Abstract
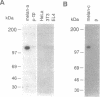
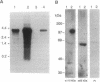
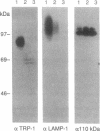
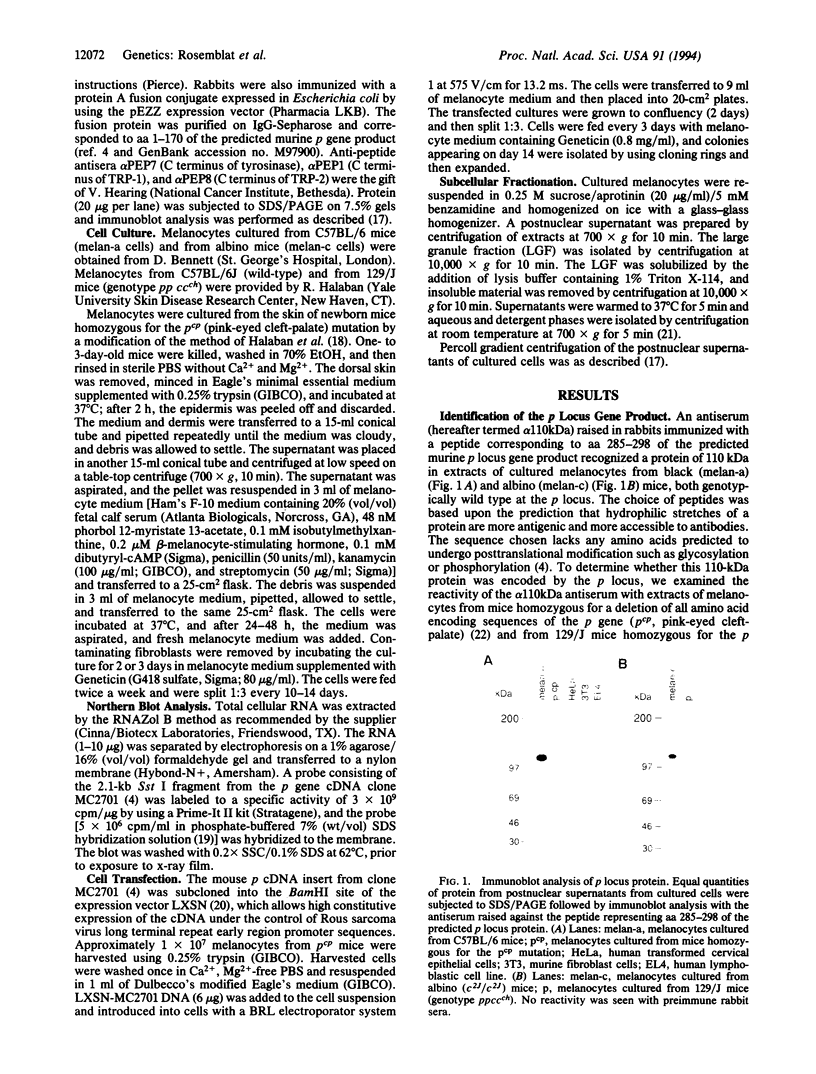
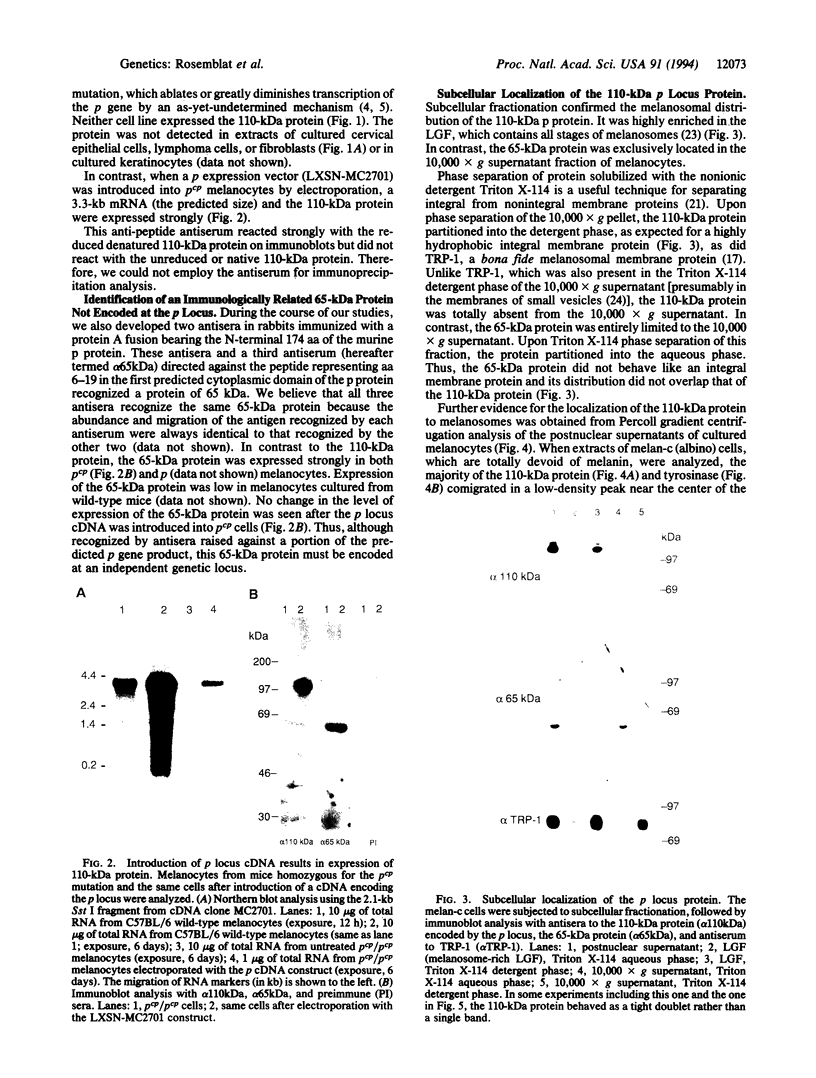
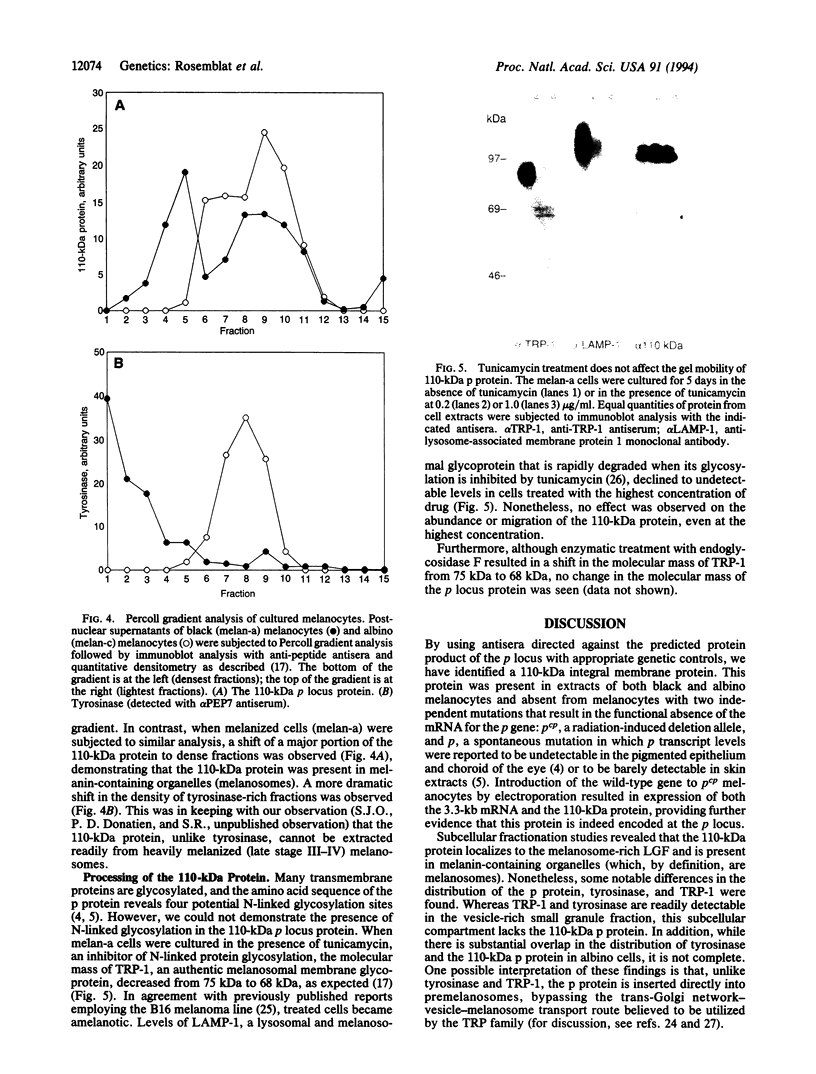
The pink-eyed dilution (p) locus in the mouse is critical to melanogenesis; mutations in the homologous locus in humans, P, are a cause of type II oculocutaneous albinism. Although a cDNA encoded by the p gene has recently been identified, nothing is known about the protein product of this gene. To characterize the protein encoded by the p gene, we performed immunoblot analysis of extracts of melanocytes cultured from wild-type mice with an antiserum from rabbits immunized with a peptide corresponding to amino acids 285-298 of the predicted protein product of the murine p gene. This antiserum recognized a 110-kDa protein. The protein was absent from extracts of melanocytes cultured from mice with two mutations (pcp and p) in which transcripts of the p gene are absent or greatly reduced. Introduction of the cDNA for the p gene into pcp melanocytes by electroporation resulted in expression of the 3.3-kb mRNA and the 110-kDa protein. Upon subcellular fractionation of cultured melanocytes, the 110-kDa protein was found to be present in melanosomes but absent from the vesicular fraction; phase separation performed with the nonionic detergent Triton X-114 confirmed the predicted hydrophobic nature of the protein. These results demonstrate that the p gene encodes a 110-kDa integral melanosomal membrane protein and establish a framework by which mutations at this locus, which diminish pigmentation, can be analyzed at the cellular and biochemical levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brilliant M. H., Gondo Y., Eicher E. M. Direct molecular identification of the mouse pink-eyed unstable mutation by genome scanning. Science. 1991 Apr 26;252(5005):566–569. doi: 10.1126/science.1673574. [DOI] [PubMed] [Google Scholar]
- COLEMAN D. L. Effect of genic substitution on the incorporation of tyrosine into the melanin of mouse skin. Arch Biochem Biophys. 1962 Mar;96:562–568. doi: 10.1016/0003-9861(62)90337-5. [DOI] [PubMed] [Google Scholar]
- Chiu E., Lamoreux M. L., Orlow S. J. Postnatal ocular expression of tyrosinase and related proteins: disruption by the pink-eyed unstable (p(un)) mutation. Exp Eye Res. 1993 Sep;57(3):301–305. doi: 10.1006/exer.1993.1128. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham-Pierre D., Gardner J. M., Nakatsu Y., King R. A., Francke U., Ching A., Aquaron R., del Marmol V., Brilliant M. H. African origin of an intragenic deletion of the human P gene in tyrosinase positive oculocutaneous albinism. Nat Genet. 1994 Jun;7(2):176–179. doi: 10.1038/ng0694-176. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Nakatsu Y., Gondo Y., Lee S., Lyon M. F., King R. A., Brilliant M. H. The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science. 1992 Aug 21;257(5073):1121–1124. doi: 10.1126/science.257.5073.1121. [DOI] [PubMed] [Google Scholar]
- Gondo Y., Gardner J. M., Nakatsu Y., Durham-Pierre D., Deveau S. A., Kuper C., Brilliant M. H. High-frequency genetic reversion mediated by a DNA duplication: the mouse pink-eyed unstable mutation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):297–301. doi: 10.1073/pnas.90.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R., Ghosh S., Duray P., Kirkwood J. M., Lerner A. B. Human melanocytes cultured from nevi and melanomas. J Invest Dermatol. 1986 Jul;87(1):95–101. doi: 10.1111/1523-1747.ep12523594. [DOI] [PubMed] [Google Scholar]
- Harris E. B., Prabhakaran K. Uptake of radioactive DOPA by Mycobacterium leprae in vitro. Microbios. 1975;12(49):119–124. [PubMed] [Google Scholar]
- Lee S. T., Nicholls R. D., Bundey S., Laxova R., Musarella M., Spritz R. A. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994 Feb 24;330(8):529–534. doi: 10.1056/NEJM199402243300803. [DOI] [PubMed] [Google Scholar]
- Mishima Y., Imokawa G. Selective aberration and pigment loss in melanosomes of malignant melanoma cells in vitro by glycosylation inhibitors: premelanosomes as glycoprotein. J Invest Dermatol. 1983 Aug;81(2):106–114. doi: 10.1111/1523-1747.ep12542192. [DOI] [PubMed] [Google Scholar]
- Moyer F. H. Genetic variations in the fine structure and ontogeny of mouse melanin granules. Am Zool. 1966 Feb;6(1):43–66. doi: 10.1093/icb/6.1.43. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y., Tyndale R. F., DeLorey T. M., Durham-Pierre D., Gardner J. M., McDanel H. J., Nguyen Q., Wagstaff J., Lalande M., Sikela J. M. A cluster of three GABAA receptor subunit genes is deleted in a neurological mutant of the mouse p locus. Nature. 1993 Jul 29;364(6436):448–450. doi: 10.1038/364448a0. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Boissy R. E., Moran D. J., Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993 Jan;100(1):55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Boissy R. E., Moran D. J., Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993 Jan;100(1):55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Chakraborty A. K., Pawelek J. M. Membrane glycoproteins common to vesicles and melanosomes in mouse melanoma cells. Pigment Cell Res. 1992;Suppl 2:162–170. doi: 10.1111/j.1600-0749.1990.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Zhou B. K., Chakraborty A. K., Drucker M., Pifko-Hirst S., Pawelek J. M. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol. 1994 Aug;103(2):196–201. doi: 10.1111/1523-1747.ep12392743. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K. Assay of diphenoloxidase--difficulties and how to avoid them. Indian J Lepr. 1990 Jul-Sep;62(3):360–362. [PubMed] [Google Scholar]
- Rinchik E. M., Bultman S. J., Horsthemke B., Lee S. T., Strunk K. M., Spritz R. A., Avidano K. M., Jong M. T., Nicholls R. D. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993 Jan 7;361(6407):72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Russell E S. A Quantitative Histological Study of the Pigment Found in the Coat-Color Mutants of the House Mouse. IV. the Nature of the Effects of Genic Substitution in Five Major Allelic Series. Genetics. 1949 Mar;34(2):146–166. doi: 10.1093/genetics/34.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIJI M., SHIMAO K., BIRBECK M. S., FITZPATRICK T. B. Subcellular localization of melanin biosynthesis. Ann N Y Acad Sci. 1963 Feb 15;100:497–533. [PubMed] [Google Scholar]
- Sidman R. L., Pearlstein R., Waymouth C. Pink-eyed dilution (p) gene in rodents: increased pigmentation in tissue culture. Dev Biol. 1965 Aug;12(1):93–116. doi: 10.1016/0012-1606(65)90023-0. [DOI] [PubMed] [Google Scholar]
- Tamate H. B., Hirobe T., Wakamatsu K., Ito S., Shibahara S., Ishikawa K. Levels of tyrosinase and its mRNA in coat-color mutants of C57BL/10J congenic mice: effects of genic substitution at the agouti, brown, albino, dilute, and pink-eyed dilution loci. J Exp Zool. 1989 Jun;250(3):304–311. doi: 10.1002/jez.1402500310. [DOI] [PubMed] [Google Scholar]
- Witkop C. J., Jr, Hill C. W., Desnick S., Thies J. K., Thorn H. L., Jenkins M., White J. G. Ophthalmologic, biochemical, platelet, and ultrastructural defects in the various types of oculocutaneous albinism. J Invest Dermatol. 1973 Jun;60(6):443–456. doi: 10.1111/1523-1747.ep12702920. [DOI] [PubMed] [Google Scholar]