage (45). On the other hand, in our experimental setting, the accumulation of Gr-1dimCD11b+ cells correlated better with weight loss than with Mtb burden; TB severity and lethality are not directly associated with Mtb burden (13, 46). Thus, the role for Mtb-derived signals in hematopoietic shifts and generation MDSCs during TB is yet to be determined.
Possible roles of Gr-1dimCD11b+ cells in TB pathogenesis
Other interesting questions raised by our data are: what is the role for Gr-1dimCD11b+cells in TB pathogenesis, i.e., does the accumulation of Gr-1dimCD11b+ cells cause TB progression, witness it or result from it? Why fatal TB is always accompanied by the accumulation of Gr-1dimCD11b+ cells? While our data have documented a strong association between the accumulation of Gr-1dimCD11b+ cells and TB fatality, the role, which these cells play in TB pathogenesis, is to be determined.
Based on our data on the ability of Gr-1dimCD11b+ cells to suppress IFN-γ response, the main host protective mechanism, it is logical to suppose that Gr-1dimCD11b+ cells can directly contribute to TB fatality. However, the pathways whereby Gr-1dimCD11b+ cells are involved in TB pathogenesis may be more complicated.
It is now understood that un-controlled T-cell responses are deleterious (47). Thus, Gr-1dimCD11b+ cells may have a regulatory function and their accumulation at the advanced stage of disease may represent the last attempt of the host to dampen exacerbated immune responses. This concept is supported by the fact that the generation of MDSCs is driven by pro-inflammatory factors, and their activation is induced by IFN-γ (16). Thus, MDSCs are generated and activated in the circumstances when the host critically needs to balance immune responses.
It is also possible that the generation of Gr-1dimCD11b+ cells contributes to TB progression indirectly, for example, through the concomitant disappearance of neutrophils. As shown in this study, the accumulation of Gr-1dimCD11b+ cells is always accompanied by the disappearance of neutrophils, and neutrophils have been implicated in TB protection (see above).
Besides suppressing and/or regulating T-cell responses, Gr-1dimCD11b+ cells may also play other roles. For example, they may secrete pro-inflammatory factors and/or serve as a reservoir for Mtb (our preliminary observations). These functions of Gr-1dimCD11b+ cells need further investigations.
Finally, we cannot rule out the possibility that the accumulation of Gr-1dimCD11b+ cells does not contribute to TB pathogenesis, but simply witness it. Indeed, pro-inflammatory factors, the major inductors of MDSCs and TB pathology, are abundantly produced during severe TB infection. The accumulation of Gr-1dimCD11b+ cells may therefore represent a side effect of overwhelming inflammatory response.
An intriguing question is whether there is an association between the generation of Gr-1dimCD11b+ cells and TB susceptibility, i.e., whether susceptible mice have intrinsically higher propensity to generate Gr-1dimCD11b+ cells compared to more resistant mice. We believe that the generation and the accumulation of Gr-1dimCD11b+ cells is rather a consequence than an initial cause of TB progression. Initial pathways leading to rapid TB progression are likely different in genetically different hosts. Such pathways may include poor ability of the host to restrict Mtb growth; overwhelming inflammatory response to mycobacteria, e.g., due to inflammatory hyper-reactivity of host macrophages (13); high reactivity of neutrophils to inflammatory stimuli (6) et al. When the disease progresses, all these pathways converge to induce un-controlled inflammation characterized by over-production of pro-inflammatory factors. These factors cause accelerated hematopoiesis, which leads to the generation of high numbers of Gr-1dimCD11b+ immature myeloid cells and to the disappearance of mature Gr-1hi neutrophils. Gr-1dimCD11b+ cells and lack of neutrophils may further interfere with the host immune response and exacerbate the infection. This concept implies that mechanisms driving TB disease at the initial stages of infection are diverse in different individuals. In contrast, mechanisms driving TB progression at the late stage of infection are common and include exacerbated inflammation, the generation of immature myeloid cells and their accumulation in the periphery. If this concept is confirmed in humans, it may have an important practical outcome: it indicates that there is no need to search for exact cause that has driven severe disease in each individual; it might be possible to slow down disease progression by targeting MDSC cells and/or inflammatory reactions. With this respect, co-treatment of host with anti-Mtb and anti-inflammatory drugs opens new perspectives for efficient TB treatment (48).
In conclusion, our study for the first time demonstrates that Gr-1-expressing cells abundantly accumulating at pre-lethal stage of TB do not belong to mature granulocytes, but rather represent immature myeloid cells able to suppress T cell responses. The accumulation of Gr-1dimCD11b+ cells represents a hallmark of fatal TB infection in mice and may contribute to TB pathogenesis. These results change our view of mechanisms driving TB progression and suggest new potential cellular targets for TB immunotherapy.
Acknowledgments
We are grateful to Drs William Telford and Gary Winslow for critically reading the manuscript; N. Kolokolova and P. Tugusheva for technical assistance.
This work was supported in part by CTRI RAMS, NIH grant #AIRO1078899 (to IL), RFBR grant #13-04-01906 (to IL).
Abbreviations
- BM
bone marrow
- iNOS
inducible NO-synthase
- LN
lymph nodes
- MDSCs
myeloid-derived suppressor cells
- MFI
mean fluorescence intensity
- MNC
mononuclear cells
- Mtb
Mycobacterium tuberculosis
- PMN
polymorphonuclear cells
- ROI
reactive oxygen intermediates
- SC
spleen cells
- TB
tuberculosis
References
- 1.Barry S, Breen R, Lipman M, Johnson M, Janossy G. Impaired antigen-specific CD4(+) T lymphocyte responses in cavitary tuberculosis. Tuberculosis (Edinburgh, Scotland) 2009;89:48–53. doi: 10.1016/j.tube.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland JS, Jeffries DJ, Donkor S, Walther B, Hill PC, Adetifa IMO, Adegbola RA, Ota MOC. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis (Edinburgh, Scotland) 2009;89:398–404. doi: 10.1016/j.tube.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf HJ, Hanke K, Gross O, Ruland J, Kaufmann SHE. The adaptor molecule CARD9 is essential for tuberculosis control. The Journal of Experimental Medicine. 2010;207:777–792. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eruslanov EB, I, Lyadova V, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, Apt AS. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infection and Immunity. 2005;73:1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller C, Hoffmann R, Lang R, Brandau S, Hermann C, Ehlers S. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infection and Immunity. 2006;74:4295–4309. doi: 10.1128/IAI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host & Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infection and Immunity. 2002;70:4591–4599. doi: 10.1128/IAI.70.8.4591-4599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyadova IV. Inflammation and Immunopathogenesis of Tuberculosis Progression. Understanding Tuberculosis - Analyzing the Origin of Mycobacterium Tuberculosis Pathogenicity. 2012:19–42. [Google Scholar]
- 10.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, Stenger S. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. Journal of Immunology (Baltimore, Md: 1950) 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 11.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. Journal of Clinical Investigation. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu LM, Liu CH, Chen P, Dai AG, Li CX, Xiao K, Chen Y, Cao J, Chen YR. Multidrug-resistant tuberculosis is associated with low plasma concentrations of human neutrophil peptides 1–3. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2011;15:369–374. [PubMed] [Google Scholar]
- 13.Lyadova IV, Tsiganov EN, Kapina MA, Shepelkova GS, Sosunov VV, Radaeva TV, Majorov KB, Shmitova NS, van den Ham HJ, Ganusov VV, De Boer RJ, Racine R, Winslow GM. In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs. PLoS One. 2010;5:e10469. doi: 10.1371/journal.pone.0010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–8. [PubMed] [Google Scholar]
- 15.Dilek N, Vuillefroy de Silly R, Blancho G, Vanhove B. Myeloid-derived suppressor cells: mechanisms of action and recent advances in their role in transplant tolerance. Front Immunol. 2012;3:208. doi: 10.3389/fimmu.2012.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 18.Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol. 2011;11:789–93. doi: 10.1016/j.intimp.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Ginderachter JA, Beschin A, De Baetselier P, Raes G. Myeloid-derived suppressor cells in parasitic infections. Eur J Immunol. 2010;40:2976–85. doi: 10.1002/eji.201040911. [DOI] [PubMed] [Google Scholar]
- 20.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–8. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goni O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int Immunol. 2002;14:1125–34. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- 22.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–72. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 23.Mencacci A, Montagnoli C, Bacci A, Cenci E, Pitzurra L, Spreca A, Kopf M, Sharpe AH, Romani L. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J Immunol. 2002;169:3180–90. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- 24.Tsiganov EN, Razinkova EV, Radaeva TV, Nikitina IY, Sosunov VV, Lyadova IV. Immature myeloid Gr-1dim cells in tuberculosis progression. In: Cooper AM, Wilkinson RJ, editors. Keystone Symposia: Host Response in Tuberculosis. Keystone Symposia; Whistler, British Columbia, Canada: 2013. p. 90. [Google Scholar]
- 25.Eruslanov EB, Majorov KB, Orlova MO, Mischenko VV, Kondratieva TK, Apt AS, Lyadova IV. Lung cell responses to M. tuberculosis in genetically susceptible and resistant mice following intratracheal challenge. Clin Exp Immunol. 2004;135:19–28. doi: 10.1111/j.1365-2249.2004.02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyadova IV, Eruslanov EB, Khaidukov SV, Yeremeev VV, Majorov KB, Pichugin AV, Nikonenko BV, Kondratieva TK, Apt AS. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. J Immunol. 2000;165:5921–31. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]
- 27.Kapina MA, Shepelkova GS, Mischenko VV, Sayles P, Bogacheva P, Winslow G, Apt AS, Lyadova IV. CD27low CD4 T lymphocytes that accumulate in the mouse lungs during mycobacterial infection differentiate from CD27high precursors in situ, produce IFN-gamma, and protect the host against tuberculosis infection. J Immunol. 2007;178:976–85. doi: 10.4049/jimmunol.178.2.976. [DOI] [PubMed] [Google Scholar]
- 28.Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol. 2003;170:2046–52. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 29.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–408. [PubMed] [Google Scholar]
- 30.Satake S, Hirai H, Hayashi Y, Shime N, Tamura A, Yao H, Yoshioka S, Miura Y, Inaba T, Fujita N, Ashihara E, Imanishi J, Sawa T, Maekawa T. C/EBPbeta is involved in the amplification of early granulocyte precursors during candidemia-induced “emergency” granulopoiesis. J Immunol. 2012;189:4546–55. doi: 10.4049/jimmunol.1103007. [DOI] [PubMed] [Google Scholar]
- 31.Biermann H, Pietz B, Dreier R, Schmid KW, Sorg C, Sunderkotter C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J Leukoc Biol. 1999;65:217–31. doi: 10.1002/jlb.65.2.217. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 34.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikonenko BV, Averbakh MM, Jr, Lavebratt C, Schurr E, Apt AS. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuber Lung Dis. 2000;80:15–25. doi: 10.1054/tuld.1999.0225. [DOI] [PubMed] [Google Scholar]
- 36.Mitsos LM, Cardon LR, Ryan L, LaCourse R, North RJ, Gros P. Susceptibility to tuberculosis: a locus on mouse chromosome 19 (Trl-4) regulates Mycobacterium tuberculosis replication in the lungs. Proc Natl Acad Sci U S A. 2003;100:6610–5. doi: 10.1073/pnas.1031727100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191:17–23. doi: 10.4049/jimmunol.1300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–9. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyadova IV. Understanding Tuberculosis - Analyzing the Origin of Mycobacterium Tuberculosis Pathogenicity. 2012. Inflammation and Immunopathogenesis of Tuberculosis Progression. [Google Scholar]
- 40.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends in Immunology. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–92. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–89. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 45.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186:5367–75. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koo MS, Manca C, Yang G, O’Brien P, Sung N, Tsenova L, Subbian S, Fallows D, Muller G, Ehrt S, Kaplan G. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One. 2011;6:e17091. doi: 10.1371/journal.pone.0017091. [DOI] [PMC free article] [PubMed] [Google Scholar]