Abstract
The optimal T cell attributes for adoptive cancer immunotherapy are unclear. Recent clinical trials of ex vivo expanded tumor-infiltrating lymphocytes indicated that differentiated T effector cells can elicit durable anti-tumor responses in some cancer patients, with their anti-tumor activity tightly correlated with their persistence in the host. Thus, there is great interest in the definition of intrinsic biomarkers that can predict the conversion of short-lived tumor antigen-specific T effector cells into long-lived T memory cells. Long-term persistence of ex vivo expanded tumor specific CD8+ T effector clones has been reported in refractory metastatic melanoma patients after adoptive T cell transfer. By utilizing highly homogeneous clone populations from these preparations, we performed a comparative transcriptional profiling to define pre-infusion molecular attributes that can be ascribed to an effector-to-memory transition. Through this route, we discovered that pre-infusion T cell clones which expressed the IL-7 receptor (IL-7R) and c-myc were more likely to persist longer after adoptive transfer to patients. The predictive value of these two biomarkers was strengthened by utilizing IL-7R protein, IL-7 induced pSTAT5, and c-myc mRNA expression to prospectively identify human tumor-specific T effector clones capable of engraftment into immunodeficient mice. Overall, our findings reveal IL-7R and c-myc expression as intrinsic biomarkers that can predict the fate of effector CD8+ T cells after adoptive transfer.
Keywords: Adoptive immunotherapy, CTL clones, persistence, IL-7 receptor, c-myc
Introduction
A central pursuit in the adoptive immunotherapy of cancer and viral diseases is the repopulation of the host immune system with antigen specific T cells that can mediate potent effector function, yet also, establish long term memory. Clinical trials transferring ex vivo expanded autologous tumor infiltrating lymphocytes have provided evidence that differentiated effector T cells can mediate objective tumor responses in patients with a variety of solid tumors (1–4). Retrospective analyses of these trials have associated the ability of the transferred cells to persist in vivo with their anti-tumor efficacy (3, 5, 6). Thus, in an effort to improve treatment outcomes, there has been active interest in identifying intrinsic markers that can predict whether antigen specific effector T cells develop into long-lived memory cells versus undergoing programmed cell death after transfer into patients. However, a significant challenge in defining the precise cellular attributes associated with these dichotomous fates stems from the heterogeneity of the polyclonal cell products infused in prior human trials. A clinical strategy to address this problem involves the adoptive transfer of highly characterized monoclonal populations of tumor specific T cells. We recently reported the long term persistence of ex vivo-expanded melanocyte differentiation antigen (MDA) specific CD8+ T effector clones in refractory metastatic melanoma patients after adoptive T cell transfer (NCT00665470 and NCT01495572) (7, 8). By virtue of the genetically unique TCR sequences expressed in these individual clones, we were able to accurately track their in vivo engraftment and survival without the interpretive ambiguity associated with bulk polyclonal cell infusions. Interestingly, although all of the infused clones were highly differentiated, lytic CD8+ effector T cells, several clones were able to establish long term memory and repopulate the immune repertoire of patients (8). In the current study, we sought to define the cellular and molecular attributes associated with this effector to memory transition by comparative transcriptional profiling of CD8+ T effector clones that could persist versus those that could not. Here, we report that the pre-infusion clone expression levels of the IL-7R and the proto-oncogene, c-myc, directly correlated with the level of persistence of these CD8+ effector T cell clones after adoptive transfer in humans. These clinical observations were experimentally validated upon an independently isolated set of CD8+ effector T cell clones by controlled adoptive transfer into highly immunodeficient mice. These findings support that IL-7R and c-myc expression may serve as valuable cell intrinsic markers that can predict the fate of effector CD8+ T cells after adoptive transfer.
Materials and Methods
Patients and clinical protocol
HLA-A2+ patients with metastatic melanoma were treated with either gp100-specific CD8+ T cell clones or MART-1-specific CD8+ T cell clones at the Surgery Branch, National Cancer Institute (NCI), between January 2009 and January 2013 on two consecutive phase II clinical protocols (NCT00665470 and NCT01495572) (8) approved by the Institutional Review Board and U.S. Food and Drug Administration. All patients gave informed consent for treatment in accordance with the Declaration of Helsinki. The patients were required to be 18 years of age or older and have measurable metastatic melanoma that expressed gp100 or MART-1 and Major Histocompatibility Complex Class I by immunohistochemistry. Prior to clone infusion, patients were transiently lymphoablated with a nonmyeloablative lymphodepleting regimen including intravenous administration of cyclophosphamide (60 mg/kg) for 2 days followed by fludarabine (25 mg/m2) for 5 days as previously described (3). One day after completion of their lymphodepleting regimen, patients received expanded CD8+ T cell clones intravenously, either with or without high-dose IL-2 (720,000 IU/kg) every 8 hours to tolerance.
Media and cell culture
Human cultured cell lines, including T2 cells (HLA-A2+ peptide transporter-associated protein deficient T-B hybrid) and melanoma tumor lines, 526 mel (HLA-A2+/gp100+/MART+) and 888 mel (HLA-A2−/gp100+/MART+), were routinely cultured in complete medium (CM) as previously described (8). The melanoma cell lines, 526mel and 888mel, were obtained from the cell production facility in the Surgery Branch, NCI. The tumor cells had been characterized to confirm tumor morphology, antigen, and HLA expression by immunohistochemistry; they were obtained and used within six months of testing. Human PBMCs used in this study were obtained by leukapheresis from HLA-A2+ metastatic melanoma patients evaluated on IRB approved protocols at the Surgery Branch, National Cancer Institute (NCI, National Institutes of Health, Bethesda, MD) and cultured in CM with 10% heat-inactivated human AB serum (Gemini Bio-Products).
Microarray and gene expression analysis of T cell clones
Total RNA from pre-infusion CD8+ effector T cell clones was isolated using an RNeasy kit (Qiagen) as per the manufacturer’s instructions and quality was validated with Agilent Bioanalyzer. About 100 ng of total RNA samples were reverse transcribed and labeled by biotin. Biotin-labeled cDNA was hybridized to Affymetrix Human Genome U133 Plus 2.0 Array (Santa Clara, CA) according to manufacturer's instructions. Washing, staining, and scanning of the microarray were carried out under strictly controlled conditions with the Affymetrix Fluidics Station and Scanner. Raw data from the generated cell-intensity files (.cel' extension) were imported into Partek Genomics Suite with normalization performed by robust multichip analysis (RMA) algorithm. Genes with differences in expression between persisting and non-persisting clones were identified by two-way analysis of variance (Partek) with a primary stringent p value < 0.01. Genes with differences in expression were filtered by the Benjamini-Hochberg false-discovery rate procedure (P < 0.05) and a between–group 'fold-change' criterion of over 2.0 (P < 0.05). The microarray data have been deposited in the National Center for Biotechnology Information under accession number GSE65627. The Ingenuity Pathway Analysis online software was used for pathway analysis of the list of differentially expressed genes. Relative mRNA quantitation for selected genes was determined by robust multichip analysis (RMA)-normalized intensity. qRT-PCR validation of microarray findings was performed using TaqMan primer/probe sets (Applied Biosystems). Results are presented relative to β-actin expression.
Adoptive transfer into NSG mice
NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were purchased from Jackson Laboratories and housed at the animal facility at the NCI (Bethesda) in pathogen-free conditions. Mouse experiments were approved by the NCI Animal Care and Use Committee (ACUC) and performed in accordance with NIH guidelines. Cohorts of NSG mice were adoptively transferred with equal numbers of CD8+ T cell clones (5e6 cells per mouse) via tail vein injection. At the time of adoptive transfer, mice also received concomitant intraperitoneal administration of human recombinant IL-15 (1 µg) and every alternate day for the duration of the experiment. At day 21 post transfer, mice were euthanized by CO2 asphyxiation and spleens were harvested to quantitate the persistence of the transferred clones by flow cytometry.
Additional methods have been described in the supplementary section
Results
Tumor specific CD8+ T effector clones have highly variable levels of engraftment after adoptive transfer in humans
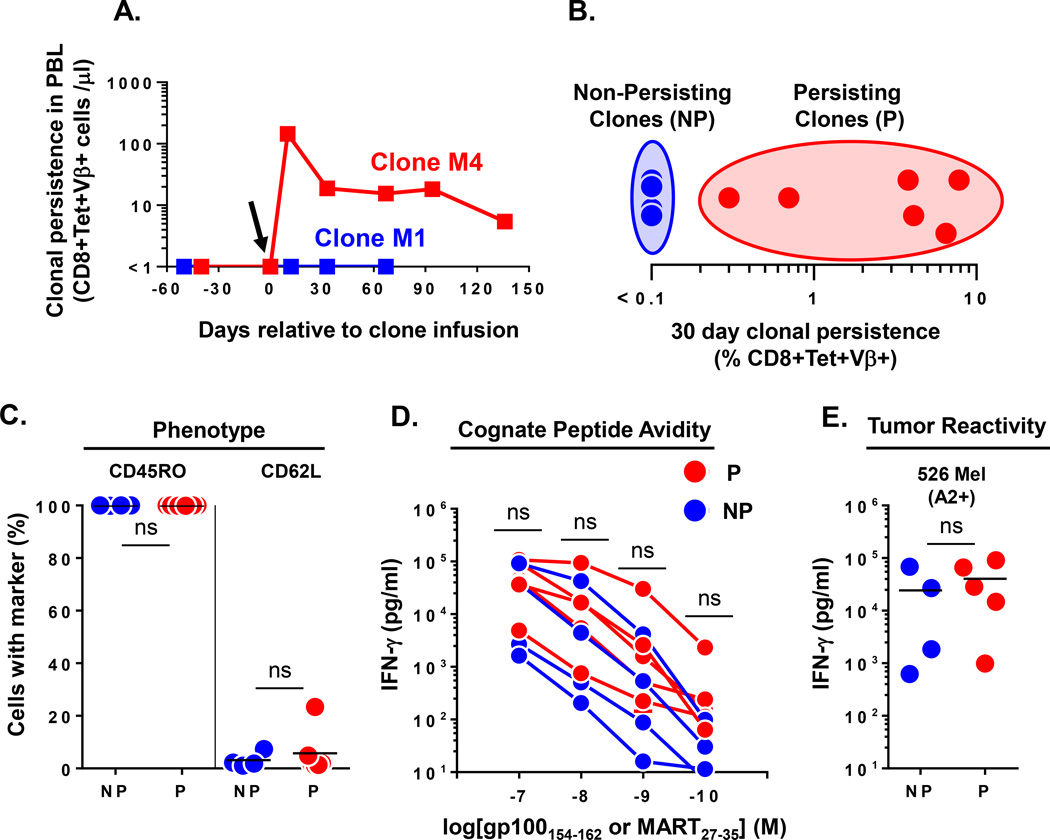
We analyzed samples of ten unique MDA specific CD8+ T cell clones which had been cryopreserved immediately prior to infusion into nine HLA-A*0201+ patients with refractory metastatic melanoma. All patients had undergone a non-myeloablative lymphodepleting conditioning regimen as previously described (8). The characteristics of the analyzed cohort of patients and their clone therapy are shown in Table 1. The first four patients were treated with CD8+ T cell clones specific for the gp100154-62 epitope and the next five patients received CD8+ T cell clones specific for the MART27-35 epitope. The TCR clonotype for each clone was defined by complete molecular sequencing of the beta chain variable region (Vβ). Each patient received a single clonotype except for patient M2 who received two unique clonotypes. To evaluate the in vivo persistence of the transferred clones, peripheral blood samples, obtained prior to and one month after cell infusion, were compared by flow cytometry for the percentage of CD8+ T cells that were tetramer positive. Further, each CD8+tetramer+ population was FACS sorted to >99% purity to allow complete TCR Vβ molecular sequencing to determine the antigen specific clonotypes that were present in the peripheral blood before and after clone infusion. The presence of the infused clonotype after infusion, but not before infusion, was defined as clonal persistence. The percentage of this clonotype among total circulating CD8+ T cells was used to define the persistence frequency. Using this stringent criterion, we detected highly variable levels of clonal engraftment ranging from undetectable (<0.1%) to 7.8% of all circulating CD8+ T cells at one month post infusion (Table 1). To evaluate the long-term fate of these clones, we obtained extended peripheral blood samples from patients and demonstrated the sustained persistence of some clones beyond 120 days (example: clone M4), while other clones could never be detected in the circulating PBL (example: clone M1) (Figure 1A). These persisting clones were additionally found to be functionally capable of re-responding to cognate antigen stimulation immediately ex vivo without culturing or cytokine support (7, 8). Next, to better study the dichotomous fate of the transferred clones, we categorized the infused clones as “persisting (P)” if they demonstrated peripheral blood levels of >0.1% of circulating CD8+ T cells at one month post transfer; “non-persisting (NP)” clones had engraftment levels of ≤0.1% of CD8+ T cells at one month post transfer (Figure 1B). The pre-infusion phenotype of both the NP and P set of clones demonstrated uniformly high expression of CD45RO and low expression of CD62L, consistent with their differentiated effector status (Figure 1C). Further, pre-infusion functional assays demonstrated no difference between NP and P clones in their avidity for cognate peptide pulsed on T2 target cells (Figure 1D) and naturally presented peptide on an allogeneic HLA-A2+ melanoma tumor cell line (526 Mel) (Figure 1E) by IFN-γ cytokine release assay. Cumulatively, these data suggested that the clinically administered CD8+ effector T cell clones had similar phenotypic and functional attributes, but possessed very different abilities to engraft in the host.
Table 1.
Patient and infused clone characteristics
| Age | Sex | Melanoma origin |
Disease sites | Clone ID# |
Clone specificity |
TCR Vβ clonotype |
No. infused clones (xiP) |
30 day clone persistence (%CD8+Tet+Vβ+) |
|---|---|---|---|---|---|---|---|---|
| 56 | M | Cutaneous | Lu, LN | gp1 | gp100 | 6.5 | 45.1 | 4.1 |
| 55 | F | Mucosal | Lu, Li, SQ, LN | gp5 | gp100 | 29.1 | 18.8 | <0.1 |
| 34 | F | Cutaneous | SQ, LN, Br | gp6 | gp100 | 12.3 | 10.8 | <0.1 |
| 45 | M | Cutaneous | SQ, LN | gp10 | gp100 | 4.1 | 9.5 | 0.7 |
| 65 | F | Cutaneous | Cut, SQ, LN | M1 | MART | 7.3 | 1.5 | <0.1 |
| 45 | M | Cutaneous | Br, Lu, Li, LN, ST | M2a | MART | 4.1 | 21.4 | 0.3 |
| M2b | MART | 24.1 | 8.7 | 6.5 | ||||
| 35 | M | Mucosal | Lu, Li, Bo | M3 | MART | 2 | 4.3 | <0.1 |
| 52 | F | Cutaneous | Lu, LN | M4 | MART | 4.3 | 58.3 | 7.8 |
| 42 | M | Cutaneous | Lu, Li, Bo, LN | M5 | MART | 28 | 15.1 | 3.8 |
Figure 1. Heterogeneous engraftment of CD8+ T effector clones after adoptive transfer in humans.
(A.) Dichotomous long term engraftment of M1 and M4 MART specific CD8+ T effector clones as determined by the absolute number of CD8+Tetramer+Vβ+ T cells per microliter of blood at time points relative to infusion in respective melanoma patients. Arrow denotes the day of clone infusion. (B.) Heterogeneous persistence of infused effector clones (n=10 clones infused into 9 patients) as assessed by % CD8+Tetramer+Vβ+ cells identified in PBMC at day 30 post infusion. Non-persisting (NP) clones were defined to have engraftment levels of ≤0.1% of CD8+ T cells; Persisting (P) clones had engraftment levels >0.1% of CD8+ T cells. (C.) Pre-infusion phenotype of NP (n=4) and P (n=6) effector clones as determined by FACS expression of the indicated differentiation markers (% of cells). (D.) Cognate peptide avidity and (E.) reactivity against 526 Mel (A2+) tumor cell line for NP (n=4) and P (n=5) effector clones. Shown are supernatant IFN-γ levels after subtracting background reactivity against either control peptide or 888 Mel (A2−) tumor cell line. Statistical comparison performed by unpaired T test; ns, non-significant. Bar on graphs represents mean.
Comparative gene expression profiling reveals pre-infusion transcriptional differences between persisting and non-persisting CD8+ T effector clones
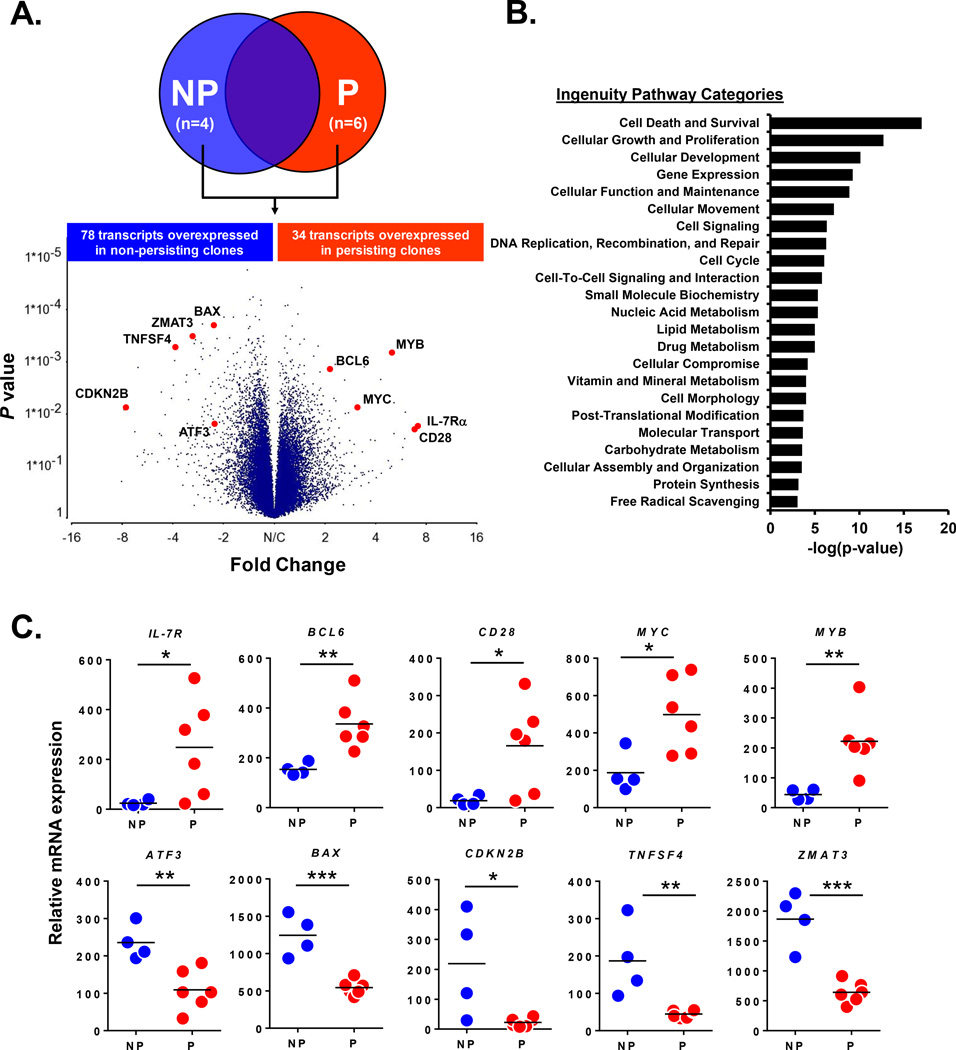
Since conventional phenotypic and functional profiling failed to reveal significant pre-infusion differences between P and NP clones, we next sought to determine if there was a distinct molecular signature that was associated with the eventual engraftment of the effector clones. We performed comparative microarray gene expression profiling on P (n=6) and NP (n=4) clones which were procured immediately prior to their adoptive transfer. Of the 54,675 transcripts analyzed, there were 112 unique genes that were differentially expressed (P<0.05 and fold difference >2) between the P and NP clones (Figure 2A and complete gene list in Supplementary Table 1). Ingenuity Pathway Analysis revealed that the dominant functional role for this gene set was related to cell death and survival (Figure 2B). The profiling specifically identified 34 genes that were overexpressed in P clones (Figure 2A) including IL-7R, BCL-6, CD28, MYC, and MYB which are known to be involved in T cell survival and proliferation (9–13). To more accurately define the expression pattern of these genes in the individual infused clones, we quantified the relative mRNA levels of the selected genes by robust multichip analysis (RMA)-normalized intensity. We observed that IL-7R, BCL-6, CD28, MYC, and MYB mRNA expression were uniformly low in the NP clones, but significantly higher in the P clones (Figure 2C). Further, there were 78 genes identified to be overexpressed in NP clones (Figure 2A) including ATF3, BAX, CDKN2B, TNFSF4, and ZMAT3 which are involved in cell cycle arrest, stress, terminal effector function and apoptotic cell death (14–18). Relative mRNA quantitation of these selected genes demonstrated uniformly low expression in P clones but significantly higher expression in the NP clones (Figure 2C). From these findings, we concluded that persisting CD8+ T effector clones have higher pre-infusion expression of genes associated with cell survival and proliferation, while non-persisting clones over-expressed genes associated with cell death.
Figure 2. Comparative gene expression profiling reveals pre-infusion transcriptional differences between persisting and non-persisting CD8+ T effector clones.
(A) Affymetrix cDNA microarray comparison of NP (n=4) and P (n=6) clones. Volcano plot highlights selected overexpressed transcripts (p <0.05 and fold difference >2) in NP and P effector clones. (B) Ingenuity Pathway Analysis of the 112 differentially expressed transcripts between NP and P effector clones; shown are functional categories ranked by statistical significance. (C) Relative mRNA quantitation by RMA normalized intensity of selected genes expressed in NP (n=4) and P (n=6) effector clones. Each dot represents an individual infused CD8+ T cell clone. *P< 0.05, ** P< 0.01, ***P< 0.001; unpaired T test. Bar on graphs represents mean.
Pre-infusion expression levels of IL-7R and c-myc by CD8+ T effector clones correlate with their level of persistence after adoptive transfer in humans
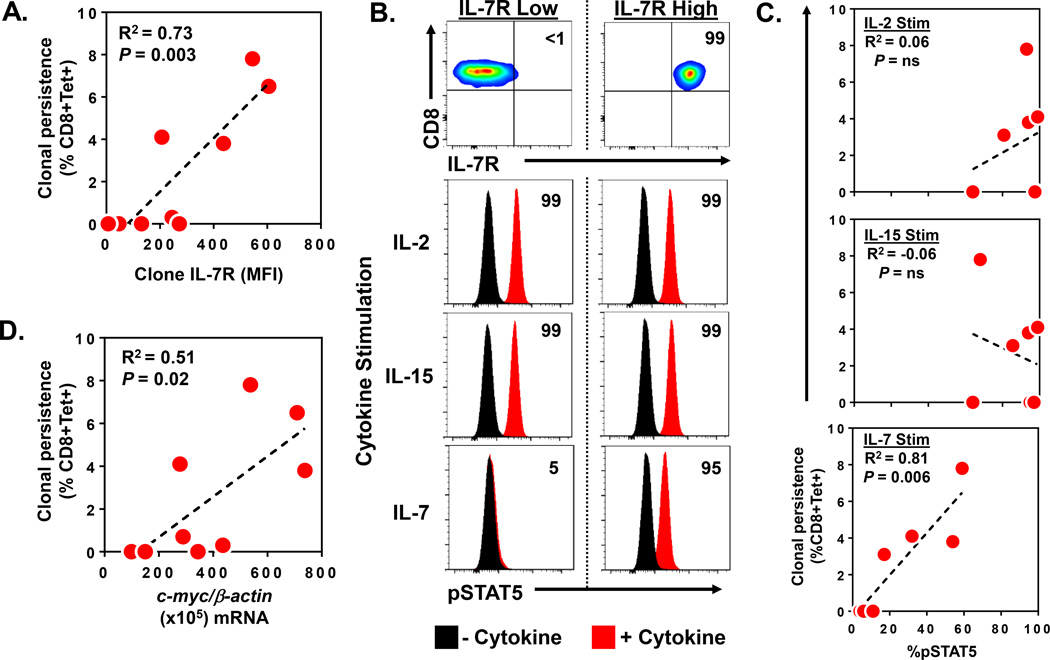
Although the microarray screening had revealed several genes that were differentially expressed between P and NP CD8+ T effector clones, we noted that the level of over-expression of each of these genes was highly variable with a significant range of expression among the individual clones (Figure 2C). Further, we also appreciated that the levels of persistence after adoptive transfer were equally variable among these same clones (Table 1). Based upon these observations, we postulated that our initial categorization of clones as either P or NP was insufficient to accurately identify the individual genes that were most strongly associated with clonal persistence. Thus, to better account for the observed heterogeneity, we next analyzed the relationship between the quantitative expression levels of each gene transcript profiled in the microarray analysis and the precise level of persistence for each individual clone. Normalized mRNA transcript levels in the pre-infusion clone samples were statistically correlated with the absolute level of persistence for that clone at one month post transfer. Pearson correlation coefficients (r) were derived for each of the correlation analyses and ranked to identify the genes with the strongest positive and negative correlation with in vivo persistence (P<0.05) (Table 2). We found that pre-infusion mRNA expression levels of ATF3 (r= −0.791) and TMEM64 (r= −0.741) were most negatively correlated with clone persistence. Conversely, the genes most positively correlated with clone persistence was IL-7R (r= +0.747) followed by MYC (r = +0.717). Prior reports from murine models had suggested that effector CD8+ T cells which selectively expressed cell surface IL-7 receptor (IL-7R) could give rise to long-lived memory cells after adoptive transfer (9). However, these findings had not been clearly demonstrated in human adoptive transfer studies. Thus, we next sought to determine whether pre-infusion IL-7R cell surface protein expression and the phosphorylation of its downstream signal transduction molecule, STAT5, were associated with human clonal persistence. IL-7Rα expression was measured by antibody staining and flow cytometric quantitation after the infused clones were rested for 24 hours in cytokine free media. Regression analysis found IL-7Rα expression (MFI and % expressing cells) on the pre-infusion clones was strongly and linearly correlated with the eventual persistence of those clones after adoptive transfer (IL-7Rα MFI: R2=0.73, P=0.003; % IL-7Rα: R2=0.71, P=0.004) (Figure 3A and Supplementary Figure 1). Interestingly, the expression of IL-7Rα was detected on a fraction of cells ranging from 0 to 12.4% of the total infused clone population. To better understand the differentiation status of the IL-7Rα+ cells, we performed comparative phenotypic profiling of the IL7Rα+ and IL7Rα− cells from the pre-infusion clone products. Flow cytometric analysis of the IL-7Rα+ subsets demonstrated these cells to have effector memory differentiation based upon high expression of CD45RO and low expression of CD62L. This expression was similar to the IL-7R− cells within the respective clones. Additional markers including cytokine receptors, co-stimulatory molecules, activation and inhibitory markers were performed and showed no difference between the IL7Rα+ and IL7Rα− cells from the pre-infusion cloned products. A representative phenotype profiling of clone M4 is shown in Supplementary Figure 2. Next, we sought to determine whether the detected IL-7 receptor on these cells could specifically and functionally respond to its cognate ligand, IL-7. We measured the phosphorylation of STAT5 (pSTAT5) by flow cytometry after independently exposing each of the infused T cell clones to a panel of common γ-chain cytokines, including IL-7, IL-2, and IL-15. An illustrative signal transduction assay performed upon two control clones with known high (99% of cells) and low (<1% of cells) IL-7R expression is shown in Figure 3B. In response to rIL-2 and rIL-15 exposure, both clones demonstrated a uniform increase in pSTAT5 expression (shift in 99% of cells) when compared to baseline levels in the absence of cytokine. These findings suggested that the clones possessed comparable levels of the IL-2 and IL-15 receptors which could functionally respond to their respective γ-chain cytokines. In contrast, when the same clones were exposed to rIL-7, the clone with high IL-7R expression demonstrated a significant increase in pSTAT5 (95% of cells), while the clone with low IL-7 receptor expression demonstrated negligible pSTAT5 (5% of cells). When this assay was performed on the clinically administered CD8+ T effector clones (n=7), we found that rIL-2 and rIL-15 induced marked pSTAT5 increases in all of the clones (range 60–99% of cells) and these levels did not correlate with clonal persistence (R2=0.06 and −0.06, respectively) (Figure 3C). However, when these same clones were exposed to rIL-7, we observed highly variable levels of pSTAT5 expression (range 4–60% of cells) which did strongly correlate with the persistence of these clones after adoptive transfer (R2=0.81, P=0.006). Next, we sought to further evaluate the other gene found by microarray screening to be associated with clonal persistence, the c-myc proto-oncogene. Quantitative mRNA analysis was performed on pre-infusion samples of the administered clones (n=9). Regression analysis revealed a significant linear correlation between normalized c-myc mRNA levels and the eventual persistence of those clones (R2=0.51, P=0.02) (Figure 3D). Collectively, these findings demonstrated that pre-infusion cell surface expression of IL-7R (MFI and %), the level of IL-7 induced pSTAT5, and c-myc mRNA expression by CD8+ T effector clones were all strongly associated with the engraftment capability of these cells after adoptive transfer in humans.
Table 2.
Correlation between gene expression and level of in vivo clonal persistence
| NEGATIVE CORRELATION with CLONE PERSISTENCE | ||
| Gene Symbol | Gene Title | r |
| ATF3 | activating transcription factor 3 | −0.791 |
| TMEM64 | transmembrane protein 64 | −0.741 |
| PTGDR | prostaglandin D2 receptor (DP) | −0.710 |
| TNFAIP3 | tumor necrosis factor, alpha-induced protein 3 | −0.698 |
| ATP10D | ATPase, class V, type 10D | −0.668 |
| GADD45A | growth arrest and DNA-damage-inducible, alpha | −0.666 |
| ZMAT3 | zinc finger, matrin-type 3 | −0.656 |
| MCC | mutated in colorectal cancers | −0.650 |
| TNFSF9 | tumor necrosis factor (ligand) superfamily, member 9 | −0.635 |
| PHLDA3 | pleckstrin homology-like domain, family A, member 3 | −0.632 |
| CLECL1 | C-type lectin-like 1 | −0.626 |
| POSITIVE CORRELATION with CLONE PERSISTENCE | ||
| Gene Symbol | Gene Title | r |
| IL7R | interleukin 7 receptor | +0.747 |
| MYC | v-myc myelocytomatosis viral oncogene homolog (avian) | +0.717 |
| SIK1 | salt-inducible kinase 1 | +0.695 |
| CASP4 | caspase 4, apoptosis-related cysteine peptidase | +0.692 |
| OSM | oncostatin M | +0.682 |
| AHI1 | Abelson helper integration site 1 | +0.643 |
| CD28 | CD28 molecule | +0.637 |
| MYB | v-myb myeloblastosis viral oncogene homolog (avian) | +0.634 |
| IRF4 | interferon regulatory factor 4 | +0.630 |
| CHD7 | chromodomain helicase DNA binding protein 7 | +0.630 |
| SYCP2 | synaptonemal complex protein 2 | +0.628 |
| EQR1 | early growth response 1 | +0.625 |
Figure 3. Pre-infusion expression levels of IL-7R and c-myc by CD8+ T effector clones correlate with their level of persistence after adoptive transfer in humans.
(A) Correlation between pre-infusion IL-7R cell surface expression (MFI) on infused CD8+ T effector clones (n=9) and peripheral blood persistence (% CD8+Tet+) at day 30 after adoptive transfer. (B) Representative STAT5 phosphorylation (pSTAT5) assay performed upon effector clones with low and high IL-7R expression. FACS plots show expression of CD8 and IL-7Rα in effector clones. Numbers in FACS plots represent % CD8+IL-7Ra+ cells compared to isotype control staining. pSTAT5 was measured by FACS before (no cytokine) and after clones were exposed to either 10ng/ml of rIL-2, rIL-15, or rIL-7 cytokine for 15 min. Numbers in histograms represent % of cells induced to express pSTAT5 by respective cytokine compared to no cytokine baseline. (C) Correlation between cytokine induced pSTAT5 (% of cells) in infused CD8+ T effector clones (n=7) and peripheral blood persistence (% CD8+Tet+) at day 30 after adoptive transfer. (D) Correlation between pre-infusion c-myc mRNA (per 105 b-actin copies) expressed in infused CD8+ T effector clones (n=9) and peripheral blood persistence (% CD8+Tet+) at day 30 after adoptive transfer.
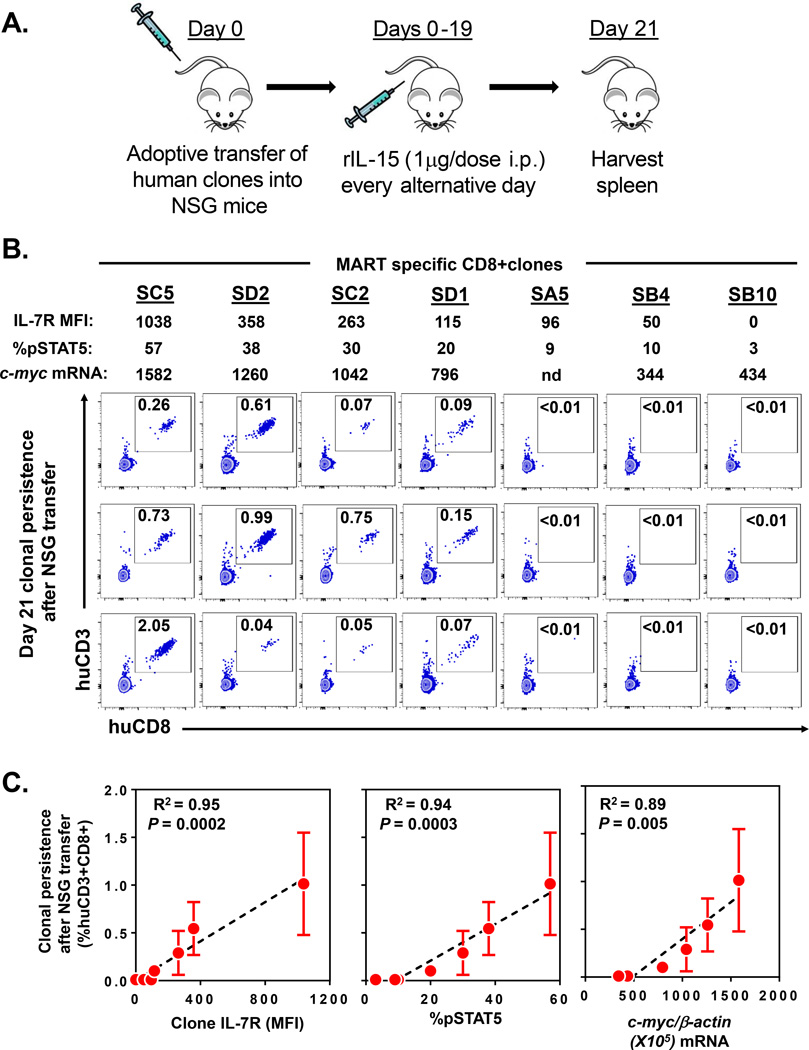
Pre-infusion expression levels of IL-7R and c-myc by CD8+ T effector clones predict their level of persistence after adoptive transfer in NSG mice
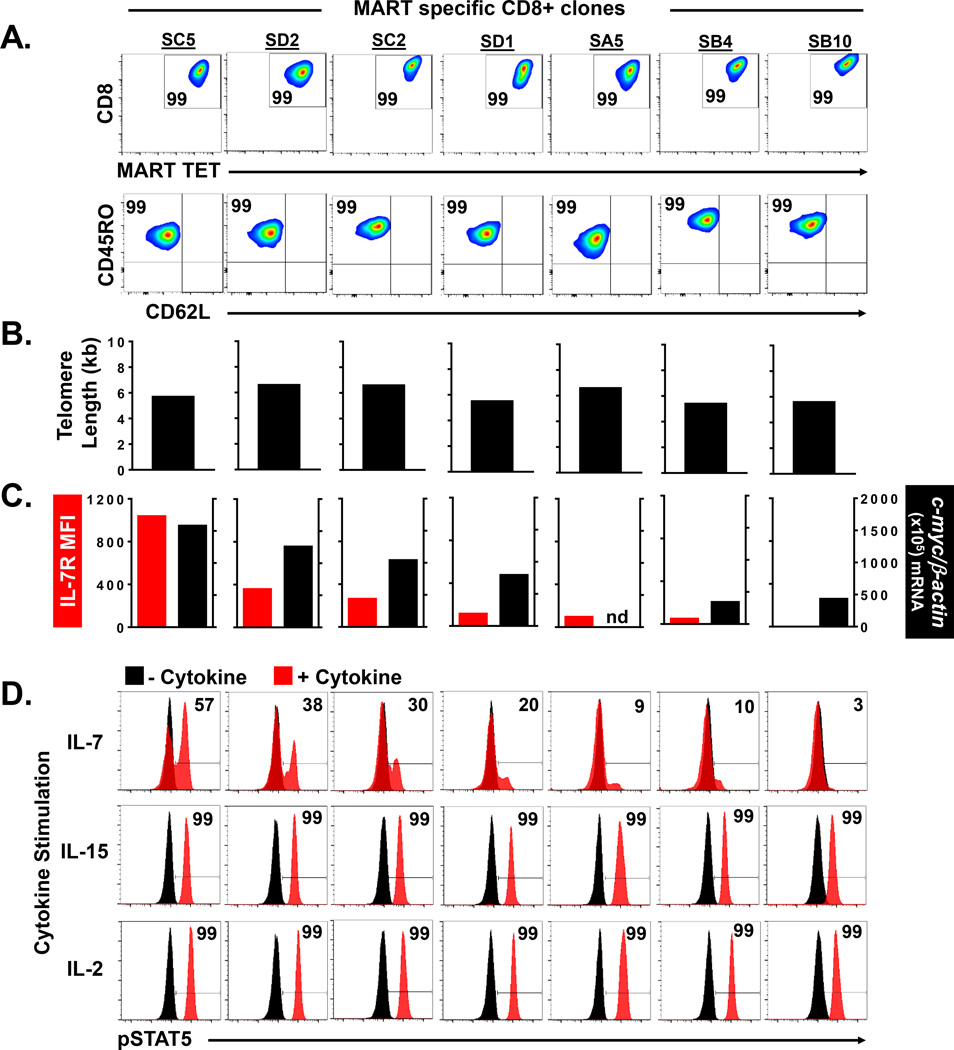
The development of T cell memory responses in patients undergoing adoptive transfer therapy may be confounded by the inherent heterogeneity among recipient host immune systems. Patient specific variables that might have influenced clonal engraftment in our clinical trials include varying levels of homeostatic cytokines after cell transfer, differences in antigen load and presentation, presence of immune regulatory cells, and other unique host factors. Thus, to more precisely define the intrinsic cellular attributes associated with the engraftment of CD8+ effector T cells, we sought to experimentally eliminate host heterogeneity by studying the fate of human effector clones after highly controlled adoptive transfer into immunodeficient NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. We prospectively isolated an independent cohort of MART specific CD8+ T effector clones by high throughput in vitro sensitization and limiting dilution cloning (7, 8, 19) from a single patient with metastatic melanoma. Each of the expanded “sister” clones (n=7) demonstrated monoclonal antigen specificity by anti-CD8 and MART MHC tetramer staining (Figure 4A). Further, all of the clones possessed the typical phenotypic appearance of highly differentiated antigen experienced CD8+ T effector cells with uniform high expression of CD45RO and low expression of CD62L (Figure 4A). The replicative histories of the clones were also highly similar based upon telomere length assessment which ranged from 5.4 to 6.6 kb (Figure 4B). However, when we assessed the cell surface expression of IL-7R on these clones, we found significant heterogeneity with MFI values ranging from 0 to 1038 (Figure 4C, left). Further, these same clones demonstrated highly variable c-myc mRNA transcript levels (range 344–1582 copies per 105 β-actin copies) (Figure 4C, right). Linear regression analysis found the c-myc mRNA levels were highly correlated with the IL-7R expression in these clones (R2=0.78, P=0.018) (Supplementary Figure 3). Next, to evaluate the functionality of the IL-7R, we analyzed pSTAT5 expression after exposure of the clones to γ-chain cytokines (Figure 4D). We found that rIL-2 and rIL-15 induced uniform pSTAT5 expression (99% of cells) in each of the clones suggesting no difference in the expression of their respective receptors. However, when these same clones were exposed to rIL-7, we observed highly variable levels of pSTAT5 expression (range 3–57% of cells) (Figure 4D) which strongly and linearly correlated with their respective IL-7R MFI expression (R2=0.86, P=0.0025) (Supplementary Figure 4). Based upon our prior correlation studies involving the patient infused clones (Figure 3), we hypothesized that these independently isolated effector clones would also have differing engraftment capabilities that could be predicted based upon their IL-7R and c-myc expression. Thus, we next evaluated the persistence of these sister clones after controlled prospective adoptive transfer into NSG mice. In this NSG xenograft model, we and others have found that human CD8+ T cells require human cytokines to support their engraftment (20, 21). In multiple independent experiments comparing the efficacy of IL-2, IL-7, and IL-15 to support the engraftment of human cells into NSG mice, we found engraftment only in the presence of administered rIL-15 (data unpublished). Therefore, our experimental model included repeated administration of human rIL-15 cytokine after the transfer of the individual effector clones into the NSG mice (n=3 for each clone) (Figure 5A). The persistence of each of the infused clones was evaluated at day 21 post transfer by determining the frequencies of human CD3+CD8+ T cells present in the spleens of replicate mice (Figure 5B). Notably, there was no detectable engraftment of the CD8+ T effector clones that had low pre-infusion expression of IL-7R (MFI<96), low IL-7 induced pSTAT5 (<10% of cells), and low c-myc expression (<434 copies per 105 β-actin copies). In contrast, the transfer of the effector clones with higher expression of these factors resulted in reproducible engraftment in the animals. When the mean level of persistence was determined for each clone, we found a strong predictive correlation with the clone’s pre-infusion IL-7R MFI expression (R2=0.95, P=0.0002), IL-7 induced pSTAT5 expression (R2=0.94, P=0.0003), and c-myc mRNA expression (R2=0.89, P=0.005) (Figure 5C). These results strongly paralleled our retrospective clinical trial findings in humans and further supported that pre-infusion expression level of IL-7R and c-myc could be used to predict the level of persistence of CD8+ T effector clones after adoptive transfer.
Figure 4. Prospective characterization of effector CD8+ T cell clones based upon by IL-7R and c-myc expression.
(A) Phenotype of individual MART specific CD8+ T effector clones (n=7) isolated by limiting dilution from the peripheral blood of a metastatic melanoma patient. Numbers in FACS plot represent % CD8+Tetramer+ cells (upper panel) and % CD45RO+CD62L− cells (lower panel) compared to isotype. (B) Telomere length of isolated effector clones. (C) Cell surface IL-7R expression (MFI) and c-myc mRNA (per 105 b-actin copies) expression in isolated effector clones. (D) Cytokine induced pSTAT5 expression (% of cells) in isolated CD8+ effector clones (n=7). Numbers in histograms represent % of cells induced to express pSTAT5 by respective cytokine compared to no cytokine baseline.
Figure 5. Pre-infusion expression levels of IL-7R and c-myc by CD8+ T effector clones predict their level of persistence after adoptive transfer in NSG mice.
(A) Experimental xenograft model to evaluate the persistence of human CD8+ effector T cell clones after adoptive transfer into immunodeficient NSG mice. (B) Persistence in NSG mice of human MART specific CD8+ T effector clones with varying pre-infusion expression levels of IL-7R, induced pSTAT5, and c-myc. FACS dot plots demonstrate the % of huCD3+huCD8+ cells within the harvested spleens of mice (n=3 mice per clone group; shown in columns) at day 21 post transfer. Each FACS plot represents splenocytes harvested from an individual replicate animal. (C) Respective correlation analyses between pre-infusion IL-7R MFI expression, IL-7 induced pSTAT5 expression, and c-myc mRNA (per 105 b-actin copies) expression in infused CD8+ T effector clones and mean clonal persistence (% huCD3+huCD8+) at day 21 after adoptive transfer into NSG mice (n=3). Linear regression plots demonstrate mean clonal persistence ± SEM (error bars). Experiment is representative of 2 independently performed adoptive transfer experiments.
Discussion
The optimal T cell attributes for the adoptive immunotherapy of cancer and viral diseases are currently unclear. Murine models of adoptive transfer have suggested that less differentiated memory populations have superior ability to persist and mediate tumor regression after infusion when compared to more differentiated effector cells (20, 22, 23). With prolonged in vitro culturing, CD8+ T cells were found to progressively lose in vivo proliferative potential, and subsequently become senescent and undergo apoptosis (24, 25). These observations led to the prevailing theory that in vitro effector cell differentiation was the dominant explanation for the poor long-term persistence and limited anti-tumor efficacy of extensively expanded CD8+ effector T cells administered in prior adoptive transfer clinical trials. However, this hypothesis has been difficult to reconcile with human clinical trial data which has demonstrated that differentiated effector T cells can mediate complete and durable tumor responses in patients with metastatic melanoma (3). Furthermore, the administration of less differentiated T cells in human cancer therapy trials has been technically challenging given the obligatory in vitro expansion, and consequent cellular differentiation, that occurs with the generation of a T cell product for adoptive transfer. A potential solution to this problem is the identification and administration of differentiated T cells that have potent effector function but also possess intrinsic properties which can facilitate their long-term survival. We previously reported that selected tumor specific CD8+ T effector clones could engraft and persist for prolonged durations in patients with metastatic melanoma despite these cells having undergone massive ex vivo expansion and differentiation (8). In the current study, we compared the pre-infusion transcriptional profile of effector clones with varying engraftment capabilities to help identify intrinsic cellular attributes that may predict cellular persistence in future adoptive transfer clinical efforts. We found the pre-infusion clone mRNA expression levels of IL-7R and the proto-oncogene, c-myc, directly correlated with the level of persistence of these clones after adoptive transfer in humans. The predictive value of these markers was confirmed by utilizing IL-7R protein, IL-7 induced pSTAT5, and c-myc mRNA expression to prospectively identify human tumor specific effector clones that could engraft after adoptive transfer into NSG mice.
The mechanistic role of IL-7R and c-myc in effector to memory development is still unclear. Although the requirement of IL-7 for the survival of naïve and memory cells has been well described (26–28), its importance in differentiated effector cells has been more difficult to elucidate. In previously reported murine studies, selective expression of IL-7R identified a subset of antigen-experienced differentiated effector cells that could preferentially survive and establish long-term immunological memory (9). Furthermore, adoptive transfer of this IL-7R expressing population in cytokine knockout mouse models suggested that IL-7 was functionally required for their in vivo survival (9). However, in follow up studies, the constitutive expression of IL-7R was unable to alter the fate and rescue effector cells from activation induced cell death (AICD), suggesting a more permissive rather than instructive role for the IL-7/IL-7R axis in memory cell development (29). Further, multiple lines of evidence have recently supported the role of IL-15, in the absence of IL-7, in promoting effector to memory cell development (30, 31). These findings are consistent with our observation that IL-7 cytokine support was not required for the persistence of effector clones in our NSG model. Based upon these findings, we hypothesize that IL-7R expression in effector clones may represent a correlative marker for other more direct pro-survival pathways.
Our finding that the levels of c-myc mRNA expression also correlated with the engraftment fate of human effector T cells has not been previously described. The transcription factor Myc is known to be involved in regulating expression of 15% of all genes including several that control cell cycle, growth, proliferation, and differentiation (32). Further, in recent years, the contributing role of Myc in directing pluripotent cell fates has been exploited to re-program differentiated cells (33, 34). Deregulation of Myc occurs through several mechanisms and is one of the most common oncogenic events in human malignancies (35, 36). In the setting of hematologic malignancies, a clinically aggressive subset of B-cell lymphomas can result from chromosomal breakpoint translocations of the loci encoding for c-Myc and either of the anti-apoptotic proteins, BCL2 or BCL6. These genetic alterations result in constitutive activation of these genes in the so called “double and triple hit” lymphomas (37). Interestingly, in our comparative transcriptional profiling of CD8+ effector T cells, we found both c-myc and BCL6 mRNA to be overexpressed in “non-malignant” T cells that could survive and persist after adoptive transfer (Figure 2). Recent studies of primary T cells have suggested a robust physiological role for Myc in global transcriptional amplification (12), proliferation (38), reprogramming of the cellular metabolic state upon T cell activation (39), but paradoxically also increased apoptosis (40). We hypothesize that the overexpression of Myc in our CD8+ effector clones may be balanced by the anti-apoptotic and pro-survival effects of BCL6 and/or IL-7R (41) to direct the memory fate of effector cells. However, further mechanistic studies are necessary to define this interaction. In sum, based upon our current analysis, we can conclude that IL-7R and c-myc have a strong predictive association with effector T cell persistence after adoptive transfer. These findings have direct implications on the selection of CD8+ T cells for future adoptive immunotherapy studies.
Supplementary Material
Acknowledgements
We thank the Surgery Branch cell production facility and the immunotherapy clinical and support staff for their contributions. We thank Arnold Mixon and Shawn Farid for assistance with cell sorting by flow cytometry. This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Footnotes
Conflicts of Interest: None
Reference List
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, et al. The stoichiometric production of IL-2 and IFN-gamma mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med. 2012;4:149ra120. doi: 10.1126/scitranslmed.3004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandran SS, Paria BC, Srivastava AK, Rothermel LD, Stephens DJ, Dudley ME, et al. Persistence of CTL clones targeting melanocyte differentiation antigens was insufficient to mediate significant melanoma regression in humans. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 10.Ichii H, Sakamoto A, Arima M, Hatano M, Kuroda Y, Tokuhisa T. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int Immunol. 2007;19:427–433. doi: 10.1093/intimm/dxm007. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184:452–465. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 12.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern JB, Smith KA. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986;233:203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- 14.Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- 15.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 16.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Windgassen D, Papoutsakis ET. Comparative analysis of transcriptional profiling of CD3+, CD4+ and CD8+ T cells identifies novel immune response players in T-cell activation. BMC Genomics. 2008;9:225. doi: 10.1186/1471-2164-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellborg F, Qian W, Mendez-Vidal C, Asker C, Kost-Alimova M, Wilhelm M, et al. Human wig-1, a p53 target gene that encodes a growth inhibitory zinc finger protein. Oncogene. 2001;20:5466–5474. doi: 10.1038/sj.onc.1204722. [DOI] [PubMed] [Google Scholar]
- 19.Kammula US, Serrano OK. Use of high throughput qPCR screening to rapidly clone low frequency tumour specific T-cells from peripheral blood for adoptive immunotherapy. J Transl Med. 2008;6:60. doi: 10.1186/1479-5876-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 27.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 29.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel JH, Loboda AP, Showe MK, Showe LC, McMahon SB. Analysis of genomic targets reveals complex functions of MYC. Nat Rev Cancer. 2004;4:562–568. doi: 10.1038/nrc1393. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Cole MD. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- 36.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120:3884–3895. doi: 10.1002/cncr.28899. [DOI] [PubMed] [Google Scholar]
- 38.Link JM, Hurlin PJ. The activities of MYC, MNT and the MAX-interactome in lymphocyte proliferation and oncogenesis. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagrm.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 41.Seckinger P, Milili M, Schiff C, Fougereau M. Interleukin-7 regulates c-myc expression in murine T cells and thymocytes: a role for tyrosine kinase(s) and calcium mobilization. Eur J Immunol. 1994;24:716–722. doi: 10.1002/eji.1830240334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.