Abstract
Rationale
Bipolar disorder (BD) is a disabling and life-threatening disease characterized by states of depression and mania. New and efficacious treatments have not been forthcoming partly due to a lack of well-validated models representing both facets of BD.
Objectives
We hypothesized that cholinergic- and dopaminergic-pharmacological manipulations would model depression and mania respectively, each attenuated by lithium treatment.
Methods
C57BL/6J mice received the acetylcholinesterase inhibitor physostigmine or saline before testing for ‘behavioral despair’ (immobility) in the tail-suspension test (TST) and forced-swim test (FST). Physostigmine effects on exploration and sensorimotor gating were assessed using the cross-species behavioral pattern monitor (BPM) and prepulse inhibition (PPI) paradigms. Other C57BL/6J mice received chronic lithium drinking water (300, 600, or 1200 mg/l) before assessing their effects alone in the BPM or with physostigmine on FST performance. Another group was tested with acute GBR12909 (dopamine transporter inhibitor) and chronic lithium (1000 mg/l) in the BPM.
Results
Physostigmine (0.03 mg/kg) increased immobility in the TST and FST without affecting activity, exploration, or PPI. Lithium (600 mg/l) resulted in low therapeutic serum concentrations and normalized the physostigmine-increased immobility in the FST. GBR12909 induced mania-like behavior in the BPM of which hyper-exploration was attenuated, though not reversed, after chronic lithium (1000 mg/ml).
Conclusions
Increased cholinergic levels induced depression-like behavior and hyperdopaminergia induced mania-like behavior in mice, while chronic lithium treated some, but not all, facets of these effects. These data support a cholinergic-monoaminergic mechanism for modeling BD aspects and provide a way to assess novel therapeutics.
Keywords: Acetylcholine, dopamine transporter, lithium, bipolar disorder, prepulse inhibition, depression, mania
INTRODUCTION
Bipolar disorder (BD) is a severely disabling mental illness affecting 1–2 % of the global population for BD type I (Merikangas et al. 2011). The seriousness of the disorder is indicated by an increased suicide mortality rate (Osby et al. 2001) where one in three patients attempt suicides (Novick et al. 2010) and lifetime costs of persons with BD amounting to $24 billion US (Begley et al. 2001).
BD is a unique mood disorder defined by periods of depression and mania during which symptoms of patients differ markedly (Belmaker and Bersudsky 2004). In fact, symptoms can be largely opposite from each other with hyperactivity being a hallmark feature of mania (DSM-V 2013), whereas lethargy or psychomotor retardation characterize depression. Lithium is commonly used to treat aspects of mania and depression as well as maintain a patient’s state between periods (Malhi et al. 2012), but its effects are limited and it has a low acceptability profile (Cipriani et al. 2011). Greater understanding of the mechanisms underlying and/or overlapping different phases of BD may improve the development of novel and more efficacious therapeutics.
An improved understanding of mechanisms contributing to each aspect of BD may also lead to better animal models with which to test therapeutics targeted at these mechanisms. The current paucity of treatments may be due in part to the dearth of valid animal models targeting the etiologies of BD (Malkesman et al. 2009; Young et al. 2011a). Indeed, animal models being used to reproduce a BD-like phenotype are usually specific only to the manic phase of the disorder (Gould et al. 2001; Roybal et al. 2007; van Enkhuizen et al. 2013a). These models typically reproduce aspects of hyperactivity (Shaldubina et al. 2002), with some measuring impaired risk-taking (Young et al. 2011b), and increased reward seeking (van Enkhuizen et al. 2013c). An awareness exists for the necessity to model the full spectrum of BD (depression and mania) in animals in order to find novel treatments for the full disorder (Machado-Vieira et al. 2004), but the complexity of the matter has prevented such an outcome as yet (Young and Dulcis 2015).
Key difficulties in recreating the range of BD from depression to mania are the evidence of differing mechanisms underlying each aspect of the disorder. Recently, a human imaging study implicated elevated acetylcholine (ACh) levels across all brain regions in both acutely depressed and recovered subjects (Saricicek et al. 2012). Importantly, the same has also been suggested in BD patients during periods of depression (Hannestad et al. 2013). Early observations in animals support depression-relevant behavior in a selectively bred line of rats with increased sensitivity to acetylcholinesterase (AChE; the primary enzyme hydrolyzing ACh), the Flinders Sensitive Line (Overstreet 1993). More recently, the AChE inhibitor physostigmine induced depression-like behavior in mice (Mineur et al. 2013) consistent with physostigmine-induced severe depression and psychomotor retardation in marijuana-intoxicated humans (El-Yousef et al. 1973) as well as increased symptoms of depression in patients with mania, depression, schizoaffective disorder (Janowsky et al. 1974), and healthy subjects (Risch et al. 1981). This physostigmine-induced immobility of mice in the forced swim test (FST) was reversed by antidepressant treatment (Mineur et al. 2013), although the same dose reduced mobility which could have confounded the selectivity of effect. These data provide putative support that increasing ACh levels models the behavior and etiology of depression that is relevant to BD, renewing interest in the cholinergic hypothesis of depression (Janowsky et al. 1972; van Enkhuizen et al. 2015).
The biological underpinnings of BD mania likely involve mechanisms other than those of depression however, putatively catecholaminergic in nature (Janowsky et al. 1972; van Enkhuizen et al. 2015). Previously, we demonstrated that mice with reduced dopamine transporter (DAT) functioning model exploratory profiles of BD mania (Perry et al. 2009). Both DAT knockdown (KD) mice and mice receiving the selective DAT inhibitor GBR12909 exhibit hyperactivity, increased exploration, and straight paths of movement as quantified by the mouse behavioral pattern monitor (BPM) (Young et al. 2010a; b) similar to patients with BD mania (Minassian et al. 2011; Perry et al. 2010) and euthymia (Henry et al. 2013) in a human BPM. Although some of the abnormal behavior observed in these GBR12909-treated mice, such as impaired decision-making (van Enkhuizen et al. 2013b), is also present in depressed patients with BD performing similar human tasks (Adida et al. 2011), their hyperactivity, increased motivation (Young and Geyer 2010), and reduced immobility times in the tail suspension test (TST) (Sarkisyan et al. 2010) and FST (Esumi et al. 2013) more closely model the manic phase of BD. Furthermore, dysfunctional sensorimotor gating of the startle reflex as measured by reduced prepulse inhibition (PPI) has been observed in manic patients with BD (Perry et al. 2001), but not in depressed subjects (Perry et al. 2004; Quednow et al. 2006), suggesting that dysfunctional PPI is state dependent (Kohl et al. 2013). These PPI deficits can be recreated in rodents (Geyer et al. 2002) and have been observed in rodent models of BD mania (van Enkhuizen et al. 2013c), including mice challenged with GBR12909 (Kwek and van den Buuse 2013).
These animal data may have etiological relevance to BD because reduced striatal DAT levels are observed in unmedicated patients with BD (Anand et al. 2011) as well as in postmortem tissue (Rao et al. 2012). Thus, hyperdopaminergia caused by reduced DAT levels has been used to model mania-like behavior in mice.
To date, the effect of the same treatments approved for depression and mania have yet to be determined on behaviors induced by physostigmine or reductions in DAT function. Chronic lithium-treatment can treat both mania and depression. We hypothesized that 1) physostigmine would induce depression-like effects measured by “behavioral despair” in mice by using the TST (Cryan et al. 2005) and FST (Petit-Demouliere et al. 2005) and that 2) lithium would attenuate these effects in the FST. To address mania-relevant behaviors, we used human and rodent cross-species tests of exploration in the BPM and PPI in the acoustic startle test (Henry et al. 2010), predicting that 3) physostigmine would not recreate mania-relevant behaviors while 4) GBR12909 treatment would recreate mania-relevant behaviors, an effect attenuated by lithium treatment.
METHODS
Animals
Male (n=136) and female (n=51) C57BL/6J mice were used in these studies (Fig. 1). Mice were maintained in a temperature-controlled vivarium (21±1°C) on a reversed day-night cycle (lights on at 7.00 PM, off at 7.00 AM) and were tested during the dark phase between 8.00 AM and 5.00 PM. Mice were group housed (four/cage), weighed between 20–40 g, and were 3–4 months old at the time of testing. Mice had ad libitum access to water and food (Harlan, Madison, WI, USA) except during testing with lithium (see below). All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Figure 1. Timeline of the testing procedures for the current studies.
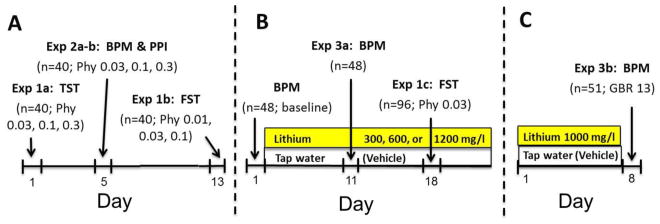
One group of male mice (n=40; a) were tested in the TST, BPM, PPI, and FST respectively. Over the four tests, each animal received each drug dose or saline once. Drug or saline was administered 30 min prior to testing in the TST and FST, and 10 and 55 min prior to the BPM and PPI test respectively. Another group of male mice (n=96; b) was treated with either lithium in their drinking water (300, 600, or 1200 mg/l) or normal tap water (vehicle) after baseline-matching. After 10 days of treatment, half of each group was tested in the BPM. One week later and 17 days of lithium treatment, half of each group received saline or physostigmine in counter-balanced order 30 min prior to testing in the FST. Other naïve mice (n=51; c) received vehicle or 1000 mg/l lithium solution for 7 days. Half of each group was administered saline or GBR12909 immediately prior to testing in the BPM. Doses are in mg/kg unless indicated otherwise.
Drug treatment
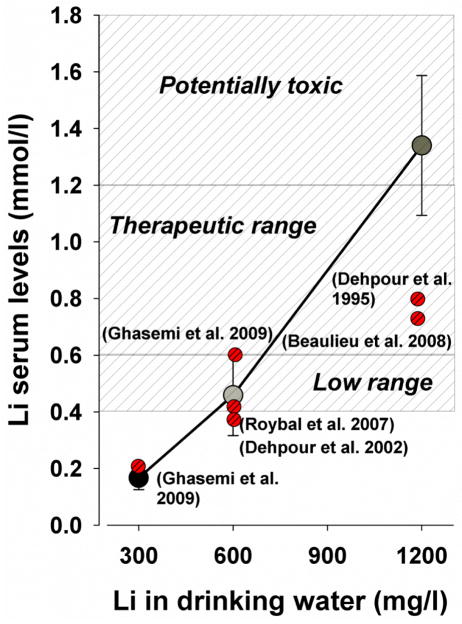
GBR12909 dihydrochloride and physostigmine sulfate (Sigma-Aldrich, St Louis, MO, USA) were both dissolved in 0.9% saline vehicle (10 ml/kg), GBR12909 after sonicating for 2–4 h at 40 °C (van Enkhuizen et al. 2013a; Young et al. 2010b). GBR12909 (13 mg/kg) was administered by i.p. injection immediately prior to the experiment (Loos et al. 2010; van Enkhuizen et al. 2013a; van Enkhuizen et al. 2013b) and physostigmine (0.01, 0.03, 0.1, and 0.3 mg/kg) by i.p. injection 30 min prior to the experiment as done previously (Clark et al. 2005; Dunstan and Jackson 1977; Gard et al. 2012). Initially, lithium chloride (Sigma-Aldrich, St Louis, MO, USA) was dissolved into the drinking water at 300, 600, or 1200 mg/l and given for 10–17 days (Fig. 1). This procedure was chosen based on previous studies using procedures with 600 mg/l and 1200 mg/l to achieve serum levels approaching human therapeutic concentrations (Fig. 2) (Dehpour et al. 1995; Roybal et al. 2007). After we acquired serum levels with these doses, we tested 1000 mg/l lithium dissolved into the drinking water for the BPM and GBR12909 experiment hypothesizing this dose would produce therapeutic serum levels. Control animals received tap water. Drug doses were calculated as the weight of the free-base, except for lithium chloride.
Figure 2. Serum concentrations of lithium after 17 days of different doses of lithium treatment in drinking water.
Lithium (Li) at 300 mg/l resulted in low serum levels (0.17 ± 0.04 mmol/l), while 1200 mg/l resulted in high/toxic Li concentrations (1.34 ± 0.25 mmol/l). Drinking water with Li 600 mg/l resulted in low therapeutic serum concentrations for bipolar disorder (0.46 ± 0.14 mmol/l). Comparative lithium levels are provided from earlier publications, the variability from which is discussed below. Data are presented as mean ± S.E.M.
Serum lithium measurements
Mice were decapitated and trunk blood was collected. Blood was left to clot for approximately 15 min and then centrifuged for 10 min. Serum was removed and frozen. Samples were analyzed by using spectrophotometry performed by UCSD Medical Center (USA).
Mouse behavioral pattern monitor
Locomotor behavior and exploration were examined in eight mouse BPM chambers (San Diego Instruments, USA) as described previously (Risbrough et al. 2006; Tanaka et al. 2012). In brief, each Plexiglas arena consists of a 30.5 × 61 × 38 cm area with three floor and eight wall holes (three in each long wall and one in each short wall; 1.25 cm in diameter, 1.9 cm from the floor), each equipped with an infrared photobeam to detect holepoking. Each chamber is enclosed in an outer box with an internal white house-light above the arena (350 lux in the center and 92 lux in the four corners) that minimizes external light and noise. Activity was obtained from a grid of 12 × 24 infrared photobeams 1 cm above the floor (2.5 cm apart; 24 × 12 X-Y array), recording the location of the mouse every 0.1 s, with its position defined across nine unequal regions (four corners, four walls, and center (Geyer et al. 1986)). Another set of 16 photobeams, placed 2.5 cm above the floor, was used to detect rearing behavior. At the start of the session, mice were placed in the bottom left-hand corner of the arena and the test session started immediately. The primary outcome measures were transitions across the defined regions and center entries (locomotor activity), holepoking and rearing (exploratory behavior), and spatial d (dimensionality of locomotor patterns). Spatial d measures the degree to which the animal makes more straight-line movements versus more circumscribed paths of movement. It quantifies the geometric dimensionality of the locomotor path, where a value closer to 1 reflects a one-dimensional straight path, and values closer to 2 indicating highly circumscribed small-scale movements (Paulus and Geyer 1991).
Acoustic startle testing
Startle and PPI testing were examined in eight startle chambers (SR-LAB, San Diego Instruments, USA), using an experimental session as described previously (van Enkhuizen et al. 2013c). Each chamber consists of a Plexiglas cylinder, 5 cm in diameter, resting on a platform in a ventilated sound-attenuating outer box. Speakers mounted 33 cm above the cylinders produced all acoustic stimuli and movements of the animal were transduced by piezoelectric accelerometers mounted under the cylinders and stored and digitized by an interface and computer assembly. Mice were placed into the startle chambers and testing started after a 5 min acclimation period. Mice were exposed to a 65 dB background sound and light, located on the ceiling of the chamber, continuously throughout the session. Startle pulses were 40 ms and prepulses were 20 ms in duration. The inter-trial interval (ISI) between stimulus presentations ranged between 3–12 s (7 s average). The acoustic startle sessions included five blocks. The first block consisted of five 120 dB pulses. The second block included prepulse trials (69, 73, and 81 dB) preceding a 120 dB pulse. The third block included acoustic startle responding only (80, 90, 100, 110, and 120 dB pulses). The fourth block varied the ISI, consisting of 73 dB prepulses preceding 120 dB pulses by 25, 50, 100, 200, and 500 ms. The fifth and final block delivered five 120 dB pulses and together with 120 dB pulses in each block served to assess habituation. PPI was calculated as a percentage score for each prepulse intensity based on the 120 dB pulse within that block: %PPI = 100 − [(startle magnitude for prepulse + pulse / startle magnitude for pulse alone) × 100].
Tail suspension test
Assessing immobility in the TST is commonly used to screen for compounds with antidepressant efficacy and is also used to identify depression-like behavior in mice (Cryan et al. 2005). Mice were gently suspended by the tip of the tail attached with a piece of adhesive tape to a metal bar placed horizontally 50 cm above the tabletop. Videotapes were scored by an experimenter blind to the experimental treatment for time spent immobile over 6 min. The primary outcome measure was immobility defined as no movement except for respiration.
Forced swim (Porsolt) test
Consistent with the TST, the FST was devised to screen for compounds with antidepressant efficacy and is based on observing so-called “behavioral despair”. Mice were placed in a clear glass beaker with a diameter of 15 cm, a height of 24 cm, and 15 cm of tap water at 25 °C. The duration of the test was 6 min and videotapes were scored for all 6 min by a trained investigator blind to the experimental treatment. The primary outcome measure was immobility defined as no movement except minor movement required to keep afloat. After the test, the animal was dried and returned to its home cage. The water was replaced between every 5–6 animals in order to retain correct temperature and cleanliness.
Experiments
For a more detailed description of the animals used, and the experimental timeline, see Fig. 1. Fig. 2 provides information on the serum levels produced by 300, 600, or 1200 mg/l of lithium as well as compared with other studies.
Exp 1a: Assessing the effects of physostigmine on immobility in the TST
Mice received saline or 0.03, 0.1, or 0.3 mg/kg physostigmine 30 min prior to testing (n=10/group).
Exp 1b: Assessing the effects of physostigmine on immobility in the FST
After observing a strong lethargy-inducing effect with 0.3 mg/kg physostigmine treatment in the BPM, we investigated a lower dose of this compound in the FST. Mice received saline or 0.01, 0.03, or 0.1 mg/kg physostigmine 30 min prior to testing (n=10/group).
Exp 1c: Assessing the effects of lithium on physostigmine-induced increased immobility in the FST
Mice (n=96) received chronic lithium (300, 600, or 1200 mg/l) or regular drinking water (vehicle) for 17 days. Because mice treated with lithium 1200 mg/l had experienced severe side-effects (including some deaths), only mice treated with lithium 300 or 600 mg/l were tested. These mice then received saline or 0.03 mg/kg physostigmine 30 min prior to testing (n=12/group).
Exp 2a: Assessing the effects of physostigmine on exploration in the BPM
Mice were tested in the BPM for 45 min and received saline or 0.03, 0.1, or 0.3 mg/kg physostigmine 10 min prior to testing (n=10/group).
Exp 2b: Assessing the effects of physostigmine on PPI in the acoustic startle test
Immediately after experiment 2a, mice were tested in the acoustic startle test. Hence, these mice had received saline or 0.03, 0.1, or 0.3 mg/kg physostigmine 55 min prior to testing (n=10/group).
Exp 3a: Assessing the effects of lithium on exploration in the BPM
Mice (n=48) were tested first without drug in the BPM for 30 min to match their baseline behavior based on transitions, holepoking, rearing, and spatial d. They were then counterbalanced into groups that received regular tap water (vehicle; n=12) or lithium solution (n=12/dose). After 10 days of treatment, mice were tested in the BPM for 60 min.
Exp 3b: Assessing the effects of lithium on GBR12909-induced hyper-exploration in the BPM
Treatment naive female C57BL/6 mice received tap water (vehicle; n=24) or 1000 mg/l lithium solution (n=27). After 7 days of treatment, half of these groups received saline or GBR12909 (13 mg/kg; n ≈ 12/group) immediately prior to assessing their exploration in the BPM for 60 min.
Statistical analyses
We first confirmed that all data was distributed normally and displayed equal variances. Data from the BPM and acoustic startle test were analyzed using two- or three-way analyses of variance (ANOVA), with GBR12909 and lithium treatment as between-subject factors and time-period (15 min), prepulse intensity, pulse intensity, ISI, or habituation block as within-subject factors. TST and FST data were analyzed using a one- or two-way ANOVA with drug and dose of lithium treatment as between-subject factors. Tukey post hoc analyses of main or interaction effects were performed where applicable. The animals’ body weights were compared using an independent samples t-test. All BPM and startle data were analyzed using the BMDP statistical software (Statistical Solutions Inc., USA), while TST and FST data were analyzed using SPSS (19.0, Chicago, IL, USA). The α level was set to 0.05.
RESULTS
Experiment 1a: The effects of physostigmine on immobility in the TST
Three saline-treated mice were excluded from analyses, due to climbing their tail. Physostigmine significantly increased immobility time (F(3,33)=16.3, p<0.001; Fig. 3a), at each dose compared to saline (p<0.001).
Figure 3. Time spent immobile in the TST and FST after physostigmine administration and its reversal by lithium in the FST.
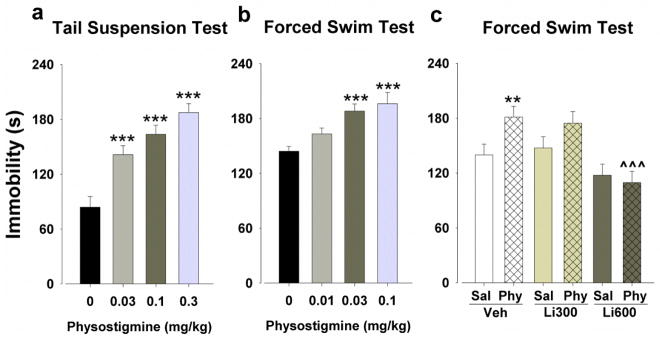
Each dose of physostigmine increased immobility times compared to saline in the TST (a). Both 0.03 and 0.1 mg/kg increased immobility times compared to saline in the FST, but 0.01 mg/kg had no effect (b). In another group of mice, physostigmine (Phy; 0.03 mg/kg) increased immobility times compared to saline (Sal) in the vehicle-treated mice (Veh), but after a more focused re-analysis this physostigmine-induced immobility was not observed in the mice treated with lithium 600 mg/l (c). Data are presented as mean + S.E.M. n = 10–12 animals per group. ** p<0.01 and *** p<0.001 when compared to saline and ^^^ p<0.001 when compared to vehicle.
Experiment 1b: The effects of physostigmine on immobility in the FST
Consistent with the TST, physostigmine increased immobility time in the FST (F(3,36)=8.0, p<0.001; Fig. 3b), at 0.03 and 0.1 mg/kg (p<0.001), but not 0.01 mg/kg (p>0.05), compared to saline.
Experiment 1c: The effects of lithium on physostigmine-induced increased immobility in the FST
A few mice were excluded from analyses because of unforeseen death (due to circumstances not related to lithium treatment). When analyzed including both lithium doses, there was a main effect of lithium (F(2,60)=10.4, p<0.001) and physostigmine (F(1,60)=4.2, p<0.05), but no interaction between both (F(2,60)=2.3, p>0.05). Given the numerical distinction that the main effect was at 600 mg/l, the data were re-analyzed comparing lithium 600 mg/l to saline treatment. A main effect of lithium (F(1,42)=15.4, p<0.001) and lithium by physostigmine interaction (F(1,42)=4.2, p<0.05; Fig. 3c) were observed, without a main effect of physostigmine (F(1,42)=4.2, p>0.05). Hence, despite the evidence that lithium exerted overall antidepressant-like effects, the focused analysis revealed that this effect was driven by 600 mg/l lithium treatment in physostigmine-treated mice. Post hoc analyses confirmed that physostigmine increased immobility time compared to saline in the vehicle- (p<0.01), but not the lithium-treated animals (p>0.05). Hence, treatment with lithium 600 mg/l attenuated the physostigmine-induced increased immobility (p<0.001). Lithium did not affect mice receiving saline (p>0.05). We also observed the mice for potential adverse effects of lithium. No adverse effects on health were reported; neither were differences in weight observed between vehicle-treated mice (28.0 g) and mice treated with lithium 300 mg/l (27.3 g; t(46)=1.0, p>0.05) or lithium 600 mg/l (26.0 g; t(45)=1.5, p>0.05).
Experiment 2a: The effects of physostigmine on exploration in the BPM
Locomotor behavior
Mice receiving physostigmine exhibited significantly reduced transitions (F(3,36)=55.0, p<0.001; Fig. 4a) and center entries (F3,36)=26.1, p<0.001; Fig. 4b),
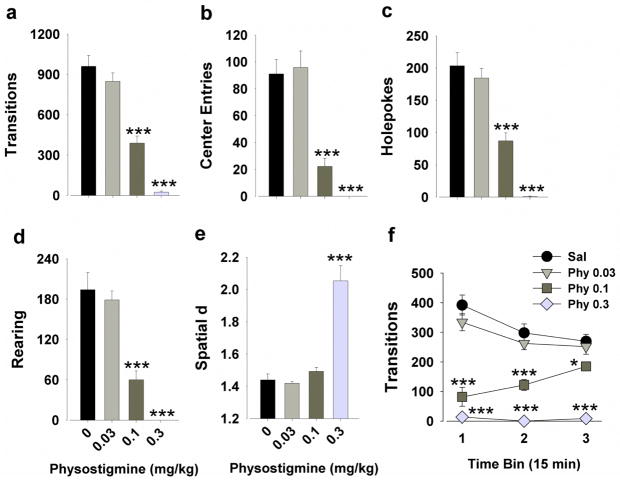
Figure 4. Effects of physostigmine on the exploratory profile of mice in the BPM.
Physostigmine (0.1 and 0.3 mg/kg) decreased activity as measured by transitions (a) and center entries (b). Physostigmine (0.1 and 0.3 mg/kg) decreased exploration as measured by holepoking (c) and rearing (d). The highest dose greatly increased spatial d values to the limit of its range, likely due to severely depressed activity compromising the calculation of spatial d (e). Mice administered 0.1 mg/kg physostigmine exhibited increased activity over time, approaching normal levels by the end of the session (f). The lowest dose (0.03 mg/kg) had no effect on any of the measures (a–f). Data are presented as mean ± S.E.M. n = 10 animals per group. * p<0.05 and *** p<0.001 when compared to saline.
Exploratory behavior
Physostigmine significantly reduced holepoking (F3,36)=44.9, p<0.001; Fig. 4c) and rearing (F3,36)=33.5, p<0.001; Fig. 4d) in mice.
Locomotor patterns
Physostigmine significantly increased spatial d in mice (F3,36)=34.1, p<0.001; Fig. 4e). Only 0.3 mg/kg physostigmine increased spatial d compared to saline (p<0.001), although the low activity limits the exact measurement of spatial d.
Post hoc analyses confirmed that only the two highest doses of physostigmine (0.1 and 0.3 mg/kg) reduced transitions, center entries, holepokes, and rearing (p<0.001). The lowest dose (0.03 mg/kg) did not affect any of the above measures compared with vehicle (p>0.05). When split by three 15 min time bins, there were significant time by physostigmine interactions for transitions (F(6,72)=12.2, p<0.001), center entries (F(6,72)=2.3, p<0.05), holepoking (F(6,72)=7.4, p<0.001), and rearing (F(6,72)=5.5, p<0.001), reflecting that mice treated with 0.1 mg/kg physostigmine approached control levels of behavior over time (Fig. 4f).
Experiment 2b: The effects of physostigmine on PPI in the acoustic startle test
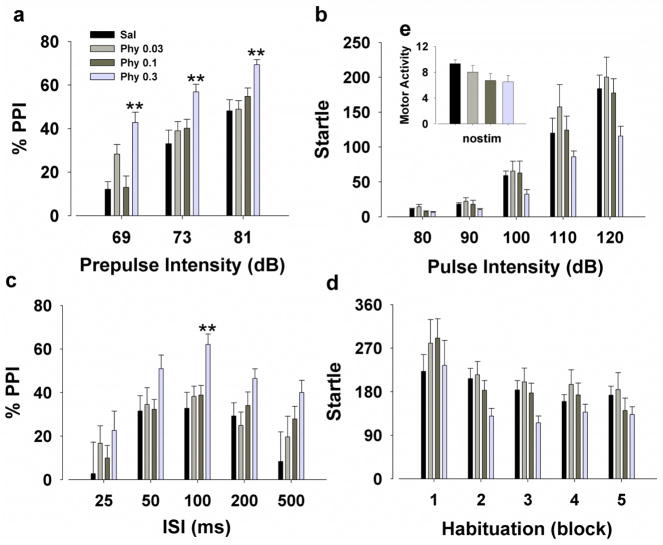
A main effect of prepulse (F(2,72)=129.0, p<0.001; Fig. 5a) revealed that PPI improved with higher prepulse intensities, providing construct validity. A main effect of physostigmine (F(3,36)=8.7, p<0.001) and interaction with prepulse (F(6,72)=3.3, p<0.01) were observed. Post hoc analyses revealed that 0.3 mg/kg physostigmine increased PPI at each prepulse intensity (p<0.01), while 0.03 mg/kg tended to increase PPI only at a 69 dB prepulse intensity (p<0.1). There was also a trend effect of physostigmine on startle amplitude (F(3,36)=2.6, p<0.1; Fig. 5b) and a pulse by physostigmine interaction (F(12,144)=1.8, p<0.05). No post hoc effects were observed however. When split by ISI, there was a main effect of physostigmine (F(3,36)=3.6, p<0.05; Fig. 5c). Post hoc analyses revealed that consistent with its effects on PPI at varying prepulse intensity levels, 0.3 mg/kg physostigmine increased PPI at ISI 100 (p<0.01) and tended to at ISI 500 (p<0.1). All mice habituated over time (F(4,144)=15.5, p<0.001; Fig. 5d), without effect of physostigmine treatment (F(3,36)=1.6, p>0.05). No effect of physostigmine was observed on movement when no stimuli were presented (F(3,36)=1.9, p>0.05; Fig. 5e). Finally, in mice matched for startle reactivity (n=14), there was still a main effect of physostigmine on PPI (F(3,30)=6.0, p<0.01) with 0.3 mg/kg increasing PPI at each prepulse intensity (p<0.05).
Figure 5. Effects of physostigmine on sensorimotor gating of the acoustic startle response of mice.
The highest dose of physostigmine (0.3 mg/kg) increased PPI compared to saline (Sal) whereas 0.03 mg/kg only tended to increase PPI at the lowest prepulse (a). Physostigmine had no effect on overall amplitude of the startle response (b). When split by ISI, 0.3 mg/kg physostigmine increased PPI at ISI 100 (c). All mice exhibited habituation over time, without effect of physostigmine treatment (d). No difference between drug and saline was observed when no stimulus was presented (e). Data are presented as mean + S.E.M. n = 10 animals per group. ** p<0.01 when compared to saline.
Experiment 3a: The effects of different doses lithium on exploration in the BPM
Locomotor behavior
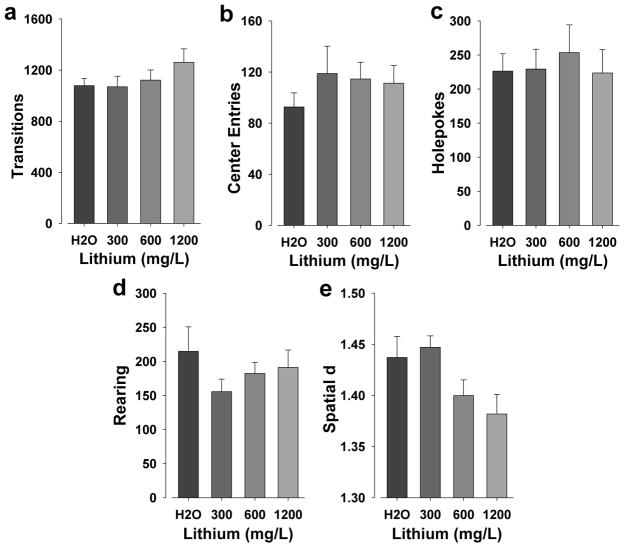
There were no main effects of lithium on transitions, nor on center entries (F<1, p>0.05; Fig. 6a–b). Post hoc analyses revealed no differences between doses of lithium and vehicle treatment.
Figure 6. The effects of chronic lithium on the exploratory profile of mice in the BPM.
There were no significant effects of lithium on transitions (a) or center entries (b). No differences between any dose of lithium and vehicle treatment were observed for holepoking (c) or rearing (d). Although there was a main effect of lithium on spatial d, no differences between doses of lithium and vehicle treatment were observed (e). Data are presented as mean + S.E.M. n = 12 animals per group.
Exploratory behavior
There were no effects of lithium on holepoking or rearing (F<1, p>0.05; Fig. 6c–d).
Locomotor patterns
There was a main effect of lithium on spatial d (F(3,44)=3.3, p<0.05; Fig. 6e). Post hoc analyses revealed no differences between lithium and vehicle treatment however.
We also examined the potential adverse effect of lithium on the animals’ body weights and observed no difference between vehicle-treated mice (27.5 g) and mice treated with lithium 300 mg/l (26.8 g; t(22)<1, p>0.05) or lithium 600 mg/l (25.4 g; t(21)=1.1, p>0.05). Mice treated with lithium 1200 mg/l had significantly lower body weights compared to vehicle however (23.9 g; t(22)=3.2, p<0.005).
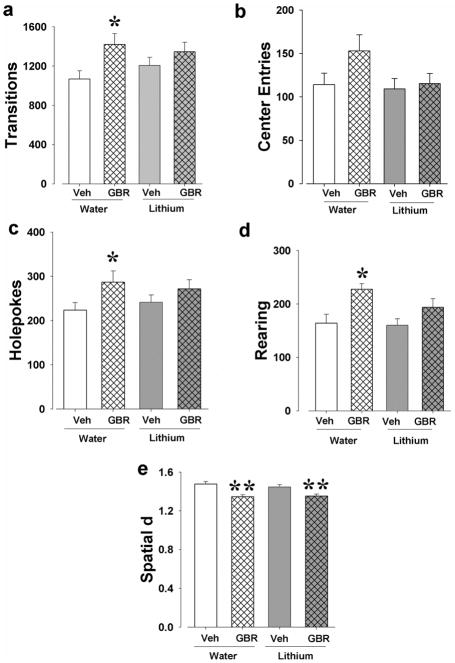
Experiment 3b: The effects of lithium 1000 mg/l on GBR12909-induced hyper-exploration in the BPM
Locomotor behavior
A main effect of GBR12909 was observed for transitions (F(1,47)=7.0, p<0.05; Fig. 7a), but not center entries (Fig. 7b). There were no main effects of or interactions with lithium treatment for transitions or center entries. Post hoc analyses revealed that GBR12909 increased transitions in the vehicle-treated (p<0.05), but not in the lithium-treated mice.
Figure 7. Chronic lithium-induced attenuation of DAT blockade-induced mania-like exploratory behavior in the BPM.
GBR12909 significantly increased activity as measured by transitions in both groups although only in vehicle- but not chronic-lithium treated mice in post hoc tests (a). There were no effects on center entries (b). GBR12909 induced hyper-exploration as measured by increased holepoking (c) and rearing (d) overall, but not in lithium-treated mice after post hoc assessment. Treatment with GBR12909 resulted in more linear patterns of movement as deduced from lower spatial d overall in both vehicle- and lithium-treated mice (e). Data are presented as mean + S.E.M. n ≈ 12 animals per group. ** p<0.05 and *** p<0.01 when compared to saline.
Exploratory behavior
GBR12909 significantly increased holepoking (F(1,47)=5.4, p<0.05; Fig. 7c) and rearing (F(1,47)=11.5, p<0.01; Fig. 7d). There were no main effects of or interactions with lithium treatment for holepoking or rearing. Post hoc analyses revealed that GBR12909 increased holepoking and rearing in the vehicle-treated (p<0.05), but not in the lithium-treated mice.
Locomotor patterns
There was a main effect of GBR12909 for spatial d (F(1,47)=26.5, p<0.001; Fig. 7e), but no lithium effect or interaction between both. Post hoc analyses revealed that GBR12909 reduced spatial d in both vehicle-treated mice and lithium-treated mice (p<0.05).
When body weight was examined, it was observed that mice treated with lithium 1000 mg/l (23.8 g) had significantly lower body weights compared to vehicle-treated mice (25.4 g) (t(49)=2.2, p<0.05).
DISCUSSION
We examined the potential use of cholinergic and dopaminergic manipulations to model BD facets of depression and mania in mice respectively. The AChE inhibitor physostigmine induced depression-like behavior in mice similar to human observations and consistent with the increased ACh levels observed in the pathophysiology of depressed patients. Importantly for BD research, this increased ACh-induced “behavioral despair” was normalized by the mood-stabilizer lithium. Reducing DAT functioning via GBR12909 treatment induced a mania-like state in mice as measured by hyper-exploration. Similar to its reversal of physostigmine-induced depression-like behavior, chronic lithium attenuated GBR12909-induced hyper-exploration, although it did not alter the effect of GBR12909 on behavioral organization. Thus, by targeting diverse etiologically relevant mechanisms, separate models of both depression and mania can be generated pharmacologically that are both partially responsive to lithium treatment.
Physostigmine administration induced depression-like behavior in mice, replicating its depression-inducing effects in humans (Risch et al. 1981). This depression-like behavior was interpreted from physostigmine increasing immobility times in the TST and FST at doses that were not sedative, an effect consistent with previous reports of physostigmine-induced immobility in mice (Mineur et al. 2013) and rats (Hasey and Hanin 1991) that may be hippocampally mediated (Mineur et al. 2013). Mineur et al. reported that 0.5 mg/kg physostigmine induced depression-like behavior without affecting motor activity. In contrast, we observed that 0.03 mg/kg increased immobility without affecting locomotion, while 0.3 mg/kg severely depressed locomotion and immobility. The sources of the differences between these dosing reports remain unclear, although reduced locomotor activity has been observed before with physostigmine at doses above 0.1 mg/kg (Dunstan and Jackson 1977), but not as low as 0.03 mg/kg. Here, 0.03 mg/kg physostigmine increased immobility in the TST and FST, without affecting activity in the BPM, supporting the conclusion that physostigmine induced “behavioral despair”/ depression-like behavior rather than an overall suppression of activity. Higher physostigmine doses decreased activity and exploration (0.1 and 0.3 mg/kg), and produced severely localized movements (0.3 mg/kg). To determine the cross-species relevance of these findings, current studies are investigating the human exploratory profile of patients with both unipolar and bipolar depression in the BPM.
At high doses, physostigmine increased PPI in the acoustic startle test, consistent with previous results at similar doses (Clark et al. 2005). This effect is unlikely due to sedation since increases in PPI were also seen in mice matched for startle reactivity. In support of a non-sedating effect explaining physostigmine-induced increases in PPI, locomotor activity had normalized by the time its effects on PPI were tested (Fig. 4f). The immobility-inducing dose of 0.03 mg/kg did not affect PPI. Although PPI has yet to be studied in depressed bipolar patients, PPI is unaffected in unipolar depression (Perry et al. 2004; Quednow et al. 2006), supporting the disease-relevance of these findings. Future studies will investigate sensorimotor gating across the spectrum of BD, but it is likely that active manic symptoms and/or acute psychosis are necessary to exhibit PPI deficits (Kohl et al. 2013).
Previously, we observed a consistent pattern of increased activity, increased exploration, and more linear patterns of movement in mice administered GBR12909 or DAT KD mice in the mouse BPM (Perry et al. 2009; Young et al. 2010a; b), mimicking the behavior of manic (Minassian et al. 2011) and euthymic BD patients (Henry et al. 2013). In the current studies, GBR12909 at 13 mg/kg again produced this mania-like behavior in mice. Recently, we reported that chronic valproate at human therapeutic blood levels for BD attenuated the mania-like pattern induced by GBR12909 (van Enkhuizen et al. 2013a). Moreover, GBR12909 increased measures of motivation (van Enkhuizen et al. 2013b) and sped responding in mice (Young and Geyer 2010), while low doses (5 mg/kg) also reduced immobility time in the FST (Esumi et al. 2013) and impaired PPI (Kwek and van den Buuse 2013), consistent with reported PPI deficits in patients with BD mania (Perry et al. 2001). Increasing DA functional activity by inhibiting the DAT thus results in different facets of behavior in rodents relevant to mania in humans.
Previously, we showed that valproate only attenuated GBR12909-induced hyperactivity compared with vehicle-treated mice, without affecting their specific exploration (van Enkhuizen et al. 2013a). Earlier studies with lithium in chow (1.2 and 2.4%) were terminated due to concerns for the health of the mice (e.g. polydipsia and severe weight loss, despite making saline available; unpublished observations). Lithium 600 mg/l administered chronically in drinking water resulted in low therapeutic blood concentrations consistent with previous studies of 10–21 days lithium administration (Fig. 2) (Dehpour et al. 2002; Ghasemi et al. 2009; Roybal et al. 2007). Lithium at 300 mg/l resulted in serum concentrations below therapeutic levels and was not effective in reversing immobility. No adverse health or weight effects were observed with 300 or 600 mg/l. Lithium at 1000 mg/l however, resulted in a slight weight loss (1.5 g). Lithium treatment at 1200 mg/l resulted in toxic blood concentrations, and a more severe loss of weight (3.6 g). Other studies examining 1200 mg/l of lithium resulted in lower concentrations likely because measurements were taken from brain tissue (Beaulieu et al. 2008) and after only 10 days of treatment (Dehpour et al. 1995). Future behavioral studies with chronic lithium should include pretreatment weights in their design and include a bottle of saline for mice in order to better regulate the potential lithium-induced electrolyte imbalance. Based on our serum concentration studies, a dose of 1000 mg/l would likely yield serum concentrations in the therapeutic range for BD to block switching to a manic state. Lithium at 1000 mg/l indeed attenuated the effects of GBR12909 on exploration and activity, although significant differences compared to GBR12909 + vehicle pretreatment were not observed, in contrast to results with valproate (van Enkhuizen et al. 2013a). Lithium did not attenuate GBR12909-induced effects on behavioral organization of these mice as measured by spatial d. In contrast, lithium counteracted amphetamine-induced hyperactivity (Gould et al. 2007), an effect that may have been mediated by reductions in catecholamine synthesis and release (Berggren 1985). In terms of depression-like behavior, chronic lithium treatment at 600 mg/l reduced overall immobility as indicated by a main effect, consistent with previous findings (Bersudsky et al. 2007; Can et al. 2011). Specifically however, lithium normalized the physostigmine-induced immobility without affecting vehicle-treated mice. Physostigmine-induced immobility was similarly reversed by acute treatment with the antidepressant fluoxetine (Mineur et al. 2013), although immobility was also reduced in control mice. The ‘antidepressant’ effect of lithium observed here may be due to compensation of physostigmine-induced reduction of AChE levels, since lithium can increase AChE levels (Varela et al. 2013). Lithium also increases cholinergic functioning (Jope 1979) and augments seizure- and catalepsy-inducing effects of pilocarpine in rats however (Jope and Morrisett 1986; Lerer 1985). Clearly, a comprehensive explanation of the mechanism of action of lithium is unclear, but likely entails parallel effects on the catecholamine and acetylcholine neurotransmitter systems. Ultimately, lithium prevents mania relapses in only approximately 50% of patients (Geddes et al. 2004), hence further investigation and better treatments are required. Future studies examining other behaviors relevant to bipolar depression and mania are required.
The present studies support the premise of a cholinergic/catecholaminergic mechanism that differentiates states of BD (van Enkhuizen et al. 2015). Research investigating how the same subjects may cycle between these different neurobiological states during depression and mania is required. Altered light exposure has been theorized to contribute to these extreme behaviors (Sherman 2012) and indeed changing light exposure can switch neurotransmitter levels in rats (Dulcis et al. 2013). Utilizing this environmental manipulation alongside animals with a genetic susceptibility to BD (Malkesman et al. 2009) may provide a model animal with which to test novel therapeutics to treat the entire spectrum of BD (Young and Dulcis 2015). Thus, instead of reproducing depression- and mania-like behaviors using two separate manipulations, these combined challenges may mimic BD more accurately in a single model animal.
For future testing, improving the techniques used to measure depression-like behavior would prove useful. Although increased immobility times are commonly used to identify depression-like behavior, there is a debate as to whether the FST and TST can only be used to screen for antidepressant-like activity and may not reflect depressive states per se. Future studies could assess more complex behaviors related to cognition given its importance to functional outcome for patients with BD (Green 2006). For example, examining dynamic decision-making in models of BD mania and depression could be useful. Patients across the spectrum exhibit poor decision-making (Adida et al. 2011), but this effect may be driven by a hypersensitivity to rewards during the manic phase (Brambilla et al. 2012; van Enkhuizen et al. 2014a), yet a hypersensitivity to punishments during the depressed phase (Adida et al. 2011). Future tests should also assess other treatments available such as antipsychotics and antidepressants in these models of BD depression- and mania-like behavior. Finally, although most studies reported here used males, females were used to develop evidence of lithium-induced blockade of GBR12909-induced mania-relevant profiles, demonstrating treatment efficacy across sexes. Considering BD affects both males and females, these studies require testing in both sexes in future studies.
In conclusion, we described separate animal models of both depressive- and mania-like facets of BD by manipulating the cholinergic and dopaminergic pathways respectively and assessing the efficacy of chronic treatment with lithium. These models support the hypotheses that the neurobiological underpinnings may vary dependent upon the phases of BD (van Enkhuizen et al. 2014b). Understanding the mechanism(s) under which the neurobiology of patients change will be vital for blocking the cycling that occurs in patients (Young and Dulcis 2015). Meanwhile, the currently described models provide a way of testing novel therapeutics targeted at symptoms exhibited during different stages of BD.
Acknowledgments
We thank Drs. Berend Olivier, William Perry, and Arpi Minassian for their support. These studies were supported by NIH grants R01-MH071916, and R01-MH104344, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Conflict of Interest
Dr. van Enkhuizen and Ms. Milienne-Petiot report no conflict of interest. Dr. Geyer has received consulting compensation from Abbott, Dart, Lundbeck, Neurocrine, Omeros, Otsuka, and Sunovion, and holds an equity interest in San Diego Instruments. Dr. Geyer also has research grant support from Intracellular Therapeutics, Johnson & Johnson, NIDA, NIMH, and the U.S. Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. Dr. Young has received consulting compensation from Amgen and Arena Pharmaceuticals as well as research grant support from Cerca, Omeros, Lundbeck Ltd, NIMH, and the U.S. Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The aforementioned support did not direct any research presented here.
References
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, Hutchins GD, Normandin MD, Yoder KK. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disorder. 2011;13:406–13. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–36. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Begley CE, Annegers JF, Swann AC, Lewis C, Coan S, Schnapp WB, Bryant-Comstock L. The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics. 2001;19:483–95. doi: 10.2165/00019053-200119050-00004. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Bersudsky Y. Bipolar disorder: Mania and depression. Discov Med. 2004;4:239–45. [PubMed] [Google Scholar]
- Berggren U. Effects of chronic lithium treatment on brain monoamine metabolism and amphetamine-induced locomotor stimulation in rats. J Neural Transm. 1985;64:239–50. doi: 10.1007/BF01256470. [DOI] [PubMed] [Google Scholar]
- Bersudsky Y, Shaldubina A, Belmaker RH. Lithium’s effect in forced-swim test is blood level dependent but not dependent on weight loss. Behav Pharmacol. 2007;18:77–80. doi: 10.1097/FBP.0b013e32801416ed. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perlini C, Bellani M, Tomelleri L, Ferro A, Cerruti S, Marinelli V, Rambaldelli G, Christodoulou T, Jogia J, Dima D, Tansella M, Balestrieri M, Frangou S. Increased salience of gains versus decreased associative learning differentiate bipolar disorder from schizophrenia during incentive decision making. Psychological Medicine. 2012:1–10. doi: 10.1017/S0033291712001304. [DOI] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O’Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes Brain Behav. 2011;10:434–43. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, Purgato M, Spineli LM, Goodwin GM, Geddes JR. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378:1306–15. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- Clark MG, Sun W, Myers TM, Bansal R, Doctor BP, Saxena A. Effects of physostigmine and human butyrylcholinesterase on acoustic startle reflex and prepulse inhibition in C57BL/6J mice. Pharmacology, Biochemistry and Behavior. 2005;81:497–505. doi: 10.1016/j.pbb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neuroscience and Biobehavioral Reviews. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dehpour AR, Farsam H, Azizabadi-Farahani M. Inhibition of the morphine withdrawal syndrome and the development of physical dependence by lithium in mice. Neuropharmacology. 1995;34:115–21. doi: 10.1016/0028-3908(94)00121-8. [DOI] [PubMed] [Google Scholar]
- Dehpour AR, Sadr SS, Azizi MR, Namiranian K, Farahani M, Javidan AN. Lithium inhibits the development of physical dependence to clonidine in mice. Pharmacol Toxicol. 2002;90:89–93. doi: 10.1034/j.1600-0773.2002.900206.x. [DOI] [PubMed] [Google Scholar]
- DSM-V. Diagnostic and statistical manual of mental health disorders: DSM-5. 5. American Psychiatric Publishing; 2013. [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–53. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Dunstan R, Jackson DM. The demonstration of a change in responsiveness of mice to physostigmine and atropine after withdrawal from long-term haloperidol pretreatment. Journal of Neural Transmission. 1977;40:181–9. doi: 10.1007/BF01300132. [DOI] [PubMed] [Google Scholar]
- El-Yousef MK, Janowsky DS, Davis JM, Rosenblatt JE. Induction of severe depression by physostigmine in marihuana intoxicated individuals. British Journal of Addiction to Alcohol and Other Drugs. 1973;68:321–5. doi: 10.1111/j.1360-0443.1973.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Esumi S, Sagara H, Nakamoto A, Kawasaki Y, Gomita Y, Sendo T. Effect of GBR12909 on affective behavior: distinguishing motivational behavior from antidepressant-like and addiction-like behavior using the runway model of intracranial self-stimulation. Behavioural Brain Research. 2013;243:313–21. doi: 10.1016/j.bbr.2012.10.051. [DOI] [PubMed] [Google Scholar]
- Gard PR, Naylor C, Ali S, Partington C. Blockade of pro-cognitive effects of angiotensin IV and physostigmine in mice by oxytocin antagonism. European Journal of Pharmacology. 2012;683:155–60. doi: 10.1016/j.ejphar.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. American Journal of Psychiatry. 2004;161:217–22. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Molecular Psychiatry. 2002;7:1039–53. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacology, Biochemistry and Behavior. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Sadeghipour H, Poorheidari G, Dehpour AR. A role for nitrergic system in the antidepressant-like effects of chronic lithium treatment in the mouse forced swimming test. Behav Brain Res. 2009;200:76–82. doi: 10.1016/j.bbr.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–33. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. Journal of Clinical Psychiatry. 2006;67(Suppl 9):3–8. discussion 36–42. [PubMed] [Google Scholar]
- Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z, Seibyl JP, McClure-Begley TD, Picciotto MR, Esterlis I. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol Psychiatry. 2013;74:768–76. doi: 10.1016/j.biopsych.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasey G, Hanin I. The cholinergic-adrenergic hypothesis of depression reexamined using clonidine, metoprolol, and physostigmine in an animal model. Biological Psychiatry. 1991;29:127–38. doi: 10.1016/0006-3223(91)90041-j. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, Geyer MA, Perry W. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. J Affect Disord. 2013;150:948–54. doi: 10.1016/j.jad.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neuroscience and Biobehavioral Reviews. 2010;34:1296–306. doi: 10.1016/j.neubiorev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosomatic Medicine. 1974;36:248–57. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–5. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Jope R. Effects of lithium treatment in vitro and in vivo on acetylcholine metabolism in rat brain. J Neurochem. 1979;33:487–95. doi: 10.1111/j.1471-4159.1979.tb05179.x. [DOI] [PubMed] [Google Scholar]
- Jope RS, Morrisett RA. Neurochemical consequences of status epilepticus induced in rats by coadministration of lithium and pilocarpine. Exp Neurol. 1986;93:404–14. doi: 10.1016/0014-4886(86)90200-1. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. Journal of Psychiatric Research. 2013;47:445–52. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kwek P, van den Buuse M. Modafinil disrupts prepulse inhibition in mice: strain differences and involvement of dopaminergic and serotonergic activation. European Journal of Pharmacology. 2013;699:132–40. doi: 10.1016/j.ejphar.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Lerer B. Studies on the role of brain cholinergic systems in the therapeutic mechanisms and adverse effects of ECT and lithium. Biol Psychiatry. 1985;20:20–40. doi: 10.1016/0006-3223(85)90132-5. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–24. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:209–24. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Das P, Berk M. The science and practice of lithium therapy. Australian and New Zealand Journal of Psychiatry. 2012;46:192–211. doi: 10.1177/0004867412437346. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Chen G, Manji HK. Reverse translational strategies for developing animal models of bipolar disorder. Dis Model Mech. 2009;2:238–45. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. Public Library of Science One. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3573–8. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick DM, Swartz HA, Frank E. Suicide attempts in bipolar I and bipolar II disorder: a review and meta-analysis of the evidence. Bipolar Disord. 2010;12:1–9. doi: 10.1111/j.1399-5618.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Archives of General Psychiatry. 2001;58:844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D. Prepulse inhibition in patients with non-psychotic major depressive disorder. Journal of Affective Disorders. 2004;81:179–84. doi: 10.1016/S0165-0327(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biological Psychiatry. 2001;50:418–24. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178:84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Westheide J, Kuhn KU, Werner P, Maier W, Hawellek B, Wagner M. Normal prepulse inhibition and habituation of acoustic startle response in suicidal depressive patients without psychotic symptoms. Journal of Affective Disorders. 2006;92:299–303. doi: 10.1016/j.jad.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. Journal of Affective Disorders. 2012;136:63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Sitaram N, Gillin JC, Murphy DL. Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Research. 1981;4:89–94. doi: 10.1016/0165-1781(81)90012-3. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. American Journal of Psychiatry. 2012;169:851–9. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan G, Roberts AJ, Hedlund PB. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behavioural Brain Research. 2010;209:99–108. doi: 10.1016/j.bbr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaldubina A, Einat H, Szechtman H, Shimon H, Belmaker RH. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J Neural Transm. 2002;109:433–40. doi: 10.1007/s007020200035. [DOI] [PubMed] [Google Scholar]
- Sherman JA. Evolutionary origin of bipolar disorder-revised: EOBD-R. Medical Hypotheses. 2012;78:113–22. doi: 10.1016/j.mehy.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behavioural Brain Research. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int J Neuropsychopharmacol. 2013a;16:1021–31. doi: 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task : Relevance to mania. Psychopharmacology. 2013b;225:661–74. doi: 10.1007/s00213-012-2854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, Geyer MA, Young JW. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014a;39:3112–22. doi: 10.1038/npp.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW, Geyer MA. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. Eur J Pharmacol. 2014b doi: 10.1016/j.ejphar.2014.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW, Geyer MA. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. Eur J Pharmacol. 2015;753:114–26. doi: 10.1016/j.ejphar.2014.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Minassian A, Young JW. Further evidence for ClockDelta19 mice as a model for bipolar disorder mania using cross-species tests of exploration and sensorimotor gating Behavioural. Brain Research. 2013c;249:44–54. doi: 10.1016/j.bbr.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela RB, Valvassori SS, Lopes-Borges J, Fraga DB, Resende WR, Arent CO, Zugno AI, Quevedo J. Evaluation of acetylcholinesterase in an animal model of mania induced by D-amphetamine. Psychiatry Res. 2013;209:229–34. doi: 10.1016/j.psychres.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Young JW, Dulcis D. Investigating the mechanism(s) underlying switching between states in bipolar disorder. Eur J Pharmacol. 2015;759:151–162. doi: 10.1016/j.ejphar.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biol Psychiatry. 2010;67:784–7. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208:443–54. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010b;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. British Journal of Pharmacology. 2011a;164:1263–84. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. Journal of Psychopharmacology. 2011b;25:934–43. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]