Summary
Long-lived ‘memory-like’ NK cells have been identified in individuals infected by human cytomegalovirus (HCMV), but little is known about how the memory-like NK cell pool is formed. Here, we have shown that HCMV-infected individuals have several distinct subsets of memory-like NK cells that are often deficient for multiple transcription factors and signaling proteins, including tyrosine kinase SYK, for which the reduced expression was stable over time and correlated with epigenetic modification of the gene promoter. Deficient expression of these proteins was largely confined to the recently discovered FcRγ-deficient NK cells that display enhanced antibody-dependent functional activity. Importantly, FcRγ-deficient NK cells exhibited robust preferential expansion in response to virus-infected cells (both HCMV and influenza) in an antibody-dependent manner. These findings suggest that the memory-like NK cell pool is shaped and maintained by a mechanism that involves both epigenetic modification of gene expression and antibody-dependent expansion.
Introduction
NK cells constitute a critical component of innate immunity and serve as a first line of defense against malignancy and viral infections, particularly herpesvirus infections(Biron et al., 1989; Orange, 2002; Vivier et al., 2011). Many recent studies have revealed adaptive immune or ‘memory-like’ properties of NK cells, including long-term persistence and enhanced functional responsiveness, following pathogen infection or exposure to other stimuli(Beziat et al., 2012; Cooper et al., 2009; Foley et al., 2012; Guma et al., 2004; Lopez-Verges et al., 2011; O'Leary et al., 2006; Paust et al., 2010; Petitdemange et al., 2011; Sun et al., 2009). Although some of these characteristics may be transient or reflect a pre-activation state, it is also possible that some NK cells have undergone stable changes that serve to maintain memory-like properties, analogous to changes that occur during the differentiation of memory T cells(Farber et al., 2014). However, little is known about such changes that may stably alter the transcriptional programs of memory-like NK cells.
In humans, elevated and variable frequencies of memory-like NK cells, characterized by the expression of the activation receptor NKG2C, have been observed in association with prior infection by human cytomegalovirus (HCMV) (Guma et al., 2004; Guma et al., 2006b; Monsivais-Urenda et al., 2010; Muntasell et al., 2013; Noyola et al., 2012), a common herpesvirus that establishes life-long latent infection in the majority of human populations(Dowd et al., 2009). It has also been observed that NKG2C+ NK cells expand in number in transplant patients experiencing HCMV reactivation and persist long-term, even after clearance of active infection(Della Chiesa et al., 2012; Foley et al., 2012; Lopez-Verges et al., 2011). NKG2C may be a useful marker for identifying memory-like NK cells, but more recent studies have shown that HCMV-infected individuals also have expanded populations of NK cells that persist long-term and express certain activation forms of killer-cell immunoglobulin-like receptors (KIR), including KIR2DS2 and KIR2DS4, even in the absence of NKG2C(Beziat et al., 2013; Della Chiesa et al., 2014). Thus, the memory-like NK cell pool in HCMV-infected individuals is likely to include a variety of expanded NK cell subsets expressing different activation receptors. Yet, despite the association with HCMV infection, there has been no direct evidence that these receptors themselves are responsible for activation of NK cells in response to HCMV-infected target cells. In fact, NKG2C+ NK cells display poor functional responses toward HCMV-infected cells(Magri et al., 2011; Petersen et al., 2010; Zhang et al., 2013).
Infection of HCMV-seropositive individuals by certain other viruses, including hantavirus, HIV-1 or EBV, is associated with further elevation of NKG2C+ NK cell frequencies(Bjorkstrom et al., 2011; Brunetta et al., 2010; Mela and Goodier, 2007; Petitdemange et al., 2011; Saghafian-Hedengren et al., 2013), illustrating the potential impact of other viral infections on the expansion of the memory-like NK cell pool in HCMV-infected individuals. Again, there is no direct evidence that NKG2C is responsible for activation of NK cells in response to these viral infections. Importantly, these memory-like NK cells differ from conventional NK cells in their turnover rates and functional responses to tumor cells and cytokines(Beziat et al., 2012; Beziat et al., 2013), suggesting there is a fundamental difference between these cells. Currently, it is unclear what role, if any, HCMV-infection plays in the formation of the memory-like NK cell pool, or what role other infections may have. Additionally, little is known regarding the mechanisms underlying the phenotypic and functional differences between these memory-like NK cells and conventional NK cells.
From healthy individuals with prior exposure to HCMV, we have recently discovered a distinct subset of NK cells characterized by deficiency in expression of FcRγ (also known as FcεRIγ)(Hwang et al., 2012b; Zhang et al., 2013), a signaling adaptor associated with the Fc receptor CD16(Lanier, 2008). These FcRγ-deficient (FcRγ-) NK cells, termed “g-NK cells”, express normal amounts of CD3ζ, another signaling adaptor associated with CD16, and often display predominant expression of NKG2C or particular KIR, reflecting clonal-like expansion(Hwang et al., 2012b; Zhang et al., 2013). Compared to conventional NK cells, FcRγ-NK cells express lower amounts of major tumor recognition receptors, NKp30 and NKp46, and show generally poor responsiveness to tumor targets(Hwang et al., 2012b). FcRγ-NK cells are stably maintained and constitute as much as 85% of the total NK cell population, but persist at variable frequencies depending on the donor. Despite the association with HCMV infection, FcRγ-NK cells respond poorly to HCMV-infected cells, reminiscent of the poor response of NKG2C+ NK cells(Magri et al., 2011; Petersen et al., 2010; Zhang et al., 2013). However, FcRγ-NK cells display greatly enhanced functional responsiveness to HCMV-infected cells in the presence of HCMV-specific antibody (Ab), superior to conventional NK cells(Zhang et al., 2013), indicating that the response of FcRγ-NK cells toward HCMV-infected cells is not through direct recognition, but through Ab-dependent recognition via CD16. Compatible with our finding, a more recent study showed that NKG2C+ NK cells also display enhanced functional responses to HCMV-infected cells in an Ab-dependent manner(Wu et al., 2013). Importantly, the discovery of FcRγ-NK cells illustrates a specific protein deficiency associated with pathogen infection in the human body.
Because FcRγ-deficiency can identify Ab-dependent memory-like NK cells that exist within either NKG2C+ or NKG2C- populations(Zhang et al., 2013), FcRγ-deficiency itself provides a molecular signature for memory-like cells in HCMV-infected individuals. In this study, we sought to identify molecular changes in transcriptional programs that are stably maintained in memory-type NK cells. Our study revealed multiple transcription factor and signaling protein deficiencies associated with HCMV infection, including SYK-deficiency that is associated with epigenetic modifications and persists long-term. Finally, we demonstrate that expansion of these memory-like NK cells occurs in response to HCMV-infected, as well as influenza virus-infected, target cells in an Ab- dependent manner.
Results
Gene expression profiling of FcRγ-NK and conventional NK cells
As a starting point for exploring potential differences in gene expression between conventional NK and FcRγ-NK cells, and to screen for genes that might contribute to the enhanced CD16 responsiveness of FcRγ-NK cells, we performed gene expression profiling studies on sorted samples of NK cells. Since intracellular staining of FcRγ requires cell fixation, we used cell surface markers that had predominant expression on FcRγ-NK cells in individual donors; NKG2C for donor #214, and KIR2DL2 for donor #221 (Figure 1A). By using this strategy and two different markers to avoid generating skewed data associated with a specific marker, CD56+CD3- NK cells were sorted into enriched FcRγ-NK and conventional NK cell samples, which were used to generate gene-expression profiles. This comparison revealed 407 transcripts in common that were differentially expressed (>1.75 fold different; 218 lower and 189 higher in FcRγ-NK cells) between FcRγ-NK and conventional NK cells within the same donor (Table S1), including many transcripts encoding cell surface markers. Consistent with microarray data, we found that on the surface of FcRγ-NK cells, ITGA6, SIGLEC7, CD7, PECAM1, and TIM-3 were expressed at lower amounts, while FAS, CD2, and ILT2 were expressed at higher amounts in all donors (n=7) examined (Figure 1B). These data illustrate distinct transcriptional differences that contribute to the phenotypic characteristics of FcRγ-NK cells compared to conventional NK cells.
Figure 1. Gene expression analysis and identification of SYK-deficiency in FcRγ- NK cells.
(A) PBMCs from two individual donors were stained with Ab against surface molecules, then fixed prior to intracellular staining for FcRγ. Dot plots depict sorting strategies for enrichment of CD56+CD3-CD19-CD14- FcRγ-NK cells and conventional NK cells using cell surface markers; NKG2C (Donor #214, 94% conventional NK and 89% FcRγ-NK cell purity) or KIR2DL2 (Donor #221, 85% conventional NK and 76% FcRγ-NK cell purity).
(B) Based on microarray analysis, several cell surface markers were selected for protein expression analysis. Histograms show fluorescence on FcRγ-NK cells (bold line) and conventional NK cells (thin line) compared to control staining (shaded peak), and are representative of at least 7 donors analyzed in at least two independent experiments.
(C) Bar graph shows fold differences in transcripts encoding the indicated proteins that function downstream of CD16 signaling between FcRγ-NK and conventional NK cells from two donors, e.g., SYK mRNA in FcRγ-NK cells from Donor #214 amounted to less than 5% of that in conventional NK cells.
(D) Analysis of intracellular expression of SYK and ZAP70 proteins in FcRγ-NK and conventional NK cells. Histograms show expression amounts of indicated proteins in a control donor (#204), and donors #214 and #221; FcRγ-NK cells (bold line), conventional NK cells (thin line), and control (shaded peak).
SYK expression varies widely in NK cells
To gain insight into the mechanism of FcRγ-NK cell enhanced responsiveness to CD16 stimulation, we looked for differences in expression of genes encoding signaling molecules that function downstream of CD16(Colucci et al., 1999; Lanier, 2008). The gene profiling data showed that the amount of transcript encoding the protein tyrosine kinase SYK was less than 5% in FcRγ-NK cells compared to conventional NK cells in the donor #214 (Figure 1C). However, there was minimal difference in mRNA amounts for SYK in donor #221, or for other signaling proteins, including ZAP70 and PLC-γ2, in either donor. Thus, among CD16 downstream signaling molecules, SYK expression can be quite different between FcRγ-NK and conventional NK cells, but may also vary greatly between individuals.
To examine SYK protein expression, we performed flow cytometric analysis following intracellular staining with anti-SYK mAb. We observed abundant expression of SYK in NK cells from many donors (e.g., donor #204) (Figure 1D). The majority of FcRγ- NK cells in donor #214 were completely deficient for the expression of SYK, while in donor #221, nearly all FcRγ-NK cells expressed SYK, consistent with the gene profiling analysis. These data confirm that there was a distinct difference in SYK transcript and protein amounts between FcRγ-NK and conventional NK cells in donor #214, and that FcRγ-NK cells can express variable amounts of SYK. In contrast, all NK cells, including SYK-deficient NK cells, in these donors expressed the other SYK family kinase ZAP70 at uniformly high abundance (Figure 1D).
SYK-deficient NK cells are associated with HCMV infection
Analysis of our cohort of 62 healthy donors revealed that SYK-deficient NK cells were present in many donors, constituting as much as 60% of the total NK cell pool (Figure 2A). However, we found no evidence of ZAP70-deficient NK cells in these donors. Given the association of FcRγ-NK cells with HCMV infection, we suspected there might also be a correlation between SYK-deficient NK cells and HCMV infection. Testing of plasma samples for HCMV-specific Ab revealed that all donors with SYK-deficient NK cells (using 3% as the detection threshold), except for one, were seropositive for HCMV IgG (Figure 2B). The exceptional donor, in which SYK-deficient NK cells constituted 5% of total NK cells, had substantial memory T cells specific for HCMV pp65 antigen(Zhang et al., 2013), demonstrating that all donors with SYK-deficient NK cells were previously exposed to HCMV. In contrast, SYK-deficient NK cells were not associated with HSV-1 or HSV-2 infection. Further serological analysis indicated that SYK-deficient NK cells were detectable in HCMV-infected donors regardless of HSV-1 and HSV-2 infection (P < 0.05) (Figure 2C). Thus, the presence of SYK-deficient NK cells is associated with prior infection by HCMV, but not by HSV-1 or HSV-2.
Figure 2. Association of SYK-deficient NK cells with prior HCMV infection.
(A) PBMCs from many donors were analyzed for the presence of SYK-deficient (SYK-) or ZAP70-deficient (ZAP70-) NK cells. Dot graphs show the frequency of SYK-deficient (n=62) and ZAP70-deficient (n=36) cells among total NK cells.
(B) Frequencies of SYK-deficient NK cells within individual donors (n=62) grouped according to IgG serological status for HCMV, HSV-1, or HSV-2 infection.
(C) Frequencies of SYK-deficient NK cells within individual donors (n=62) grouped according to IgG serological status for one, two, or three specific herpesvirus infections. ns, not significant, * P < 0.05, ** P < 0.005.
SYK-deficiency is associated with epigenetic modifications
To determine whether SYK-deficient NK cells are a transient or stable population, we performed longitudinal studies. Analysis of samples collected from several donors at different time points showed no noticeable decrease in the frequencies of SYK-deficient NK cells (Figure 3A), suggesting that the SYK-deficiency is stably maintained.
Figure 3. Stability of SYK-deficient phenotype associated with DNA hyper-methylation.
(A) Frequency of SYK-deficient NK cells collected at the initial time point and indicated months later from 8 healthy donors.
(B) Dot plot shows IFN-γ production by indicated subsets of NK cells from a representative donor among 15 individuals after stimulation with immobilized anti-CD16 for 7 h. Numbers represent the percentage of FcRγ-NK and conventional NK cells. Dot graphs show the percentage of SYK-expressing NK cells and SYK-deficient NK cells that produced IFN-γ. Circles connected by a line designate the same donor sample (n=15) ** P < 0.01.
(C) NK cell clones were generated via limiting dilution of sorted NK cells, then tested for functionality after CD16 stimulation. Dot plots show IFN-γ production by two representative clones from five independent experiments. Numbers represent the percentage of cells that produced IFN-γ. Dot graph shows the percentage of cells that produced IFN-γ following stimulation of SYK-expressing (○) and SYK-deficient clones (●) (n=20 each). Each dot represents an individual clone and bars indicate the mean +/- SEM percentage of IFN-γ producing cells for each group. ** P < 0.01.
(D) Schematic diagram of the SYK gene including the promoter-associated CpG island and translation start codon. Arrow represents the transcription initiation site. Expanded region details the location of 20 specific CpG dinucleotides as potential methylation sites. Bar graphs below show the percentage of methylation detected at each individual site in SYK-expressing and SYK-deficient NK cell clones. Data shown is from one donor; similar patterns were observed from 2 additional donors. See also Figure S1.
Given the importance of Syk in CD16 signaling in mouse NK cells(Colucci et al., 1999), we sought to examine whether SYK-deficiency affects the functional responsiveness to CD16 engagement in human NK cells. Despite SYK-deficiency, crosslinking of CD16 led to production of interferon-γ (IFN-γ) by SYK-deficient NK cells at amounts significantly higher than those produced by SYK-expressing NK cells in peripheral blood mononuclear cells (PBMC) (P < 0.01) (Figure 3B), indicating that SYK- deficiency does not impair NK cell responsiveness to CD16 in human NK cells.
To examine if SYK-deficiency is maintained following cell division, NK cell clones were generated under limiting dilution conditions, and analyzed for phenotype and functional attributes. The status of SYK expression (either present or absent) did not change over the course of several weeks of culturing (data not shown). Upon CD16 crosslinking, SYK-deficient NK cell clones displayed higher IFN-γ production compared to SYK-expressing NK cell clones (Figure 3C), consistent with observations of fresh NK cells. Thus, these data indicate that SYK-deficient NK cells are stably maintained even after several rounds of cell division, and their functional characteristics are inheritable by daughter cells.
As a potential mechanism underlying the SYK-deficiency, we examined the methylation status of the SYK promoter region and found evidence of hyper-methylation within a CpG-rich region in which methylation has been correlated with reduced expression of SYK in human tumor cell lines(Goodman et al., 2003). In our studies, methylation was detected at several cytosines within the CpG island proximal to the SYK transcription initiation site in SYK-deficient NK cell clones, but not SYK-expressing clones (Figure 3D and S1). In contrast, cytosines further upstream did not show such a difference. These data indicate that the SYK-deficiency is associated with hyper- methylation of a specific region in the SYK promoter DNA sequences, suggesting that epigenetic modifications lead to silencing of the SYK gene in memory-like NK cells.
SYK-deficiency is predominantly confined to the FcRγ-NK cell subset
Considering the association of SYK-deficient, FcRγ-NK, and NKG2C+ NK cells with HCMV infection(Guma et al., 2004; Zhang et al., 2013), we co-stained cells to further examine their relationship. This analysis showed nearly all SYK-deficient NK cells were also deficient for FcRγ expression in most donors (Figure 4A and S2A), indicating that the majority of SYK-deficient NK cells are present within the FcRγ-NK cell subset. The SYK-deficient NK cells constituted the major population of FcRγ-NK cells in some donors, but FcRγ-NK cells expressed SYK frequently in other donors. Although a low frequency of SYK-deficient NK cells that expressed FcRγ were detectable in several donors, these cells generally had lower amounts of FcRγ than the conventional NK cells (Figure 4A and S2A). Thus, the NK cell population could be divided into three major subsets, conventional NK cells, FcRγ-NK cells that are SYK-deficient, or FcRγ-NK cells that are SYK-expressing, and these subsets were present at variable frequencies between different donors. With respect to NKG2C expression, SYK-deficient NK cells were often present within the NKG2C+ population (Figure S2B). However, in many donors, substantial proportions of SYK-deficient NK cells did not express NKG2C and, in two donors among our cohort, all SYK-deficient NK cells completely lacked expression of NKG2C (Figure S2B and S2C), indicating there was not a strong relationship between SYK-deficiency and NKG2C expression. Nonetheless, our data demonstrate that the SYK-deficiency is largely confined to FcRγ-NK cells, but not NKG2C+ NK cells, revealing a close relationship between SYK-deficiency and FcRγ- deficiency.
Figure 4. Association and functional impact of SYK deficiency with FcRγ deficiency in NK cells.
(A) Flow cytometric analysis of FcRγ vs. SYK expression in CD56+CD3-CD19-CD14- NK cells from 4 representative donors.
(B) Dot plots show IFN-γ production by indicated subsets of NK cells from a representative donor among 10 individuals after CD16 stimulation. Numbers represent the percentage of NK cells within the designated quadrants. Dot graphs show the percentage of SYK-expressing conventional NK cells (FcRγ+SYK+; I), SYK-expressing FcRγ-NK cells (FcRγ-SYK+; II), or SYK-deficient FcRγ-NK cells (FcRγ-SYK-; III) that produced IFN-γ from several donors. Circles connected by a line designate the same donor sample (n=10).
(C) Cell surface expression of CD107a was determined following stimulation as in (B). Numbers represent the percentage of NK cells within the designated quadrants. Dot graph shows the percentage of SYK-expressing conventional NK cells (I), SYK- expressing FcRγ-NK cells (II), or SYK-deficient FcRγ-NK cells (III) that displayed CD107a (n=10).
(D) PBMCs were cultured for 3 days with mock- or HCMV-infected MRC-5 cells, with the last 6 hours in the presence or absence of autologous plasma as indicated. Dot plots show IFN-γ production by NK cells from one representative donor among 9 individuals, and dot graph shows the percentage of NK cells that produced IFN-γ in SYK-expressing conventional NK cells (I), SYK-expressing FcRγ-NK cells (II), or SYK-deficient FcRγ-NK cells (III) from several donors (n=9) +/- autologous plasma. ns, not significant; * P < 0.05 and ** P < 0.01.
SYK Deficiency and Lack of NKG2C Expression Does Not Impair CD16 Responsiveness
We next sought to examine whether SYK-deficiency affects the functional responsiveness to CD16 engagement in FcRγ-NK cells. Crosslinking of CD16 led to production of IFN-γ by SYK-deficient FcRγ-NK cells at amounts significantly higher than those produced by SYK-expressing FcRγ-NK cells (P < 0.05) as well as conventional NK cells (P < 0.01), although there was substantial variation between donors (Figure 4B). However, there was no significant difference between these subsets in the degranulation response, as measured by cell surface expression of CD107a (Figure 4C).
We also examined the responses of these NK cell subsets to viral infection, and observed that direct stimulation of NK cells with HCMV-infected target cells did not induce substantial IFN-γ production in any subset (Figure 4D, upper panels). Considering the robust responses of SYK-deficient NK cells to Ab-mediated CD16 crosslinking, we examined whether the presence of naturally occurring Ab against HCMV would impact the NK cell responses to HCMV-infected cells. Addition of autologous plasma containing anti-HCMV IgG led to a dramatic production of IFN-γ by SYK-deficient FcRγ-NK cells at amounts significantly higher (P < 0.01) than those produced by conventional NK cells (Figure 4D, lower panels). These data indicate that SYK-deficient FcRγ-NK cells poorly respond to virus-infected targets cells directly, but instead, respond strongly to infected target cells in the presence of virus- specific Ab. However, the difference between SYK-deficient FcRγ-NK cells and SYK- expressing FcRγ-NK cells was not significant in this target cell-based system (Figure 4D). Importantly, the production of IFN-γ by SYK-deficient FcRγ-NK cells was higher than conventional NK cells in either system, indicating that SYK itself does not play a positive role in CD16-induced cytokine production in human NK cells, and that SYK- deficiency can contribute in part to the enhanced CD16 responsiveness of the FcRγ-NK cell population. Moreover, when NK cells were categorized with respect to the presence or absence of NKG2C expression, FcRγ-NK cells also displayed superior responsiveness compared to conventional NK cell subsets regardless of NKG2C expression (Figure S3).
FcRγ-NK cells are deficient for several proteins expressed in conventional NK cells
Based on the gene profiling data and evidence of epigenetic modification in FcRγ-NK cells, we suspected that there might be additional differences in factors influencing the transcriptional programs or signaling between FcRγ-NK and conventional NK cells. To explore the possibility, we examined protein expression of several other genes that showed differential mRNA amounts between FcRγ-NK and conventional NK cells (Table S1). These included the transcription factors PLZF (also known as ZBTB16) and IKZF2 (also known as HELIOS), and signaling molecules DAB2 and EAT-2(Mathew et al., 2012; Perez-Quintero et al., 2014; Shapira et al., 2014; Thornton et al., 2010).
The majority of conventional NK cells expressed PLZF (Figure 5A). In contrast, essentially all FcRγ-NK cells were deficient in PLZF expression. We noted that there were PLZF-deficient NK cells that expressed FcRγ in some donors, but these cells tended to express FcRγ at intermediate amounts. Similar to the pattern of PLZF expression, the signaling molecules DAB2 and EAT-2 were expressed by the majority of conventional NK cells (Figure 5B and 5C). However, FcRγ-NK cells generally displayed deficient expression of DAB2 and variable deficiency patterns of EAT-2. Furthermore, the majority of conventional NK expressed IKZF2 (Figure 5D). The pattern of IKZF2 expression in NK cells was more heterogeneous and complex, with evidence of multiple subsets in the FcRγ-NK cells, including a fraction of FcRγ-NK cells that expressed this transcription factor at amounts even higher than conventional NK cells. Thus, our study reveals the presence of several distinct subsets that are deficient for multiple transcription factors and signaling molecules. Importantly, these deficiencies are largely confined to the FcRγ-NK cell population, indicating a relationship between FcRγ- deficiency and PLZF, DAB2, EAT-2, and IKZF2 deficiencies, resembling the relationship with SYK-deficiency. Considering the heterogeneous expression of these proteins, we performed gene expression profiling on enriched NK cell samples sorted from 2 additional donors, which revealed many consistent differences between the populations of enriched FcRγ-NK cells and conventional NK cells (Table S2). Importantly, direct analysis of FcRγ-NK and conventional NK cells from multiple donors at the single cell level revealed protein expression differences that were often more dramatic than observed differences in mRNA at the population level.
Figure 5. Multiple protein deficiencies correlate with FcRγ-deficiency.
PBMCs were co-stained for FcRγ and transcription factor PLZF (A), signaling molecules DAB2 (B) and EAT-2 (C). Shown are dot plots depicting CD56+CD3-CD19-CD14- NK cells from a representative HCMV seropositive donor among at least 7 HCMV- seropositive individuals. Dot graphs show mean fluorescence intensity (MFI) of indicated proteins in CD3+CD56- T cells (T), conventional NK cells (NK) and FcRγ-NK cells from HCMV seronegative and seropositive donors. MFIs were each normalized by subtraction of the MFI from control staining. Symbols connected by lines are from the same donor sample.
(D) PBMCs were co-stained for FcRγ and transcription factor IKZF2. Shown are dot plots depicting CD56+CD3-CD19-CD14- NK cells from one HCMV seronegative and three representative HCMV seropositive donors as indicated. All data are representative of at least two independent experiments.
Ab-dependent expansion of FcRγ-NK cells occurs in response to HCMV-infected cells
Hyper-methylation of the SYK promoter in SYK-deficient NK cells suggested that other protein deficiencies, including FcRγ-deficiency in FcRγ-NK cells, were due to changes in DNA methylation. Since changes in methylation patterns on these target genes occur presumably in a stochastic manner, our observation that SYK-deficient NK cells, as well as other protein deficient cells, were largely confined to the FcRγ-NK cell population suggested potential effect of FcRγ-deficiency, possibly through enhanced CD16 responsiveness, on the formation of memory-like NK cell pool.
As an attempt to address this possibility, we co-cultured PBMCs containing FcRγ- NK cells with HCMV-infected or mock-infected cells for 11-13 days in the presence or absence of autologous plasma containing anti-HCMV IgG. Measurement of the relative frequencies of FcRγ-NK cells showed that there was no increase in the frequency of FcRγ-NK cells in the presence of HCMV-infected cells compared to mock-infected cells (Figure 6A). In fact, the relative frequencies of FcRγ-NK cells generally decreased after culturing with HCMV-infected cells when compared to their frequencies before culture. Moreover, the absolute numbers of FcRγ-NK cells obtained following co-culture with HCMV-infected cells were not higher than those obtained from the culture with mock- infected cells for the majority of the donors examined, although some increases in the number of FcRγ-NK cells in a few donors after culture with HCMV-infected cells were observed (Figure 6B). However, the addition of autologous plasma to the HCMV culture led to significant increases in both relative frequencies (P < 0.01) and absolute numbers (P < 0.01) of FcRγ-NK cells, compared to those resulting from co-culturing with either mock-infected cells, HCMV-infected cells, or mock-infected cells plus plasma (Figure 6A and 6B). Importantly, under this condition, FcRγ-NK cells also underwent significant preferential expansion over conventional NK cells, which was apparently due to active proliferation of FcRγ-NK cells in a later phase (> 6 days) of the culture period (Figure S4). Moreover, functional analysis of the expanded FcRγ-NK cells indicated that these cells maintained the enhanced ability to produce IFN-γ in response to CD16 crosslinking (Figure S5). Taken together, these data demonstrate that FcRγ-NK cells can expand robustly upon encounter of HCMV-infected cells in the presence, but not in the absence, of seropositive plasma, and expanded FcRγ-NK cells maintain enhanced CD16 responsiveness.
Figure 6. Ab-dependent expansion of FcRγ-NK cell population in response to HCMV-infected target cells.
(A) PBMCs were cultured in the presence of mock- or HCMV-infected target cells with or without autologous plasma as indicated. Dot plots show NK cells from one representative donor among 14 individuals before and after 11-13 d culture in indicated conditions. Numbers represent the percentage of FcRγ-NK cells. Dot graph depicts the change in frequencies of conventional (○) and FcRγ-NK cells (●) compared to initial frequencies for individual donors after culturing for 11-13 d as indicated (n=14).
(B) Bar graph depicts absolute numbers of conventional (○) and FcRγ-NK cells (●) from one representative donor among 14 individuals after culture in indicated conditions as described in (A). Dot graph shows the fold change in the absolute number of NK cells based on the absolute number of NK cells obtained from the control condition (PBMCs cultured with mock-infected cells without plasma) for each individual donor (n=14). The mean +/- SEM are indicated.
(C) NK cells were sorted from PBMCs and cultured as described in (A). Dot plots show NK cells from one representative donor among 9 individuals cultured for 11 d as indicated. Purified IgG (Ab) was also tested. Numbers represent percentages of FcRγ- NK cells.
(D) Dot graph depicts the fold change in the absolute number of NK cells based on the absolute number of NK cells obtained from the control condition (PBMCs cultured with mock-infected cells plus plasma) for each individual donor (n=9, ** P < 0.01).
To determine whether the plasma-dependent FcRγ-NK cell expansion requires other leukocytes, NK cells were sorted from PBMCs and co-cultured with mock- or HCMV-infected cells in the presence or absence of autologous plasma. Similar to the data obtained with PBMC cultures, inclusion of both seropositive plasma with HCMV- infected target cells yielded substantial increase in the frequencies and numbers of FcRγ-NK cells compared to either the condition containing mock-infected cells with plasma or the condition containing HCMV-infected cells without plasma (Figure 6C, 6D and not shown). Moreover, when compared to conventional NK cells, FcRγ-NK cells displayed significant preferential expansion under this condition (P < 0.01). Finally, the addition of purified IgG to the HCMV culture also led to preferential and dramatic expansion of FcRγ-NK cells at amounts comparable to those obtained with plasma, indicating that the plasma effect was primarily mediated by Ab. Taken together, these data demonstrate that FcRγ-NK cells can undergo dramatic and preferential expansion in response to HCMV-infected cells in the presence of Ab, regardless of other immune cells. Importantly, these data support the idea that, while HCMV infection drives the stochastic epigenetic changes leading to FcRγ-deficiency, as well as other deficiencies, FcRγ-deficiency itself may be an important part of a mechanism to select and promote FcRγ-NK cell expansion in the presence of virus-specific Ab during HCMV reactivation.
Ab-dependent expansion of FcRγ-NK cells occurs in response to influenza virus-infected cells
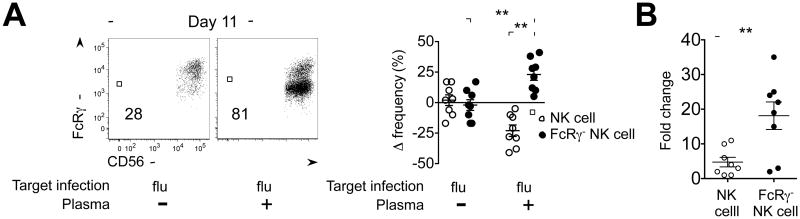
Considering the Ab-dependent expansion capability of FcRγ-NK cells in response to HCMV-infected cells, we sought to test FcRγ-NK cells in the setting of another common virus, influenza (flu). Using flow cytometry, we found that plasma from many healthy donors contained Ab that could bind to flu-infected, but not mock-infected, target cells (data not shown). Through experiments analogous to the HCMV co-culture system, we observed that the frequencies of FcRγ-NK cells increased in the presence of both flu- infected cells and plasma, but not in the absence of plasma, when compared to their frequencies before culture (Figure 7A). Importantly, the relative increase in absolute number of FcRγ-NK cells was significantly greater than that of conventional NK cells in the presence of both flu-infected cells and plasma (P < 0.01), compared to the control condition containing flu-infected cells without plasma (Figure 7A and 7B). Thus, similar to in vitro HCMV infection conditions, FcRγ-NK cells displayed robust and preferential expansion over conventional NK cells in response to flu-infected cells in an Ab-dependent manner.
Figure 7. Ab-dependent expansion of FcRγ-NK cells in response to flu-infected target cells.
(A) PBMCs were co-cultured with flu-infected target cells in the presence (+) or absence (-) of autologous plasma as indicated. Dot plots show NK cells from one representative donor among 8 individuals after 11 d culture in indicated conditions. Numbers represent the percentage of FcRγ-NK cells. Dot graph depicts the change in frequencies of conventional NK (○) and FcRγ-NK cells (●) compared to initial frequencies for individual donors after culture for 11 d in indicated conditions (n=8).
(B) Dot graph shows the fold change in the absolute number of conventional NK (○) and FcRγ-NK (●) cells after culture in indicated conditions as described in (A). Fold change is based on the absolute number obtained from the control culture (PBMCs cultured with flu-infected cells without plasma) for each individual donor. The mean +/- SEM are indicated (n=8, ** P < 0.01).
Discussion
Multiple lines of evidence support the concept that NK cells in humans and mice can exhibit several memory-like properties. These properties may be acquired through certain molecular changes to maintain the memory-like state. Here, as a model for memory-like NK cells in humans, we have presented a stably maintained molecular signature of FcRγ-NK cells, which includes not only FcRγ-deficiency, but also differences in expression of multiple signaling molecules (SYK, DAB2 and EAT-2) and transcription factors (PLZF and IKZF2). For this study, the differences we observed were clear reductions from normal amounts, and therefore are referred to as ‘deficiencies’ for clarity. Unlike conventional NK cells that express these proteins, the majority of FcRγ-NK cells are deficient for PLZF and DAB2, while substantial, yet variable, fractions of FcRγ-NK cells are deficient for SYK, EAT-2 and IKZF2. Multi-protein-deficient FcRγ-NK cells were found almost exclusively in the HCMV-infected individuals of our cohort. Considering the prevalence of HCMV infection(Dowd et al., 2009), and the observation that FcRγ-NK cells are readily detectable in the majority of HCMV-seropositive donors in our cohort, we estimate that more than two billion people worldwide have substantial numbers of these multi-protein-deficient memory-like NK cells.
Our study has also shown that SYK-deficiency is associated with promoter DNA hyper-methylation, suggesting that epigenetic modifications are responsible for SYK- deficiency. Consistent with our data, association between DNA hypermethylation and FcRγ and EAT-2 deficiencies has been reported (Bryceson, 2014 AAI meeting). Thus, epigenetic modification may be a common mechanism for altered expression of these signaling proteins and transcription factors. Since epigenetic modifications occur presumably in a stochastic manner, our expansion data suggest that a subset of NK cells with particular epigenetic changes, specifically FcRγ-deficiency, might be further selected and expanded preferentially in an Ab-dependent manner, especially during reactivation of HCMV or perhaps even during secondary infections by certain other viruses. It is possible that this process could continue with additional epigenetic changes during subsequent rounds of HCMV reactivation and Ab-dependent FcRγ-NK cell expansion, resulting in multiple protein deficiencies within the FcRγ-NK cell population. Taken together, we propose a model whereby HCMV infection directs stochastic epigenetic modifications, and subsequent Ab-dependent expansion further selects and shapes the pool of memory-like NK cells.
Given that the majority of NKG2C+ NK cells belong to the FcRγ-NK cell population, the Ab-dependent expansion also provides a potential explanation for why HCMV-infected individuals with co-infections of other viruses had elevated frequencies of NKG2C+ NK cells(Bjorkstrom et al., 2011; Brunetta et al., 2010; Mela and Goodier, 2007; Petitdemange et al., 2011; Saghafian-Hedengren et al., 2013). These viral infections may induce HCMV reactivation, which can in turn promote the expansion of NKG2C+ NK cells directly, or in collaboration with HCMV-specific Ab. Other chronic or repeat viral infections may also have an impact on the size of the memory-like NK cell pool by promoting Ab-dependent expansion. Still, we expect that the degree of FcRγ-NK cell expansion may depend on the nature of the infection, as certain infections that are localized, or that do not support sustained surface expression of antigens for Ab interaction may not be sufficient for substantial or detectable FcRγ-NK cell expansion. Furthermore, we speculate during primary infection, precursor cells of FcRγ-NK cells may respond directly to HCMV-infected target cells by utilizing multiple activation receptors, including NKG2C, NKp46, DNAM1, and activating KIRs(Beziat et al., 2013; Guma et al., 2006a; Long et al., 2013; Magri et al., 2011), and perhaps CD16 itself(Grier et al., 2012; Mandelboim et al., 1999), leading to expansion and differentiation into FcRγ-NK cells through epigenetic mechanisms that involve DNA methylation. It is also possible that other epigenetic mechanisms, such as chromatin remodeling or changes in transcription factor expression such as PLZF(Mathew et al., 2012), contribute to the formation of the memory-like NK cell pool. We are only beginning to observe and understand dramatic and stable epigenetic alterations of the innate immune system following infection by a human pathogen.
As part of this study, analysis of mice that were infected with mouse cytomegalovirus (MCMV) yielded no evidence for the presence of FcRγ- or Syk-deficient NK cells within the MCMV-specific Ly49H+ memory NK cell pool (data not shown), revealing a difference between human and mouse memory NK cells. Nonetheless, the development of an animal model or in vitro infection model will be useful for addressing key issues, such as the specific role of HCMV infection in the differentiation of these memory-like NK cells, and functional impact of specific protein deficiencies. In addition to decreased gene expression, there are many factors that are more abundantly expressed in FcRγ-NK cells. Further exploration of our gene profiling data will likely reveal more differences between memory-like FcRγ-NK and conventional NK cells. Finally, the superior Ab-dependent expansion and functional capabilities of FcRγ-NK cells suggest that these memory-like NK cells may function to control HCMV reactivation from latency. Through these capabilities, FcRγ-NK cells may also aid in the control of multiple chronic or recurrent viral infections, especially other herpesviruses, thereby providing important protection for the host through an intriguing symbiotic relationship.
Experimental Procedures
Human subjects and blood samples
PBMCs were obtained from healthy volunteer donors with informed consent or from de- identified leukocyte reduction filters (American Red Cross), as approved by the Michigan State University Biomedical and Health Institutional Review Board.
Antibodies and reagents
Serological status of donor plasma was determined using virus-specific ELISA kits (MP Biomedicals) according to the manufacturer's instructions. The list of Ab used in this study is included in the Supplemental Experimental Procedures.
Flow cytometric analysis of NK cells and T cells
For analysis of cell surface markers, FcRγ, SYK, IFN-γ, and CD107a expression, PBMCs or cultured cells were stained as previously described(Hwang et al., 2012b). For the analysis of DAB2 and EAT-2 intracellular molecules, cells were stained with anti- DAB2 or anti-EAT-2 Ab followed by fluorochrome-conjugated secondary Ab. For analysis of IKZF2 and PLZF, cells were fixed and permeabilized using Transcription Factor Buffer Set (BD). LIVE Cell Stain Kit (Invitrogen) was used to exclude dead cells from all analyses of NK cells (CD56+CD3-CD19-CD14-) and T cells (CD3+CD14-CD19- CD56-).
Functional and expansion assays of NK cells
NK cell functional assays using immobilized anti-CD16 mAb or HCMV-infected target cells were performed as previously described (Zhang et al., 2013). For expansion assay, MRC-5 lung fibroblast cells were cultured in 96-well plates, and either mock- infected, infected with HCMV (Towne strain, MOI=1)(Zhang et al., 2013) or Influenza virus (PR8 strain, 500 hemagglutination units per well)(Hwang et al., 2012a) for 2 h, and then washed with PBS to remove unabsorbed virus before addition of PBMCs or sorted NK cells and cultured for 11-13 d in the presence of recombinant human IL-2 (10 U/ml). Plasma or purified IgG was added 2 d after plating of cells and half of culture media was removed and replenished with media containing IL-2 and plasma or IgG every 3-4 d.
Microarray for gene profiling analysis
NKG2C+ and NKG2C- NK (CD56+ CD3-CD19-CD14-) cells were sorted from the PBMCs of donor #214. KIR2DL2+ and KIR2DL2- NK cells were sorted from PBMC of donor #221. Purified RNA from these cell preparations was analyzed by using 4×44K Agilent human arrays (design ID 026652) in the RTSF at Michigan State University.
NK cell cloning
PBMCs were enriched for CD56+CD3-CD19- cells using NK Cell Isolation Kit (Miltenyi Biotec). Isolated NK cells were distributed into 96-well plates by limiting dilution in NK cell cloning medium [RPMI1640 supplemented with 10% Fetal Bovine Serum (Hyclone), 5% pooled human AB serum (Cellgro), 500 U/mL IL-2, sodium pyruvate (Sigma), non- essential amino acids (Sigma), and PHA] along with feeder cells comprised of allogeneic PBMCs and RPMI 8866 cells which had been irradiated at 50Gy using X-RAD 320 Irradiation System.
DNA extraction and methylation analysis
DNA was extracted from SYK+ and SYK- NK clones using DNeasy Blood &Tissue Kit (Qiagen) and bisulfite treated with the EZ DNA Bisulfite Kit (ZYMO). Nested PCR was performed on treated DNA to amplify the promoter region of the SYK gene using primers and conditions previously described(Goodman et al., 2003).
Statistics
The Wilcoxon matched-pairs signed-rank test was used for comparison of the frequency change and fold change during NK cell expansion and functional assays. The Chi- squared test was used for comparison of ELISA data. Differences were considered significant when P < 0.05 (GraphPad Prism).
Supplementary Material
Highlights.
SYK-deficient NK cells are present in HCMV-infected individuals.
SYK-deficiency is associated with hyper-methylation of the gene promoter.
Memory-like NK cells have protein deficiencies in combination with FcRγ-deficiency.
FcRγ-deficient NK cells expand preferentially in an antibody-dependent manner.
Acknowledgments
We thank E. Gardner (Michigan State University) for influenza virus stocks; S. Hong, D. Bieber, J. Conwell, S. Sheikh, and L. King for technical assistance; and W. Esselman for discussion and advice. Supported by the National Institutes of Health (5R21CA149476), Michigan State University and a National Agenda Project grant from the Korea Research Council of Fundamental Science and Technology, Republic of Korea.
Footnotes
Author Contributions: J.L. and J.M.S. performed experiments, interpreted data, and prepared the paper; T.Z. and I.H. conceived the study, performed experiments and interpreted data; A. K. performed experiments and prepared the paper; L.N. and M.K. performed experiments; Y.K. and L.L.L. designed, performed experiments, and interpreted data; S.K. conceived the study, interpreted results, supervised all aspects of the work and prepared the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debre P, et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- Beziat V, Liu L, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, Retiere C, Sverremark-Ekstrom E, Traherne J, Ljungman P, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013 doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. The Journal of experimental medicine. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- Colucci F, Turner M, Schweighoffer E, Guy-Grand D, Di Bartolo V, Salcedo M, Tybulewicz VL, Di Santo JP. Redundant role of the Syk protein tyrosine kinase in mouse NK cell differentiation. Journal of immunology. 1999;163:1769–1774. [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Chiesa M, Falco M, Bertaina A, Muccio L, Alicata C, Frassoni F, Locatelli F, Moretta L, Moretta A. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C-/- umbilical cord blood. Journal of immunology. 2014;192:1471–1479. doi: 10.4049/jimmunol.1302053. [DOI] [PubMed] [Google Scholar]
- Della Chiesa M, Falco M, Podesta M, Locatelli F, Moretta L, Frassoni F, Moretta A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119:399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman PA, Burkhardt N, Juran B, Tibbles HE, Uckun FM. Hypermethylation of the spleen tyrosine kinase promoter in T-lineage acute lymphoblastic leukemia. Oncogene. 2003;22:2504–2514. doi: 10.1038/sj.onc.1206313. [DOI] [PubMed] [Google Scholar]
- Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, Pandey R, Campbell KS, Orange JS. Human immunodeficiency- causing mutation defines CD16 in spontaneous NK cell cytotoxicity. The Journal of clinical investigation. 2012;122:3769–3780. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus- infected fibroblasts. Blood. 2006a;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- Guma M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, Lopez-Botet M. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006b;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- Hwang I, Scott JM, Kakarla T, Duriancik DM, Choi S, Cho C, Lee T, Park H, French AR, Beli E, et al. Activation mechanisms of natural killer cells during influenza virus infection. PLoS One. 2012a;7:e51858. doi: 10.1371/journal.pone.0051858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRγamma and specialized for antibody-dependent immune functions. Int Immunol. 2012b;24:793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual review of immunology. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri G, Muntasell A, Romo N, Saez-Borderias A, Pende D, Geraghty DE, Hengel H, Angulo A, Moretta A, Lopez-Botet M. NKp46 and DNAM-1 NK-cell receptors drive the response to human cytomegalovirus-infected myeloid dendritic cells overcoming viral immune evasion strategies. Blood. 2011;117:848–856. doi: 10.1182/blood-2010-08-301374. [DOI] [PubMed] [Google Scholar]
- Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Seiler MP, Scanlon ST, Mao AP, Constantinides MG, Bertozzi-Villa C, Singer JD, Bendelac A. BTB-ZF factors recruit the E3 ligase cullin 3 to regulate lymphoid effector programs. Nature. 2012;491:618–621. doi: 10.1038/nature11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela CM, Goodier MR. The contribution of cytomegalovirus to changes in NK cell receptor expression in HIV-1-infected individuals. J Infect Dis. 2007;195:158–159. doi: 10.1086/509811. author reply 159-160. [DOI] [PubMed] [Google Scholar]
- Monsivais-Urenda A, Noyola-Cherpitel D, Hernandez-Salinas A, Garcia-Sepulveda C, Romo N, Baranda L, Lopez-Botet M, Gonzalez-Amaro R. Influence of human cytomegalovirus infection on the NK cell receptor repertoire in children. Eur J Immunol. 2010;40:1418–1427. doi: 10.1002/eji.200939898. [DOI] [PubMed] [Google Scholar]
- Muntasell A, Lopez-Montanes M, Vera A, Heredia G, Romo N, Penafiel J, Moraru M, Vila J, Vilches C, Lopez-Botet M. NKG2C zygosity influences CD94/NKG2C receptor function and the NK-cell compartment redistribution in response to human cytomegalovirus. Eur J Immunol. 2013;43:3268–3278. doi: 10.1002/eji.201343773. [DOI] [PubMed] [Google Scholar]
- Noyola DE, Fortuny C, Muntasell A, Noguera-Julian A, Munoz-Almagro C, Alarcon A, Juncosa T, Moraru M, Vilches C, Lopez-Botet M. Influence of congenital human cytomegalovirus infection and the NKG2C genotype on NK-cell subset distribution in children. Eur J Immunol. 2012;42:3256–3266. doi: 10.1002/eji.201242752. [DOI] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Quintero LA, Roncagalli R, Guo H, Latour S, Davidson D, Veillette A. EAT-2, a SAP-like adaptor, controls NK cell activation through phospholipase Cgamma, Ca++, and Erk, leading to granule polarization. The Journal of experimental medicine. 2014;211:727–742. doi: 10.1084/jem.20132038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Petersen CC, Moller-Larsen A, Hokland ME. Short-term exposure to human cytomegalovirus-infected fibroblasts induces a proportional increase of active CD94/NKG2A(+) natural killer cells. Hum Immunol. 2010;71:29–35. doi: 10.1016/j.humimm.2009.09.355. [DOI] [PubMed] [Google Scholar]
- Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM, Vieillard V. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7:e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghafian-Hedengren S, Sohlberg E, Theorell J, Carvalho-Queiroz C, Nagy N, Persson JO, Nilsson C, Bryceson YT, Sverremark-Ekstrom E. Epstein-Barr virus coinfection in children boosts cytomegalovirus-induced differentiation of natural killer cells. Journal of virology. 2013;87:13446–13455. doi: 10.1128/JVI.02382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira KE, Hirschhorn T, Barzilay L, Smorodinsky NI, Henis YI, Ehrlich M. Dab2 inhibits the cholesterol-dependent activation of JNK by TGF-beta. Mol Biol Cell. 2014;25:1620–1628. doi: 10.1091/mbc.E13-09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, Schirmbeck R, Mertens T. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. Journal of virology. 2013;87:7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. Journal of immunology. 2013;190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.