Abstract
Ocular involvement can be quite symptomatic in patients with chronic graft-versus-host disease (GVHD). The prevalence of and risk factors for ocular GVHD and its impact on quality of life (QOL) in patients with chronic GVHD were studied in a prospective, multicenter, longitudinal, observational study. This study enrolled 342 patients with 1,483 follow-up visits after allogeneic hematopoietic cell transplantation. All patients in this analysis were diagnosed with chronic GVHD requiring systemic treatment and enrolled within 3 months of chronic GVHD diagnosis. The symptom burden of ocular GVHD was based on the degree of dry eye symptoms, frequency of artificial tear usage, and impact on activities of daily living. Patients’ quality of life (QOL) was measured by self-administered questionnaires. Variables associated with ocular GVHD at enrollment and subsequent new onset ocular GVHD, and the associations with QOL were studied. Of the 284 chronic GVHD patients, 116 (41%) had ocular GVHD within 3 months of chronic GVHD diagnosis (“early ocular GVHD”). Late ocular GVHD (new onset > 3 months after chronic GVHD diagnosis) occurred in 64 patients. Overall cumulative incidence at 2 years was 57%. Female gender (p=0.005), higher acute GVHD grade (p=0.04) and higher prednisone dose at study entry (p=0.04) were associated with early ocular GVHD. For patients who did not have ocular GVHD within 3 months of chronic GVHD diagnosis, presence of prior grade I-IV acute GVHD (HR 1.78, P=0.04) was associated with shorter time to late ocular GVHD, while female donor into male recipient (HR 0.53, P=0.05) was associated with longer time to late ocular GVHD onset. Utilizing all visit data, patients with ocular GVHD had worse quality of life as measured by FACT-BMT (P=0.002), and greater chronic GVHD symptom burden, as measured by the Lee symptom overall score excluding the eye component (P<0.001) compared to patients who did not have ocular GVHD. In conclusion, this large multicenter, prospective study shows that ocular GVHD affects 57% of patients within 2 year of chronic GVHD diagnosis. Women, patients on higher doses of prednisone at study entry, and those with a history of acute GVHD were at higher risk for ocular GVHD. Strong evidence suggests that ocular GVHD is associated with worse overall health-related QOL.
Introduction
Since the first successful bone marrow transplantation in 1968, allogeneic hematopoietic cell transplantation (HCT) is used with increasing frequency for treatment of hematological disorders (both malignant and non-malignant) with curative intent.1,2 Graft-versus-host disease (GVHD) caused by alloreactive donor T cells remains an important cause of non-relapse mortality and morbidity after allo-HCT.3 Chronic GVHD typically starts more than 3 months after transplant, and may persist for many years. Chronic GVHD most often involves the mouth and skin, but it can also affect multiple sites, such as eyes, gastrointestinal tract, liver, lungs, and genital tract.4,5
Ocular GVHD reportedly occurs in more than 50% of allogeneic HCT recipients with chronic GVHD.5 The most frequent ocular symptoms are a sensation of dryness, irritation, redness, and photophobia. Ocular GVHD can affect all parts of the eye, but the ocular surface is the most common level of involvement.6–8 Conjunctival involvement has been associated with higher mortality9 and worse quality of life10 in prior single-centered and small-grouped reports.
Quality of Life (QOL) is defined as the general well-being of individuals and societies.11 It is a multidimensional concept, and health is one of the important dimensions of overall quality of life. The QOL in patients with ocular disorders can be affected by ocular symptoms, such as blurred vision, severe photophobia, or having difficulties in keeping eyes open due to discomfort.12–14 Affected patients might not be able to drive, read, watch television, or perform other common activities in their routine daily life. In this study we describe the epidemiology of ocular GVHD symptoms in patients with chronic GVHD, perform a risk factor analyses, and describe the impact of ocular involvement on QOL.
Materials and Methods
Data were derived from the Chronic GVHD Consortium, a multicenter observational cohort study of chronic GVHD-affected HCT recipients. The rationale and design of this cohort study have been previously described.16 Patients with chronic GVHD requiring systemic treatment were enrolled if the time from onset of GVHD was 2 years or less. Cases were defined as incident if onset of chronic GVHD was within 3 months of study enrollment and prevalent if onset of chronic GVHD was > 3 months of study enrollment. For this analysis, only incident cases were included. The study protocol was approved by the institutional review board of each participating center, and all participants or their guardians gave written informed consent. Patients had detailed assessment by the transplant clinician or trained provider at pre-specified time-points as required by the protocol using a variety of assessment tools.16 Scoring of chronic GVHD was done using the NIH Consensus Criteria.15 Ocular involvement is scored on a 0–3 scale and is dependent on the degree of dry eye symptoms, frequency of eye drops or intervention for dry eye, and impact on activities of daily living.17 Patients completed detailed QOL questionnaires, including the Functional Assessment of Cancer Therapy Bone Marrow Transplantation (FACT-BMT)18,19 the Short Form (36) Health Survey, which provides a Physical Component Score (SF36 PCS) and Mental Component Score (SF36 MCS);20,21 the Human Activity Profile providing a Maximum Activity Score (HAP MAS),22 Adjusted Activity Score (HAP AAS), and modified HAP AAS23; and the Lee symptom score24 at these visits. The QOL metrics were not designed specifically for ocular disorders but measure a person’s multi-dimensional QOL.
Definition
For this study, the ocular GVHD group was defined as eye involvement reported by both the clinician (NIH 0–3 eye score > 0) and patient (eye worst 0–10 score > 1 or Lee symptom eye score > 20)25. It is likely that risk factors associated with ocular GVHD may vary depending on time of onset of ocular GVHD in relation to development of systemic chronic GVHD. Thus, early ocular GVHD was defined as the onset of ocular involvement at study enrollment and late ocular GVHD was defined as onset three or more months after the diagnosis of chronic GVHD. Ocular assessments prior to HCT or prior to study enrollment were not routinely performed.
Statistical analysis
Descriptive statistics, including continuous variables and categorical variables, were calculated and reported in Table 1 and Table 2. All the variables we analyzed were as below: age at HCT, patient gender, donor and patient gender combination, preparative regimen intensity, donor match status, source of stem cells, prior acute GVHD, maximum acute GVHD grade (overall, liver, gastrointestinal, and skin), underlying diseases, disease status, platelet count at onset and enrollment, bilirubin level at onset and enrollment, prednisolone dose at onset and enrollment, worst Schirmer’s test at enrollment, study site (FHCRC vs. others), and duration from treatment to enrollment. Univariable and multivariable logistic regression models were fit to identify risk factors that were associated with ocular GVHD at enrollment. To identify the risk factors that are associated with development of ocular GVHD at subsequent follow up, Cox regressions were fit for patients who did not have ocular GVHD at enrollment (N=168). Relapse and death before the onset of ocular GVHD were treated as competing events. Cox regressions were also fit for overall survival (OS) and nonrelapse mortality (NRM) of the two cohorts (chronic GVHD with and without ocular GVHD at study enrollment). Covariates significantly associated with outcomes were included for adjustment, including platelet count (<100,000/µl or >=100,000/µl), donor gender combination (female into male or others), bilirubin (≤2 mg/dL or higher), and NIH global severity (mild or less, moderate, or severe). Linear mixed models with random patient effect were fit for evaluating the ocular involvement associated with QOL, including functional and symptom measures, adjusted for significant clinical covariates. The covariates included study site (FHCRC or others), months since enrollment, platelet count (<100,000/µl or >=100,000/µl), NIH global severity (mild or less, moderate, or severe), bilirubin (≤2 mg/dL or higher), overlap or classic, and prior acute GVHD. A p-value below 0.05 was considered statistically significant.
Table 1.
Patient characteristics at study enrollment (N=342)
| Variable | N (%) |
|---|---|
| Age (years), median (range) | 51 (19–79) |
| Recipient gender | |
| Male | 190 (56%) |
| Female | 152 (44%) |
| Male recipient/female donor | 92 (27%) |
| Transplant type | |
| Myeloablative | 196 (58%) |
| Non-myeloablative | 143 (42%) |
| Donor status | |
| Matched related | 145 (43%) |
| Matched unrelated | 142 (42%) |
| Mismatched | 53 (15%) |
| Prior acute GVHD | |
| Yes | 224 (66%) |
| No | 118 (34%) |
| Ocular GVHD | |
| Yes | 116 (34%) |
| No | 168 (49%) |
| Unclassifiable at enrollment (Missing either clinician or patient information) | 58 (17%) |
Table 2.
Ocular characteristics of early ocular GVHD* patients (N=116)
| Variable | N (%) |
|---|---|
| National Institute of Health (NIH) eye score | |
| 0 | 0 (0%) |
| 1 | 82 (71%) |
| 2 | 32 (27%) |
| 3 | 2 (2%) |
| Schirmer’s test (worst eye) | |
| >10 mm | 30 (26%) |
| 5–10 mm | 29 (25%) |
| <5 mm | 30 (26%) |
| Missing | 27 (23%) |
early ocular GVHD: ocular GVHD onset within 3 months after diagnosis of cGVHD
Statistical analyses were performed using SAS/STAT software, version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
General characteristics of the patients included in this study are presented in Table 1. This study included 342 adult patients who had 1,483 visits. The median follow-up time was 28 months from enrollment in the study (range 4–66 months). The cumulative incidence of all ocular GVHD at 2 years after enrollment was 57% (95% CI 52%–63%). The incidence of ocular GVHD between FHCRC and other centers was also analyzed and no significance was noted (p value = 0.49).
Risk factor analysis of early ocular GVHD
Of the 342 patients, 284 (83%) patients had complete clinician and patient-reported ocular data at study enrollment. Of the 284 patients, 116 (41%) had ocular GVHD confirmed by both clinician and patient-report within 3 months of chronic GVHD diagnosis (“early ocular GVHD”) (Table 2). Most of the early ocular GVHD patients (71%) had mild ocular symptoms (NIH eye score: 1). Among the 116 early ocular GVHD patients, Schirmer’s test was measured in 89 patients (77%), of which 59 patients (66%) had a mean value of 10 mm or less. In a multivariable analysis, female sex (odds ratio [OR]=2.14, 95% CI 1.26–3.63 P=0.005), overall maximum acute grade (OR=1.32, 95% CI 1.01–1.73 P=0.04) and higher prednisone dose at study entry (OR=2.04, 95% CI 1.04–4.00, P=0.04) were associated with early ocular GVHD (Table 3).
Table 3.
Multivariable analysis of risk factors associated with early ocular GVHD* involvement in chronic GVHD patients at enrollment (N=116)
| Covariate | Effect | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
| Patient gender | Female vs. Male | 2.14 | 1.26–3.63 | 0.005 |
| Max acute GVHD grade: overall | Continuous 0–4 | 1.32 | 1.01–1.73 | 0.04 |
| Prednisone dose at enrollment | Continuous | 2.04 | 1.04–4.00 | 0.04 |
early ocular GVHD: ocular GVHD onset within 3 months after diagnosis of chronic GVHD
Development and risk factor analysis of late ocular GVHD
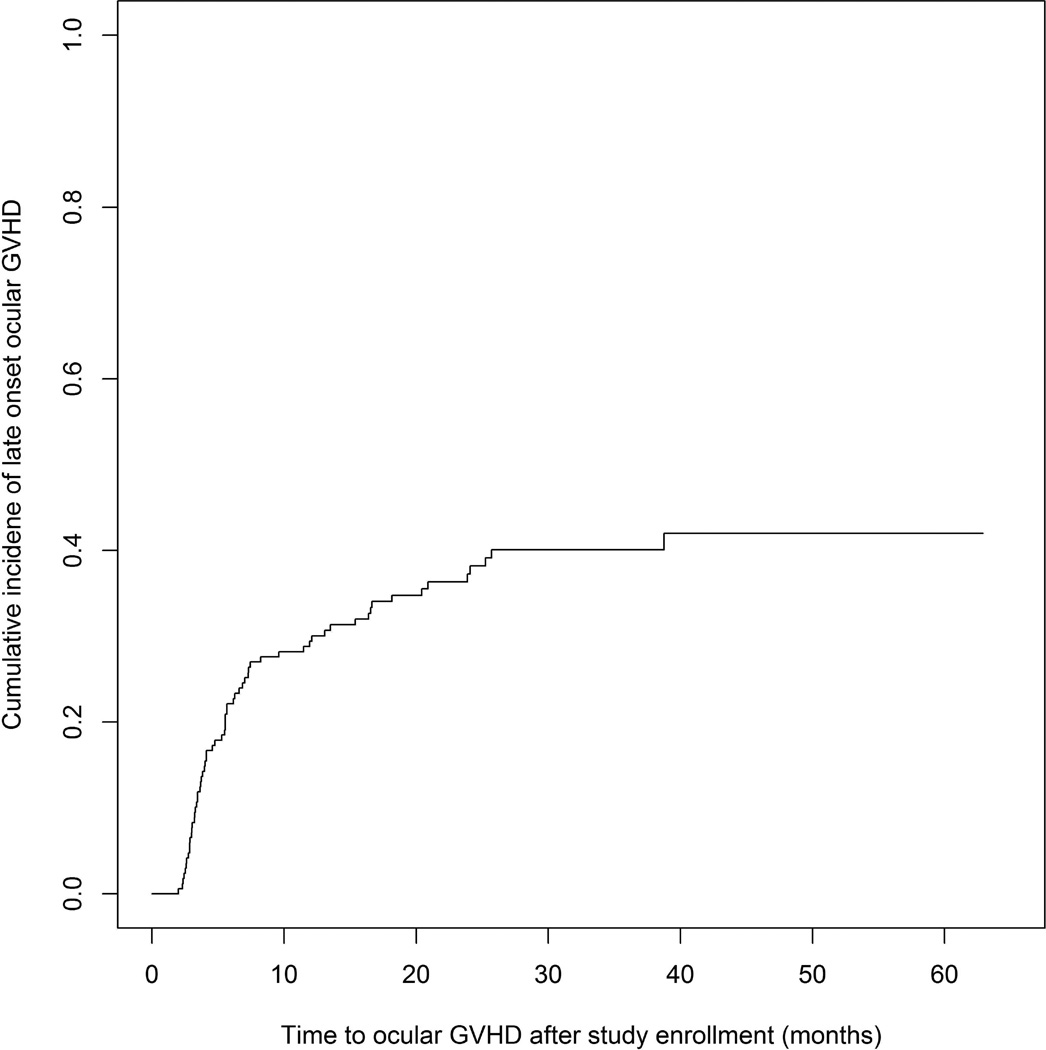
Late ocular GVHD (new onset after study enrollment) occurred in 64 patients who were confirmed not to have ocular GVHD at enrollment. The cumulative incidence of late ocular GVHD at 2-year post enrollment was 37% (95% CI 30%–46%) (Figure 1). In multivariable modeling, presence of prior grade I-IV acute GVHD (HR 1.78, P=0.04) was associated with shorter time to late ocular GVHD, while female donor into male recipient (HR 0.53, P=0.05) was associated with longer time to late onset ocular GVHD (Table 4).
Figure.
Cumulative incidence of subsequent late ocular GVHD* among patient without ocular GVHD at study enrollment (N=64) (*late ocular GVHD: ocular GVHD onset > 3 months after diagnosis of chronic GVHD)
Table 4.
Multivariable analysis of risk factors associated with late ocular GVHD* involvement (N=168, Number of events=64)
| Covariate | Effect | HR | 95% CI | P-value |
|---|---|---|---|---|
| Prior grade I–IV acute GVHD | Yes vs. No | 1.78 | 1.04–3.20 | 0.04 |
| Donor/patient gender combination | Female into male vs. Others | 0.53 | 0.27–0.96 | 0.05 |
late ocular GVHD: ocular GVHD onset > 3 months after diagnosis of chronic GVHD
Eighteen of the 58 patients (31%) whose ocular GVHD could not be classified at enrollment because of missing clinician or patient information subsequently had confirmed ocular GVHD later in their course. These patients were excluded from the analysis of late ocular GVHD predictors since we are uncertain if they had early or late onset ocular GVHD.
Survival and non-relapse mortality
In multivariate Cox models with ocular GVHD as a time-varying covariate, ocular GVHD was not associated with survival (HR 1.08, P=0.76) or subsequent non-relapse mortality (HR 1.27, P=0.48)
Analysis between quality of life and ocular GVHD
Table 5 shows the association of ocular GVHD with patient-reported QOL, using all available visit data adjusted for center effect, months since enrollment, platelet count, NIH severity, bilirubin, prior acute GVHD, and overlap vs. classic chronic GVHD. The association between QOL and ocular involvement is significant, especially in the FACTBMT score (P<0.01) despite adjusting for the clinical factors. The Lee symptom score overall (excluding the eye component to avoid bias from eye symptoms) is also highly associated with ocular involvement (P <0.001). The other QOL questionnaires, including SF36 and HAP, did not show significant association with ocular involvement (P >0.05).
Table 5.
The association between quality of life (QOL) and ocular GVHD in chronic GVHD patients
Estimate 0-reference category;
Lee symptom score-eye component excluded
Discussion
This study describes the high incidence of symptomatic ocular GVHD in patients with established chronic GVHD in a longitudinal prospective cohort. In our cohort, 41% of patients had ocular GVHD symptoms within 3 months after diagnosis of chronic GVHD and another 23% developed ocular GVHD by 2 years after chronic GVHD onset for a 2-year cumulative incidence of 57%. Thus, patients with chronic GVHD who do not have ocular GVHD at onset of chronic GVHD remain at significant risk of subsequently developing eye symptoms later and should be carefully monitored.
Some study limitations should be noted. We categorized ocular GVHD according to the tools recommended by the 2005 NIH Consensus Conference on Criteria for Clinical Trials in Chronic GVHD. These assessments were designed so they could be collected from transplant providers and patients, and not require subspecialists.15,28 Indeed, many of the patients in our study were managed by their transplant physicians and not by ophthalmologists. Although, Schirmer’s test was performed in the majority of the patients, results are not used in severity scoring, as it is known to be unreliable.25–27 The change in light, humidity and temperature and patient anxiety may interfere with the tear reflex.26 The paper’s contact with eyelashes and the position of the paper placed on the lid margin are also the factors which can explain the large discrepancies in the reported repeatability of the Schirmer’s test.27 We acknowledge that other etiologies besides ocular GVHD could contribute to dry eye symptoms in our population and could confound the analyses. A comprehensive assessment by ophthalmologists would provide more detailed, and likely more accurate, information about ocular involvement, but referral is not uniformly practiced. In addition, multiple consensus criteria for severity scoring have been proposed by the ophthalmology community but none have been validated, suggesting that while the evaluation may be more complete, the ability to use that information to quantify the severity of involvement is not robust yet.17 A recent analysis compared the Japanese dry eye score and the Dry Eye Workshop Score (DEWS) to the NIH eye score,28 and found excellent correlation among the 3 scoring systems, lending support to the accuracy of transplant physicians’ in assessing severity. However, a prospective study using ophthalmology examinations as the gold standard is still necessary and important for further evaluation of ocular GVHD.
Risk factors for ocular GVHD have been reported in retrospective studies. In addition to global GVHD, prior acute skin GVHD and male recipients of female donors were reported as two independent risk factors for ocular GVHD.29 A higher incidence of ocular GVHD after peripheral blood stem cell transplantation than bone marrow transplantation (BMT) or cord blood transplantation was reported by Uchino M, et al. in 2012.30 In our prospective study, independent risk factors for early ocular GVHD included female sex, more severe acute GVHD, and higher prednisone dose at enrollment. Female predilection for early ocular GVHD in this study (median age 51 years old) is compatible with the predisposing factors for dry eye syndrome, such as middle age and female gender.31 The biological reasons for these associations remain unknown, but may involve common pathways. Interestingly, it seems that acute GVHD could predict development of early ocular GVHD as well as late ocular GVHD. In multivariable modeling, presence of prior grade I-IV acute GVHD was associated with shorter time to late ocular GVHD, while female donor into male recipient was associated with longer time to late ocular GVHD onset. These results should help define risk stratification of patients in future clinical trials addressing prevention of ocular GVHD.
Quality of life measures capture the impact of medical conditions on physical, social, emotional, cognitive, work- or role-related, and spiritual aspects of life. GVHD has consistently been shown to have a major impact on patients’ quality of life. Lee et al.32 showed that both grades II-IV acute and chronic GVHD were associated with worse QOL after HCT. Pallua and colleagues33 also found that patients suffering from chronic GVHD showed worse role functioning and global QOL, and had a higher likelihood of taking chronic medications. Pidala et al.34 reported that chronic GVHD severity defined by NIH criteria was independently associated with QOL. Riemens et al.10 used two questionnaires (VFQ-25: visual function questionnaire-25 and OSDI: ocular surface disease index) to assess vision-related QOL in 2014. Their results showed that patients who developed ocular GVHD (N=14) have impaired visual QOL.
In this study, we focused on the relationship between ocular GVHD and health-related QOL instead of vision-related QOL, to study the impact of dry eye symptoms on general QOL. Several different questionnaires, including FACT-BMT, SF36, HAP, and Lee symptom score were used to assess the QOL of ocular GVHD patients. FACT-BMT is an outcome measure for health-related quality of life. SF-36 is a measure more about general health and HAP is for functional performance and activity limitation in adults. We found that FACT-BMT and Lee symptom score (without the eye subscale) showed significant differences between patients with and without ocular GVHD symptoms, adjusting for other clinical factors. The association of ocular GVHD and FACT-BMT and no association with SF-36 and HAP might be because of the characteristic of ocular GVHD which is an ocular surface disorder causing a lot of eye discomforts but not necessarily affecting vision to impact activity and functional performance. To our knowledge, this is the first prospective study investigating the association of ocular GVHD and health-related QOL. There was no correlation with SF36 and HAP.
In conclusion, we found that the patients with more severe acute GVHD developed ocular GVHD coincident with their diagnosis of chronic GVHD and remained at higher risk for subsequent late onset ocular GVHD. Approximately a third of patients with chronic GVHD had ocular GVHD at diagnosis, while almost a quarter developed ocular GVHD at later time points. A significant relationship between ocular GVHD and non-vision related QOL and non-ocular GVHD symptoms was also revealed in this study. Ocular GVHD may cause permanent destruction of lacrimal glands, resulting from increase in number of stromal fibroblasts and excessive fibrosis in the extracellular matrix of the lacrimal glands.35 Early initiation of topical cyclosporine-A 0.05% in ocular GVHD patients could relieve the patients’ dry eye symptoms as well as might reduce the inflammatory response in the lacrimal glands.36 Ideally, prophylactic or pre-emptive clinical trial (NCT00755040, pending analyses) strategies prior to onset of irreversible dry eye syndrome are needed. Comprehensive ophthalmologic assessment should be undertaken in patients at high risk for ocular GVHD, and in patients with ocular symptoms. Preventive evaluation at set time-points after transplant to screen of asymptomatic ocular GVHD is encouraged.
Highlights.
We report aA prospective, multicenter study correlating about the impact of ocular GVHD on quality of life.
The risk factors of early developing ocular GVHD are female gender, more severe prior acute GVHD, and higher prednisone dose at enrollment.
Early referral to an ophthalmologist for high-risk patients is highly recommended for the prevention of permanent damage by ocular GVHD.
Acknowledgments
Financial Support: This work was supported by grants CA118953 and CA163438 from the National Institutes of Health. The Chronic GVHD Consortium (U54 CA163438) is a part of the National Institutes of Health Rare Diseases Clinical Research Network, supported through collaboration between the National Institutes of Health Office of Rare Diseases Research at the National Center for Advancing Translational Science, the National Cancer Institute, and the Fred Hutchinson Cancer Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicting relationship exists for any author
References
- 1.Appelbaum FR, Forman SJ, Negrin RS, Blume KG. Thomas’ hematopoietic cell transplantation. 4th ed. Oxford, England: Wiley-Blackwell; 2009. pp. 705–1199. [Google Scholar]
- 2.Gratwohl A, Baldomero H, Demirer T, et al. Hematopoetic stem cell transplantation for solid tumors in Europe. Ann Oncol. 2004;15:653–660. doi: 10.1093/annonc/mdh142. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Vogelsang G, Flowers MED. Chronic graft versus host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 5.Westeneng AC, Hettinga Y, Lokhorst H, Verdonck L, van Dorp S, Rothova A. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea. 2010;29:758–763. doi: 10.1097/ICO.0b013e3181ca321c. [DOI] [PubMed] [Google Scholar]
- 6.Rojas B, Cuhna R, Zafirakis P, et al. Cell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81:313–325. doi: 10.1016/j.exer.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:S19–S27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- 8.Tabbara KF, Al-Ghamdi A, Al-Mohareb F. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009;116:1624–1629. doi: 10.1016/j.ophtha.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Jabs DA, Wingard J, Green WR, et al. The eye in bone marrow transplantation. III. Conjunctival graft-versus-host disease. Arch Ophthalmol. 1989;107:1343–1348. doi: 10.1001/archopht.1989.01070020413046. [DOI] [PubMed] [Google Scholar]
- 10.Riemens A, Te Boome LC, Kalinina Ayuso V, et al. Impact of ocular graft-versus-host disease on visual quality of life in patients after allogeneic stem cell transplantation: questionnaire study. Acta Ophthalmol. 2014;92:82–87. doi: 10.1111/aos.12047. [DOI] [PubMed] [Google Scholar]
- 11.Schipper H, Clinch J, Olweny C. Quality of life studies: definitions and conceptual issues. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd edn. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 11–23. [Google Scholar]
- 12.Aydin Kurna S, Altun A, Gencaga T, Akkaya S, Sengor T. Vision related quality of life in patients with keratoconus. J Ophthalmol. doi: 10.1155/2014/694542. Epub 2014 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellanti JA, Settipane RA. Quality of life issues ranging from the burden of ocular and nasal allergies to the anxiety associated with having to carry self-injectable epinephrine for insect sting allergy. Allergy Asthma Proc. 2014;35:195–196. doi: 10.2500/aap.2014.34.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Hong J, Wei A, et al. Vision-related quality of life in patients with infectious keratitis. Optom Vis Sci. 2014;91(3):278–283. doi: 10.1097/OPX.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Chronic GVHD Consortium. Rationale and design of the chronic GVHD cohort study: improving outcomes assessment in chronic GVHD. Biol Blood Marrow Transplant. 2011;17:1114–1120. doi: 10.1016/j.bbmt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa Y, Kim SK, Dana R, et al. International Chronic Ocular Graft-versus-Host Disease (GVHD) Consensus Group: proposed diagnostic criteria for chronic GVHD (Part I) Sci Rep. 2013;3:3419. doi: 10.1038/srep03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 19.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–368. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 21.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: A Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 22.Daughton DM, Fix AJ, Kass I, Bell CW, Patil KD. Maximum oxygen consumption and the ADAPT quality-of-life scale. Arch Phys Med Rehabil. 1982;63:620–622. [PubMed] [Google Scholar]
- 23.Herzberg PY1, Heussner P, Mumm FH, et al. Validation of the human activity profile questionnaire in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1707–1717. doi: 10.1016/j.bbmt.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:444–452. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- 25.Inamoto Y, Chai X, Kurland B, et al. Validation of measurement scales in ocular graft-versus-host disease. Ophthalmology. 2012;119:487–493. doi: 10.1016/j.ophtha.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms with dry eye disease. Cornea. 2004;23:762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 27.Feldman F, Wood MM. Evaluation of the Schirmer tear test. Can J Ophthalmol. 1979;14:257–259. [PubMed] [Google Scholar]
- 28.Tetematsu Y, Ogawa Y, Abe T, et al. Grading criteria for chronic ocular graft-versus-host disease: comparing the NIH eye score, Japanese dry eye score, and DEWS 2007 score. Sci Rep. 2014;4:6680. doi: 10.1038/srep06680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs R1, Tran U, Chen H, et al. Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant. 2012;47:1470–1473. doi: 10.1038/bmt.2012.56. [DOI] [PubMed] [Google Scholar]
- 30.Uchino M, et al. Comparison of stem cell sources in the severity of dry eye after allogeneic haematopoietic stem cell transplantation. Br J Ophthalmol. 2012;96:34–37. doi: 10.1136/bjophthalmol-2011-300514. [DOI] [PubMed] [Google Scholar]
- 31.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Kim HT, Ho VT, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2006;38:305–310. doi: 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]
- 33.Pallua S1, Giesinger J, Oberguggenberger A, et al. Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation. Bone Marrow Transplant. 2010;45:1534–1539. doi: 10.1038/bmt.2010.5. [DOI] [PubMed] [Google Scholar]
- 34.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa Y, Yamazaki K, Kuwana M, et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001;42:111–119. [PubMed] [Google Scholar]
- 36.Malta JB, Soong HK, Shtein RM, et al. Treatment of ocular graft-versus-host disease with topical cyclosporine 0.05% Cornea. 2010;29:1392–1396. doi: 10.1097/ICO.0b013e3181e456f0. [DOI] [PubMed] [Google Scholar]