Abstract
Human cord blood (CB) is enriched in circulating endothelial colony forming cells (ECFC) that display high proliferative potential and in vivo vessel forming ability. Since diminished ECFC survival is known to dampen the vasculogenic response in vivo, we tested how long implanted ECFC survive and generate vessels in three-dimensional (3D) type I collagen matrices in vitro and in vivo. We hypothesized that human platelet lysate (HPL) would promote cell survival and enhance vasculogenesis in the 3D collagen matrices. We report that the percentage of ECFC co-cultured with HPL that were alive was significantly enhanced on days 1 and 3 post- matrix formation, compared to ECFC alone containing matrices. Also, co-culture of ECFC with HPL displayed significantly more vasculogenic activity compared to ECFC alone and expressed significantly more pro-survival molecules (pAkt, p-Bad and Bcl-xL) in the 3D collagen matrices in vitro. Treatment with Akt1 inhibitor (A-674563), Akt2 inhibitor (CCT128930) and Bcl-xL inhibitor (ABT-263/Navitoclax) significantly decreased the cell survival and vasculogenesis of ECFC co-cultured with or without HPL and implicated activation of the Akt1 pathway as the critical mediator of the HPL effect on ECFC in vitro. A significantly greater average vessel number and total vascular area of human CD31+ vessels were present in implants containing ECFC and HPL, compared to the ECFC alone implants in vivo. We conclude that implantation of ECFC with HPL in vivo promotes vasculogenesis and augments blood vessel formation via diminishing apoptosis of the implanted ECFC.
Keywords: Endothelial colony forming cells (ECFC), Human platelet lysate (HPL), Three dimensional (3D) collagen matrix, Vasculogenesis, Apoptosis
INTRODUCTION
The progenitor cells for the endothelial lineage play critical roles in vascular homeostasis and regeneration in adult subjects [1,2]. Endothelial colony forming cells (ECFC) are derived from a rare circulating subset that may arise from resident endothelium of established blood vessels in man [2]. We have successfully isolated human peripheral blood (PB) or umbilical cord blood (CB) derived circulating ECFC that display a hierarchy of proliferative potential through the use of single cell clonogenic and functional assays [3–5]. Human CB derived ECFC also display de novo vessel forming ability in nude or Non-obese diabetic/ severe combined immunodeficient mice (NOD/SCID) in vivo after subcutaneous implantation of ECFC in type I collagen matrices and upon perfusion with host murine vessels, become a part of the systemic host circulation [6–9].
Previous studies have reported that human ECFC-derived vessels formed in subcutaneous implants were found as early as day 1 or 2 after implantation in NOD/SCID mice [10]. However, these premature vessels were not properly perfused with murine red blood cells of the host circulatory system at those early time points. Proof of human ECFC cell-lined vessel connection to host circulation system after 3–4 days of implantation in vivo was subsequently reported [10]. It has been reported that up to 60% of human umbilical vein endothelial cells (HUVECs) undergo apoptosis in the first 24 hours after suspension in collagen gels and there is further loss of vascular structures in 3D type I collagen matrices after 24 hours of culture [11]. In our previous study, we demonstrated that human CB ECFC containing collagen matrices displayed ECFC undergoing apoptosis on day 1 following implantation [12]. After 2–3 days, most ECFC died with only 1–3% of the total implanted cells demonstrating viability in the subcutaneous implants [12]. Since the stability of newly formed vessels requires systemic blood flow, these data suggest that implanted endothelial cells must survive at least 3–4 days in vivo to form a stable perfused capillary network connected to the host circulatory system.
The microvascular network has the intrinsic capacity to remodel itself through changes in endothelial cell differentiation, growth, migration, and matrix modification [13,14]. Previous studies have reported that cytokines, matrix proteins, and integrins are important factors in capillary endothelial network formation and remodeling in various matrix models (Matrigel and type I collagen matrix systems) [7,15–20]. The absence of peri-endothelial support cells or lack of endothelial cell adhesion to matrix proteins causes destabilization and regression of capillary structures within Matrigel or 3D type I collagen matrices [16,17,21,22]. Apoptosis of endothelial cells has been observed in the context of dynamic capillary network remodeling in the various matrix models both in vitro and in vivo and is thought to play a necessary role for optimal network formation [17,21]. On the other hand, the generation of anti-apoptotic signals is also required to maintain integrity of the vascular network following realignment of certain matrix proteins and cell-cell or cell-matrix interactions during the process [17,21]. Thus, understanding the balance between pro-apoptotic and pro-survival signals is a critical point in capillary structure formation and the remodeling process.
Apoptosis is a cellular process in tissue development that generally culminates with the sequential activation of caspases, the cysteine proteases for cleavage of proteins [23,24]. There are two pathways that result in apoptosis depending on the apoptotic signal. The extrinsic pathway is initiated by ligand binding to membrane receptors of the death receptor family, whereas the intrinsic pathway is mediated by stress-mediated damage such as alterations in temperature, osmolality, DNA damaging agents, free radical generation compounds, removal of nutrients, and oxygen deprivation [23,24]. The activation of the two pathways leads to the same type of apoptotic response, causing release of cytochrome c (Cyt-C) from mitochondria and activation of the executioner caspase-3 [23,24]. In the normal state, Akt activation (phosphorylated Akt, pAkt) by various growth factors induces cell survival via protection of mitochondrial integrity and inhibition of Cyt-C release [25,26]. Akt has also been reported to stimulate the expression of anti-apoptotic Bcl-2 proteins, such as Bcl-xL that prevents permeablization of the mitochondrial membrane by inhibiting activation of Bax/Bak [27]. However, under apoptotic stimulation, pro-apoptotic members of the Bcl-2 family (Bax, Bak and Bad) are activated and induce the release of Cyt-C from mitochondria by binding and inactivating anti-apoptotic proteins [28]. As the penultimate event, caspase-3 is activated to cleaved-caspase-3 to generate all the biochemical and morphologic hallmarks of cell apoptosis.
Platelets regulate the balance between apoptosis and cell survival in numerous cells involved in the repair of injured tissues [29,30]. Since platelets have a critical role in regulation of cell death and survival and contains various growth factors and cytokines [29–31], we hypothesized that human platelet lysate (HPL) would increase ECFC survival and enhance ECFC vascularization by modulating the balance between apoptosis and cell survival of ECFC in 3D collagen matrices.
MATERIALS AND METHODS
Culture of human umbilical cord blood (UCB) derived ECFC
ECFC were isolated and cultured as previously described [3]. ECFC colonies appeared between 5 and 22 days of culture and were noted to form colonies of adherent cells with cobblestone morphology. After approximately 10 days of culture, the ECFC-derived ECs were released from the culture dish by TrypLE™ Express (Gibco) and replated onto 25/75 cm2 tissue culture flasks pre-coated with Type I rat-tail collagen (BD Biosciences) for subsequent passage.
Preparation of three-dimensional (3D) collagen matrices
All of the type I collagen oligomers and associated polymerization reagents were purchased from GeniPhys [32]. Stock oligomer was diluted in 0.01N hydrogen chloride (HCl) and neutralized according to manufacturer’s recommendations to achieve a final oligomer concentration of 1.37mg/ml (200Pa matrix stiffness). ECFC (1 × 105/ 60µl or 1 × 106/ 250µl) were suspended in the collagen solution with or without human platelet lysate (HPL- 10% of final concentration) at 4°C. The HPL was purchased from Gemeinnützige Salzburger Landeskliniken Betriebsges (SALK) Graz, Austria. The HPL was prepared from pooled platelet-rich plasma derived from a minimum of 40 whole blood donations [33]. The collagen-cell suspensions were plated on 96-well or 48-well plates and allowed to polymerize at 37°C for 30 minutes and covered with complete endothelial cell growth medium (EGM-2, Lonza) with 10% defined fetal bovine serum (Hyclone) for incubation for one to three days at 37°C, 5% CO2.
Toluidine blue staining of 3D collagen matrices and quantification of in vitro vascular structures
For analysis of in vitro vascular structure formation in 3D collagen gel, cellularized collagen matrices were fixed with 4% paraformaldehyde for 20 minutes on Day 1 or Day 3 after culture. The matrices were stained with 0.1% toluidine blue O dye (30% methanol) for 25 minutes at room temperature and washed with PBS for 30 minutes for three times at room temperature. Vascular structures including vacuoles, lumens and tube formation were quantified using ImageJ image analysis software (National Institutes of Health (NIH)). Vacuoles and lumens were defined as areas completely surrounded by a toluidine blue labeled endothelial cell membrane. A single image of the entire well was captured at a depth of 10 microns from the surface of the matrix and then additional places 150 microns apart for each of triplicate samples of each group. Images of vascular structures were captured at 10× and 20× levels of magnification.
Assessment of apoptosis of ECFC in 3D collagen matrices
Apoptosis was assessed by examining the percentage of human CD31+ ECFC that bound Annexin V and propidium iodide and was performed as per the manufacturer’s instruction (Apoptosis Detection kit; eBioscience). ECFC (1 × 105/ 60µl) were suspended in collagen matrices with/without HPL (10% of final concentration), with dimethyl sulfoxide (DMSO) vehicle (same volume of solvent for each inhibitor) or with signaling pathway inhibitors, plated in 96-well plates and incubated for one to three days as above. As previously described [12], matrices were recovered from one to three days after culture and incubated in 250 µl Collagenase Type I (0.25%) (Stemcell technology) for 20 minutes at 37°C. Akt1 inhibitor (A-674563) and Bcl-xL inhibitor (ABT-263/Navitoclax) were purchased from Selleckchem and Akt2 inhibitor (CCT128930) was purchased from Santa Cruz biotechnology. Cell dissociation buffer was added to stop the enzymatic reaction (Invitrogen). Cells were centrifuged at 500 g for 5 minutes at room temperature. Cell pellets were suspended in staining buffer and stained with anti-human CD31 antibody conjugated to phycoerythrin (PE) for 15 minutes (clone WM-59, BD Biosciences Pharmingen). Cells were incubated and stained for Annexin V-allophycocyanin (APC) and propidium iodide (PI) in binding buffer for 10 minutes at room temperature in the dark. Stained cells were analyzed by FlowJo software. Three different clones of ECFCs were analyzed in each experiment and each experiment repeated one or two times.
Western Blot
Cellular protein lysates were extracted from the ECFC in collagen matrices and electrophoresed using sodium dodecyl sulfate–polyacrylamide matrix electrophoresis (SDS-PAGE), transferred to nitrocellulose, and probed with monoclonal antibodies that included anti-human Bcl-xL (clone 54H6, Cell signaling), cleaved caspase-3 (clone 3G2, Cell signaling), Akt1 (clone 2H10, Cell signaling), Akt2 (clone L79B2, Cell signaling), phospho-Akt1 (pAkt1 Ser473) (clone D7F10, Cell signaling), phospho-Akt2 (pAkt2 Ser474) (clone D3H2, Cell signaling) and phospho-Bad (pBad Ser136) (clone D25H8, Cell signaling). Anti-human β-actin (mAbcam 8226, Abcam) and GAPDH (clone 14C10, Cell signaling) antibodies were used to probe for loading control proteins. Three different clones of ECFCs were analyzed in each experiment on at least two occasions.
Implantation of human cord blood derived ECFC into NOD/SCID mice
Cellularized matrix implants were cast as noted above. Cultured ECFC (1 × 106/ 250µl) were suspended in the collagen mixture with or without HPL (10% of final concentration). Then 250 µL of the cell suspension was pipetted into one well of a 48-well tissue culture plate, allowed to polymerize at 37°C for 30 minutes, and covered with 500 µL EGM-2 medium for 30minute incubation at 37°C, in 5% CO2. Matrices were implanted into the flanks of anesthetized 6- 9-week-old NOD/SCID mice. After 5 days or 14 days, the mice were sacrificed; the grafts were excised and analyzed by histology and immunohistochemistry (N=6 each group) as previously described [5].
Histology and Immunohistochemistry
Sections were stained as previously described [5]. Briefly, paraffin-embedded tissue sections were deparaffinized and then either directly stained with hematoxylin and eosin (H&E) or immersed in retrieval solution (Dako) for 20 minutes at 90–99°C. Slides were incubated at room temperature for 30 minutes with anti-human CD31 antibody (clone JC70/A, Abcam) followed by a 10-minute incubation with LASB2 link-biotin and streptavidin-HRP (Vector Laboratories), then developed with DAB (Vector Laboratories) solution for 5 minutes. Slides were analyzed by microscope under 20× magnification.
Statistical Analysis
Results are expressed as mean ± the standard error of the mean (SEM) for the study variables. The assessment of apoptosis of ECFC, in vitro vascular structure quantification and the quantification of western blot data points (ECFC versus ECFC+HPL), western blot data points (ECFC treated with DMSO, Akt1 inhibitor, Akt2 inhibitor or Bcl-xL inhibitor) were assessed by unpaired t-test. A statistically significant difference was set at *P<0.05, **P<0.001, or ***P<0.0001.
RESULTS
Human cord blood (CB) derived ECFC with human platelet lysate (HPL) significantly reduces apoptosis of ECFC in three dimensional (3D) collagen matrices
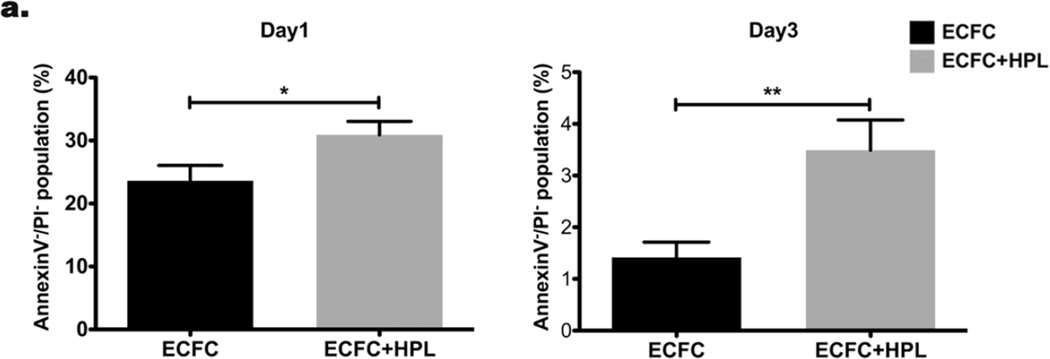
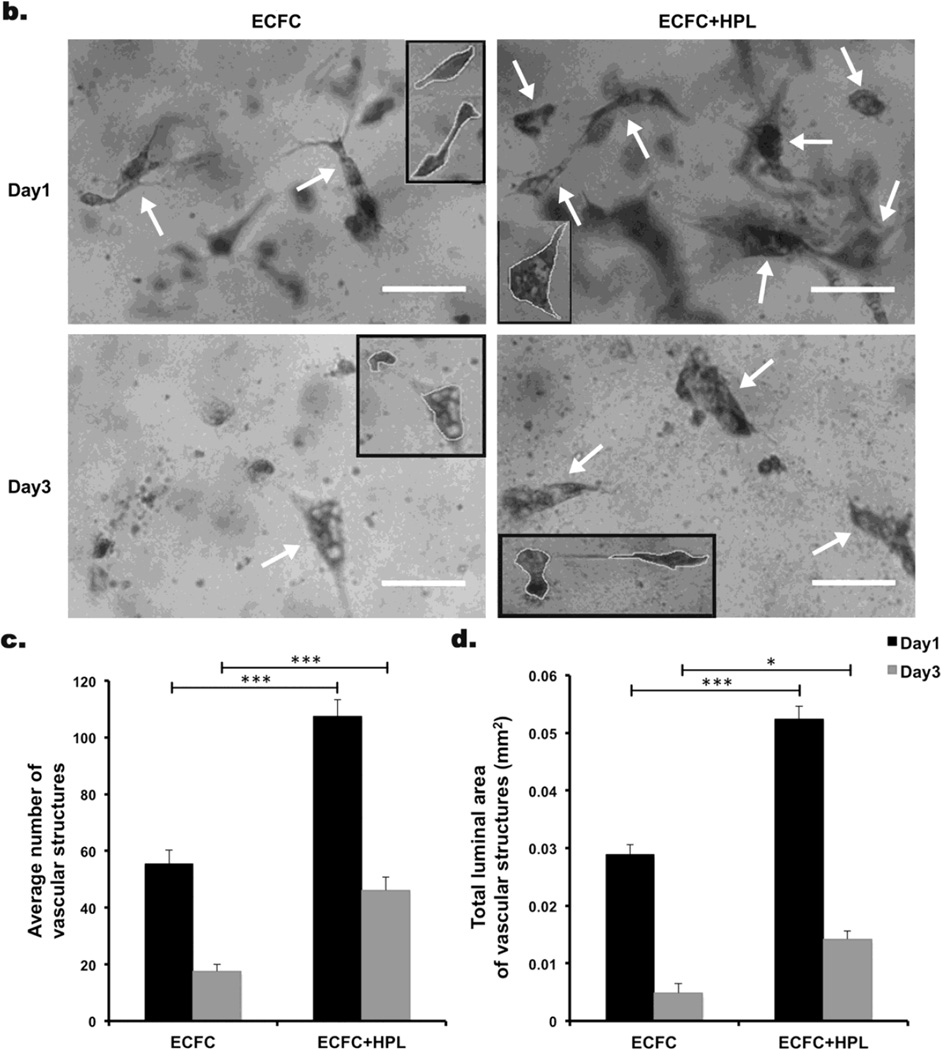
Our previous study demonstrated that cultured ECFC started undergoing apoptosis on day 1 and a significant majority of ECFC died on day 2 and 3 in 3D collagen matrices after implantation [12]. We first examined whether the presence of HPL altered ECFC proliferation in 3D collagen matrices and observed no effect (data not shown). We then tested whether the co-culture of ECFC with HPL would decrease apoptosis of human CB ECFC in the 3D collagen matrices in vitro. After 1–3 days, ECFC were recovered from 3D collagen matrices and assessed for apoptosis of ECFC stained with anti-human CD31, annexinV and propidium iodide (PI). CD31+ ECFC were selected from the total cell population and separated into annexinV+/− and PI+/− expressing subsets (Supplementary Fig. 1). Cultured ECFC without HPL started undergoing apoptosis on day 1 and a significant amount of the cells died by day 3 after matrix formation in vitro (Fig 1a). In contrast, ECFC co-culture with HPL revealed a significantly higher percentage of the AnnexinV−/PI− viable cell population compared to the control (ECFC alone) (Fig. 1a). We investigated whether co-culture of ECFC with HPL would enhance more vascular structures in 3D collagen matrices in vitro. ECFC-derived vascular structures were defined as endothelial cells with vacuoles and lumenized structures were defined as areas completely surrounded by a toluidine blue labeled endothelial cell membrane (Fig. 1b). A significantly greater average number of vascular structures (Fig. 1c) and total luminal area of vascular structures (mm2) (Fig. 1d) were quantified in ECFC + HPL group compared to the control group on both day 1 and 3 of the in vitro study. These data suggest that ECFC + HPL co-culture significantly promotes vasculogenesis by inducing greater cell survival of ECFC in 3D collagen matrices in vitro.
Figure 1. Co-culture of ECFC with human platelet lysate (HPL) diminishes apoptosis of ECFC in 3D collagen matrices in vitro.
(a) The Annexin V−/PI− viable population of co-cultured ECFC with HPL was significantly higher on day 1 and 3 compared to ECFC alone culture. N=3 *P < 0.05. (b) Representative images of vascular structures of ECFC cultured with or without HPL in collagen matrices on day 1 and 3 (white arrows indicate the structures and small boxes indicates representative counted structures). Scale bar represents 0.05µm. Co-culture of ECFC with HPL resulted in a significant increase in (c) the average number of vascular structures per collagen matrix surface area and (d) total luminal area of vascular structures per matrix surface area (mm2) compared to controls. N=10 each group *P < 0.05, **P<0.001 or ***P<0.0001.
Co-culture of ECFC with HPL modulates the balance between apoptosis and cell survival in 3D collagen matrices
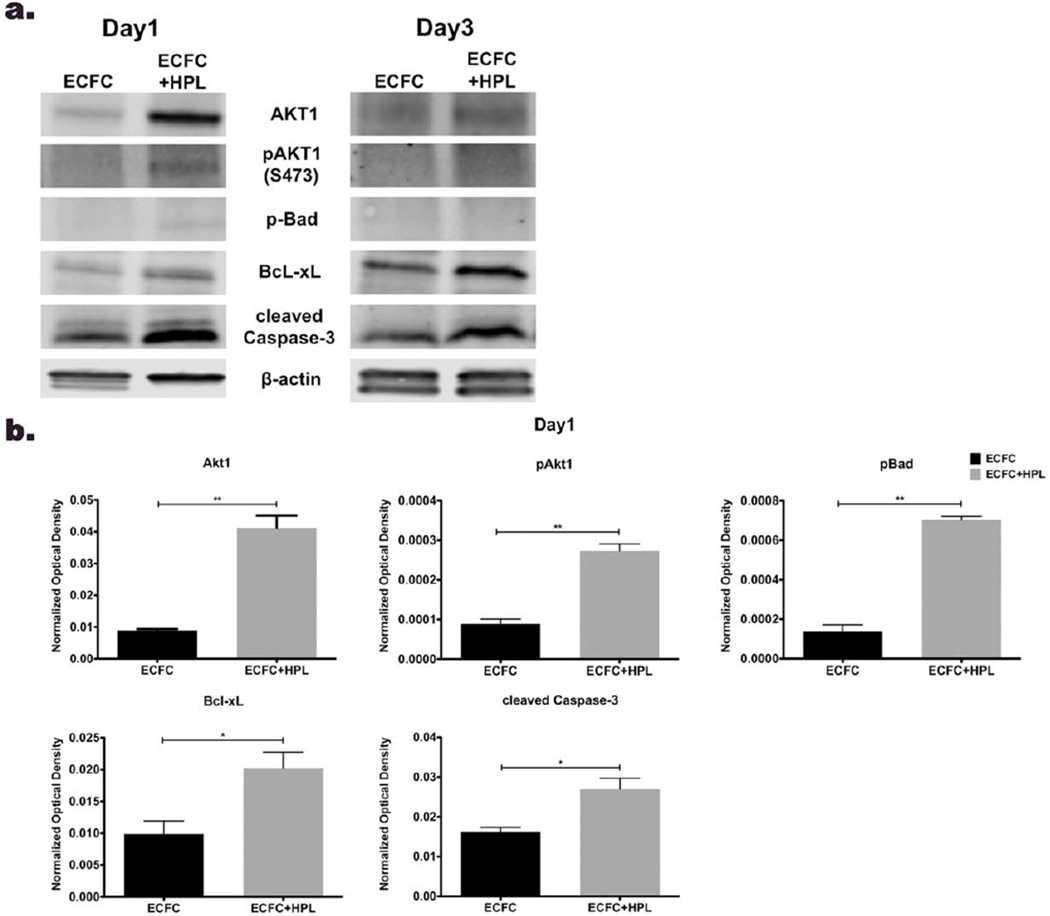
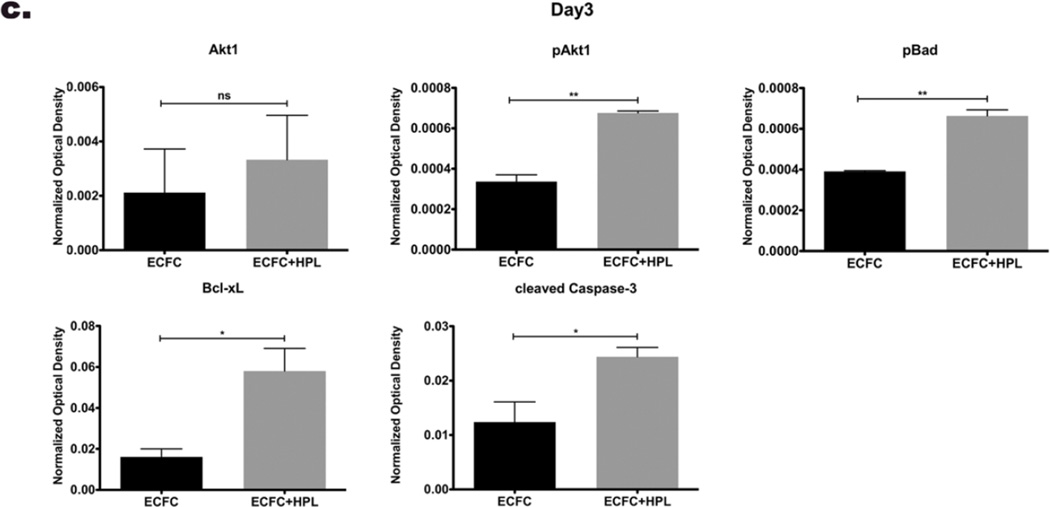
Knowing that HPL treatment can modulate the balance between apoptosis and cell survival [29,30], we subsequently tested whether co-culture of ECFC with HPL would alter the protein expression level of pro-apoptotic, anti-apoptotic and pro-survival molecules in vitro. On day 1 and 3 after casting the matrices, expression of pro-survival molecules (Akt1 and phosphorylated Akt1 (pAkt1)) and an anti-apoptotic molecule (Bcl-xL) were upregulated in co-culture of ECFC with HPL compared to ECFC without HPL group (Fig. 2a and b). The pro-apoptotic molecule, Bad was phosphorylated (pBad) to an inactive form that blocks apoptosis and the expression level of pBad was upregulated in co-culture ECFC with HPL compared to control group on day 1 and 3 in vitro (Fig. 2a and b). The expression level of active cleaved caspase-3 was enhanced in co-culture of ECFC with HPL compared to control samples on day 1 and 3 (Fig. 2a and b). We also examined protein expression level of Akt2 and pAkt2, but these proteins were not detected in either group on day 1 or 3 (data not shown). Since HPL contains various cytokines and growth factors that are involved in pro-survival and anti-apoptotic signaling [34], HPL may induce more pro-survival/anti-apoptotic signaling to resist the apoptotic stimuli that activities of caspase-3 (cleaved caspase-3). These data suggest that co-culture of ECFC with HPL in vitro promotes cell survival of ECFC by modulating the balance between pro-apoptotic and anti-apoptotic/pro-survival molecules.
Figure 2. Human platelet lysate (HPL) in ECFC co-culture stimulates protein expression of apoptotic/ cell survival related molecules.
(a) Representative immunoblots of pro-survival (Akt1, pAkt1, pBad and Bcl-xL) and apoptotic (cleaved caspase-3) proteins from ECFC and ECFC with HPL in 3D matrices. Lower panels show quantification of repeated experiments on day 1 (b) and day 3(c) after culture (protein expression levels were normalized using β-actin). Pro-survival molecules and apoptotic molecule were significantly upregulated on co-culture of ECFC with HPL compared to ECFC alone on day 1 and day 3. N=2 each group ns=no significance, *P < 0.05 or **P<0.001.
Co-implantation of ECFC with HPL significantly enhances vasculogenesis of ECFC in vivo
Co-culture of ECFC with HPL induced more formation of vascular structures in vitro in 3D collagen matrices compared to control matrices (Fig 1). We subsequently tested whether co-implantation of ECFC with HPL would promote vasculogenesis in an in vivo model. Specific anti-human CD31 antibody staining revealed human CB ECFC cell-lined microvessels that were perfused with murine red blood cells in the grafts (Fig 3a). The quantification of murine erythrocyte-containing human CD31+ microvessels revealed a significant increase of average vessel numbers (Fig. 3b) and total vessel area (mm2) (Fig. 3c) in ECFC + HPL implants compared to ECFC alone implants. In addition, the size distribution of the microvessels differed between ECFC implants with HPL and ECFC alone implants (Fig. 3d). Noticeably, only co-implantation of ECFC with HPL gave rise to the largest vascular elements (1001–4000µm2). These data suggest that co-implantation of ECFC with HPL enhanced vasculogenesis in vivo as evidenced by more ECFC-derived vessel formation with an enlarged vascular bed area.
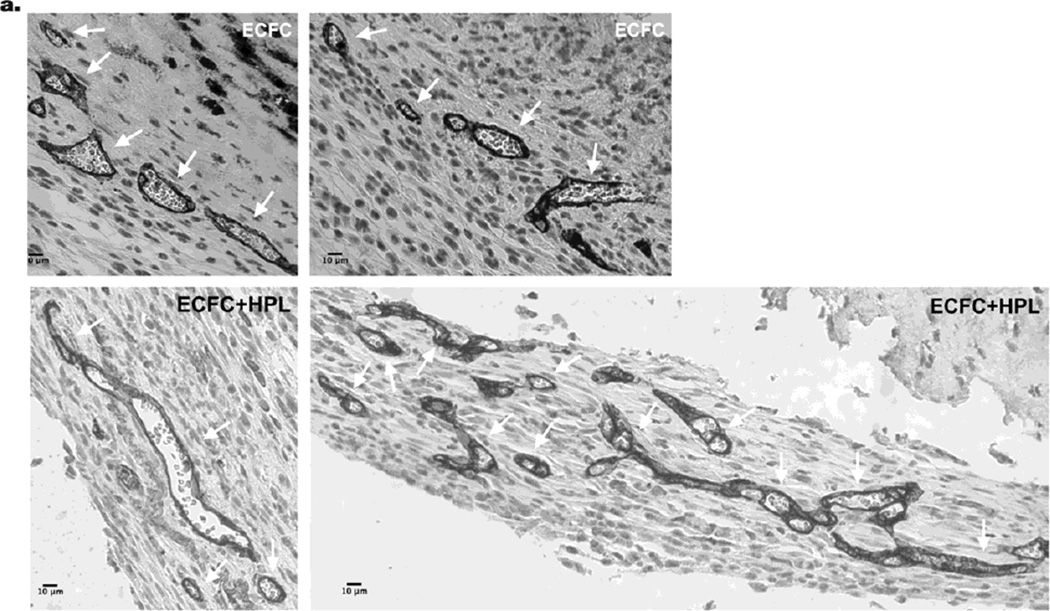
Figure 3. Co-implantation of ECFC with HPL induces the formation of functional vessels in vivo.
(a) Human ECFC-derived vessels were identified by rat anti-human CD31 staining (white arrows indicate ECFC-derived CD31+ vessels). The two top panel images show representative images of ECFC-derived human CD31+ vessels. The two lower panel images show representative images of ECFC with HPL derived human CD31+ vessels. Scale bar represents 10µm. There was a significant increase in (b) average vessel numbers per unit sectional area and (c) total vessel area (mm2) per unit sectional area in the ECFC with HPL implants. (d) In addition, only co-implantation of ECFC with HPL formed the largest sized vessels (# indicates 1001–4000µm2) in the size distribution of hCD31+ microvessels perfused with murine red blood cells. N=10 mice each group *P < 0.05 or **P<0.001.
Inhibition of pro-survival/anti-apoptotic signaling enhances apoptosis of ECFC by modulating protein expression of pro-survival molecules in 3D collagen matrices
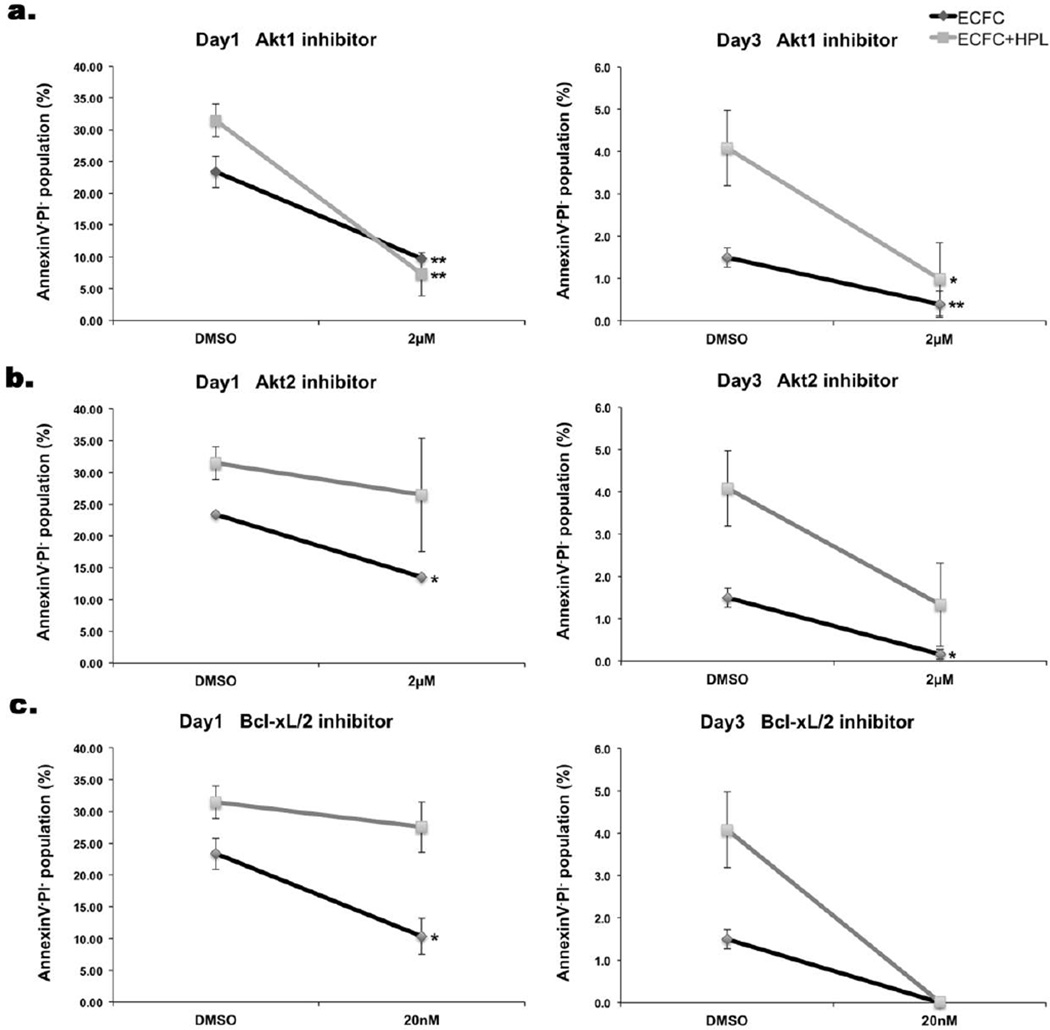
Since ECFC cell survival was significantly increased when ECFC were co-cultured with HPL by balancing the anti-apoptotic/pro-survival (Akt1, pAkt1, pBad and Bcl-xL) and pro-apoptotic signals (cleaved caspase-3), we evaluated whether inhibition of these pro-survival and anti-apoptotic molecules would alter cell survival of ECFC in 3D collagen matrices. Akt is a critical regulator of PI3K-mediated cell survival [35]. Since, Akt1 and Akt2 are the upstream molecules in PI3K-mediated cell survival and have been reported to display compensatory functions between isoforms [36,37], Akt1 and Akt2 inhibitors were selected to examine the importance of these signaling pathways on ECFC pro-survival signaling. Akt1 inhibitor blocks the phosphorylation of the Akt downstream target pBad to result in increased apoptosis [38,39]. Akt2 inhibitor has been reported as a novel small molecule that inhibits cell proliferation by inducing cell cycle arrest and triggers apoptosis [40]. Also, the inhibitor of Bcl-xL was selected for blocking anti-apoptotic signaling in ECFC in 3D collagen matrices. Bcl-xL inhibitor binds to apoptosis suppressor Bcl-xL and prevents binding to the apoptotic effectors Bax and Bak proteins [41,42]. The concentration of each inhibitor was chosen based on dose-dependent optimization experiments (Supplementary Fig. 2). ECFC and ECFC + HPL groups were treated with DMSO as vehicle control, 2µM of Akt1 inhibitor (Fig.4a), 2 µM of Akt2 inhibitor (Fig.4b) and 20nM of Bcl-xL inhibitor (Fig.4c). The percentage of the Annexin V−/PI− viable population was significantly reduced in both groups of ECFC alone and ECFC with HPL when exposed to the Akt1 inhibitor on both day 1 and 3 of culture. While cell survival of ECFC alone group was significantly decreased on day 1, both groups of ECFC and ECFC + HPL displayed zero cell survival on day 3 when treated with the Bcl-xL inhibitor. Akt2 inhibitor treatment significantly reduced cell survival of ECFC alone group on day 1 and 3. However, there was not a statistical significant difference in cell survival of ECFC in the ECFC + HPL group when treated with Akt2 inhibitor on day 1 and 3 because of high variability among the repeated experiments. As a result, only Akt1 inhibition of ECFC displayed a significant reduction of ECFC cell survival in both groups on day 1 following culture. These results implicate the Akt1 pathway as the most important pro-survival pathway promoting ECFC survival in the 3D matrices.
Figure 4. Inhibition of pro-survival molecules reduces cell survival in 3D collagen matrices on day 1 and day 3.
The Annexin V−/PI− viable population of inhibitor treatment of ECFC alone or ECFC with HPL in 3D collagen matrices on day 1 and day 3: DMSO as vehicle control, (a) Akt1 inhibitor, 2µm, (b) Akt2 inhibitor 2µm and (c) Bcl-xL inhibitor 20nM. Percentage of Annexin V−/PI− viable population was significantly decreased by inhibition of Akt1, Akt2 and Bcl-xL. Of note, the most profound decrease in viability was observed in the ECFC + HPL matrices that contained the Akt1 inhibitor on day 1. N=3 each group*P < 0.05 or **P<0.001.
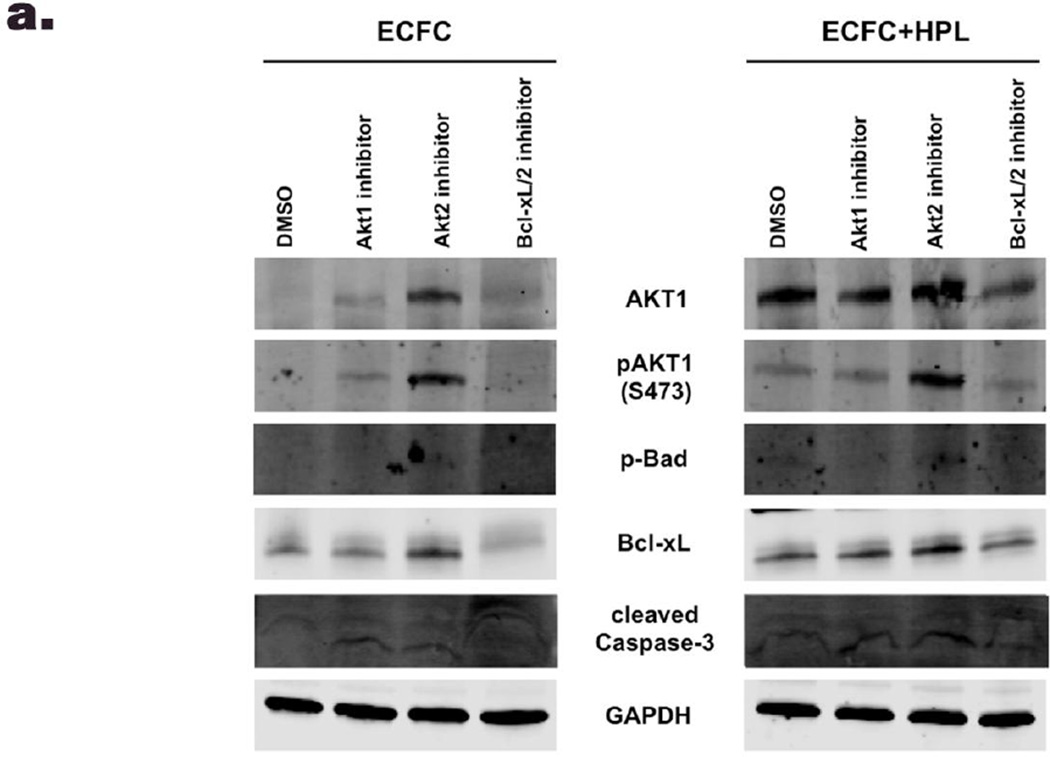
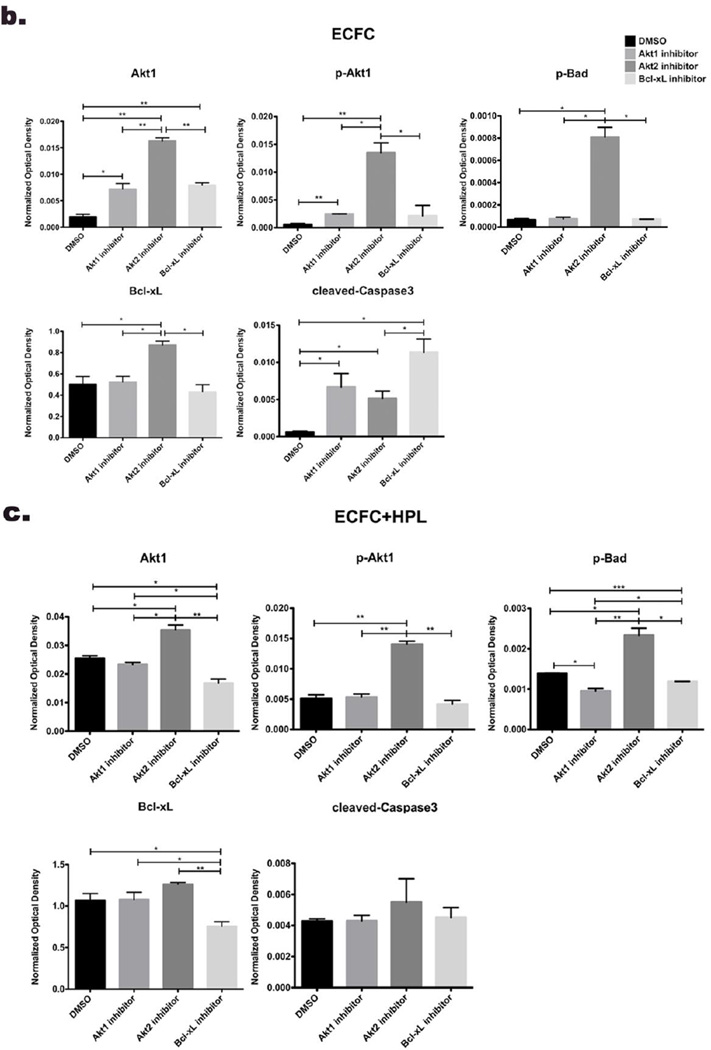
Next, we examined whether the inhibitor treatments would alter the protein expression level of pro-survival/anti-apoptotic or pro-apoptotic molecules in ECFC in 3D collagen matrices on day 1. We tested only day 1 samples because cell survival of ECFC was close to zero by all inhibitor treatments on day 3. When treated with the Akt1 inhibitor, pBad (inactive form that blocks apoptosis) was barely detected in ECFC alone group and significantly decreased in ECFC + HPL group compared to DMSO control group. The level of Bcl-xL (anti-apoptotic molecule) was not changed in both co-culture of ECFC and ECFC + HPL matrices with the inhibitor compared to DMSO control samples. When exposed to the Bcl-xL inhibitor, the level of pBad and Bcl-xL was significantly decreased in the both groups compared to DMSO controls. On the other hand, Akt2 inhibition caused a significant increase of expression level of Akt1/ pAkt1, Bcl-xL (anti-apoptotic molecule) and pBad (inactivated pro-apoptotic molecule) in both groups compared to DMSO group (Fig. 5). Cleaved caspase-3 (pro-apoptotic molecule) was increased with treatment of the three inhibitors in ECFC alone group compared to ECFC with DMSO group (Fig. 5b). However, cleaved caspase-3 expression was not changed in ECFC + HPL groups with the three inhibitors compared to ECFC + HPL treated with DMSO samples (Fig. 5c). Thus, inhibition of Akt1 and Bcl-xL (pro-survival/ anti-apoptotic molecules) promotes apoptosis of ECFC by decreasing pBad (inactivated pro-apoptotic molecule) and Bcl-xL (pro-survival molecule), which are Akt downstream substrates, in both ECFC alone and ECFC + HPL 3D groups. In summary, only Akt1 inhibition significantly diminished cell survival of ECFC by decreasing protein level of pBad in co-culture of ECFC with HPL, even though cleaved caspase-3 expression was not concomitantly changed.
Figure 5. Inhibition of pro-survival molecules affects protein expression of molecules involved in the apoptotic/cell survival signaling in 3D collagen matrices.
(a) Representative immunoblots of inhibitor treatment of ECFC or ECFC with HPL in 3D collagen matrices (DMSO as vehicle control, Akt1 inhibitor, 2µm, Akt2 inhibitor 2µm and Bcl-xL inhibitor 20nM). Lower panels show quantification of repeated experiments of the inhibitor treatment ECFC alone (b) and ECFC with HPL (c) in 3D collagen matrix on day 1 after culture (protein expression levels were normalized using GAPDH). N=2 each group *P < 0.05, **P<0.001 or ***P<0.0001.
In addition, we also determined that ECFC treated with the Akt1, Akt2 and Bcl-xL inhibitors on day 1 and 3 displayed significantly diminished vascular structures on day 1 and absence of vascular structures on day 3 (date not shown). Collectively, these observations have demonstrated that inhibition of the Akt1 signaling pathway significantly diminished cell survival and vasculogenesis of ECFC by altering expression level of pro-apoptotic and anti-apoptotic/pro-survival molecules.
DISCUSSION
The microvascular network has dynamic structural plasticity. Vasculogenesis, angiogenesis and microvascular remodeling are complex processes following endothelial cell growth, migration and differentiation [43–45]. Many of theses in vivo processes can be modeled and examined in vitro. Human CB ECFC or human umbilical vein endothelial cells (HUVEC) participate in cell-cell and/or cell-matrix interactions and form capillary network within the first 24hrs on 2D Matrigel coated dishes. The capillary network formation shows tremendous structural plasticity and dynamic processes of not only new network formation but also network regression. By blocking cell-matrix interactions with colchicine or anti-human avb3 antibody, HUVEC failed to form a capillary network and a significant increase of endothelial cell death was observed [21]. Thus, cell-matrix interactions are necessary for cell viability and the formation of a capillary network on Matrigel coated dishes [21,31]. Bcl-2 over expression in HUVEC enhanced cell viability and promoted stabilization of the capillary network formed on Matrigel [21]. This study suggested that the formation of capillary networks is dependent on the balance between pro-apoptotic and pro-survival of endothelial cells on 2D Matrigel substrates. We suggest that the balance between pro-apoptotic and anti-apoptotic/pro-survival signals play a critical role in promoting cell survival of ECFC and enhancing vasculogenesis when ECFC are co-implanted with HPL in in vitro and in vivo models (Fig. 1, 2 and 3).
Previous studies have been reported that HPL contains various cytokines and growth factors that are involved in various cellular events, particularly cellular migration, proliferation, differentiation, extracellular matrix organization and remodeling, and cell survival [31,34,46,47]. Growth factors in HPL, especially, VEGF and angiopoietin-1, stimulate Akt to promote cell survival and ensure adequate vascular development in cardiovascular functions [48–53]. Akt also has been reported to stimulate the expression of anti-apoptotic Bcl-2 proteins, such as Bcl-xL through the activation of NF-κB [27,50]. Constitutive activation of Akt signaling protects cardiomyocytes against apoptosis [54]. In addition, chronic activation of Akt/PI3K pathway in endothelial cells enhances cell survival of endothelial cells (ECs) and promotes more vasculogenesis of ECs in absence of growth factors and serum [55]. Since Akt1 is generally the predominant expressed isoform (Akt1, Akt2 and Akt3) and compensates Akt2 functions for the inhibition of Akt2 (Ak2 knockout) [36,37], such compensation may explain the why inhibition of Akt2 may not effectively alter expression of pro-survival/anti-apoptotic survival signaling molecules in the ECFC in 3D matrices (Fig.4 and 5). Only Akt1 inhibition significantly decreased the downstream targets, pBad and significantly diminished cell survival of ECFC on both day 1 and day 3 in our in vitro model. Thus, growth factors in HPL may enhance cell survival of ECFC by activating Akt1 and enhancing expression of pro-survival/anti-apoptotic molecules (pAkt1, pBad and Bcl-xL) against the apoptotic signal (cleaved caspase-3) mediated by environmental stress in our experimental models.
CONCLUSIONS
In summary, we have demonstrated that co-implantation of human cord blood ECFCs with HPL improves cell survival of ECFC and promotes robust formation of human ECFC-derived microvessels with increased average vessel number, total vessel area and enlarged vessel sizes in vivo. By activating Akt1 pro-survival signaling in the ECFC, HPL modifies the balance of pro-apoptotic and anti-apoptotic/ cell survival signaling in the implanted cells to enhance vasculogenesis.
Supplementary Material
Representative dot plots of apoptosis analysis of co-culture of ECFC alone and ECFC with HPL on day 1 and 3 of in vitro culture.
Percentage of the Annexin V−/PI− viable population indicates the dose-dependent inhibition of cell survival in ECFC via treatment of DMSO, (a) Akt1 inhibitor, (b) Akt2 inhibitor or (c) Bcl-xL inhibitor.
Highlights.
Human platelet lysate (HPL) significantly increases ECFC survival in collagen gel.
ECFC with HPL significantly promotes vasculogenesis of ECFC in vitro and in vivo.
HPL modulates protein expression of pro-survival molecules in Akt1 signaling.
ACKNOWLEDGEMENTS
This work was supported in part by funding from the National Institutes of Health HL109602 (SV-H and MY) and the Riley Children’s Foundation (MY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Dr. Sherry L. Voytik-Harbin is a founder of GeniPhys (Zionsville, Indiana). No other conflicts of interest are identified for any of the other authors.
Contributor Information
Hyojin Kim, Email: kimhyo@iu.edu.
Nutan Prasain, Email: nprasain@iu.edu.
Sasidhar Vemula, Email: svemula@iu.edu.
Michael J. Ferkowicz, Email: mferkow@iu.edu.
Momoko Yoshimoto, Email: myoshimo@iu.edu.
Sherry L. Voytik-Harbin, Email: harbins@purdue.edu.
Mervin C. Yoder, Email: myoder@iu.edu.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Critser PJ, Grimes BR, Yoder MC. Human umbilical cord blood plasma can replace fetal bovine serum for in vitro expansion of functional human endothelial colony-forming cells. Cytotherapy. 2011;13:712–721. doi: 10.3109/14653249.2010.548380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng G, Liao S, Kit Wong H, Lacorre DA, di Tomaso E, et al. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. 2011;118:4740–4749. doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 10.Allen P, Kang KT, Bischoff J. Rapid onset of perfused blood vessels after implantation of ECFCs and MPCs in collagen, PuraMatrix and fibrin provisional matrices. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1803. [DOI] [PubMed] [Google Scholar]
- 11.Ilan N, Mahooti S, Madri JA. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci. 1998;111(Pt 24):3621–3631. doi: 10.1242/jcs.111.24.3621. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Huang L, Critser PJ, Yang Z, Chan RJ, et al. Notch ligand Delta-like 1 promotes in vivo vasculogenesis in human cord blood-derived endothelial colony forming cells. Cytotherapy. 2015 doi: 10.1016/j.jcyt.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- 14.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 15.Ranta V, Mikkola T, Ylikorkala O, Viinikka L, Orpana A. Reduced viability of human vascular endothelial cells cultured on Matrigel. J Cell Physiol. 1998;176:92–98. doi: 10.1002/(SICI)1097-4652(199807)176:1<92::AID-JCP11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 17.Fukai F, Mashimo M, Akiyama K, Goto T, Tanuma S, et al. Modulation of apoptotic cell death by extracellular matrix proteins and a fibronectin-derived antiadhesive peptide. Exp Cell Res. 1998;242:92–99. doi: 10.1006/excr.1998.4076. [DOI] [PubMed] [Google Scholar]
- 18.Sacharidou A, Stratman AN, Davis GE. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cells Tissues Organs. 2012;195:122–143. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stratman AN, Davis MJ, Davis GE. VEGF and FGF prime vascular tube morphogenesis and sprouting directed by hematopoietic stem cell cytokines. Blood. 2011;117:3709–3719. doi: 10.1182/blood-2010-11-316752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittington CF, Yoder MC, Voytik-Harbin SL. Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol Biosci. 2013;13:1135–1149. doi: 10.1002/mabi.201300128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollman MJ, Naumovski L, Gibbons GH. Endothelial cell apoptosis in capillary network remodeling. J Cell Physiol. 1999;178:359–370. doi: 10.1002/(SICI)1097-4652(199903)178:3<359::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–1062. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 25.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 27.Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 29.Gambim MH, do Carmo Ade O, Marti L, Verissimo-Filho S, Lopes LR, et al. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 31.Shih DT, Chen JC, Chen WY, Kuo YP, Su CY, et al. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011;51:770–778. doi: 10.1111/j.1537-2995.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- 32.Bailey JL, Critser PJ, Whittington C, Kuske JL, Yoder MC, et al. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers. 2011;95:77–93. doi: 10.1002/bip.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp. 2009 doi: 10.3791/1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fekete N, Gadelorge M, Furst D, Maurer C, Dausend J, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 36.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 39.Zhu QS, Ren W, Korchin B, Lahat G, Dicker A, et al. Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45 alpha. Cancer Res. 2008;68:2895–2903. doi: 10.1158/0008-5472.CAN-07-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap TA, Walton MI, Hunter LJ, Valenti M, de Haven Brandon A, et al. Preclinical pharmacology, antitumor activity, and development of pharmacodynamic markers for the novel, potent AKT inhibitor CCT128930. Mol Cancer Ther. 2011;10:360–371. doi: 10.1158/1535-7163.MCT-10-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 42.Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–3277. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 43.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 44.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 45.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 46.Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med. 2007;4:e234. doi: 10.1371/journal.pmed.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 48.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 49.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 51.Kontos CD, Stauffer TP, Yang WP, York JD, Huang L, et al. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18:4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 53.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 54.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative dot plots of apoptosis analysis of co-culture of ECFC alone and ECFC with HPL on day 1 and 3 of in vitro culture.
Percentage of the Annexin V−/PI− viable population indicates the dose-dependent inhibition of cell survival in ECFC via treatment of DMSO, (a) Akt1 inhibitor, (b) Akt2 inhibitor or (c) Bcl-xL inhibitor.