Abstract
Rationale
Emerging preclinical evidence suggests that imidazoline I2 receptor ligands may be effective analgesics. Quantitative analysis of the combined I2 receptor ligands and opioids is needed for the justification of combination therapy.
Objective
This study systematically examined the anti-hyperalgesic and response rate-suppressing effects of selective I2 receptor ligands (2-BFI and phenyzoline) alone and in combination with oxycodone in rats.
Methods
Von Frey filament test was used to examine the anti-hyperalgesic effects of drugs in a rat model of complete Freund's adjuvant (CFA)-induced inflammatory pain. Schedule-controlled responding was used to assess the rate-altering effects of study drugs. Duration of actions of individual drugs (2-BFI, phenyzoline and oxycodone) alone or in combination were studied. Dose-addition analysis was employed to assess the anti-hyperalgesic interactions between drugs.
Results
Oxycodone (0.1-3.2 mg/kg, i.p.), 2-BFI (1-17.8 mg/kg, i.p.) and phenyzoline (17.8-56 mg/kg, i.p.) all dose-dependently produced significant antinociceptive effects. When studied as combinations, 2-BFI and oxycodone produced additive interactions while phenyzoline and oxycodone produced supra-additive interactions under all fixed ratios. The same drug combinations did not alter or significantly reduced the operant responding depending on the ratios of the drug combinations.
Conclusions
Quantitative analysis of the anti-hyperalgesic effects of I2 receptor ligands strongly supports the therapeutic potential of I2 receptor ligands against inflammatory pain. In addition, the data reveal that phenyzoline is superior to the prototypic I2 receptor ligand 2-BFI for the management of pain and warrants further consideration as a novel analgesic.
Keywords: imidazoline I2 receptor, oxycodone, complete Freund's adjuvant, time course, dose-addition analysis, rats
INTRODUCTION
Pain affects more Americans than diabetes, heart disease and cancer combined (Institute of Medicine, 2011). Millions suffer from acute or chronic pain every year and the annual national economic cost associated with pain is estimated to be $560-635 billion (Institute of Medicine, 2011). Unfortunately, currently available analgesics are not adequate to meet the clinical needs, leaving a large population with undertreated pain. Thus, there is a dire clinical need to develop novel and effective pharmacotherapies. A promising strategy is combination therapy, which requires combining two or more drugs as a pharmacotherapy and has been successfully practiced for treating various diseases including pain (Orrú et al., 2013; Smith, 2008). The overall aim of combination therapy is to increase the analgesic effectiveness of analgesics such as opioids and/or reduce unwanted effects as smaller doses of individual drugs may be needed (Smith, 2008; Gilron et al., 2013).
Accumulating evidence suggests that drugs that target imidazoline I2 receptors could be a novel class of analgesics, particularly for chronic pain (Ferrari et al., 2011; Li et al., 2014; Li and Zhang, 2012; Li and Zhang, 2011; Meregalli et al., 2012). Interestingly, several studies found that selective I2 receptor ligands can enhance the antinociceptive effects of the opioids morphine and tramadol in different animal models of acute nociception (Sanchez-Blazquez et al., 2000; Gentili et al., 2006; Li et al., 2011b), even under conditions that I2 receptor ligands alone have no significant effect (Sampson et al., 2012; Thorn et al., 2011). Two recent studies explored the interactions between I2 receptor ligands and morphine in animal models of persistent pain and found different results. In a rat model of postoperative pain, the novel I2 receptor ligand CR4056 enhanced the antinociceptive effects of morphine in a synergistic manner (Lanza et al., 2014). In another study, a simple additive interaction was observed between the selective I2 receptor ligand 2-BFI and morphine in a rat model of chronic inflammatory pain (Li et al., 2014). Numerous differences exist between the two studies (e.g., study drug, animal model, and dosing regimen) which preclude the generality of the findings. Nevertheless, these results suggest that I2 receptor ligands can enhance the antinociceptive effects of opioids and therefore may be suitable for combination therapy with opioids for pain treatment. However, these studies typically only use one I2 receptor ligand and recent evidence suggests that currently available I2 receptor ligands have important differences (Qiu et al., 2014; Qiu et al., 2015), thus more detailed parametric studies are needed in order to draw a decisive conclusion on this important issue. Moreover, because the nature of drug interactions for combination therapy is often determined by the amount of the individual drugs in the combination (Berenbaum, 1989), it is important to study multiple combinations with varying drug proportions.
This study utilized a quantitative analytical approach to examine the anti-hyperalgesic effects of selective I2 receptor ligands alone and in combination with the clinically widely used opioid oxycodone using the von Frey filament test in rats with complete Freund's adjuvant (CFA)-induced inflammatory pain. Dose-addition analysis was used to quantitatively examine the nature of the I2 ligand-oxycodone interactions in the same chronic pain assay. Furthermore, we examined the effects of I2 receptor ligands alone and in combination with oxycodone on food-maintained operant responding to rule out the possibility of behavioral competition with the observed antinociceptive effects.
METHODS
Subjects
Adult male Sprague-Dawley rats (n = 194) (Harlan, Indianapolis, IN) were housed individually on a 12/12-h light/dark cycle (behavioral experiments were conducted during the light period) with free access to water except during experimental sessions. All animals had free access to standard rodent chow in their home cages except those that were used in the study of food-maintained operant responding (n = 8). Rats in operant studies had restricted access to food and their body weights were maintained at 85% of their free-feeding body weights by adjusting the amount of standard rodent chow that was provided in the home cages after daily sessions. They also earned food pellets during the daily experimental sessions. Animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
Induction of inflammatory pain
Inflammatory pain was induced by CFA inoculation as previously described (Li et al., 2014). Briefly, 0.1 mL of CFA (Difco, Detroit, MI, USA) that contains 0.05 mg Mycobacterium butyricum dissolved in paraffin oil was injected in the right foot pad (hind paw) of the rats under isoflurane anesthesia (2% isoflurane mixed with 100% oxygen at a flow rate of 5 L/min). The level of anesthesia was assessed by loss of righting reflex. The rats serving as controls were injected with 0.1 mL of saline.
Mechanical hyperalgesia
Mechanical hyperalgesia was measured using the von Frey filament test consisting of calibrated filaments (North Coast Medical, Morgan Hill, CA) (1.4g-26g). Rats (n=6 per group) were placed in elevated plastic boxes with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA, USA) immediately before the test. The filaments were then applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor in an ascending order beginning with the lowest filament (1.4g). A particular filament was applied until buckling of the filament occurred and maintained for approximately 2s. Mechanical thresholds (expressed in g) corresponded to the lowest force that elicits a behavioral response (withdrawal of the hind paw) with at least two out of three applications. All drug tests were conducted 24 hr after CFA treatment because this time point represents the most significant hyperalgesia as demonstrated previously (Li et al., 2014). Measurements were taken immediately prior to drug administration and every 15min until the effect of the drug dissipated. When drugs were studied in combination, they were prepared in a mixture and administered as one injection. The experimenters were not blind to the treatments; however, they received extensive training with this procedure before the initiation of this study to ensure accurate judgment of paw withdrawal responses and minimize experimenter bias.
Schedule-controlled responding
The food-maintained operant responding experiments were conducted in commercially available chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA, USA). Chambers contained two response levers; responses on the inactive (right) lever were recorded and had no programmed consequence. Data were collected using Graphic State 3.03 software and an interface (Coulbourn Instruments Inc.). Rats were trained to press a lever for food under a multiple-cycle procedure. Each cycle began with a 10-min pretreatment period, during which the chamber was dark and responses had no programmed consequence, followed by a 5-min response period, during which a light above the active (left) lever was illuminated and rats could receive a maximum of 5 food pellets (45 mg dustless precision pellets; BioServ Inc., Frenchtown, New Jersey, USA) by responding on the active lever. Initially a single response produced a food pellet; as performance improved the response requirement was progressively increased across days to a final fixed ratio of 10. The light was terminated after delivery of 5 food pellets or after 5 min had elapsed, whichever occurred first. Daily sessions consisted of 5 cycles and rats had to satisfy the following criteria for five consecutive sessions before testing began: the daily response rate, averaged across all 5 cycles within a session, did not vary by more than ± 20% of the average daily response rate of the previous 5 training sessions; and the average response rate among the 5 cycles of a daily session did not vary by more than ± 20% (An et al., 2012). After the first test, all tests were preceded by at least two consecutive training sessions that satisfied the same criteria. During testing, rats received a single injection of either a drug alone or drugs in combination within the first minute of the first cycle.
Data analyses
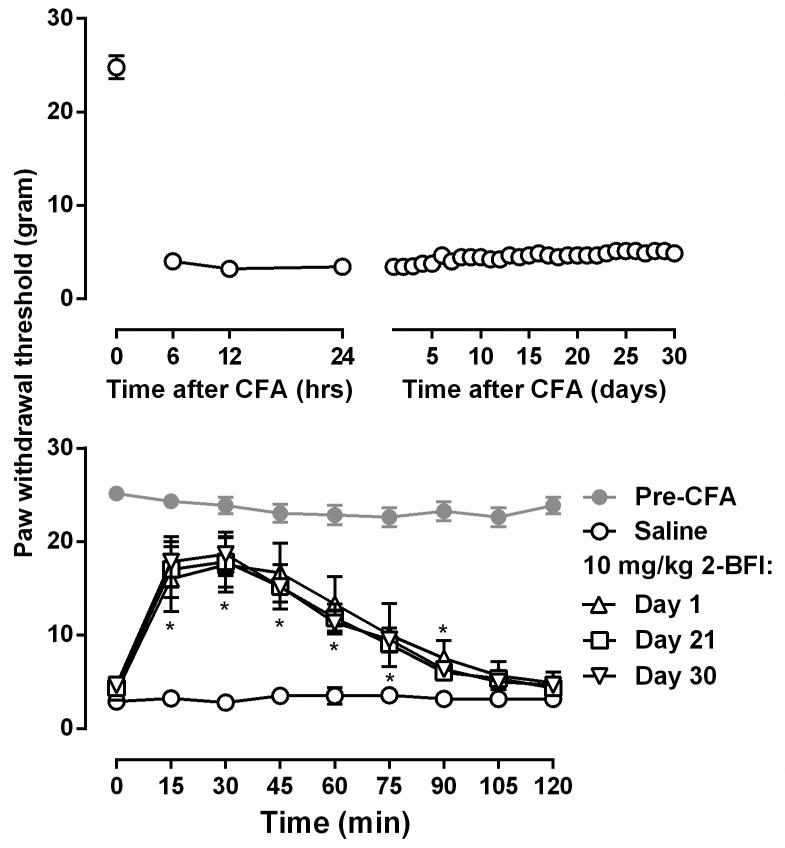
CFA-induced mechanical hyperalgesia is presented as paw withdrawal threshold (PWT) in grams. The mean values (±SEM) were calculated from individual animals and two-way analysis of variance (ANOVA) (time × treatment) followed by post hoc Bonferroni's test was used to determine statistical significance. All data from drug studies were compared with the saline control group (Fig. 1). P < 0.05 was considered statistically significant for all tests.
Figure 1.
Duration of mechanical hyperalgesia in rats after CFA treatment (upper) and the anti-hyperalgesic effects of repeated 10 mg/kg 2-BFI treatment (lower). Ordinates, paw withdrawal threshold (grams) measured by von Frey filaments; Abscissa, time (upper: hr and days; lower: min) following injection of treatment drug. Asterisks indicate significantly different from saline treatment condition.
To construct the dose-effect curves of the drugs and drug combinations, maximal effect of each dose was used (i.e., 30 min post-treatment for oxycodone, 2-BFI, phenyzoline and all combinations). Then, anti-hyperalgesic effects of the drugs studied were quantified for each animal as % maximal possible effect (MPE) for each drug dose by using the following formula: % MPE = [(post-drug value for a behavioral response (g) - pre-drug value for a behavioral response) / (pre-CFA value – pre-drug value for a behavioral response) × 100 and analyzed by log-linear regression using Prism 6.0 (GraphPad Software Inc., San Diego, CA) with the following equation: effect = slope × log (dose) + intercept. When appropriate, ED50 (± 95% confidence limits [CL]) values were estimated from the % MPE of each drug by log-linear regression.
For the study that examined the interactions between 2-BFI or phenyzoline and oxycodone, a fixed ratio dose-addition analysis method was used as described previously (An et al., 2012; Li et al., 2011a,b; Li et al., 2014). For this analysis, two drugs were combined in fixed ratios (1:3, 1:1 and 3:1) and administered as one dose of combination drugs per test. The actual doses of the individual drugs were determined based on the relative potency of the drugs and the fixed ratios used. The dose-effect curves of the drug combinations were constructed using maximal effects of each dose combination as described above. Because different doses were studied in separate groups of animals, group mean values were used for dose addition analysis as previously described (Tallarida, 2000). For drug combinations, the dose-response curves were determined and the individual ED50 values of the two drugs in the combination were calculated based upon the shared dose–response curves. The sum of the ED50 values of both drugs in the combination was defined as Zmix. The experimentally determined (actual) ED50 values (Zmix) were compared with the expected additive ED50 values (Zadd) and were considered significantly different when the 95% CL did not overlap. Zadd and Zmix values were calculated using Pharm Tools Pro version 1.1 for Windows (The McCary Group Inc., Elkins Park, PA, USA) based on the procedures described previously (Tallarida, 2000). Three fixed ratios (1:3, 1:1 and 3:1) were used in the current study. The slopes and the differences of the experimentally determined composite additive curves and the predicted composite additive curves were compared using the F-test. A non-significant F ratio for slopes and a significant F ratio for intercept show that dose–effect curves are parallel but occupy different positions on the dose axis (Koek et al., 2009). A significant leftward shift of the experimentally determined composite additive curve indicates that the drug combination produces the effect in a manner that is greater than additivity (supra-additivity) (i.e. in the presence of one drug, smaller than predicted doses of a second drug are needed to produce the same effect).
Rate of operant responding is expressed as a percentage of the control response rate. For each drug or saline test, the control response rates for individual rats were the response rates of the no drug sessions immediately prior to the test session. These percentages were averaged across 8 rats (± SEM) and plotted as a function of dose. The percentage of control response rate data was analyzed using one-way ANOVA followed by post hoc Bonferroni's test. Because the antinociceptive effects of the drugs and drug combinations reached maximum 30 min after drug administration, only data from the second cycle of each test session (25-30 min after drug administration) were presented for comparison.
Drugs
2-BFI hydrochloride and phenyzoline oxalate were synthesized according to standard procedures (Jarry et al., 1997; Ishihara and Togo, 2007). Oxycodone hydrochloride was provided by Research Technology Branch, National Institute on Drug Abuse, National Institutes of Health (Rockville, MD, USA). All drugs were dissolved in physiological saline and administered i.p. Doses are expressed as mg per kg body weight. Injection volumes were 1 ml/kg.
RESULTS
Under control conditions, rats lifted their hind paws when the force of the filament applied to the hind paw increased to nearly 26g. However, CFA injection markedly decreased the PWT to nearly 3g. Such a significant mechanical hyperalgesia sustained for at least 30 days with little variance (Fig. 1). A dose of 10 mg/kg 2-BFI significantly increased the PWT and the effect lasted for 90 min. In order to examine whether the course of hyperalgesia testing impacts the antinociceptive response of animals to 2-BFI, the same dose of 2-BFI (10 mg/kg) was re-tested 21 and 30 days after CFA treatment in the same group of rats. The antinociceptive effects of 2-BFI did not show significant differences among the frequency of tests and showed similar duration of action (Fig. 1). Two way ANOVA revealed significant time × test interactions (F [24, 120] = 6.66, P < 0.0001) and post hoc analyses found that 10 mg/kg 2-BFI significantly increased PWT between 15 and 90 min after drug administration. Because it seems that the course of hyperalgesia testing does not impact the effects of 2-BFI, all following experiments used 24 hr after CFA administration as the time of test.
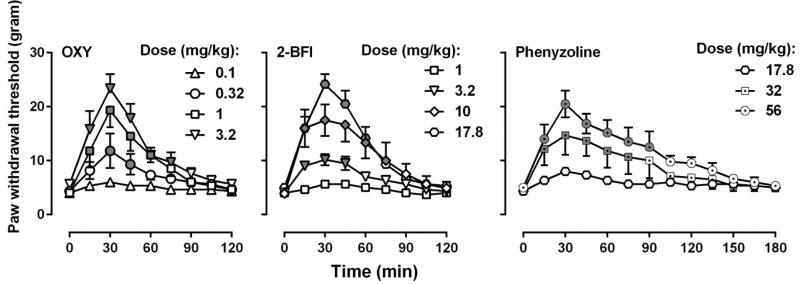
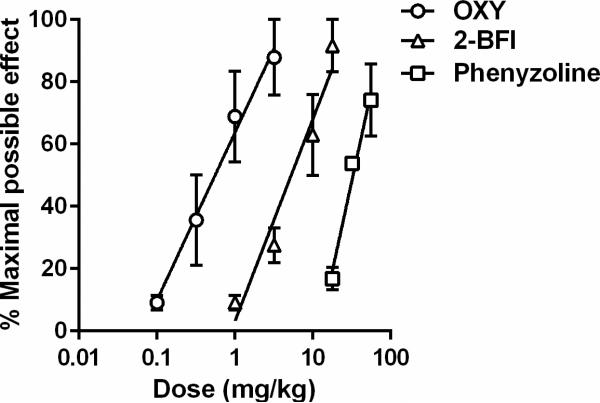
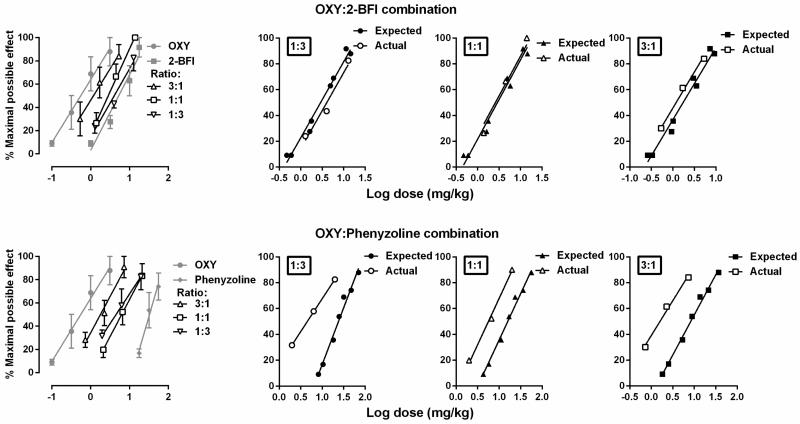
Oxycodone dose-dependently increased the PWT (two-way ANOVA: dose × time interactions (F [32, 160] = 5.79, P < 0.0001)) (Fig. 2). Post hoc analysis indicated that the dose of 1 and 3.2 mg/kg oxycodone produced significant effects 15-60 min following injection, and the dose of 0.32 mg/kg produced significant effects 30-45 min following injection as compared with vehicle. 2-BFI also dose-dependently increased the PWT (two-way ANOVA: dose × time interactions (F [32, 160] = 15.34, P < 0.0001)). Post hoc analysis indicated that the dose of 17.8 and 10 mg/kg 2-BFI produced significant effects 15-75 min following injection, and the dose of 3.2 mg/kg produced significant effects 15-45 min following injection as compared with vehicle. Similarly, phenyzoline also dose-dependently increased the PWT in CFA-treated rats (two-way ANOVA: dose × time (F [24, 120] = 3.82, P < 0.0001)). Post hoc analysis indicated that the dose of 56 mg/kg phenyzoline produced significant effects 15-90 min following injection, and the dose of 32 mg/kg produced significant effects 15-75 min following injection as compared with vehicle. The dose-effect curves of all the drugs studied were presented as MPE% in Fig. 3 and the test of parallelism showed that all the curves were not significantly deviate from parallelism. The ED50 (95% CL) values of the drugs were: oxycodone (0.50 [0.31, 0.83] mg/kg), 2-BFI (6.48[4.22, 8.08] mg/kg), and phenyzoline (32.73 [24.92, 42.28] mg/kg). Thus, the potency order of these compounds were: oxycodone > 2-BFI > phenyzoline (Fig. 3). The calculated potency ratio between 2-BFI and oxycodone was 12.98 and that between phenyzoline and oxycodone was 65.46. In all the following combination studies, these potency ratios were used to calculate the respective doses of 2-BFI and phenyzoline in the drug combination when the doses of oxycodone were given. For example, under the fixed ratio of 1:1, a dose of 0.10 mg/kg oxycodone and a dose of 1.30 mg/kg phenyzoline were administered as a mixture (upper middle panel, Fig. 4).
Figure 2.
Anti-hyperalgesic effects of oxycodone, 2-BFI, and phenyzoline on mechanical hyperalgesia in CFA-treated rats. For grayed symbols, all the data points were significantly different from vehicle treatment as analyzed by two-way ANOVA and post hoc Bonferroni's analysis (n=6 per group). OXY, oxycodone. See Fig. 1 for other details.
Figure 3.
Percent maximal possible effects of oxycodone and I2 receptor ligands. Ordinates, percentage of maximal possible effects; Abscissa, drug doses (mg/kg) expressed as log units.
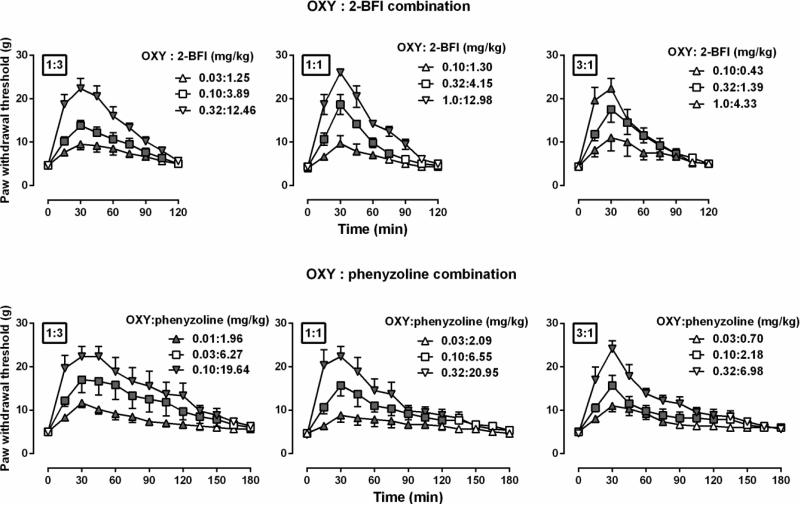
Figure 4.
Anti-hyperalgesic effects of the combined oxycodone-2-BFI (upper) and oxycodone-phenyzoline (lower) at different fixed ratios (left, 1:3; middle, 1:1; right, 3:1) in rats (n=6 per group). Symbol legends were the actual combined doses for each test. See Fig. 2 for other details.
When oxycodone was studied in combination with 2-BFI or phenyzoline under fixed ratios of 1:3, 1:1 and 3:1, the drug combinations dose-dependently increased the PWT (Fig. 4). In each panel, the filled symbols indicated significantly increased PWT as compared with vehicle control condition. For example, at the fixed ratio of 1:3 (top left panel, Fig. 4), two way ANOVA revealed significant time × dose interactions (F [24, 120] = 9.77, P < 0.0001). Post hoc analyses found that at a combined dose of 0.03 mg/kg oxycodone and 1.25 mg/kg 2-BFI (1: 3 ratio) the PWT was significantly increased 15-90 min following drug injection. The dose-effect curves of the individual drugs and drug combinations were re-constructed as shown in Fig. 5 (left panels). The combined dose-effect curves were positioned between the curves of the two individual component drugs. Generally, the higher ratio of the more potent drug was in the drug combination, the closer the dose-effect curve of the drug combination was positioned to the curve of the more potent drug when studied alone. Composite additive curve analyses were presented at the right panels of Fig. 5. For the combination of oxycodone and 2-BFI, the expected dose-effect curves of the combination were not significantly different from the experimentally-determined (actual) dose-effect curves (slopes and intercepts were not significantly different) under all fixed ratios, suggesting that the interaction between oxycodone and 2-BFI was additive (top right, Fig. 5). In contrast, for the combination of oxycodone and phenyzoline, the expected dose-effect curves of the combination were significantly different from the experimentally-determined curves under all the three fixed ratios: (F [1, 6] = 38.15, P < 0.01) for 1:3 ratio, (F [1, 6] = 186.80, P < 0.0001) for 1:1 ratio and (F [1, 6] = 326.30, P < 0.0001) for 3:1 ratio. These results were consistent with the comparison of the ED50 (95% CL) values between the expected and experimentally-determined conditions (Table 1). Thus, the experimentally-determined dose-effect curves of the oxycodone-phenyzoline combination were shifted 5.30-fold, 2.81-fold and 4.26-fold to the left of the expected curves, suggesting synergistic interaction (Table 1).
Figure 5.
Dose-addition analyses of the combined antinociceptive effects of oxycodone-2-BFI (upper) and oxycodone-phenyzoline (lower) in rats. Left: Dose-effect curves of the individual drugs and drug combinations. Right: composite additive curves of the expected and experimentally-determined (actual) antinociceptive effects at different fixed ratios.
Table 1.
Expected additive ED50 values (Zadd), actual (experimentally determined) ED50 values (Zmix) and, and the ratio of expected/actual ED50 values for drug combinations for antinociception in rats.
| Combination Relative dose (ratio) | Zadd (95% CL) | Zmix (95% CL) | Ratio Zadd/Zmix |
|---|---|---|---|
| OXY:2-BFI | |||
| 1:3 | 4.10 (3.42, 4.78) | 4.04 (3.01, 5.29) | 1.01 |
| 1:1 | 2.92 (2.47, 3.37) | 2.83 (1.63, 4.92) | 1.03 |
| 3:1 | 1.74 (1.51, 1.97) | 1.18 (0.95, 1.46) | 1.47 |
| OXY:phenyzoline | |||
| 1:3 | 23.85 (21.25, 26.79) | 4.50 (3.76, 5.27)* | 5.30 |
| 1:1 | 15.90 (14.43, 17.53) | 5.65 (4.87, 6.54)* | 2.81 |
| 3:1 | 8.63 (8.04, 9.22) | 1.18 (0.94, 1.46)* | 4.26 |
The 95% CL of Zmix did not overlap with the 95% CL of Zadd. OXY, oxycodone.
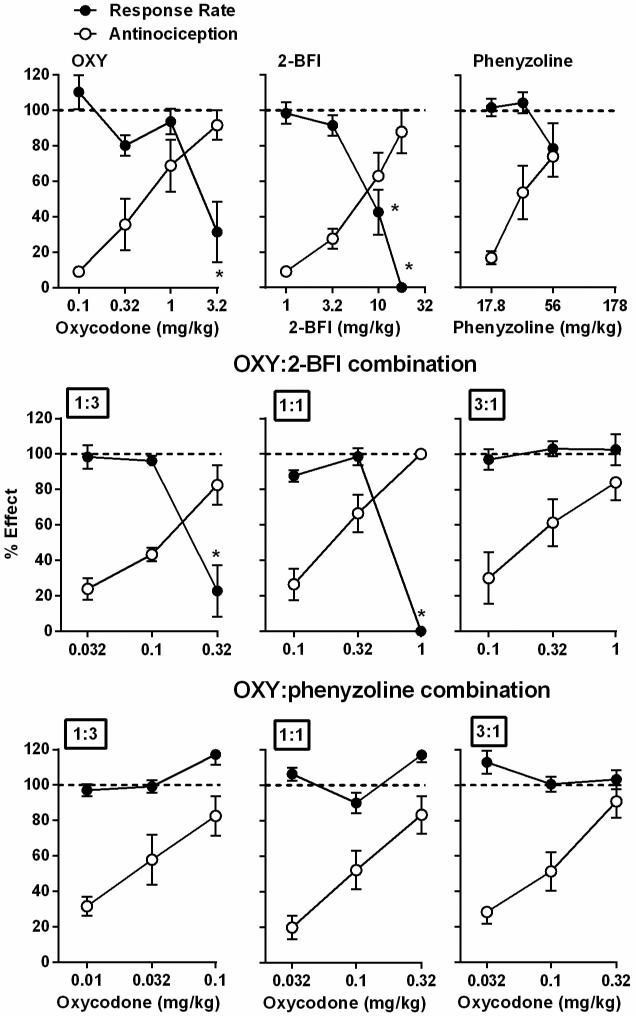
When studied alone, phenyzoline failed to produce statistically significant effects on the rate of responding under a fixed ratio 10 schedule of food presentation within the dose range that produced antinociception (Fig. 6). However, oxycodone and 2-BFI dose-dependently decreased responding rate (one-way ANOVA: F [F [4, 28] = 8.78, P < 0.01 for oxycodone and F [F [4, 28] = 32.59, P < 0.001). Post hoc analysis indicated that oxycodone produced a significant effect at the dose of 3.2 mg/kg and 2-BFI produced significant effects at doses of 10 and 17.8 mg/kg 2-BFI. When studied in combination, the fixed ratio of 1:3 oxycodone-2-BFI combination significantly decreased response rate (one-way ANOVA: F [3, 21] = 18.56, P < 0.001) and post hoc analysis revealed statistical significance at the dose of 0.32 mg/kg oxycodone in the presence of 2-BFI. The fixed ratio of 1:1 oxycodone-2-BFI combination dose-dependently decreased response rate (one-way ANOVA: F [3, 21] = 207.90, P < 0.0001) and post hoc analysis revealed statistical significance at the dose of 1 mg/kg oxycodone in the presence of 2-BFI. However, the fixed ratio of 3:1 oxycodone-2-BFI combination failed to significantly alter response rate. In contrast, the oxycodone-phenyzoline combination failed to decrease the rate of responding in rats under all studied ratios (Fig. 6).
Figure 6.
Effects of oxycodone, 2-BFI, phenyzoline and drug combinations on rate of food-maintained responding (filled circles) in rats responding under a fixed ratio 10 schedule of food presentation. The anti-hyperalgesic effects (open circles) of these drugs were also plotted as a comparison. Ordinate, percentage effect in correspondence to percentage of control responding rate (filled circles) or percentage of maximal possible effect (open circles); Abscissa, dose of drug (milligrams per kilogram). * P < 0.05 as compared to vehicle responding rate.
DISCUSSION
The primary findings of the current study were that both selective imidazoline I2 receptor ligands produced significant antinociceptive effects in a commonly used rat model of chronic inflammatory pain. In addition, the combination of I2 receptor ligands 2-BFI with the opioid oxycodone produced additive while the combination of phenyzoline with oxycodone produced supra-additive (synergistic) interactions for their antinociceptive effects. Importantly, phenyzoline demonstrated superior synergistic interactions with oxycodone for antinociception at doses that had no effects on food-maintained operant behaviors. These results substantially extend previous findings and further support the notion that I2 receptor ligands may be valuable novel analgesics for pain management, either used alone or in combination with other analgesics (e.g., opioids).
CFA treatment is a well characterized model of chronic inflammatory pain. When CFA is injected into the hind paw of an animal, long-lasting inflammation develops accompanying mechanical and thermal hyperalgesia that lasts for weeks (Nagakura et al., 2003). Consistent with the literature and our previous findings (Li et al., 2014), we found that CFA injection produced marked mechanical hyperalgesia as measured using the von Frey filament test that lasted for at least 30 days. In our previous studies (Li et al., 2014) the drug effects were examined 24 hr after CFA treatment. One can argue that the pain remains in the acute phase and may not model the chronicity of clinical inflammatory pain such as arthritis. To rule out the possibility that the course of pain might impact the effects of analgesics, we tested the same dose of 10 mg/kg 2-BFI, a dose that has previously been shown to have significant anti-hyperalgesic effect (Li et al., 2014), to see whether the effect of 2-BFI changes over time. No change was observed. Thus, it is clear that 2-BFI has marked antinociceptive action for chronic inflammatory pain. Thus, we kept the pain test at 24 hr after CFA administration for all the following studies, consistent with a previous report (Li et al., 2014).
Oxycodone, 2-BFI and phenyzoline all dose-dependently produced anti-hyperalgesic effects (Fig. 1). The effectiveness of the I2 receptor ligands was similar to that of oxycodone. However, the potencies and duration of action differed between the drugs tested. All drugs produced significant antinociception within the first 15 min following drug injection. In addition, the I2 receptor ligands both produced effects lasting longer than that of oxycodone. The I2 receptor ligands do not have bona fide anti-inflammatory effect as repeated 2-BFI treatment does not decrease the paw thickness in CFA-treated rats (Li et al., 2014). In contrast, intrathecal administration of BU224, another I2 receptor ligand, reduces the nociceptive responses of dorsal horn neurons and inhibits C-fiber evoked responses, suggesting that I2 receptor ligands may exert their anti-hyperalgesic effect by directly acting on the pain processing pathway (Diaz et al., 1997). Overall, these results are consistent with and substantially extend our previous studies to strongly suggesting that pharmacologically modulating (activating) I2 receptors are effective in pain management and that I2 receptor ligands may be a novel class of efficacious analgesics.
In previous studies, we reported that the selective I2 receptor ligand 2-BFI significantly enhances the antinociceptive effects of morphine in a warm water tail withdrawal procedure (Thorn et al., 2011) and a 5% hypertonic saline-induced writhing test in a supra-additive manner (Li et al., 2011b). Furthermore, we reported that 2-BFI enhances the antinociceptive effects of morphine in a model of chronic pain in an additive manner (Li et al., 2014). However, two other studies reported that the I2 receptor ligand CR4056 enhances the antinociceptive effects of morphine in a supra-additive manner in rat models of capsaicin-induced mechanical hyperalgesia and postoperative pain (Ferrari et al., 2011; Lanza et al., 2014). It was unclear of the cause of the discrepancy, as these studies used different I2 receptor ligands (2-BFI vs. CR4056), different models of pain (CFA-induced inflammatory pain vs. capsaicin-induced pain or postoperative pain), different routes of drug administration (i.p. vs. oral), and different dosing protocols (cumulative dosing vs. single dosing). It is also unclear whether I2 receptor ligands also enhance the antinociceptive effects of opioids other than morphine, such as oxycodone, in a chronic pain model. Thus, this study was designed to use the same model of pain to examine the antinociceptive effects of two I2 receptor ligands alone or in combination with the opioid oxycodone.
Dose addition analysis is a powerful approach to systematically examine the drug interactions when both drugs produce similar pharmacological effects (Tallarida, 2000). It is clear that when studied in combination, the I2 receptor ligand 2-BFI produced additive interaction with oxycodone for decreasing mechanical hyperalgesia. This is consistent with our previous study which showed that 2-BFI and morphine produce additive interaction when both drugs are used at a fixed ratio of 1:1 (Li et al., 2014) and also extends to that the additive interaction holds true across a broad range of drug proportions. However, the combination also significantly decreased operant responding at a dose that produced near maximal antinociceptive effect at ratios of 1: 3 and 1:1, suggesting the possibility that the observed effects of paw withdrawal suppression might be partially attributed to nonspecific behavioral suppression. Interestingly, operant suppression was not observed at a fixed ratio of 3:1, suggesting that this ratio may achieve additive analgesia with less untoward effects and could be an optimal combination for the management of pain.
Although both 2-BFI and phenyzoline are highly selective for I2 receptors and both produce similar hypothermic effects that are sensitive to idazoxan blockade (Nutt et al., 1995; Thorn et al., 2012; Qiu et al., 2015), they have important differences under certain conditions. For example, in rats discriminating 10 mg/kg CR4056, a novel selective I2 receptor ligand, phenyzoline fully while 2-BFI partially substitute for CR 4056 (Qiu et al., 2014). In rats discriminating 32 mg/kg phenyzoline, 2-BFI only partially substitutes for phenyzoline (Qiu et al., 2015). More importanly, phenyzoline did not show significant substitution for 2-BFI in rats discriminating 5.6 mg/kg 2-BFI (unpublished), suggesting that the pharmacological mechanisms mediating the discrimiantive stimulus effects of 2-BFI and phenyzoline are different. These are examples that the commonly used selective I2 receptor ligands as defined by in vitro receptor binding assays are different when tested in in vivo assays such as drug discrimination (Qiu et al., 2015). Given the different discriminative stimulus effects between 2-BFI and phenyzoline, testing their antinociceptive effects and their interactions with oxycodone could provide a new perspective to understand how I2 receptor components function. Both phenyzoline and 2-BFI only have weak antinociceptive effect in a rat warm water tail withdrawal test (Sampson et al., 2012). Interestingly, oxycodone and phenyzoline produced supra-additive interactions for antinociception at all the proportions studied, which is different from the interactions between 2-BFI and oxycodone. The I2 receptors do not exist as a homogeneous receptor. I2 receptors as identified by [3H] 2-BFI or [3H] idazoxan binding are highly heterogeneous and consist of multiple proteins (Escriba et al., 2009; Regunathan and Reis, 1996). Current study and previous drug discrimination studies (Qiu et al., 2014; Qiu et al., 2015) increasingly suggest that the frequently used selective I2 receptor ligands have important functional differences, which may be due to their different binding activities on the multiple components of the binding sites that are collectively named I2 receptors. Very few studies have systematically compared the different I2 receptor ligands in in vivo functional assays. Such studies are needed and are important as they can eventually disentangle the respective functions of the multiple components of I2 receptors, which may lead to component-selective I2 receptor ligands with preferable therapeutic (e.g., analgesic and antidepressant) effects and good safety profiles. Although the discrepancy between 2-BFI and phenyzoine for their interactions with oxycodone is unclear, a parsimonious interpretation is that both drugs have different binding and functional (activate or inhibit) activities on the multiple components of I2 receptors, with certain components producing different antinociceptive activities. This speculation seems consistent with the fact that both CR4056 and phenyzoline share similar discriminative stimulus effects (Qiu et al., 2014) and produce antinociceptive synergy with opioids (Meregalli et al., 2012; current study) while 2-BFI does not. Regardless, the interaction between opioids and I2 receptor ligands does not seem to be a pharmacokinetic interaction, as 2-BFI attenuates the development of tolerance to morphine antinociception in a tail flick test (Boronat et al., 1998) and we also found that 2-BFI and BU224 attenuated the discriminative stimlus effects of morphine (unpublished).
Schedule-controlled responding was also examined using the doses of drugs alone or in combination that produced antinociception. When studied alone, phenyzoline did not alter the rate of responding for food (Fig. 6). Oxycodone and 2-BFI decreased the responding rate at higher doses (current study; An et al., 2012). However, it is important to note that the dose of 3.2 mg/kg produced antinociception with no effect on response rate. When studied as combinations, the 1:3 and 1:1 fixed ratios of oxycodone-2-BFI combination decreased response rate at the highest combination doses tested. Similar to when 2-BFI was studied alone, the intermediate dose of these combinations produced significant antinociception while lacking effects on response rate. In contrast, the combination of oxycodone and phenyzoline did not produce significant alterations in rate of responding for food with the doses that produced antinociception. These results suggest that the antinociceptive effects of drugs alone or in combination in this study are behaviorally specific.
In summary, this study found that selective imidazoline I2 receptor ligands have marked antinociceptive effects and also significantly enhanced the antinociceptive effects of oxycodone in a rat model of chronic inflammatory pain. Dose addition analysis indicates that the interactions between I2 receptor ligands and oxycodone are highly dependent on the proportions of the individual drugs used in the combination studies. Because phenyzoline enhanced the antinociceptive effects of oxycodone supra-additively within a broad range of proportions (ranging from 25% [1:3] to 75% [3:1]), these results suggest that phenyzoline may represent a more preferable combination therapy candidate with opioids to treat pain. Taken together, these results support the therapeutic potential of combining imidazoline I2 receptor ligands with opioids for pain treatment.
Acknowledgements
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award no. R01DA034806) and by a grant from National Natural Science Foundation of China (81373390). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
David A. Thorn, Department of Pharmacology and Toxicology, University at Buffalo, Buffalo, New York, USA
Justin N. Siemian, Department of Pharmacology and Toxicology, University at Buffalo, Buffalo, New York, USA
Yanan Zhang, Research Triangle Institute, Research Triangle Park, North Caroline, USA.
Jun-Xu Li, Department of Pharmacology and Toxicology, University at Buffalo, Buffalo, New York, USA.
References
- An XF, Zhang Y, Winter JC, Li JX. Effects of imidazoline I(2) receptor agonists and morphine on schedule-controlled responding in rats. Pharmacol Biochem Behav. 2012;101(3):354–359. doi: 10.1016/j.pbb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41(2):93–141. [PubMed] [Google Scholar]
- Boronat MA, Olmos G, Garcia-Sevilla JA. Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands. Br J Pharmacol. 1998;125:175–185. doi: 10.1038/sj.bjp.0702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Mayet S, Dickenson AH. BU224 produces spinal antinociception as an agonist at imidazoline I2 receptors. Eur J Pharmacol. 1997;333:9–15. doi: 10.1016/s0014-2999(97)01118-7. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Pharmacologic characterization of imidazoline receptor proteins identified by immunologic techniques and other methods. Ann N Y Acad Sci. 1999;881:8–25. doi: 10.1111/j.1749-6632.1999.tb09336.x. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili F, Cardinaletti C, Carrieri A, Ghelfi F, Mattioli L, Perfumi M, Vesprini C, Pigini M. Involvement of I2-imidazoline binding sites in positive and negative morphine analgesia modulatory effects. Eur J Pharmacol. 2006;553(1-3):73–81. doi: 10.1016/j.ejphar.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12(11):1084–1095. doi: 10.1016/S1474-4422(13)70193-5. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (U.S.) Relieving pain in America: A blueprint for transforming prevention, care, education and research. National Academies Press; Washington, D.C.: 2011. [PubMed] [Google Scholar]
- Ishihara M, Togo H. Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine. Tetrahedron. 2007;63(6):1474–1480. [Google Scholar]
- Jarry C, Forfar I, Bosc J, Renard P, Scalbert E, Guardiola B. 5-(Arloxymethyl) oxazoline. 1997 US Patent 5,686,477, Adir eCompagnie.
- Koek W, Mercer SL, Coop A, France CP. Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl(diethoxymethyl) phosphinic acid (CGP35348) J Pharmacol Exp Ther. 2009;330(3):876–883. doi: 10.1124/jpet.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol. 2014;171(15):3693–3701. doi: 10.1111/bph.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Crocker C, Koek W, Rice KC, France CP. Effects of serotonin 5-HT1A and 5-HT2A receptor agonists on schedule-controlled responding in rats: drug combination studies. Psychopharmacology (Berl) 2011a;213(2-3):489–497. doi: 10.1007/s00213-010-2136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Anti-hyperalgesic effects of imidazoline I2 receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171(6):1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658(2-3):49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Emerging drug targets for pain treatment. Eur J Pharmacol. 2012;681(1-3):1–5. doi: 10.1016/j.ejphar.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur J Pharmacol. 2011b;669(1-3):59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–167. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306(2):490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, French N, Handley S, Hudson A, Husbands S, Jackson H, Jordan S, Lalies MD, Lewis J, Lione L, Mallard N, Pratt J. Functional studies of specific imidazoline-2 receptor ligands. Ann New York Acad Sci. 1995;763:125–139. doi: 10.1111/j.1749-6632.1995.tb32397.x. [DOI] [PubMed] [Google Scholar]
- Orrù A, Marchese G, Casu G, Casu MA, Kasture S, Cottiglia F, et al. Withania somnifera root extract prolongs analgesia and suppresses hyperalgesia in mice treated with morphine. Phytomedicine. 2014;21(5):745–752. doi: 10.1016/j.phymed.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Qiu Y, He XH, Zhang Y, Li JX. Discriminative stimulus effects of the novel imidazoline I2 receptor ligand CR4056 in rats. Sci Rep. 2014;4:6605. doi: 10.1038/srep06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhang Y, Li JX. Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. Eur J Pharmacol. 2015;749:133–141. doi: 10.1016/j.ejphar.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan S, Reis DJ. Imidazoline receptors and their endogenous ligands. Annu Rev Pharmacol Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- Sampson C, Zhang Y, Del Bello F, Li JX. Effects of imidazoline I2 receptor ligands on acute nociception in rats. Neuroreport. 2012;23(2):73–77. doi: 10.1097/WNR.0b013e32834e7db3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Boronat MA, Olmos G, Garcia-Sevilla JA, Garzon J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmacol. 2000;130(1):146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS. Combination opioid analgesics. Pain Physician. 2008;11(2):201–214. [PubMed] [Google Scholar]
- Tallarida RJ. The composite additive curve. In: Tallarida RJ, editor. Drug synergism and dose-effect data analysis. Chapman & Hall; CRC Boca Raton: 2000. pp. 77–89. [Google Scholar]
- Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX. Effects of imidazoline I2 receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur J Pharmacol. 2011;670(2-3):435–440. doi: 10.1016/j.ejphar.2011.09.173. [DOI] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I2 receptor agonists in rats. Br J Pharmacol. 2012;166(6):1936–1945. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]