Abstract
Signaling through plexin, the major cell surface receptor for semaphorin, plays critical roles in regulating processes such as neuronal axon guidance, angiogenesis and immune response. Plexin is normally kept inactive in the absence of semaphorin. Upon binding of semaphorin to the extracellular region, plexin is activated and transduces signal to the inside of the cell through its cytoplasmic region. The GTPase Activating Protein (GAP) domain in the plexin cytoplasmic region mediates the major intracellular signaling pathway. The substrate specificity and regulation mechanisms of the GAP domain have only been revealed recently. Many intracellular proteins serve as either upstream regulators or downstream transducers by directly interacting with plexin. The mechanisms of action for some of these proteins also start to emerge from recent studies. We review here these advances in the mechanistic understanding of plexin intracellular signaling from a structural perspective.
1. Introduction
Plexins are the major cell surface receptors for the axon guidance proteins semaphorins (Tamagnone et al., 1999; Winberg et al., 1998). The over 20 semaphorins are divided according to sequence conservation into eight classes (Kolodkin et al., 1993; Semaphorin_Nomenclature_Committee, 1999). Some semaphorins are secreted proteins, while others are cell surface attached through a transmembrane region or a glycosylphosphatidylinositol-linker (Semaphorin_Nomenclature_Committee, 1999). Invertebrates have two plexins (Plexins A and B), whereas the nine vertebrate plexins are organized into four classes (A, B, C and D) (Tamagnone et al., 1999). Some class A plexins require the co-receptor neuropilin (neuropilin 1 or 2) to form holo-receptors for secreted class III semaphorins (Takahashi et al., 1999).
Signals through semaphorin/plexin play essential roles in many aspects of the development of the nervous system, including axon guidance, fasciculation, branching and synapse formation (reviewed in (Kruger et al., 2005; Tran et al., 2007)). Some plexin family members are expressed in adult tissues, playing roles in controlling tissue homeostasis and regeneration after injury to the nervous system (Shim et al., 2012). While in general the semaphorin/plexin signal is repulsive in axon guidance, it can be attractive under some circumstances (reviewed in (Kruger et al., 2005; Tran et al., 2007)). In addition to their roles in the nervous system, plexins are involved in regulating angiogenesis and cardiovascular development (reviewed in (Gu and Giraudo, 2013; Sakurai et al., 2012)). Other functions of plexins include regulation of immunity and bone homeostasis (reviewed in (Kang and Kumanogoh, 2013; Takamatsu and Kumanogoh, 2012)). Genetic knockout of plexins or semaphorins are often embryonically lethal, causing severe defects in the development of the nervous and cardiovascular systems (reviewed in (Worzfeld and Offermanns, 2014)). Malfunction of the plexin pathway has been implicated in human diseases, including neurological disorder and cancer (reviewed in (Gu and Giraudo, 2013; Sakurai et al., 2012; Tamagnone, 2012; Worzfeld and Offermanns, 2014)).
In the past few years, the understanding of the mechanisms governing the regulation and signaling of plexin has grown tremendously (reviewed in (Jones, 2015)). This progress is owned largely to insights from structural studies of both the extracellular and intracellular regions of several plexin family members in various states. The N-terminal Sema domain in the plexin extracellular region binds semaphorin, on it’s own or together with the extracellular region of the neuropilin co-receptor (Janssen et al., 2012; Janssen et al., 2010; Liu et al., 2010; Nogi et al., 2010). The cytoplasmic region of neuropilin is short (~30 residues) and appears non-essential for semaphorin signaling (Nakamura et al., 1998). The activating signal initiated by semaphorin binding propagates through the multiple membrane proximal domains and the transmembrane helix to the cytoplasmic region of plexin. The cytoplasmic region is responsible for triggering intracellular signaling cascades, which ultimately lead to a variety of cellular responses that underlie the biological functions of plexin. Therefore, the cytoplasmic region of plexin and proteins associated with it have been subjected to extensive investigations to elucidate the signaling mechanisms of plexin. These aspects of plexin signaling will be the focus of this review.
2. Overall architecture and signaling mechanisms of the plexin cytoplasmic domain
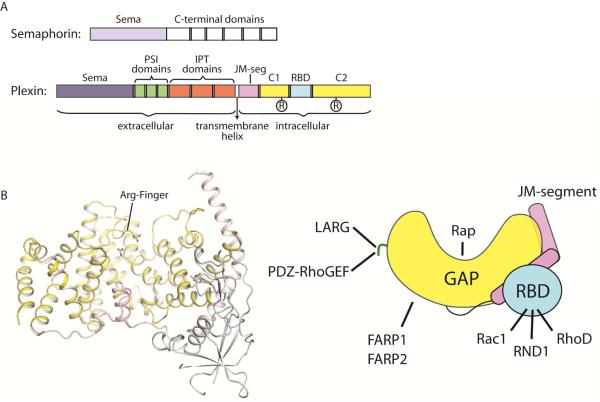
The ~600-residue cytoplasmic regions of the plexin family members are highly conserved and share a common architecture. It was discovered over a decade ago that this region contains two segments (C1 and C2) that show sequence similarity to GTPase Activating Proteins (GAPs) for Ras (Figure 1A) (Hu et al., 2001; Rohm et al., 2000). The two segments are interrupted by an insertion region. Despite this interruption, structural studies have shown that the two GAP-homology segments fold together into one intact GAP domain, which indeed structurally resembles RasGAPs such as p120GAP and neurofibromin (Figure 1B) (He et al., 2009; Tong et al., 2009). There are two conserved arginine residues in the plexin GAP domain, one in each of the two segments, corresponding to the two catalytically essential arginine residues in RasGAPs (Figure 1). Mutating these arginine residues abolishes plexin activity both in vitro and in vivo, demonstrating the essential role of the GAP domain in plexin function (Rohm et al., 2000; Worzfeld et al., 2014).
Figure 1. Overall structure of the plexin cytoplasmic region.
(A) Domain structures of semaphorin and plexin. The cartoons describe the general architectures of plexin and semaphorin, with variations among different family members omitted. (B) Structure of the cytoplasmic region of plexin. The structure of mouse PlexinA3 (PDB ID: 3IG3) is shown as a representative. The right panel shows a schematic of the structure. The color scheme is the same as in (A). The green line represents the C-terminal tail in class B plexins that interacts with LARG and PDZ-RhoGEF. The two GAP homology regions, C1 and C2 in (A), fold together to form one GAP domain. The two conserved arginine residues in the GAP domain are highlighted. The catalytic arginine finger residue is labeled “Arg-Finger”. JM-segment: juxtamembrane segment.
The identification of the GAP domain in plexins was of particular significance for establishing the fundamental signaling mechanisms underlying plexin function. Small GTPases such as Ras and Rac1 are master regulators of many fundamental cellular processes such as proliferation and cytoskeletal dynamics. They act as molecular switches, cycling between the GDP-bound inactive and GTP-bound active states. GAPs turn off GTPases by accelerating hydrolysis of the bound GTP to GDP, whereas guanine nucleotide exchange factors (GEFs) activate them by promoting the exchange of GDP for GTP. The presence of the GAP domain makes it possible for plexins to directly control the activity of small GTPases, consistent with their roles in regulating cell morphology and migration. To date, plexins remain unique as the only group of cell surface receptors known to contain a GAP domain.
The ~200-residue insertion segment between the two GAP homology regions forms an independent domain that packs against one side of the GAP domain (Figure 1B) (He et al., 2009; Tong et al., 2007; Tong et al., 2009). This domain interacts with Rho family small GTPases such as Rac1, RND1 and RhoD, and is therefore referred to as the RhoGTPase Binding Domain (RBD) (Driessens et al., 2001; Hu et al., 2001; Oinuma et al., 2003; Rohm et al., 2000; Tong et al., 2007; Turner et al., 2004; Vikis et al., 2002; Vikis et al., 2000; Zanata et al., 2002). The RBD/RhoGTPase interaction plays a regulatory role in plexin signaling. In addition to the GAP domain and the RBD, the crystal structures also revealed a juxtamembrane segment at the N-terminus of the plexin cytoplasmic region (Figure 1), which is an essential regulatory element (He et al., 2009; Wang et al., 2013).
Over 20 proteins have been reported to interact with the cytoplasmic region of plexins and contribute to signaling. Some plexin family members contain unique protein-interaction sites that mediate member-specific signaling pathways. For example, Class B plexins (PlexinB1, B2 and B3) specifically interact with two related GEFs, PDZ-RhoGEF and LARG (leukemia-associated RhoGEF) (Figure 1B). The interaction is mediated by a conserved C-terminal “VTDL” motif in class B plexins and the PDZ (PSD-95, Dlg-1 and ZO-1) domains in PDZ-RhoGEF and LARG (Aurandt et al., 2002; Driessens et al., 2002; Hirotani et al., 2002; Oinuma et al., 2003; Perrot et al., 2002; Swiercz et al., 2002). PDZ-RhoGEF and LARG recruited by Class B plexins activate RhoA, mediating an important branch of the signaling pathway (Worzfeld et al., 2014). Class A plexins interact with two other highly related GEFs FARP1 (FERM, RhoGEF, and pleckstrin homology domain protein 1) and FARP2 (Figure 1B) (Toyofuku et al., 2005; Zhuang et al., 2009). FARP1 and FARP2 contribute to plexin-mediated dendrite growth and axonal repulsion, respectively. FARP2 has also been implicated in osteoprotection by Semaphorin3A/PlexinA1 (Hayashi et al., 2012). FARPs are normally autoinhibited by adopting a closed conformation that blocks the GEF active site (He et al., 2013). The mechanism by which plexin binds FARPs and regulates their activity is not clear at present.
For comprehensive discussions on the interactions between plexins and their intracellular binding partners, please refer to recent review articles (Gay et al., 2011; Hota and Buck, 2012). Here we will focus on the GAP domain and the RBD of plexins, because they constitute the core signaling and regulatory components, and their mechanisms of action are better understood in light of recent structural studies.
3. Substrate specificity of the plexin GAP domain
Since the discovery of the GAP domain in plexin over a decade ago, many studies have been devoted to identifying its small GTPase substrate and experimentally demonstrating its catalytic activity (reviewed in (Kruger et al., 2005)). The two studies that originally identified the GAP domain did not report GAP activity to any small GTPase (Hu et al., 2001; Rohm et al., 2000). Later, a study showed that PlexinB1 is active specifically to the Ras homolog R-Ras but not Ras (Oinuma et al., 2004). This study further showed that plexin signaling is critically dependent on inactivation of R-Ras. The GAP activity, assessed by the levels of GTP-bound R-Ras in cells expressing PlexinB1, appeared to rely on concomitant binding of plexin by both semaphorin and the RhoGTPase RND1. By using the crude membrane fraction of cells over-expressing PlexinB1, GAP activity towards R-Ras was also observed in vitro. The same group later found that the PlexinB1 GAP was also active to another Ras homolog, M-Ras (Saito et al., 2009). In contrast, Sakurai et al, using similar assays, failed to detect GAP activity of PlexinD1 to R-Ras, although binding between PlexinD1 and R-Ras was observed through co-immunoprecipitation (Sakurai et al., 2010). Similarly, another study showed that the purified cytoplasmic region of PlexinB1 had no GAP activity towards R-Ras, while it displayed weak binding to R-Ras in a manner independent of the form of the bound nucleotide (Tong et al., 2009). More recently, Worzfeld et al provided strong in vivo genetic evidence that the developmental functions of PlexinB2 and PlexinD1 are not mediated by inactivation of R-Ras or M-Ras (Worzfeld et al., 2014).
A systematic analysis of most plexin family members from mouse demonstrated that the purified plexin cytoplasmic region displayed no GAP activity to either R-Ras or M-Ras (Wang et al., 2012). Rather, these plexins displayed robust GAP activity to the Rap proteins, a distinct sub-family of Ras homologs. There are five members in the Rap sub-family: Rap1A, 1B, 2A, 2B and 2C. Rap1B and Rap2A were examined, and both responded to the plexin GAP. The RapGAP activity varies among different plexin family members, but in general is quite low when compared with the RasGAP activity of p120GAP. The low basal activity and its activation by dimerization (see below) are consistent with the role of plexin as an off/on signaling switch under the control of its semaphorin ligand. In addition, the low activity may be required for plexin to induce localized inhibition of Rap without perturbing the overall pool of active Rap in the cell, which is involved in many other signaling processes independent of the plexin pathway (Wang et al., 2012).
Importantly, the RapGAP activity of plexins can be stimulated by semaphorin in cells, and is required for plexin-induced neuronal growth cone collapse (Wang et al., 2012). GTP-bound Rap is an activator of integrin for promoting cell-matrix adhesion (Gloerich and Bos, 2011). Taken together, these analyses establish that Rap is a bona fide substrate of the plexin GAP domain, and plexin-mediated Rap inactivation constitutes the core plexin signaling pathway. Inactivation of Rap by the plexin GAP would weaken integrin-mediated cell-matrix adhesion, contributing to repulsive axon guidance and other cell morphological changes. Moreover, Rap is known to inhibit RhoA activity through activating RhoGAPs (Jeon et al., 2010; Yamada et al., 2005). Inactivation of Rap by plexin may relieve the inhibition on RhoA, leading to stress fiber formation and growth cone collapse.
4. The non-canonical catalytic mechanism and unique specificity of plexin for Rap
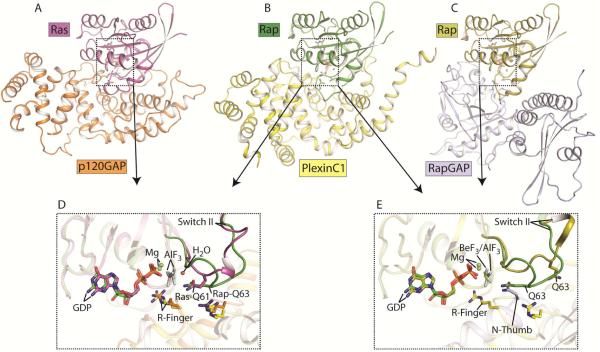
The RapGAP activity of plexin was unexpected because the plexin GAP domain structurally belongs to the RasGAP family, and is unrelated to canonical RapGAPs. RasGAPs such as p120GAP all adopt the same structure fold and contain two conserved arginine residues critically required for facilitating GTP hydrolysis for Ras (Scheffzek et al., 1997). One of the two arginine residues is termed the “arginine finger”, which plays an essential role in catalysis by neutralizing the negative charge on the leaving γ-phosphate group. The second arginine residue stabilizes the conformation of the loop in which the arginine finger resides. Concomitantly, Ras provides in cis an essential catalytic residue, Gln61, which coordinates the nucleophilic water (Figure 2A and 2D). All known RasGAPs also act on R-Ras and M-Ras using the same catalytic mechanism (Bos et al., 2007; Li et al., 1997; Ohba et al., 2000; Quilliam et al., 1999; Scheffzek et al., 1997; Scheffzek et al., 1998). This is not surprising, as Ras, R-Ras and M-Ras are virtually identical in the regions that interact with the GAPs. Rap is a unique member in the Ras family, containing a threonine at position 61 that lacks the ability to coordinate a nucleophilic water. Canonical RapGAPs are structurally unrelated to RasGAPs and do not use an arginine finger for catalysis. Instead, they contain a conserved asparagine residue, referred to as the “asparagine thumb”, which fulfills the water coordination role of Gln61 in Ras (Figure 2C and 2E) (Scrima et al., 2008).
Figure 2. Catalytic mechanisms of GAPs for Ras and Rap.
(A) Structure of the p120GAP/Ras complex (PDB ID: 1WQ1). (B) Structure of the PlexinC1/Rap1B complex (PDB ID: 4M8N). (C) Structure of the RapGAP/Rap1B complex (PDB ID: 3BRW). (D) Comparison of the active site between the p120GAP/Ras and PlexinC1/Rap1B complexes. (E) Comparison of the active site between the PlexinC1/Rap1B and RapGAP/Rap1B complexes. Ras and Rap1B are used as the references for the superimpositions in (D) and (E). In the p120GAP/Ras and PlexinC1/Rap1B structures, aluminum fluoride (AlF3) and GDP are the transition state analog of the GTP hydrolysis reaction. The RapGAP/Rap1B complex contains the ground state analog GDP and beryllium fluoride (BeF3).
The plexin GAP domain structurally resembles RasGAPs and contains two conserved arginine residues but not an asparagine thumb, appearing unsuited for facilitating GTP hydrolysis for Rap. The first clue to the catalytic mechanism of the plexin GAP came from a comparison of plexin with several GAPs that show dual GAP activity towards both Ras and Rap. These include SynGAP (Synaptic GAP), and three GAP1 family members RASAL (Ras-GTPase-activating-like protein), CAPRI (Ca2+-promoted Ras inactivator) and GAP1IP4BP (tetrakisphosphate binding protein) (Krapivinsky et al., 2004; Kupzig et al., 2006; Pena et al., 2008). The dual-specificity GAPs share the same domain fold and catalytic residues with the plexin GAP domain. They facilitate GTP hydrolysis for Ras using the same mechanism as p120GAP, but it was less clear how they act on Rap. Kinetic analyses of various structure-guided mutants of Ras, Rap, RASAL and GAP1IP4BP showed that Gln63 in Rap is critical for GTP hydrolysis catalyzed by the dual-specificity GAPs (Sot et al., 2010). These analyses suggested that the dual-specificity GAPs induce a specific conformation of Rap, allowing Gln63 to adopt the catalytic role that is normally performed by Gln61 in Ras (Sot et al., 2010). Mutating Gln63 in Rap abolishes GTP hydrolysis catalyzed by plexins, suggesting that they use the same Gln63-dependent catalytic mechanism (Wang et al., 2012).
The catalytic mechanism was elucidated recently by a crystal structure of the complex between Rap1B and the cytoplasmic region of zebrafish PlexinC1 trapped by a transition state mimic GDP/AlFx (x=3 or 4) (Wang et al., 2013). The overall binding mode between Rap and the plexin GAP domain is similar to that between Ras and p120GAP (Figure 2B) (Scheffzek et al., 1997). Rap interacts exclusively with the GAP domain of plexin, making no contacts with the juxtamembrane region or the RBD. The two conserved arginine residues in the plexin GAP domain indeed play the same roles as those in p120GAP. All small GTPases contain two so-called “switch” regions, switches I and II, which are directly involved in the binding of guanine nucleotide. The state of the bound guanine nucleotide (GTP v.s. GDP) controls the conformations of the switch regions, which determine their interactions with regulators and effectors. Similar to those in the p120GAP/Ras complex, the switch regions in Rap constitute the majority of the plexin/Rap interface. The conformations of the switch II regions are, however, strikingly different between the two structures (Figure 2D). In the p120GAP/Ras complex, the switch II adopts a smooth loop-helix structure, placing Gln61 at the tip of the loop where it is ideally positioned for coordinating the catalytic water molecule. In contrast, switch II in the plexin/Rap complex adopts a sharp hairpin-like turn, pushing residue 61 (Thr61) away from the active site. Instead, this conformation brings Gln63 into the active site, with its sidechain placed at virtually the identical location as that of Gln61 in Ras. Therefore, in plexin-catalyzed GTP hydrolysis, Gln63 in Rap indeed assumes the catalytic role analogous to Gln61 in Ras. This “Gln63-in” conformation of Rap is stabilized by interactions between Rap and several conserved residues in plexin. The dual specificity GAPs likely induce the same Gln63-in conformation to facilitate GTP hydrolysis for Rap.
The PlexinC1/Rap1B complex structure also revealed several interactions that underlie the unique specificity of the plexin GAP towards Rap, and lack of activity to Ras/R-Ras/M-Ras (Wang et al., 2013). This raises the question how the contradictory observations regarding the substrate specificity of the plexin GAP can be reconciled. P120GAP has been shown to bind tightly to GTP-bound Rap, but fail to promote GTP hydrolysis (Frech et al., 1990; Hata et al., 1990; Yatani et al., 1991). Rap therefore can act as an effective inhibitor of p120GAP. Conversion of Rap to the GDP-bound form by plexin relieves this inhibition on p120GAP, which can then decrease levels of GTP-bound Ras/R-Ras/M-Ras. This indirect effect may underlie the reported GAP activity of plexin towards R-Ras and M-Ras in the cell. Indeed, a recent study has shown that PlexinB1 uses this indirect mechanism to inhibit Ras and act as a tumor suppressor (Okada et al., 2015).
5. Autoinhibition of the plexin cytoplasmic region
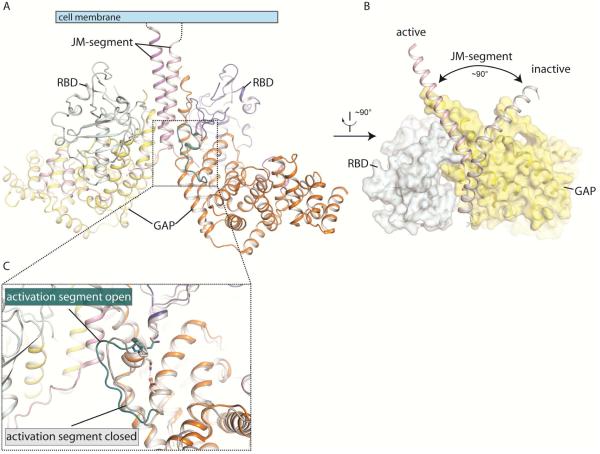
Plexin must be autoinhibited to prevent spontaneous signaling in the absence of the semaphorin ligand. A structural comparison with the p120GAP/Ras complex has suggested that the GAP domain of the monomeric cytoplasmic region of PlexinA3 adopts a closed conformation that cannot accommodate its small GTPase substrate (He et al., 2009). The structure of the PlexinC1/Rap1B complex revealed that the major inhibitory element in monomeric plexin is a helix-loop segment in the GAP domain (Figure 3C) (Wang et al., 2013). This segment is referred to as the “activation segment”, drawing analogy from protein kinases in which the activation segment controls the activity by switching between the inactive and active conformations (Wang et al., 2013). The activation segment in the PlexinA3 structure adopts a more “closed” conformation compared to that in active PlexinC1 in complex with Rap (Figure 3C). This closed conformation is stabilized by a hydrogen bond between a pair of conserved residues, an asparagine residue (Asn1774 in mouse PlexinA3) in the activation segment and an aspartate residue (Asp1758 in mouse PlexinA3) in the following helix. Moreover, a proline residue sits on top of the helix portion of the activation segment and partially buries the asparagine and aspartate residues. The rigidity of the proline residue may further stabilize the closed conformation. Consistently, this conformation is present in all the monomeric structures of several plexin family members, suggesting that it represents a free energy minimum (Bell et al., 2011; Tong et al., 2009; Wang et al., 2012).
Figure 3. Dimerization-induced activation of the plexin cytoplasmic region.
(A) Structure of the active dimer of the cytoplasmic region of zebrafish PlexinC1 (PDB ID: 4M8N). (B) Dimer-induced conformational change of the juxtamembrane region. The PlexinC1 subunit on the left from (A) is superimposed onto the structure of the inactive, monomeric PlexinA3 (PDB ID: 3IG3). The conformational difference between the two structures is highlighted. (C) Dimer-induced conformational change of the activation segment. The conformations of the activation segment in the structure of PlexinA3 and the active dimer are compared.
Docking Rap based on the PlexinC1/Rap complex structure to the inactive plexin structures showed that the proline residue sterically clashes with Rap (Wang et al., 2013). The asparagine residue is not positioned to form the two hydrogen bonds with Rap as seen in the PlexinC1/Rap complex structure. In addition, the flexible loop region in the activation segment may sample conformations that conflict with Rap binding. In contrast, the activation segment in the PlexinC1/Rap complex structure adopts an open conformation and makes intimate interactions with the switch I region of Rap. Therefore, residues in the activation segment play dual roles. They stabilize the autoinhibited conformation to suppress the basal activity of the plexin GAP. When converted to the open conformation (by dimerization, see below for details), these residues make numerous contacts with Rap and support the catalysis of GTP hydrolysis.
6. Regulation of plexin by dimerization and oligomerization
Semaphorins dimerize through the Sema domain-mediated inter-molecular interactions, and in some cases an inter-chain disulfide bond (Antipenko et al., 2003; Klostermann et al., 1998; Koppel and Raper, 1998; Love et al., 2003). Simultaneous binding of two plexin molecules by the semaphorin dimer is the first essential step in plexin activation. Recent structural studies have elucidated the binding mode between semaphorin and plexin, mediated by the N-terminal Sema domain from both molecules (Janssen et al., 2012; Janssen et al., 2010; Liu et al., 2010; Nogi et al., 2010). For semaphorin/plexin pairs that require the neuropilin co-receptor, neuropilin serves to strengthen the interaction but does not alter the binding mode (Janssen et al., 2012). All these structures were solved by using the plexin ectodomain constructs with multiple membrane proximal domains truncated. The C-termini of the two plexin molecules in these structures are placed away from each other. In full-length plexin, this distance could in principle be bridged by the membrane proximal domains, allowing the two cytoplasmic regions to interact and form a dimer that triggers intracellular signaling. On the other hand, these structures are also consistent with a model in which the semaphorin/plexin complex activates the plexin cytoplasmic region by driving dissociation of a pre-formed inhibitory dimer of this region.
The identification of Rap as the substrate of the plexin GAP allowed robust analyses of the activity of the plexin cytoplasmic region. Engineered dimers of this region from several plexin family members displayed dramatically higher GAP activity than the corresponding monomers, supporting the model that activation of the plexin cytoplasmic region is triggered by dimerization (Wang et al., 2012). Further systematic analyses of different versions of coiled coil induced plexin dimers suggested that a certain inter-subunit orientation is optimal for activation (Wang et al., 2013). This insight led to a crystal structure of the active dimer of the cytoplasmic region from zebrafish PlexinC1, revealing the activation mechanism (Wang et al., 2013). The same dimer interface and associated active conformation is also present in the structure of the PlexinC1/Rap1B complex, in which the dimerization is not enforced by the coiled coil (Wang et al., 2013).
Formation of the active dimer induces a striking conformational change in the juxtamembrane segment (Figure 3B). The N-terminal helix portion of the juxtamembrane segment in several structures of plexins in the inactive state adopts a well-defined kinked conformation and wraps around the GAP domain (He et al., 2009; Tong et al., 2009; Wang et al., 2012). In one of the PlexinB1 structures the juxtamembrane segment is disordered (Bell et al., 2011). In the active dimer, the C-terminal part of the juxtamembrane helix unwinds, which allows the N-terminal portion to straighten and pull up in relation to the GAP domain (Figure 3B) (Wang et al., 2013). It also undergoes a 90° pivotal rotation compared to the inactive conformation. The dimer interface is formed mostly by the juxtamembrane helix in this new conformation and one helix from the GAP domain. The two juxtamembrane helices from the two subunits run in parallel, poised to connect to the transmembrane helices on the cell surface (Figure 3A).
The new conformation of the juxtamembrane helix in the active dimer is coupled to a conformational change in the activation segment (Figure 3C) (Wang et al., 2013). The coupling is mediated by interactions between the juxtamembrane segment from one subunit and the activation segment from the other subunit. Through these interactions, the juxtamembrane segment pulls the activation segment away from the GAP active site, leading to the open conformation that is able to accommodate Rap as seen in the PlexinC1/Rap1B complex structure. Residues involved in these interactions as well as other parts of the dimer interface are highly conserved in the plexin family, and are critical for the dimerization-driven activation of the plexin GAP activity and induction of cell collapse (Wang et al., 2013).
Additional inter-molecular interactions beyond the 2:2 plexin/semaphorin complex may lead to their clustering on the cell surface, offering another layer of regulation to plexin signaling (Janssen et al., 2012; Janssen et al., 2010). On the other hand, an inhibitory dimer may exist to suppress the basal activity of plexin and prevent formation of the active dimer in the absence of semaphorin (Antipenko et al., 2003). Both the extracellular and intracellular regions of plexins have been implicated in mediating inhibitory dimerization (Nogi et al., 2010; Takahashi and Strittmatter, 2001; Tong et al., 2007; Tong et al., 2009). Further studies are required to examine whether these various oligomeric forms are present on the cell surface and play roles in plexin regulation.
7. Regulation of plexin by RhoGTPases
Two early studies implicated a role for Rac1 in semaphorin signaling (Jin and Strittmatter, 1997; Kuhn et al., 1999). A series of later studies, through genetic association, yeast two-hybrid and biochemical co-purification, found that the insertion region between the two GAP-homology segments binds directly to some Rho family GTPases in a GTP-dependent manner (Driessens et al., 2001; Hu et al., 2001; Oinuma et al., 2003; Rohm et al., 2000; Turner et al., 2004; Vikis et al., 2000; Zanata et al., 2002). A systematic survey has shown that the RBD of PlexinB1 binds Rac1, Rac2, Rac3, Rnd1, Rnd2, Rnd3 and RhoD, but not RhoA, Cdc42, RhoG or Rif (Fansa et al., 2013).
Binding of the RhoGTPases by plexin may sequester them from their effectors such as the PAK kinase, indicating that the RhoGTPases are downstream of plexin in the signaling pathway (Hu et al., 2001; Vikis et al., 2002). However, the plexin/RhoGTPase interactions are rather weak, with the dissociation constant in the 3-40 µM range (Fansa et al., 2013; Hota and Buck, 2009; Tong et al., 2007; Tong et al., 2009; Wang et al., 2011). It seems unlikely such a weak interaction can effectively compete with the RhoGTPase/effector interactions, which are often tighter. Over-expression of Rac1 has been shown to increase cell surface expression of plexin and its interaction with semaphorin, suggesting an inside-out signaling mechanism in which Rac1 acts as an upstream activator of plexin (Vikis et al., 2002). The upstream role of Rac1 is further supported by a study showing that a constitutively active mutant of plexin is able to induce cell collapse independent of Rac1 (Turner et al., 2004). Strikingly, binding of over-expressed RND1 to plexin has been shown to trigger cell collapse in the absence of semaphorin, suggesting that RND1 is a more potent activator than Rac1 for plexin (Zanata et al., 2002). RND1 has also been shown to promote the interaction between PlexinB1 and PDZ-RhoGEF or LARG (Oinuma et al., 2003). Intriguingly, although RhoD binds plexin with similar affinity, and presumably in the same mode as Rac1 and RND1, it strongly inhibits, rather than activates, plexin signaling (Zanata et al., 2002).
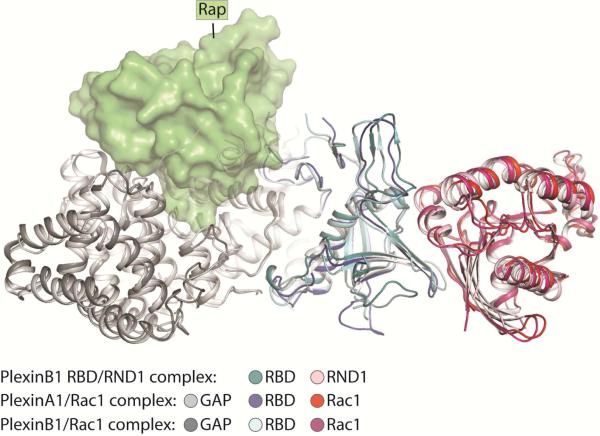
Structural analyses have revealed that the middle portion of the insertion region forms the RBD with an ubiquitin-like fold (He et al., 2009; Tong et al., 2007; Tong et al., 2009). One side of the RBD is packed against the GAP domain, whereas the other side mediates the interactions with RhoGTPases (Figure 4). The RBD/RhoGTPase interface is dominated by interactions between a hydrophobic patch on the RBD and the switch II helix of the Rho GTPases in the GTP-bound active conformation (Bell et al., 2011; Wang et al., 2011; Wang et al., 2012). Binding of the RhoGTPases does not induce any significant conformational change to plexin (Bell et al., 2011; Wang et al., 2012). The bound RhoGTPase is located far away from the active site of the GAP domain, and is therefore unlikely to affect the GAP/Rap interaction directly (Figure 4). Consistently, activity assays in solution showed that the RhoGTPases do not alter the RapGAP activity of plexin either in the monomeric or the active dimer state (Wang et al., 2012). A model of plexin activation involving RhoGTPase-mediated oligomerization has been proposed (Bell et al., 2011), but existence of this oligomeric structure in solution or on the cell surface has not been established (Siebold and Jones, 2013). These observations together suggest that the RhoGTPases may only regulate full-length plexin in the context of the cell membrane. Regulation of phospholipase C-beta by several elements has been proposed to rely on the membrane as the “conduit” of allostery (Charpentier et al., 2014). The membrane may play a similar role in plexin regulation by the RhoGTPases.
Figure 4. Interaction between the RhoGTPases and the Plexin RBD.
The structures of the PlexinA1/Rac1 complex (PDB ID: 3RYT), the PlexinB1/Rac1 complex (PDB ID: 3SUA) and the PlexinB1 RBD/RND1 complex (PDB ID: 2REX) are superimposed. Rap is docked based on the structure of the PlexinC1/Rap1B complex to indicate the location of the active site in the plexin GAP domain. It is clear that the RhoGTPases bound to the RBD are placed far away from the GAP active site.
9. Conclusion
A framework for understanding the regulation and signaling mechanisms of plexin has emerged from structural studies in the past few years (Figure 5). It is now clear how semaphorin binds plexin and induces its dimerization. This activation signal triggers the transition of the cytoplasmic region of plexin from the autoinhibited to the active dimer state. The activated cytoplasmic region transduces signal primarily by acting as a GAP for Rap. These new insights set the stage for addressing remaining mechanistic questions, including the structural basis and functional role of the inhibitory dimer/oligomer of plexin, and how exactly the RhoGTPases regulate plexin activity. More broadly, it is also important to address how the many binding partners interact with plexin, and how the interactions are coordinated to trigger multiple signaling pathways that regulate a variety of the biological processes. Answering these questions may require the combination of structural studies and biophysical analyses in the context of the lipid membrane. Finally, structural analyses of full-length plexin are ultimately required for elucidating the role of the transmembrane region, and the precise mechanism of the coupling between the extracellular and intracellular regions. This remains a major challenge, both for plexin specifically and for all single-pass transmembrane receptors in general. With recent advances in membrane protein crystallography, cryo-electron microscopy and computational approaches, the goal may be within reach in the next few years.
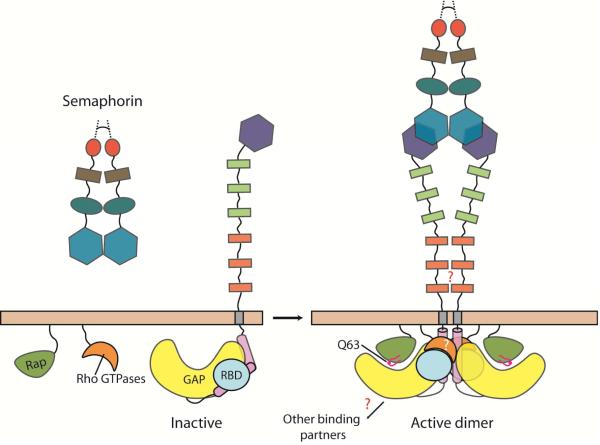
Figure 5. Overall model of plexin regulation and signaling.
The model describes semaphorin-induced transition of plexin from the inactive monomer to the active dimer state. Note that basal activity of plexin may be further suppressed by formation of an inhibitory dimer, which is not well characterized and therefore not shown. Question marks indicate unanswered mechanistic questions.
Acknowledgements
We thank members of the Zhang laboratory for critical reading of the manuscript. X.Z. is a Virginia Murchison Linthicum Scholar in Medical Research at UTSW, and supported in part by grants from the National Institutes of Health, USA (NIH) (GM088197), the Welch foundation, USA (I-1702) and the Cancer Prevention and Research Institute of Texas, USA (RP140661). H.G.P is supported in part by a cell and molecular biology training grant from the NIH (GM008203).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, Lesniak J, Barton WA, Rajashankar KR, Lu M, Hoemme C, Puschel AW, et al. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39:589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci U S A. 2002;99:12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CH, Aricescu AR, Jones EY, Siebold C. A dual binding mode for RhoGTPases in plexin signalling. PLoS Biol. 2011;9:e1001134. doi: 10.1371/journal.pbio.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Charpentier TH, Waldo GL, Barrett MO, Huang W, Zhang Q, Harden TK, Sondek J. Membrane-induced Allosteric Control of Phospholipase C-beta Isozymes. The Journal of biological chemistry. 2014;289:29545–29557. doi: 10.1074/jbc.M114.586784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- Driessens MH, Olivo C, Nagata K, Inagaki M, Collard JG. B plexins activate Rho through PDZ-RhoGEF. FEBS letters. 2002;529:168–172. doi: 10.1016/s0014-5793(02)03323-9. [DOI] [PubMed] [Google Scholar]
- Fansa EK, Dvorsky R, Zhang SC, Fiegen D, Ahmadian MR. Interaction characteristics of Plexin-B1 with Rho family proteins. Biochemical and biophysical research communications. 2013;434:785–790. doi: 10.1016/j.bbrc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Frech M, John J, Pizon V, Chardin P, Tavitian A, Clark R, McCormick F, Wittinghofer A. Inhibition of GTPase activating protein stimulation of Ras-p21 GTPase by the Krev-1 gene product. Science. 1990;249:169–171. doi: 10.1126/science.2164710. [DOI] [PubMed] [Google Scholar]
- Gay CM, Zygmunt T, Torres-Vazquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Developmental biology. 2011;349:1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends Cell Biol. 2011;21:615–623. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Gu C, Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res. 2013;319:1306–1316. doi: 10.1016/j.yexcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Kikuchi A, Sasaki T, Schaber MD, Gibbs JB, Takai Y. Inhibition of the ras p21 GTPase-activating protein-stimulated GTPase activity of c-Haras p21 by smg p21 having the same putative effector domain as ras p21s. The Journal of biological chemistry. 1990;265:7104–7107. [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- He H, Yang T, Terman JR, Zhang X. Crystal structure of the plexin A3 intracellular region reveals an autoinhibited conformation through active site sequestration. Proc Natl Acad Sci U S A. 2009;106:15610–15615. doi: 10.1073/pnas.0906923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Kuo YC, Rosche TJ, Zhang X. Structural basis for autoinhibition of the guanine nucleotide exchange factor FARP2. Structure. 2013;21:355–364. doi: 10.1016/j.str.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani M, Ohoka Y, Yamamoto T, Nirasawa H, Furuyama T, Kogo M, Matsuya T, Inagaki S. Interaction of plexin-B1 with PDZ domain-containing Rho guanine nucleotide exchange factors. Biochemical and biophysical research communications. 2002;297:32–37. doi: 10.1016/s0006-291x(02)02122-8. [DOI] [PubMed] [Google Scholar]
- Hota PK, Buck M. Thermodynamic characterization of two homologous protein complexes: associations of the semaphorin receptor plexin-B1 RhoGTPase binding domain with Rnd1 and active Rac1. Protein Sci. 2009;18:1060–1071. doi: 10.1002/pro.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Janssen BJ, Malinauskas T, Weir GA, Cader MZ, Siebold C, Jones EY. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nature structural & molecular biology. 2012;19:1293–1299. doi: 10.1038/nsmb.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CY, Kim HJ, Lee JY, Kim JB, Kim SC, Park JB. p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells. Exp Mol Med. 2010;42:335–344. doi: 10.3858/emm.2010.42.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EY. Understanding cell signalling systems: paving the way for new therapies. Philosophical transactions Series A, Mathematical, physical, and engineering sciences. 2015:373. doi: 10.1098/rsta.2013.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Seminars in cell & developmental biology. 2013;24:163–171. doi: 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lohrum M, Adams RH, Puschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. The Journal of biological chemistry. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Koppel AM, Raper JA. Collapsin-1 covalently dimerizes, and dimerization is necessary for collapsing activity. The Journal of biological chemistry. 1998;273:15708–15713. doi: 10.1074/jbc.273.25.15708. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nature reviews. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S, Deaconescu D, Bouyoucef D, Walker SA, Liu Q, Polte CL, Daumke O, Ishizaki T, Lockyer PJ, Wittinghofer A, et al. GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. The Journal of biological chemistry. 2006;281:9891–9900. doi: 10.1074/jbc.M512802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Nakamura S, Hattori S. Activation of R-Ras GTPase by GTPase-activating proteins for Ras, Gap1(m), and p120GAP. The Journal of biological chemistry. 1997;272:19328–19332. doi: 10.1074/jbc.272.31.19328. [DOI] [PubMed] [Google Scholar]
- Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nature structural biology. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, et al. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- Ohba Y, Mochizuki N, Yamashita S, Chan AM, Schrader JW, Hattori S, Nagashima K, Matsuda M. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. The Journal of biological chemistry. 2000;275:20020–20026. doi: 10.1074/jbc.M000981200. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Harada A, Negishi M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. The Journal of biological chemistry. 2003;278:25671–25677. doi: 10.1074/jbc.M303047200. [DOI] [PubMed] [Google Scholar]
- Okada T, Sinha S, Esposito I, Schiavon G, Lopez-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M, et al. The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras-MAPK signalling. Nature cell biology. 2015;17:81–94. doi: 10.1038/ncb3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena V, Hothorn M, Eberth A, Kaschau N, Parret A, Gremer L, Bonneau F, Ahmadian MR, Scheffzek K. The C2 domain of SynGAP is essential for stimulation of the Rap GTPase reaction. EMBO reports. 2008;9:350–355. doi: 10.1038/embor.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. The Journal of biological chemistry. 2002;277:43115–43120. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- Quilliam LA, Castro AF, Rogers-Graham KS, Martin CB, Der CJ, Bi C. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. The Journal of biological chemistry. 1999;274:23850–23857. doi: 10.1074/jbc.274.34.23850. [DOI] [PubMed] [Google Scholar]
- Rohm B, Rahim B, Kleiber B, Hovatta I, Puschel AW. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS letters. 2000;486:68–72. doi: 10.1016/s0014-5793(00)02240-7. [DOI] [PubMed] [Google Scholar]
- Saito Y, Oinuma I, Fujimoto S, Negishi M. Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO reports. 2009;10:614–621. doi: 10.1038/embor.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Doci C, Gutkind JS. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell research. 2012;22:23–32. doi: 10.1038/cr.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Molecular and cellular biology. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Wiesmuller L, Kabsch W, Stege P, Schmitz F, Wittinghofer A. Structural analysis of the GAP-related domain from neurofibromin and its implications. Embo J. 1998;17:4313–4327. doi: 10.1093/emboj/17.15.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima A, Thomas C, Deaconescu D, Wittinghofer A. The Rap-RapGAP complex: GTP hydrolysis without catalytic glutamine and arginine residues. Embo J. 2008;27:1145–1153. doi: 10.1038/emboj.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaphorin_Nomenclature_Committee Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Shim SO, Cafferty WB, Schmidt EC, Kim BG, Fujisawa H, Strittmatter SM. PlexinA2 limits recovery from corticospinal axotomy by mediating oligodendrocyte-derived Sema6A growth inhibition. Mol Cell Neurosci. 2012;50:193–200. doi: 10.1016/j.mcn.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold C, Jones EY. Structural insights into semaphorins and their receptors. Seminars in cell & developmental biology. 2013;24:139–145. doi: 10.1016/j.semcdb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Sot B, Kotting C, Deaconescu D, Suveyzdis Y, Gerwert K, Wittinghofer A. Unravelling the mechanism of dual-specificity GAPs. EMBO J. 2010;29:1205–1214. doi: 10.1038/emboj.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Strittmatter SM. Plexina1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends in immunology. 2012;33:127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell. 2012;22:145–152. doi: 10.1016/j.ccr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. The Journal of biological chemistry. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Hota PK, Penachioni JY, Hamaneh MB, Kim S, Alviani RS, Shen L, He H, Tempel W, Tamagnone L, et al. Structure and function of the intracellular region of the plexin-b1 transmembrane receptor. The Journal of biological chemistry. 2009;284:35962–35972. doi: 10.1074/jbc.M109.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nature neuroscience. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annual review of cell and developmental biology. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. The Journal of biological chemistry. 2004;279:33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes & development. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikis HG, Li W, He Z, Guan KL. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc Natl Acad Sci U S A. 2000;97:12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hota PK, Tong Y, Li B, Shen L, Nedyalkova L, Borthakur S, Kim S, Tempel W, Buck M, et al. Structural basis of Rnd1 binding to plexin Rho GTPase binding domains (RBDs) The Journal of biological chemistry. 2011;286:26093–26106. doi: 10.1074/jbc.M110.197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, Jiang J, Cowan CW, Zhang X. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Science signaling. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pascoe HG, Brautigam CA, He H, Zhang X. Structural basis for activation and non-canonical catalysis of the Rap GTPase activating protein domain of plexin. Elife. 2013;2:e01279. doi: 10.7554/eLife.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nature reviews Drug discovery. 2014;13:603–621. doi: 10.1038/nrd4337. [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Swiercz JM, Senturk A, Genz B, Korostylev A, Deng S, Xia J, Hoshino M, Epstein JA, Chan AM, et al. Genetic dissection of plexin signaling in vivo. Proc Natl Acad Sci U S A. 2014;111:2194–2199. doi: 10.1073/pnas.1308418111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Sakisaka T, Hisata S, Baba T, Takai Y. RA-RhoGAP, Rap-activated Rho GTPase-activating protein implicated in neurite outgrowth through Rho. The Journal of biological chemistry. 2005;280:33026–33034. doi: 10.1074/jbc.M504587200. [DOI] [PubMed] [Google Scholar]
- Yatani A, Quilliam LA, Brown AM, Bokoch GM. Rap1A antagonizes the ability of Ras and Ras-Gap to inhibit muscarinic K+ channels. The Journal of biological chemistry. 1991;266:22222–22226. [PubMed] [Google Scholar]
- Zanata SM, Hovatta I, Rohm B, Puschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron. 2009;61:359–372. doi: 10.1016/j.neuron.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]