Abstract
Palbociclib is a selective inhibitor of cyclin-dependent kinases 4 and 6 that acts by reducing phosphorylation of the tumor suppressor gene Retinoblastoma. When added to the aromatase inhibitor letrozole in a randomized phase II trial for first-line therapy of estrogen receptor-positive, HER2-negative metastatic breast cancer, palbociclib significantly increased progression-free survival compared to letrozole alone (palbociclib + letrozole: 20.2 months (95% CI 13.8-27.5), letrozole:10.2 months (95% CI 5.7-12.6); hazard ratio 0.49 (95% CI 0.32-0.75), p=0.0004). Based on these results the drug was recently granted accelerated approval by the FDA, and confirmatory studies are ongoing. Since this drug has a rational target in an oncologic pathway, concurrent biomarker development is of interest. In breast cancer, the most useful predictive biomarkers identified thus far are estrogen receptor and HER2 receptor status, although additional studies are ongoing. In this article, we review the development of palbociclib and its use in treatment of hormone receptor-positive metastatic breast cancer in the context of other FDA-approved agents in this setting.
Introduction
Targeted therapy for hormone receptor positive breast cancer has been used clinically for more than a century, primarily in the form of blocking estrogen signaling through estrogen receptor (ER) modulation or lowering circulating estrogen levels. Adjuvant endocrine therapy has improved survival rates in early stage ER+ breast cancer. However, for those who develop metastatic disease, the vast majority will experience progression of their cancer despite treatment with this targeted therapy and will ultimately succumb to the disease. Therefore, new targeted agents have been sought that are directly anti-neoplastic or that enhance the efficacy of existing therapies.
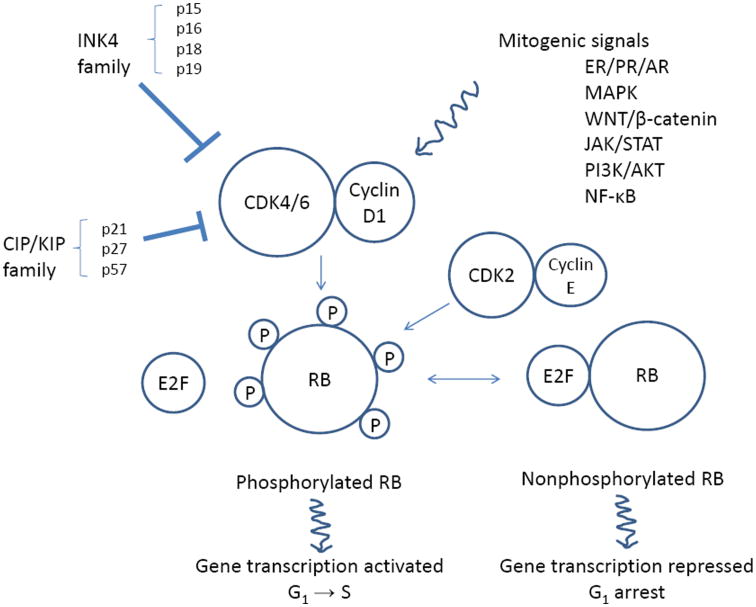
One class of targeted agents that has recently been demonstrated to have benefit for treatment of ER+ breast cancer is the cyclin-dependent kinase (CDK) 4/6 inhibitors. Cell cycle dysregulation is one of the hallmarks of cancer, and alterations in the G1-S checkpoint pathway are frequently reported in breast cancer (1, 2). One of the most studied tumor suppressors that play a role in G1-S cell cycle dysregulation is retinoblastoma (RB) protein. Loss of RB function leads to oncogenic cellular proliferation. CDK4/6 is one of the key regulators of RB and G1-S transition (Fig. 1). CDK4/6 partners with cyclin D1 to promote phosphorylation of RB, which releases transcriptional factor E2F and subsequently leads to increased transcription of genes involved in S-phase progression (3-6). The mechanisms by which CDK4/6 dysregulation may affect cellular proliferation in breast cancer often involve enhancers (cyclin D1 overexpression or amplification) and inhibitors (loss of p16 or p27) of the CDK4/6–cyclin D1 complex (2, 4). Moreover, since cyclin D1 is a transcriptional target of ER, CDK4/6 is a rational target for drug development for ER+ breast cancer.
Figure 1.
The role of CDK 4/6 in cell cycle progression. CDK4/6–cyclin D1 complex is downstream of various mitogenic signals and suppressed by INK4 and CIP/KIP families. CDK4/6–cyclin D1 complex phosphorylates RB protein, which in turn releases E2F transcriptional factor and lead to G1 to S cell cycle progression. CDK, cyclin-dependent kinases; INK4, inhibitor of kinase 4; CIP/KIP, CDK interacting protein/kinase inhibitory protein; RB, retinoblastoma; G1, gap 1 cell cycle phase; S, synthesis cell cycle phase; ER, estrogen receptor; PR, progesterone receptor; AR, androgen receptor; MAPK, mitogen-activated protein kinase; JAK, Janus kinase; STAT, signal transducer and activator of transcription; PI3K, phosphoinositide 3-kinase
Alteration of the CDK4/6–cyclin D1 complex is thought to be mutually exclusive with RB loss (2, 3, 7). RB loss is reported to occur in about 20-35% of breast cancer and has been associated with ER negative disease (2, 8). Among the ER+ breast cancers, the luminal B subtype has been more strongly associated with the RB loss gene signature compared to the luminal A subtype (9), with cyclin D1 amplification in 29% and 58% and CDK4 gain in 14% and 25% of luminal A and B, respectively (2).
Pharmacology and Preclinical Development
Palbociclib (PD-0332991) is a selective inhibitor of CDK 4/6 (Pfizer, New York, NY, USA) (10). It is orally administered with a mean bioavailability of 46% Based on pharmacokinetic studies, it is recommended that the drug be administered with food for more consistent absorption and exposure. Its peak concentration is between 6 and 12 hours, its mean plasma half-life is 29 hours, and it reaches steady state within 8 days (11). The drug undergoes hepatic metabolism with involvement of CYP3A and SULT2A1 enzymes. Thus the concomitant use of strong CYP3A modulators (inhibitors and inducers) is not recommended (11). In addition, palbociclib has been reported to have CYP3A inhibitory effect in vivo. Therefore, dose adjustment and monitoring are recommended if palbociclib is prescribed concomitantly with drugs that undergo CYP3A metabolism.
During its development, this compound was selected for its CDK4/6 specificity, and showed equivalent potency for CDK4 and CDK6 (10). In addition, as predicted it induced G1 arrest in RB-positive but not RB-negative cell lines and xenografts (10, 12, 13). The activity of the drug was associated with reduced RB phosphorylation and decreases in the Ki-67 proliferation marker. Subsequently, a panel of 47 breast cancer cell lines was treated with palbociclib, and gene expression profiles were evaluated for associations with drug response (12). The drug showed cytostatic growth inhibition of luminal ER+ as well as HER2+ cell lines. In the gene expression analyses, higher levels of RB1 and cyclin D1 and lower levels of p16 were associated with sensitivity to the drug. RB phosphorylation was significantly decreased in sensitive but not in resistant cell lines, suggesting that RB phosphorylation is a potential pharmacodynamic marker for drug activity (12).
ER can directly upregulate the cyclin D1 promoter, and endocrine therapy can induce downregulation of cyclin D (9, 14). Synergy between palbociclib and endocrine therapy was therefore examined and was demonstrated in preclinical models (12). Addition of palbociclib to either letrozole or fulvestrant increased inhibition of RB phosphorylation, reduced expression of E2F, FOXM1 and downstream target genes, and decreased cellular proliferation arrest (15). Additionally, the combination of palbociclib and letrozole showed greater tumor inhibition due to cellular senescence rather than apoptosis in a xenograft model. In endocrine-resistant cell line models, palbociclib is also able to elicit a response and induce cellular senescence (9, 12). Therefore, combining a CDK4/6 inhibitor and endocrine therapy for dual targeting of CDK4/6-cyclin D complex or to address endocrine resistance is theoretically rational.
Clinical Development
Two separate phase I trials of palbociclib monotherapy using different dose schedules have been reported. In a phase I study evaluating a 2 weeks on, 1 week off (2/1) schedule, 33 patients with RB-positive solid tumors and non-Hodgkins lymphoma were enrolled and treated with doses ranging from 100 to 225 mg (16). The most common toxicity was cytopenia. One partial response (PR) was observed in a patient with testicular cancer, and stable disease (SD) was noted in 29%. Based on the results, the recommended phase II dose (RP2D) was 200 mg (16).
In the other trial, doses ranging from 25 to 150 mg orally daily in a 3 weeks on/1 week off (3/1) schedule were evaluated in 41 patients with RB-positive solid tumors and non-Hodgkins lymphoma, including 5 (12%) patients with breast cancer (17). Twelve percent of patients experienced dose limiting neutropenia. Thirty-five percent of patients had SD, including 1 breast cancer patient. The RP2D was 125 mg daily. Both trials demonstrated that the drug was generally well-tolerated and the most common adverse events were cytopenias and fatigue. Although 1 PR was noted in patients receiving drug on the 2/1 schedule, more patients experienced durable SD when treated on the 3/1 schedule (3/1: 16% with ≥ 10 cycles vs. 2/1: 10% with ≥ 10 cycles) (16, 17).
A subsequent phase II clinical trial of 37 patients with RB-positive breast cancer was conducted using the 125 mg dose on the 3/1 schedule (18). In this trial, 7% of patients had a PR and 50% had SD. The overall clinical benefit (PR+ SD≥ 6 cycles) was 21% among 33 ER+ patients. In this heavily pretreated group (76% with ≥2 lines of therapy), median progression free survival (PFS) was 3.8 months for patients with ER+HER2- disease and 5.1 months for ER+HER2+disease. A correlative biomarker analysis examined RB expression/localization, Ki-67, p16 loss and CCND1 amplification. Only ER+ status was associated with response. At present, based on the preclinical data and the phase II trial results, ER+ or HER+ status appears to be the best predictive biomarker for palbociclib in patients with RB-positive breast cancer.
In addition to palbociclib monotherapy, palbociclib in combination with endocrine therapy has also been studied. Postmenopausal women with ER+HER2- metastatic breast cancer were treated on a phase I trial using a starting dose of 125 mg palbociclib given on the 3/1 schedule in combination with 2.5 mg daily of the aromatase inhibitor (AI) letrozole (19). No additional toxicity concerns were raised with the addition of an AI.
A randomized phase II trial of letrozole with or without palbociclib as first-line treatment (PALOMA1/TRIO1) was conducted in 165 postmenopausal women with ER+/HER2- metastatic breast cancer (20). Investigator-assessed PFS was the primary endpoint, and median follow-up was about 28 months. Initially there were two independent cohorts, patients with ER+/HER2- tumors (cohort 1) and patients who also had CCND1 amplification and/or loss of p16 (cohort 2). Unplanned interim analysis revealed that cohort selection based on the CCND1 or p16 biomarkers would not improve outcome compared to using ER+ and HER2- alone, so enrollment into cohort 2 was stopped. Pooled analysis of two cohorts revealed that the median PFS with the combination was 20.2 months (95% CI, 13.8–27.5) and with letrozole alone was 10.2 months (95% CI, 5.7–12.6), with a hazard ratio of 0.49 (95% CI, 0.32–0.75, P<0.001).The overall response rates were 43% (95% CI 32-54) vs 33% (95% CI 23-45) in favor of the combination treatment (p=0.13). Median overall survival (OS) was 37.5 months (95% CI 28.4-not estimable) for the combination group and 33.3 months (95% CI 26.4-not estimable) for the letrozole group, with a hazard ratio of 0.81 (95% CI 0.49 -1.35, p=0.42).
Based on the result of this randomized phase II trial, palbociclib received the breakthrough therapy designation in April 2013, and accelerated approval by the FDA in February 2015. The approval is contingent upon results of confirmatory studies, which are fully accrued and results are pending. The recommended starting dose is 125 mg orally daily for 21 days followed by 7 days off in 28 day cycles in combination with letrozole 2.5 mg orally daily continuously.
There were some notable findings from this trial. Overall, the patients treated on the letrozole alone arm had a PFS of 10.2 months, which is similar to that previously reported in multiple clinical trials of front-line endocrine therapy (Table 1). However, it is interesting that in cohort 1, which included unselected patients with ER+/HER2- disease, the letrozole arm had a lower than expected median PFS compared to historical data (Table 1). It is uncertain if this represents a true difference in the patients accrued to this trial relative to prior trials, or if it is an artifact due to the small sample size of this subset of patients. In a sub-analysis, the degree of benefit seen from the addition of palbociclib to letrozole was less in the biomarker-enriched cohort than in cohort 1. This suggests that p16 loss and CCND1 amplification may not be predictive for response to CDK4/6 inhibition, but may be prognostic.
Table 1. Selected trials of first-line endocrine therapy for ER+ metastatic breast cancer.
| Study drug (s) | N | Study population | Trial phase | ORR | PFS/TTP | OS | Reference |
|---|---|---|---|---|---|---|---|
| Tamoxifen (T) vs. Anastrozole (A) (combined North America and TARGET) | 1021 | ER+ or unknown receptor, postmenopausal; 60% ER+, 67% no prior endocrine | Ph III | T: 27.1% A: 29% |
T: 7 mo A: 8.5 mo; HR 1.13, p=NS ER+ subgroup: T: 6.4 mo A: 10.7 mo (p=0.02) |
T: 40.1 mo A: 39.2 mo HR 0.97 (p > 0.05) |
Bonneterre 2001 (29), Nabholtz 2003 (30) |
| Tamoxifen (T) vs. Letrozole (L) | 916 | ER+ or unknownreceptor, | Ph III | T: 21% L: 32% (p<0.01) |
T: 6 mo L: 9 mo (p<0.001) |
T: 30 mo L: 34 mo (p=NS) |
Mouridsen 2003 (31) |
| Tamoxifen (T) vs. Exemestane (E) (EORTC) | 371 | ER+ or unknownreceptor | PhIII | T: 31% E: 46% (p= 0.005) |
T: 5.8 mo E: 9.9 mo (p=0.121) |
NS | Paridaens 2008 (32) |
| Fulvestrant (F) vs. Anastrozole (A) (FIRST) | 205 | ER+ postmenopausal, 75% no prior endocrine | Ph II | F: 36% A: 35.5%; ORR 1.02 (p=0.947) |
F: Not reached A: 12.6 mo HR 0.63 (p=0.496) |
F: 54.1 mo A: 48.4 mo HR 0.70 (p=0.041) |
Robertson 2009, 2012, 2014 (33-35) |
| Anastrozole + Fulvestrant (AF) vs. Anastrozole (A) (FACT) | 514 | ER+ postmenopausal or premenopausal with ovarian suppression, 30.2-34.4% with no previous endocrine therapy; * fulvestrant at 250 mg dose | Ph III | AF: 31.8% A: 33.6% |
AF: 10.8 mo A: 10.2 mo HR 0.99 (p=0.91) |
AF: 37.8 mo A: 38.2 mo |
Bergh 2012 (36) |
| Anastrozole + Fulvestrant (AF) vs. Anastrozole (A)(SWOG) | 707 | ER+ postmenopausal, 59.7% no prior adjuvant tamoxifen; *fulvestrant at 250 mg dose | PhIII | AF: 27% A: 22% |
AF: 15 mo A: 13.5 mo HR 0.80 (p=0.007) .No prior adjuvant tamoxifen: HR 0.74 (p=0.006) Prior adjuvant tamoxifen: HR 0.89 (p= 0.37) |
AF: 47.7 mo A: 41.3 mo HR0.81 (p=0.049) No prior adjuvant tamoxifen: HR0.74 (p=0.04) Prior adjuvant tamoxifen: HR 0.91 (p= 0.22) |
Mehta 2012 (37) |
| Temsirolimus + Letrozole (TL) vs. Letrozole (L) (HORIZON) | 1112 | AI-naïve ER+, 21% HER2+ | Ph III | TL: 27% L: 27% |
TL: 9 mo L: 8.9 mo; HR 0.9 (p=0.25) |
NS | Wolff 2013 (38) |
| Fulvestrant + Letrozole +Bevacizumab vs. Fulvestrant +Letrozole (LEA) | 374 | ER+HER2- postmenopausal, 48% no prior endocrine therapy | Ph III | FLB: 41% FL: 22% (p<0.001) |
FLB: 19.3 mo FL: 14.4 mo, HR 0.83 (p=0.126) |
NS | Martin 2015 (39) |
| Palbociclib+Letrozolevs Letrozole (PALOMA-1/TRIO-18) | 165 | ER+ HER2- postmenopausal, Cohort1: First line ER+HER2-, Cohort2: First line, positive for CCND1 amplification or loss of RB, 49% de novo | PhII | Overall P+L: 43% L: 33% (p=0.13) |
Overall P+L: 20.2 mo L: 10.2 mo Cohort 1: P+L: 26.1 mo L: 5.7 mo Cohort 2: P+L: 18.1 mo L: 11.1 mo |
Overall P+L: 37.5 mo L: 33.3 mo HR 0.813 (p=0.42) |
Finn 2015 (20) |
| Palbociclib+ Fulvestrant vs Placebo+ Fulvestrant (PALOMA3) | 521 | ER+ HER2- postmenopausal or premenopausal with ovarian suppression | Ph III | Overall P+F: 10.4% Placebo+F: 6.3% |
Overall: P+F: 9.2 mo Plac+F: 3.8 mo |
Not yet reported | Turner 2015 (21) |
NS, no statistically significant difference; ER, estrogen receptor; AI, aromatase inhibitor; Ph, Phase; HR, hazard ratio; mo, months; N: number; ORR: overall response rate; OS: overall survival; TTP: time to progression; PFS: progression-free survival
Findings from the confirmatory trial PALOMA-2, a double-blind, 2:1 randomized phase III trial of palbociclib plus letrozole vs. placebo plus letrozole for the first line treatment of post-menopausal patients with ER+/HER-2-advanced breast cancer is expected to be reported later this year. In the second-line setting, palbociclib plus fulvestrant was recently demonstrated to result in an improvement in PFS compared to placebo plus fulvestrant in the PALOMA-3 phase III trial, which was stopped early for efficacy (21). The median PFS was 9.2 months (95% CI, 7.5-not estimable) for the palbociclib-containing regimen and 3.8 months (95% CI, 3.5-5.5) for the fulvestrant plus placebo arm (hazard ratio 0.42 (95% CI, 0.32-0.56, p<0.001). At the time of reporting, OS data were not mature, and doubled-blinding has been continued in the follow-up period.
In addition, palbociclib and other CDK4/6 inhibitors are being further studied as a single agent or in combination with other drugs in different clinical settings for breast cancer (Table 2), as well as for additional solid tumors and hematologic malignancies. For example, based on evidence that this class of drugs is able to penetrate the blood brain barrier and has activity in HER2+ disease, clinical trials for patients with brain metastases and with HER2+ disease have been initiated, including trials of abemaciclib (NCT02308020) and of palbociclib plus TDM-1 (NCT01976169).
Table 2. Currently ongoing studies of palbociclib in breast cancer.
| Drug (s) | Study setting | Study population | Phase | Clinicaltrials.gov ID |
|---|---|---|---|---|
| Palbociclib + letrozole (PALLET) | Neoadjuvant | ER+HER2- | II | NCT02296801 |
| Palbociclib + anastrozole | Neoadjuvant | Stage II/III ER+HER2- | II | NCT01723774 |
| Palbociclib + fulvestrant (PALOMA-3) | Advanced, after endocrine failure | HR+HER2- | III | NCT01942135 |
| Palbociclib + letrozole (PALOMA 4) | Advanced, first line, Asian | ER+HER2- | III | NCT02297438 |
| Palbociclib +endocrine therapy (AI or tamoxifen) | Adjuvant | HR+HER2- | II | NCT02040857 |
| Palbociclib + fulvestrant or tamoxifen | Advanced | ER+ breast cancer, exposed to mTOR or PI3K inhibitor | II | NCT02384239 |
| Palbociclib + endocrine therapy (PENELOPE-B) | Adjuvant in residual disease after neodajuvant | HR+HER2- | III | NCT01864746 |
| Palbociclib + PI3K inhibitor and fulvestrant | Advanced | ER any, sub-cohort with PIK3CA mutant | I | NCT02389842 |
| Palbociclib + exemestane vs. capecitabine | Advanced, refractory to non-steroidal AI | HR+HER2- | III | NCT02028507 |
| Palbociclib vs. Palbociclib + letrozole | Advanced, first line | HR+HER2- | I/II | NCT01684215 |
| Palbociclib + TDM-1 | Advanced, progression on prior anti-HER2 therapy | HER2+ | I | NCT01976169 |
Toxicity
In the phase II monotherapy trial, cytopenias were the most frequently observed adverse events: Grade 3 or 4 leukopenia 51%, neutropenia 54%, lymphopenia 30%, thrombocytopenia 19%, and anemia 35%. Dose modification occurred in 51% of patients who were on 125 mg oral dose, primarily because of neutropenia or thrombocytopenia. Of the non-hematologic toxicities, fatigue was the most common adverse event (14% Grade 2) (18).
The addition of an AI to palbociclib did not reveal any unexpected toxicities. The most common adverse events from the phase II PALOMA-1/TRIO-18 trial were neutropenia (48% Grade 3, 6% Grade4), leukopenia (19% Grade 3), fatigue (36% Grade 2, 4% Grade 3/4) (20). Notably, despite the increased incidence of neutropenia no neutropenic fever was reported. Other non-hematologic side effects reported more commonly in the combination arm included nausea, vomiting, arthralgia, alopecia, and diarrhea, although only alopecia was statistically significant. In the combination group, dose delay was required in 45%, and dose reduction was required in 40%. Because of the hematologic toxicity, blood count monitoring is recommended, and dose holds or reduction may be warranted.
Based on cross-trial comparison of phase I data of the other CDK4/6 inhibitors currently being evaluated in breast cancer (LEE001 and abemaciclib (LY2835219)), neutropenia was more frequently reported in palbociclib (all grade 66-70% in palbociclib vs. 40% in LEE001 and 16% in abemaciclib), while diarrhea was the most common adverse event reported for abemaciclib (16, 17, 22).
Treatment options for metastatic ER positive breast cancer
For ER+/HER2- metastatic breast cancer, palliative treatment with endocrine therapy is often preferred as the initial treatment of choice. Traditionally, serial treatment with different endocrine therapies has been used until the development of endocrine resistance and/or rapidly progressive disease, at which time patients are transitioned to chemotherapy. However, as more is learned about key pathways driving cancer growth, rationally designed drugs to target endocrine resistance pathways are being developed. These drugs have the potential to be less toxic and more tolerable than traditional cytotoxic chemotherapy, and may delay the time to initiation of chemotherapy.
One such example is everolimus, which targets the PI3K/AKT/MTOR pathway. The combination of everolimus and AI therapy was previously approved by the FDA for treatment of ER+ MBC resistant to AI therapy (23). There are several other therapies that target different oncogenic pathways, which are also currently being evaluated in combination with endocrine therapy (Supplementary Table S1). In addition, many of the pathways thought to be involved in endocrine resistance, such as growth factor receptors associated with mitogenic signals (EGFR, IGF-R, and HER2), are upstream of the CDK4/6–cyclin D1 complex. Therefore it is plausible that CDK4/6 inhibitors can also be combined with agents that target other pathways in order to enhance anti-tumor efficacy.
Use of CDK4/6 inhibition in combination with radiation or chemotherapy may actually result in decreased response to therapy, and therefore careful evaluation will be required. In preclinical studies, administration of palbociclib in combination with radiation therapy or the DNA damaging agent carboplatin ameliorates hematologic toxicity (24, 25). In the radiation studies, the CDK4/6-dependent cell line was resistant to the effects of radiation when treated with a CDK4/6 inhibitor. Similarly, in studies evaluating the combination of DNA-damaging carboplatin chemotherapy and CDK4/6 inhibitors using a Rb-competent murine model of breast cancer, decreased anti-tumor effect was noted with the combination compared to chemotherapy alone (25, 26). Such differential effects were not observed in an Rb-incompetent murine model. In contrast, an alternating dosing schedule of palbociclib and paclitaxel has been shown to be synergistic in a preclinical model and has shown anti-tumor activity in a phase I trial (27).
Conclusions and Future Directions
The CDK4/6 inhibitor palbociclib has single agent activity in breast cancer. In addition, palbociclib in combination with endocrine therapy has demonstrated significant PFS benefit over endocrine therapy alone. The history of palbociclib illustrates the successful identification of an oncogenic pathway in the laboratory and subsequent design and development of a drug that targets a specific molecular characteristic for use in the clinic. However, the development of this drug has also reminded us of the complexity of these pathway networks and challenges in biomarker development to predict response (28). Currently ER status is the most reliable predictive marker for palbociclib, although fewer than half of ER-positive patients in the phase II PALOMA-1 trial responded to therapy (20). It will be essential to identify additional biomarkers with demonstrated clinical utility to help guide treatment decision making.
Palbociclib is a welcome and exciting addition to an existing array of endocrine and other targeted therapies for treatment of ER+ MBC. With each new therapeutic we are making progress toward examining potential mechanisms related to endocrine resistance and translating science into the practice of precision medicine.
Supplementary Material
Acknowledgments
Grant Support: N.L. Henry was supported by the A. Alfred Taubman Medical Research Institute and in part, through her institution, by an NCI Clinical Cancer Investigator Team Leadership Award (supplement to 3-P30-CA04592).
A. Morikawa reports receiving commercial research support from Eli Lilly. N.L. Henry reports receiving commercial research funding from BioMarin Pharmaceutical, Celldex Therapeutics, and Sanofi-Aventis.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: No other potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008;8:714–24. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, Nicholson RI, et al. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–33. [PubMed] [Google Scholar]
- 5.Dickson C, Fantl V, Gillett C, Brookes S, Bartek J, Smith R, et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 1995;90:43–50. doi: 10.1016/0304-3835(94)03676-a. [DOI] [PubMed] [Google Scholar]
- 6.Caldon CE, Daly RJ, Sutherland RL, Musgrove EA. Cell cycle control in breast cancer cells. J Cell Biochem. 2006;97:261–74. doi: 10.1002/jcb.20690. [DOI] [PubMed] [Google Scholar]
- 7.Bosco EE, Wang Y, Xu H, Zilfou JT, Knudsen KE, Aronow BJ, et al. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J Clin Invest. 2007;117:218–28. doi: 10.1172/JCI28803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertel A, Dean JL, Rui H, Liu C, Witkiewicz AK, Knudsen KE, et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–63. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–45. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 11.New York: Pfizer; 2015. Full prescribing information for IBRANCE - palbociclib capsule. Internet. [cited 2015 April 20]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=2191. [Google Scholar]
- 12.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 14.Foster JS, Henley DC, Ahamed S, Wimalasena J. Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab. 2001;12:320–7. doi: 10.1016/s1043-2760(01)00436-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee NV, Yuan J, Eisele K, Cao JQ, Painter CL, Chionis J, et al. Mechanistic exploration of combined CDK4/6 and ER inhibition in ER-positive breast cancer. Cancer Res. 2014;74:LB–136. [Google Scholar]
- 16.Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1) Br J Cancer. 2011;104:1862–8. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568–76. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 18.DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 19.Finn R, Hurvitz S, Allison M, Applebaum S, Glaspy J, DiCarlo B, et al. Phase I study of PD 0332991, a novel, oral, cyclin-D kinase (CDK) 4/6 inhibitor in combination with letrozole, for first-line treatment of metastatic post-menopausal, estrogen receptor-positive (ER+), human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Cancer Res. 2009;69(24 Suppl) abstract. Abstract nr 5069. [Google Scholar]
- 20.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 21.Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015 Jun 1; doi: 10.1056/NEJMoa1505270. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Infante JR, Shapiro G, Witteveen P, Gerecitano JF, Ribrag V, Chugh R, et al. A phase I study of the single-agent CDK 4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. J Clin Oncol. 2014;32:5s. suppl; abstr 2528ˆ. [Google Scholar]
- 23.Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson SM, Torrice CD, Bell JF, Monahan KB, Jiang Q, Wang Y, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120:2528–36. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–87. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, et al. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11:2747–55. doi: 10.4161/cc.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark AS, O'Dwyer PJ, Heitjan D, Lal P, Feldman MD, Gallagher M, et al. A phase I trial of palbociclib and paclitaxel in metastatic breast cancer. J Clin Oncol. 2014;32:5s. suppl; abstr 527. [Google Scholar]
- 28.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–58. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.Nabholtz JM, Bonneterre J, Buzdar A, Robertson JF, Thurlimann B. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results. Eur J Cancer. 2003;39:1684–9. doi: 10.1016/s0959-8049(03)00326-5. [DOI] [PubMed] [Google Scholar]
- 31.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–9. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 32.Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–90. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–5. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 34.Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136:503–11. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 35.Robertson JF, Llombart-Cussac A, Feltl D, Dewar J, Jasiowka M, Hewson N, et al. Fulvestrant 500 mg versus anastrozole as first-line treatment for adnvaced brast cancer: overall survival from the phase II “first” study. Proceedings of the 2014 San Antonio Breast Cancer Symposium; 2014 Dec 8-13; San Antonio, TX. San Antonio (TX): SABCS; 2014. abstract. Abstract nr S6-04. [Google Scholar]
- 36.Bergh J, Jonsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattstrom D, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–25. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 37.Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. New Engl J Med. 2012;367:435–44. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as firstline endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin M, Loibl S, von Minckwitz G, Morales S, Martinez N, Guerrero A, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the Letrozole/Fulvestrant and Avastin (LEA) study. J Clin Oncol. 2015;33:1045–52. doi: 10.1200/JCO.2014.57.2388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.