Abstract
DCC (Deleted in Colorectal Cancer) is a single-pass transmembrane protein that belongs to the immunoglobulin superfamily. It was originally identified as a prognostic tumor marker and then subsequently found to be a receptor for netrin-1. DCC plays a key role in axon guidance and also in a number of other important cellular processes. This review describes the current progress of the structural biology of DCC with an emphasis on how DCC is involved in the dual functionality of netrin-1 as a chemo-attractant as well as a repellent in axon guidance, referred to as bi-functionality. A perspective about other DCC ligands and the signaling mechanism of the cytoplasmic tail of DCC is also recapitulated.
Keywords: DCC, Netrin-1, Axon guidance, Structure, UNC5
1. Introduction
DCC (Deleted in Colorectal Cancer) was initially identified as a prognostic tumor marker for colon cancer. It was characterized as a cell surface receptor encoded within a 370-kb region on chromosome 18q that is affected in tumors. The DCC gene was expressed in many normal tissues, but was absent in most colorectal carcinomas, hence the name DCC. Therefore at that early stage, DCC was proposed as a putative tumor suppressor gene (Fearon et al, 1990). Also expressed on spinal commissural axons, DCC was later established as a receptor for netrin-1, a neuronal axon guidance cue involved in determining the direction and extent of cell migration and axonal outgrowth in the developing nervous system (Chan et al, 1996; Keino-Masu et al, 1996).
During development, neuronal axons are guided along defined pathways by combined actions of attractive and repulsive cues in the extracellular environment. Diffusible chemoattractants attract axons to their targets, whereas repulsive guidance cues generate exclusion zones that axons avoid (Keynes & Cook, 1995; Tessier-Lavigne, 2002). For example, netrin-1, secreted by floor-plate cells in the spinal cord, diffuses in the extracellular matrix and creates a gradient that attracts growing commissural axons towards the ventral midline of the spinal cord (Kennedy et al, 1994; Serafini et al, 1994). Different guidance cue receptors on the surface of the growth cone of a neuron continually explore the environment by interacting with corresponding guidance cues released from target cells, thereby allowing the axon to correctly navigate the precise trajectory among many possible routes. Remarkably, only a relatively small number of highly conserved guidance cue families and their interacting receptors are used to ensure precise wiring of the nervous system. This suggests that a regulatory mechanism is apparently required to build and maintain the proper interactions of this neural network (Dickson, 2002). Netrin-1 is the founding member of the netrin family that was the first axon guidance cue characterized in both invertebrates and vertebrates (Kennedy et al, 1994; Serafini et al, 1994). Importantly, the netrin-1/DCC interacting pair not only remains critical for neuronal development, but also plays a role in diverse cellular processes such as tissue organization, cell adhesion, motility, proliferation, differentiation, cell survival and cancer (Cirulli & Yebra, 2007).
More recently, DCC has been functionally related to the dependence receptor family (Mehlen & Furne, 2005). Dependence receptors are membrane receptors that can mediate or inhibit cellular apoptosis depending on the absence or presence of their corresponding ligand (Bredesen et al, 2005). As a dependence receptor, in the presence of netrin-1, DCC has a dual function. It first acts as an axon guidance cue receptor and second as an oncoprotein that regulates cell survival (Arakawa, 2004). In the prolonged absence of netrin-1, the receptor does not stay inactive. Rather, it elicits an apoptotic signal through the activation of caspase-3. Therefore, cells expressing receptors like DCC are dependent on the presence of ligand, such as netrin-1, in the extracellular environment for survival (Goldschneider & Mehlen, 2010). When DCC expresses in circumstances in which netrin-1 is not available, it induces cell death. Using a recently established mouse model, it was shown that DCC indeed functions as a tumor suppressor through its ability to trigger tumor cell apoptosis. There have been increasing evidences in recent years demonstrating the function of DCC in constraining tumor progression, not just in colorectal cancer, but also in a large variety of other cancers (Castets et al, 2012), and in suppressing tumor metastasis (Krimpenfort et al, 2012). As a member of the dependence receptor family, DCC therefore has all of its diverse functions unified into one pathway. In this review we dissect the DCC molecular structure and discuss its role in axon guidance signaling.
2. The role of DCC in axon guidance
DCC is a type I transmembrane receptor that belongs to the immunoglobulin superfamily (IgSF) (Keino-Masu et al, 1996). DCC and its Drosophila homologue Frazzled (Kolodziej et al, 1996), the C. elegans homologue UNC40 (uncoordinated 40) (Chan et al, 1996), and its vertebrate homologue neogenin (Meyerhardt et al, 1997) all have a similar domain architecture. Figure 1 illustrates a schematic drawing of netrin-1 (Fig. 1a) as well as, DCC and another netrin-1 receptor UNC5 (Fig. 1b). The DCC receptor is composed of four Ig-like domains at its N-terminus, followed by six fibronectin (FN) type III domains, an approximately 50-residue long membrane-proximal stalk and a transmembrane segment. It has a very large cytoplasmic tail (around 350 residues) without a defined domain structure. Nevertheless, it was observed that there are three sequence motifs in its cytoplasmic region conserved between H. sapiens DCC, Gallus neogenin and Drosophila Frazzled (Kolodziej et al, 1996). As will be discussed later, these three motifs, termed P1, P2 and P3, play a key role in downstream signal transduction pathways.
Figure 1. The domain architecture of netrin-1 and its receptors DCC and UNC5.
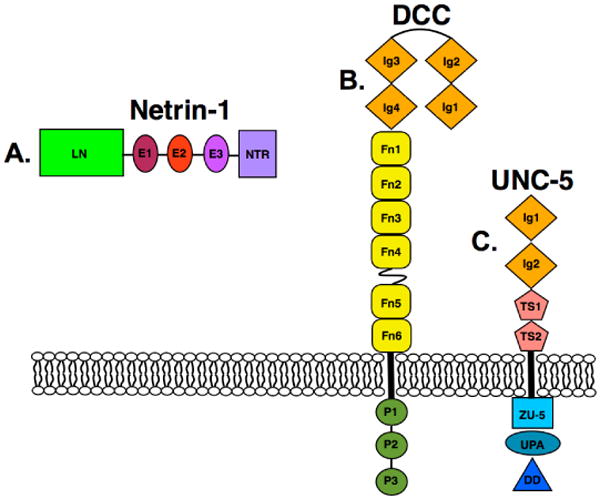
A. Domain architecture of netrin-1, depicting a laminin-like domain (LN), three epidermal growth like repeats (E1 - E3), and the C-terminal domain (NTR). B. The domain architecture of DCC, depicting its four immunoglobulin domains (Ig 1-4), six fibronectin type-3 (FN1 - FN6) domains, three P motifs (P1 - P3), and their orientation relative to the plasma membrane. C. The domain architecture of UNC5-A, depicting its two immunoglobulin domains (Ig1 - Ig2), two thrombospondin type I repeat domains (TS1 -TS2), the ZU-5 domain (as initially found in ZO-1 and UNC5), the UPA domain (which is conserved in UNC5, PIDD, and ankyrins) and the DD domain (death domain).
2.1. DCC is part of a molecular switch for axon pathfinding
In the development of the nervous system, one intriguing observation is that some guidance cues, such as netrin-1, are known to be bi-functional, explicitly having both attractive and repulsive effects (Cirulli & Yebra, 2007; Tessier-Lavigne & Goodman, 1996). Netrin-1 and other netrin family members consist of a laminin-like domain (LN), followed by three EGF repeats (E1, E2, and E3), and a C-terminal netrin-like domain (NTR) (Kennedy et al, 1994; Serafini et al, 1994) (Fig. 1a). Netrin-1 can bind to several cell surface receptors, besides DCC. The underlying mechanism of netrin-1 bi-functionality cannot be vindicated by differential netrin-1 receptors working separately for attraction and repulsion, nor can it be justified by a simple tug-of-war between attractive and repulsive responses triggered by activated netrin-1 receptors within the same growth cone. Instead, netrin-1 seems to cluster different receptors together, leading to alternative signaling outcomes. One such receptor is UNC5. Therefore, DCC plays a key role in a molecular switch to turn attraction to repulsion.
The UNC5 gene was first identified in C. elegans, and is required for guiding pioneer axons. It encodes a 919-residue transmembrane receptor, comprising two Ig-like domains followed by two thrombospondin type 1 domains in the ecto-fragment and a very large cytoplasmic segment consisting of about 550 residues (Leung-Hagesteijn et al, 1992) (Fig. 1c). Vertebrate UNC5 homologues were soon shown to be netrin-1 receptors, mediating repulsion effects (Leonardo et al, 1997). However, convincing evidence indicates that UNC5 dependent repulsion requires the presence of DCC to be expressed on the individual growth cone (Hong et al, 1999).
DCC constitutively expresses on the axonal surface. Netrin-1-binding to DCC induces chemoattraction (Keino-Masu et al, 1996). The association of netrin-1 with the extracellular portion of DCC results in the homodimerization of DCC via its very C-terminal cytoplasmic P3 motif, but not the ecto-domains (Stein et al, 2001). This recruits an intracellular signaling complex that leads to Src family kinase activation (Liu et al, 2004) and eventually the rearrangement of the cytoskeleton (Li et al, 2008), giving rise to the attraction effect. However, when UNC5 co-expresses with DCC, the attraction is switched off and repulsion is then activated. Elegant experiments carried out by Hong and colleagues demonstrated that: 1) Co-expression of UNC5 on growth cones of neurons converts netrin-mediated axon attraction to repulsion; 2) A monoclonal antibody directed against the extracellular domains of DCC blocks both attraction and repulsion, indicating the critical role of DCC in both responses; 3) In the presence of netrin-1, UNC5 co-immuno-precipitates with DCC, suggesting the formation of a ternary complex of netrin-1 with ecto-domains of DCC and UNC5; 4) Most interestingly, the ternary complex also positions the cytoplasmic tail of two receptors in close proximity for interaction. Using the yeast-two-hybrid system, it was shown that in this case, the 18-residue DCC P1 motif and its surrounding region are engaged in interactions with the DCC-binding domain (UPA) located on the cytoplasmic tail of UNC5. This induces SHP2 binding and the subsequent activation of the signal transduction pathway for repulsion (Tong et al, 2001). Functional assays confirmed the repulsion responses of axons caused by these physical interactions.
In summary, DCC binding to netrin-1 alone leads to axon attraction. Importantly, DCC has the ability to switch the netrin-1-mediated responses from attraction to repulsion when another receptor UNC5 co-exists. During navigation when the growth cones reach an intermediate target and needs to move to the next target, the switch from attraction to repulsion using DCC probably provides an economical approach, in addition to involving other guidance systems such as the Robo receptor responding to the Slit guidance cue (Kidd et al, 1998).
2.2. Structural basis for the bi-functionality of netrin-1 in association with DCC
A major progress towards answering the molecular basis of switching netrin-1 bi-functionality by its receptor, DCC, has recently been achieved in the crystal structure of human netrin-1 in complex with its binding domains of human DCC (Finci et al, 2014), referred to as the Finci model hereinbelow, (Fig. 2) and a related structure with a different DCC construct (Xu et al, 2014), refereed to as the Xu model hereinbelow. The constructs used for co-crystallization by Finci and colleagues were the laminin-like domain and three EGF domains without the C-terminal NTR domain of human netrin-1, and the two membrane-proximal domains of human DCC: FN5 and FN6. The netrin-1 molecule in the crystal structure assumes an extended conformation like a tadpole, with a typical globular laminin-like domain as the head and three consecutive skinny EGF domains tailing behind. The FN5 and FN6 domains of DCC form an abutting rod-like unit referred to as DCCFN56. Each of the FN domains adopts a common β sandwich with the ABE strands on one sheet and the C'CFG strands on the other. In the crystal structure, each netrin-1 molecule associates with two DCCFN56 molecules. The C-termini of both DCCFN56 roughly point down in the same direction, consistent with attachment to the same cellular membrane (Fig. 2). There are no direct contacts between the two bound DCCFN56 molecules. The closest distance between them is approximately 20Å. It can be envisioned that it is the netrin-1-binding event that brings the two DCC molecules into close vicinity on the cellular surface.
Figure 2. The ribbon diagram of the netrin-1/DCC56 complex.
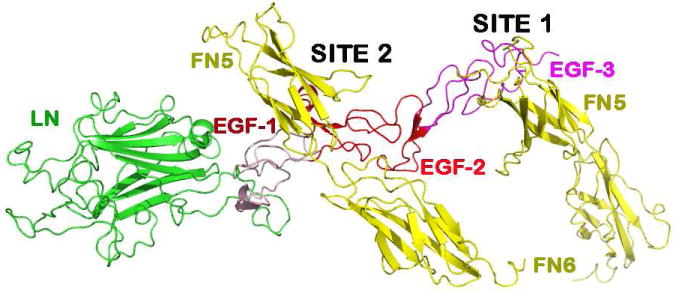
The crystal structure of the netrin-1/DCC complex is depicted with one netrin-1 molecule bound to two DCC molecules interacting at two different binding sites, designated as site 1 and site 2.
The prominent feature of this informative structure is the presence of two distinct DCC-binding sites located on netrin-1 (Finci et al, 2014). Binding site 1 exclusively involves the EGF-3 of netrin-1 and the FN5 domain of the DCCFN56 molecule. It is a highly DCC-specific site. Binding site 2 involves both the EGF-1 and the EGF-2 domains of netrin-1, and a very different portion of the FN5 domain together with an adjacent region of the FN6 domain of DCC. Binding site 2 contains few direct protein-protein interactions between netrin-1 and DCC. This characteristic two-site feature is the key for the interpretation of the bi-functionality.
Fig 3 depicts the DCC-specific binding site 1. Located at the interface, the residue Gln443 of netrin-1 EGF-3 plays a central role. Its amide group at the tip of its side chain forms two potential hydrogen bonds to the main chain carbonyl oxygen atoms of Thr932 and Met933 of the DCC FN5 domain, respectively. This fixes the aliphatic portion of the Gln443 side chain stretched into an extended conformation such that it allows for hydrophobic contacts with Met933 and Val848 of DCC, giving rise to an energetic “hot spot”. In addition, there are several potential hydrogen bonds located at the interface as shown in Fig. 3, ensuring that this interaction is indeed a DCC-specific one. By contrast, binding site 2 contains features that are quite unique for a protein-protein interface as illustrated in Fig. 4. An open-book view of the binding interface in electrostatic representation is depicted in Fig. 4a. On the netrin-1 molecule, the binding face is extremely positively charged. On the DCC molecule, it is also quite positively charged. It turns out that there are several sulfate ions mostly embedded in the netrin-1's DCC-binding area, neutralizing the two positively charged binding partners and bridging them together (Fig. 4b). A more detailed view of the interface is provided in Fig. 4c. On the right side of the figure, there appears to be an ion participating in interactions between the EGF-1 domain of netrin-1 and the FN5 domain of DCC. Notably, Asp858 and Thr856 on the AB-loop of the FN5 domain are engaged in interactions with Tyr323 of netrin-1, providing some specificity. Other interactions include the pyrole ring of Pro320 of the netrin-1 EFG-1 domain in direct contacts with His857 of DCC (not shown in Fig. 4c for clarity). On the left side of Fig. 4c, the major constituents are three arginines (Arg348, Arg349 and Arg351) from the EGF-2 domain lining up and interspersed by two sulfates. Interestingly, Arg348 from netrin-1 forms two hydrogen bonds with Thr945 from the FN6 domain of DCC, implying the indispensable role of the DCC FN6 domain in netrin-1 binding. Compared to the interdigitating and specific protein-protein interactions occupying site 1 shown in Fig. 3, site 2 is much less specific for DCC. Instead, the extremely positively charged binding surface with sulfate ions embedded may provide a platform for a similarly configured additional receptor to bind. Given that the presence of a structured cluster of sulfate ions correlates with a preference for certain proteoglycans to bind netrin-1 (Shipp & Hsieh-Wilson, 2007), and the above mentioned multiple arginines located on the EGF-2 domain of netrin-1 that are evolutionarily conserved (Finci et al, 2014), this sulfate-associated site 2 should in fact be biologically relevant. Heparan sulfate is a highly versatile extracellular scaffold molecule that binds to many secreted and cell surface proteins and has been implicated in axon guidance (Lee & Chien, 2004). This is another layer of specificity added to the already complex multitude of ligands and their binding receptors. It could be inferred that they may play a role in mediating differential receptors binding to netrin-1 at site 2, which consequently arbitrates the corresponding signaling pathway for axon guidance.
Figure 3. Binding site 1 interface.
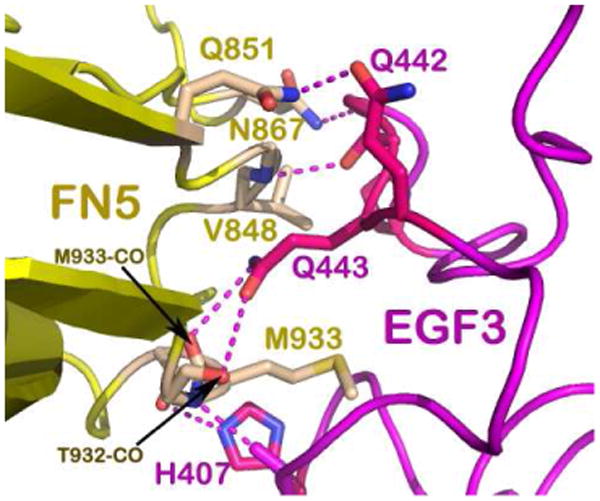
Close up view of the residues involved in the specific interactions at binding site 1. The DCC FN5 domain is colored yellow and the netrin-1 EGF-3 domain is colored in magenta. The hydrogen bonding interactions are centered around Gln443 on netrin-1 with Thr932 and Met933 on DCC that allow hydrophobic interactions with Met933 and Val848 to create an energetic hotspot. More potential hydrogen bonds are depicted using dashed lines to describe the specificity of the binding site.
Figure 4. Binding site 2 interface.
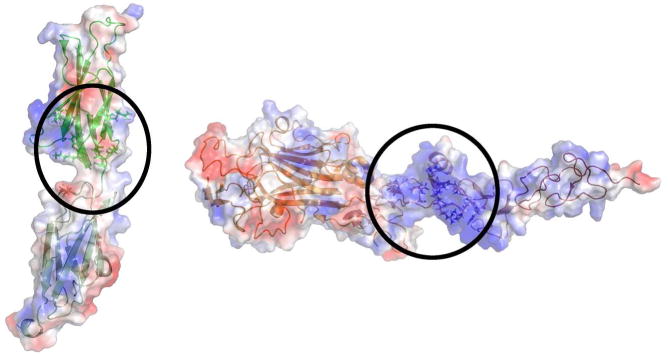
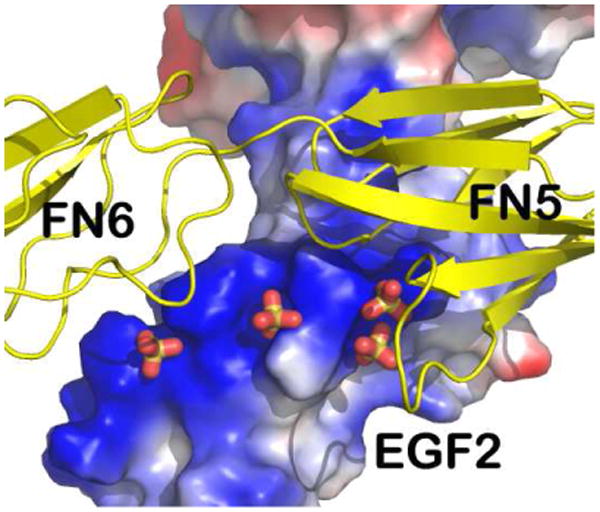
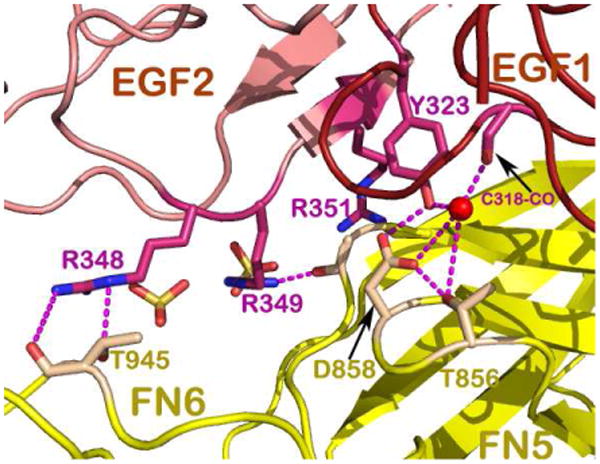
A. Open book views of the electrostatic surface potential representations of DCC and netrin-1 showing the positively charged interfaces encircled (Illustration modified form Finci et al, 2014). B. Zoom in view of binding site 2, depicting the DCC FN5 and FN6 domains in ribbon representation (colored green) and the netrin-1 EGF-1 and EGF-2 domains in electrostatic surface potential representation with sulfates mediating the interactions. C. A detailed view of binding site-2 interactions show the indispensible role of the DCC FN6 domain (the DCC FN5 and FN6 domains are both colored yellow) through interactions with arginines from the EGF-2 domain on netrin-1 (colored pink) interspersed with sulfates.
Published concurrently, the Xu model contains the same construct of netrin-1 from chicken and a different DCC fragment: the domains FN4 and FN5 from mouse (Xu et al, 2014). The most remarkable feature of this structure is that the DCC FN4 domain is bound to the laminin domain of netrin-1 (Fig. 5a). This structure shares the same feature of the FN5 domain binding to netrin-1's EGF-3 domain, designated as site 1, with the Finci model discussed above. Using the shared FN5 domain, the two structures can be overlaid on top of each other rather well, suggesting the complementarity of the two structures. Fig, 5b gives a combined model based on the superposition on the shared binding site 1. Here, the signaling unit proposed in the Finci model is depicted with green netrin-1 bound to two DCC receptors (in red and magenta). The red DCC receptor has FN4-FN5-FN6 constructed from the two models. The binding of LN on FN4 (designated as site 0 here) observed in the Xu model brings two signaling units into a cluster. This complementary figure delineates a possible clustering of DCC receptors on the cell membrane by netrin-1 ligands, which may offer a more complete picture of the netrin-1/DCC interaction biology
Figure 5. The crystal structure of the chicken netrin-1/mouse DCC45 complex and the overlay of two complex structures.
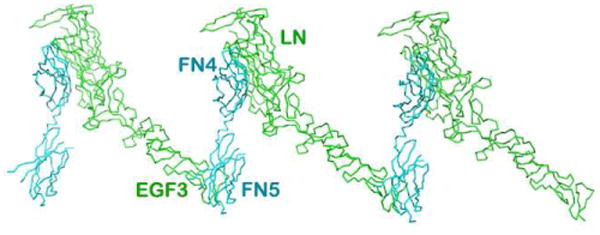
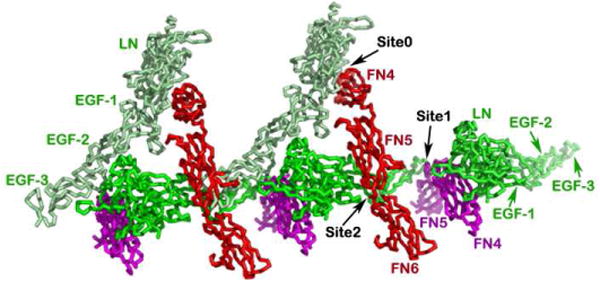
A. The netrin-1/DCC45 structure. The interactions between the DCC FN4 domain and the netrin-1 laminin-like domain along with the relative clustering seen in the crystal packing are highlighted. Note that the FN5/EGF-3 binding site is shared with what is seen in Fig. 2 at site 1. B. Overlay of the crystal structures of the chicken netrin-1/mouse DCC45 and the human netrin-1/human DCC56 highlights complementarity. All three binding sites are designated as binding site 0, binding site 1, and binding site 2. Netrin-1 molecules are colored in green and light green, respectively, whereas DCC receptors are colored in red and magenta, respectively.
2.3. Functional evidence supporting the netrin-1/DCC structural model for bi-functionality
Early genetic and biochemical experiments have suggested a functional role of DCC FN4 and FN5 domains in netrin-1 binding (Geisbrecht et al, 2003; Kruger et al, 2004). These results in fact assisted in the efforts of the construct design for the structure determination described above. The more intriguing question is how the structural observations can offer an explanation for the bi-functionality of netrin-1. Our site-directed mutagenesis experiments in combination with cell-binding assays, clustering assays, and particularly the in vivo axon guidance assay have indeed provided complementary evidences to support the molecular mechanism underlying netrin-1's bi-functionality (Finci et al, 2014).
The most significant structural observation pertinent for bi-functionality is the presence of two distinct netrin-1 binding sites on the DCC receptor. To perform the cell binding assay, the wild type or mutant DCC constructs were transfected into COS-7 cells, to test netrin-1 binding in the culture medium. An antibody against the netrin-1 LN domain was then used for immunostaining. By introducing a charged residue on DCC such as Met933Arg or a charge-reversal double mutation on netrin-1 such as Arg349Asp+Arg351Asp in the interface, the subsequent binding was perturbed.
More convincing results came from the axon guidance assay. Mouse primary neurons were cultured from the dorsal horn of the spinal cord of E15 (embryonic day 15) embryos of the wild-type or the DCC-/- mice (Rigato et al, 2011). Using the microinjection technique, the DCC wild-type or the mutant constructs, the wild-type UNC5A construct and a relevant marker were introduced into a 2-day old neuron. The heparin pre-coated beads were coated with netrin-1 and placed at one corner of the culture dish. After 24 hours of incubation, the growing directions of the axons were recorded for analyses. Fig. 6 provides the results from these experiments. It shows that 1) DCC wild-type has a clear attraction effect; 2) UNC5A has its repulsion effects only when it co-exists with DCC. UNC5A alone did not lead to any defined axon growth; 3) Mutant DCC at site 1 (Met933Arg or Val848Arg) eliminates both the repulsion and attraction responses, whereas mutant DCC at site 2 (His857Ala or Asp858Arg) did not affect the repulsion effects but instead neutralized chemo-attraction responses. These site-directed mutagenesis experiments in conjunction with the axon guidance assay imply that it is imperative to have DCC binding at site 1 to facilitate both attraction and repulsion. Since mutant DCC at site 2 did not prevent repulsion, it suggests that UNC5A must occupy a region close to this site 2 for repulsion effects. The above structural and functional data have provided experimental evidence, supporting the role of how netrin-1 exerts a bi-functionality effect and how DCC acts on its own to promote attraction and acts as a “co-receptor” for UNC5A to switch to chemorepulsion.
Figure 6. Radar plots from the axon guidance assay.
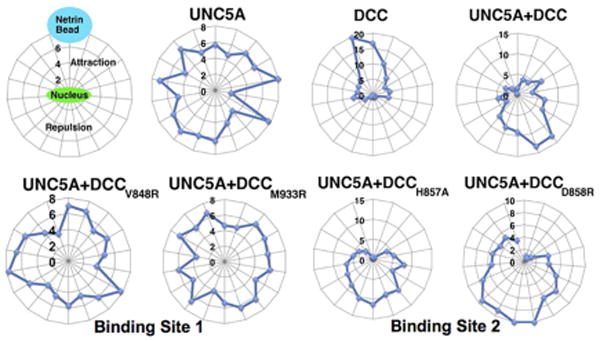
Performed with DCC -/- neurons that were microinjected with DNA from the WT DCC, the mutant DCC and the WT UNC5-A. The figure demonstrates that DCC is required for UNC5-A dependent repulsion. Mutants from DCC are designated as either binding site 1 (Val848Arg and Met933Arg) or binding site 2 (His857Ala and Asp858Arg) (Illustration modified form Finci et al, 2014).
3. The functional N-terminal four Ig-like domains of DCC
Many cell surface receptors are composed of multiple domains in tandem. This is particularly remarkable in the case of IgSF members (Wang & Springer, 1998). As mentioned above, the ecto-fragment of DCC also has four Ig-like domains followed by six FN domains. A recent structural study revealed that the N-terminal four Ig-like domains of DCC fold into a horseshoe-like conformation (Fig. 7) (Chen et al, 2013). First characterized in the insect protein hemolin (Su et al, 1998) and later in neuronal adhesion molecules, Drosophila Dscam (Meijers et al, 2007), as well as chicken axonin-1/TAG-1 (Freigang et al, 2000), this unique horseshoe conformation turns out to be a conserved structural feature in many neuronal receptors (Chen et al, 2013). In the case of Dscam, the horseshoe mediates functionally critical homophilic binding characteristics (Meijers et al, 2007). As for DCC, since netrin-1 binds to the membrane-proximal FN4-FN5-FN6 domains, it is interesting to note that the horseshoe conformation is functionally required for axon guidance mediated by netrin-1 (Chen et al, 2013). Electron microscopic (Meijers et al, 2007) and X-ray crystallographic analyses (Sawaya et al, 2008) both show that in addition to the horseshoe conformation, an 8-Ig-domain fragment of the Dscam molecule further folds into a more compact configuration. This implies that the DCC receptor might also further fold the N-terminal horseshoe close to the membrane-proximal portion of the molecule, such that the disruption of the horseshoe would affect netrin-1 binding to the membrane-proximal FN domains.
Figure 7. The DCC N-terminal horseshoe configuration.
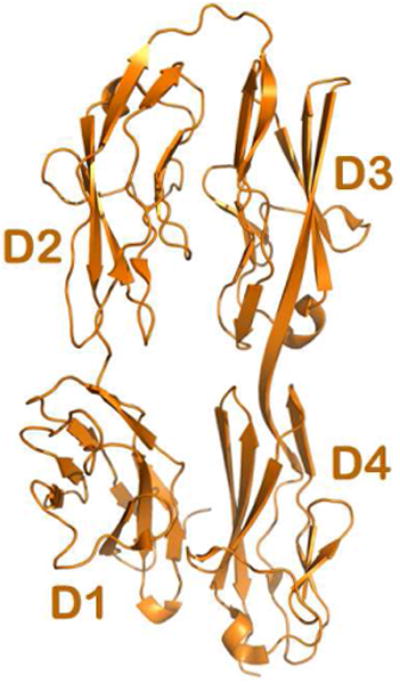
The Ribbon diagram of the crystal structure depicting the four N-terminal Immunoglobulin (Ig) domains of DCC (colored in gold). The folding of the four domains results in a horseshoe configuration, with the first two domains D1 and D2 bending over to contact the proceeding two domains, D4 and D3. This configuration was demonstrated to be functionally relevant for netrin-1 mediated axonal attraction.
There is a good indication that the knockout of either DCC or netrin-1 in mice impairs axon guidance function, and the DCC knockout also causes more severe effects compared with netrin-1 knockout mice (Fazeli et al, 1997; Serafini et al, 1996), implying the existence of additional DCC-associated guidance cue molecules. Using an unbiased large-scale protein-microarray screen, a molecule named CBLN4 (Cerebellin 4) was found to be specific for DCC. CBLN4 belongs to the C1q-tumor necrosis factor family. It competes with netrin-1 in binding to the DCC membrane-proximal FN4-FN5-FN6 region at 5-fold lower affinity (Haddick et al, 2014). Using the signal sequence trap screening technique, another new axon guidance protein, draxin (dorsal repulsive axon guidance protein) has also been identified (Islam et al, 2009). This 349-residue soluble guidance cue molecule binds to the netrin-1 receptors DCC, neogenin, UNC5 and DSCAM. The binding affinity of draxin for DCC in transfected cells is higher than that of the DCC/netrin-1 interaction in a similar assay, with an affinity of 970 pM. Interestingly, draxin-binding is preferentially at the N-terminal horseshoe region rather than being localized at the FN domains so that draxin-binding and netrin-1-binding are on separate regions of the DCC receptor (Ahmed et al, 2011). Apparently, these data add more complexity to the very sophisticated axon guidance system associated with multiple guidance cue molecules and receptors, in which DCC plays a significant role.
4. Signaling through the cytoplasmic tail of DCC
Like many other receptors, DCC as well as its counterpart, UNC5 use ecto-domains to sense their ligands in the extracellular environment, and they use their cytoplasmic segment to transduce the signal, eventually guiding the direction of axonal growth. The cytoplasmic tail of DCC is actually a very large polypeptide segment with around 350 residues. Intriguingly, this segment of the molecule does not appear to fold into a defined three-dimensional domain structure judged by the NCBI domain architecture search website. However, through sequence comparison, three conserved motifs, P1, P2 and P3 were vaguely identified, each consisting of a couple of dozen of amino acids (Kolodziej et al, 1996). We have already described how one netrin-1 ligand binds to two DCC receptors on the cell surface. The unique feature is that the two DCC receptors are almost 20Å apart from each other on the cell surface. Presumably, this is close enough to bring the very C-terminal P3 motif of the two molecules to form homodimer as a structural unit for further signaling (Finci et al, 2014). We have also discussed how one netrin-1 may bind DCC and UNC5 each, which brings the DCC P1 motif into close contact with the cytoplasmic DCC-binding domain (UPA) of UNC5 to form a heterodimer for signaling (Finci et al, 2014). However, how do these small sequence motifs function?
It can be envisioned that the flexible unstructured cytoplasmic tail of DCC provides a good specific binding platform utilized for signaling like many so-called intrinsically disordered proteins (Dyson & Wright, 2005). There have already been some experimental indications. The DCC P3 motif was found to be responsible for DCC binding to myosin X (Myo X). The binding between the DCC P3 motif and Myo X's MyTh4-FERM domains plays a role in netrin-1-guided axonal projection (Zhu et al, 2007). A more recent structural investigation demonstrates a helical conformation of the DCC C-terminal P3 motif, when it binds to MyTh4-FERM tandem (Wei et al, 2011). In striking contrast to DCC, the majority of the cytoplasmic tail of UNC5 folds into a compact three-domain structural unit, in which the DCC-binding domain (UPA) might be blocked from binding in the closed conformation (Fig. 8) (Ma et al, 2010). It will be extremely interesting to see in future studies how the DCC P1 motif engages the UNC5 UPA domain and unlocks this structural unit.
Figure 8. The crystal structure of the cytoplasmic supramodule of UNC5-B.
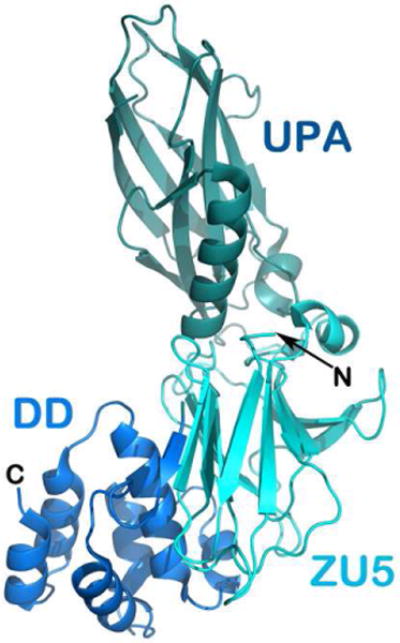
ribbon representation of the cytoplasmic supramodule of UNC5-B consisting of the ZU5 domain, the DCC binding domain that is labeled UPA, and the death domain (DD).
5. Concluding remarks
As the major receptor for netrin-1, DCC plays a key role in axon guidance and in a variety of other cellular processes. In this review, we have elaborated on the current progress in structural biology of this axon guidance receptor, focusing more on netrin-1 binding to the membrane-proximal FN region of DCC, and the mechanism of bi-functionality triggered by the netrin-1/DCC interaction. Additionally, the structure of the N-terminal Ig-region of DCC and two newly discovered DCC-associated guidance cues, CBLN4 and draxin, have been described. A brief account of the signaling mechanism of the cytoplasmic tail of DCC is also elaborated on. Many more questions regarding DCC remain to be answered, including how different heparan sulfate proteoglycans may regulate different receptors, such as UNC-5 and DSCAM along with DCC, binding to netrin-1 articulating a sophisticated function and what is the structural basis of the downstream signal transduction pathway? Another challenging issue is how three known guidance cues coordinate in binding to DCC for axon guidance. A synchronized research effort between disciplines spanning neuroscience and structural biology will unequivocally lead to exciting new insight.
Acknowledgments
This work was supported by the Ministry of Education of China to J.-H.W. and Y.Z., the National Science Foundation of China (NSFC) Major Research Grant (91132718) to Y.Z., an NIH grant (HL48675) and funds from the Peking-Tsinghua Center for Life Sciences to J-H W, and a short-term EMBO postdoctoral fellowship to L. I. F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- Ahmed G, Shinmyo Y, Ohta K, Islam SM, Hossain M, Naser IB, Riyadh MA, Su Y, Zhang S, Tessier-Lavigne M, Tanaka H. Draxin inhibits axonal outgrowth through the netrin receptor DCC. J Neurosci. 2011;31:14018–14023. doi: 10.1523/JNEUROSCI.0943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4:978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Mehlen P, Rabizadeh S. Receptors that mediate cellular dependence. Cell death and differentiation. 2005;12:1031–1043. doi: 10.1038/sj.cdd.4401680. [DOI] [PubMed] [Google Scholar]
- Castets M, Broutier L, Molin Y, Brevet M, Chazot G, Gadot N, Paquet A, Mazelin L, Jarrosson-Wuilleme L, Scoazec JY, Bernet A, Mehlen P. DCC constrains tumour progression via its dependence receptor activity. Nature. 2012;482:534–537. doi: 10.1038/nature10708. [DOI] [PubMed] [Google Scholar]
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sun X, Zhou XH, Liu JH, Wu J, Zhang Y, Wang JH. N-terminal horseshoe conformation of DCC is functionally required for axon guidance and might be shared by other neural receptors. J Cell Sci. 2013;126:186–195. doi: 10.1242/jcs.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Finci LI, Kruger N, Sun X, Zhang J, Chegkazi M, Wu Y, Schenk G, Mertens HD, Svergun DI, Zhang Y, Wang JH, Meijers R. The Crystal Structure of Netrin-1 in Complex with DCC Reveals the Bifunctionality of Netrin-1 As a Guidance Cue. Neuron. 2014;83:839–849. doi: 10.1016/j.neuron.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell. 2000;101:425–433. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J Biol Chem. 2003;278:32561–32568. doi: 10.1074/jbc.M302943200. [DOI] [PubMed] [Google Scholar]
- Goldschneider D, Mehlen P. Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene. 2010;29:1865–1882. doi: 10.1038/onc.2010.13. [DOI] [PubMed] [Google Scholar]
- Haddick PC, Tom I, Luis E, Quinones G, Wranik BJ, Ramani SR, Stephan JP, Tessier-Lavigne M, Gonzalez LC. Defining the ligand specificity of the deleted in colorectal cancer (DCC) receptor. PLoS ONE. 2014;9:e84823. doi: 10.1371/journal.pone.0084823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Islam SM, Shinmyo Y, Okafuji T, Su Y, Naser IB, Ahmed G, Zhang S, Chen S, Ohta K, Kiyonari H, Abe T, Tanaka S, Nishinakamura R, Terashima T, Kitamura T, Tanaka H. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science. 2009;323:388–393. doi: 10.1126/science.1165187. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Keynes R, Cook GM. Axon guidance molecules. Cell. 1995;83:161–169. doi: 10.1016/0092-8674(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P, Song JY, Proost N, Zevenhoven J, Jonkers J, Berns A. Deleted in colorectal carcinoma suppresses metastasis in p53-deficient mammary tumours. Nature. 2012;482:538–541. doi: 10.1038/nature10790. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Lee J, Li W, Guan KL. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J Neurosci. 2004;24:10826–10834. doi: 10.1523/JNEUROSCI.3715-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Chien CB. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nature reviews Genetics. 2004;5:923–935. doi: 10.1038/nrg1490. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–838. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- Li X, Gao X, Liu G, Xiong W, Wu J, Rao Y. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Shang Y, Wei Z, Wen W, Wang W, Zhang M. Phosphorylation of DCC by ERK2 is facilitated by direct docking of the receptor P1 domain to the kinase. Structure. 2010;18:1502–1511. doi: 10.1016/j.str.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Furne C. Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol Life Sci. 2005;62:2599–2616. doi: 10.1007/s00018-005-5191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers R, Puettmann-Holgado R, Skiniotis G, Liu JH, Walz T, Wang JH, Schmucker D. Structural basis of Dscam isoform specificity. Nature. 2007;449:487–491. doi: 10.1038/nature06147. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Look AT, Bigner SH, Fearon ER. Identification and characterization of neogenin, a DCC-related gene. Oncogene. 1997;14:1129–1136. doi: 10.1038/sj.onc.1200935. [DOI] [PubMed] [Google Scholar]
- Rigato C, Buckinx R, Le-Corronc H, Rigo JM, Legendre P. Pattern of invasion of the embryonic mouse spinal cord by microglial cells at the time of the onset of functional neuronal networks. Glia. 2011;59:675–695. doi: 10.1002/glia.21140. [DOI] [PubMed] [Google Scholar]
- Sawaya MR, Wojtowicz WM, Andre I, Qian B, Wu W, Baker D, Eisenberg D, Zipursky SL. A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell. 2008;134:1007–1018. doi: 10.1016/j.cell.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Shipp EL, Hsieh-Wilson LC. Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem Biol. 2007;14:195–208. doi: 10.1016/j.chembiol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- Su XD, Gastinel LN, Vaughn DE, Faye I, Poon P, Bjorkman PJ. Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science. 1998;281:991–995. doi: 10.1126/science.281.5379.991. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M. Wiring the brain: the logic and molecular mechanisms of axon guidance and regeneration. Harvey Lect. 2002;98:103–143. [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tong J, Killeen M, Steven R, Binns KL, Culotti J, Pawson T. Netrin stimulates tyrosine phosphorylation of the UNC-5 family of netrin receptors and induces Shp2 binding to the RCM cytodomain. J Biol Chem. 2001;276:40917–40925. doi: 10.1074/jbc.M103872200. [DOI] [PubMed] [Google Scholar]
- Wang JH, Springer TA. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunological Review. 1998;163:197–215. doi: 10.1111/j.1600-065x.1998.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Wei Z, Yan J, Lu Q, Pan L, Zhang M. Cargo recognition mechanism of myosin X revealed by the structure of its tail MyTH4-FERM tandem in complex with the DCC P3 domain. Proc Natl Acad Sci U S A. 2011;108:3572–3577. doi: 10.1073/pnas.1016567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Wu Z, Renier N, Antipenko A, Tzvetkova-Robev D, Xu Y, Minchenko M, Nardi-Dei V, Rajashankar KR, Himanen J, Tessier-Lavigne M, Nikolov DB. Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science. 2014;344:1275–1279. doi: 10.1126/science.1255149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XJ, Wang CZ, Dai PG, Xie Y, Song NN, Liu Y, Du QS, Mei L, Ding YQ, Xiong WC. Myosin X regulates netrin receptors and functions in axonal path-finding. Nature cell biology. 2007;9:184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]


