Abstract
Natural killer (NK) cells are regulated killer immunoglobulin-like receptor (KIR) interactions with HLA class I ligands. Several models of NK reactivity have been associated with improved outcomes following myeloablative allogeneic hematopoietic cell transplantation (HCT), but this issue has not been rigorously addressed in reduced-intensity conditioning (RIC) unrelated donor (URD) HCT. We studied 909 patients undergoing RIC-URD HCT. Patients with acute myeloid leukemia (AML, n=612) lacking ≥1 KIR ligands experienced higher grade III–IV acute graft-vs.-host disease (GvHD) (HR 1.6, 95%CI 1.16–2.28, p=0.005) compared to those with all ligands present. Absence of HLA-C2 for donor KIR2DL1 was associated with higher grade II–IV (HR 1.4, p=0.002) and III–IV acute GvHD (HR 1.5, p=0.01) compared to HLA-C2+patients. AML patients with KIR2DS1+, HLA-C2 homozygous donors had greater treatment-related mortality compared to others (HR 2.4, 95%CI 1.4–4.2, p=0.002), but did not experience lower relapse. There were no significant associations with outcomes for AML when assessing donor activating KIRs or centromeric KIR content, nor for any donor-recipient KIR-HLA assessments in patients with myelodysplastic syndrome (n=297). KIR-HLA combinations in RIC-URD HCT recapitulate some but not all KIR-HLA effects observed in myeloablative HCT.
Introduction
Disease relapse is a significant cause of treatment failure after allogeneic hematopoietic cell transplantation (HCT). With reduced-intensity conditioning (RIC) approaches, in particular, the graft-versus-leukemia (GVL) effect is critical for successful outcomes in patients with advanced myeloid malignancies. Therefore, strategies to optimize conditions for achieving a GVL effect will improve outcomes of RIC HCT.
The GVL effect has been attributed to donor-derived alloreactive immune cells including T-lymphocytes and natural killer (NK) cells1–4. The function of NK cells is regulated by inhibitory and activating signals mediated through cell-surface receptors including killer immunoglobulin-like receptors (KIRs). HLA-C is the main ligand for most inhibitory KIRs and is categorized into C1 and C2 groups based on a polymorphism at residue 80 in the HLA molecule5. Inhibitory KIR2DL2 and KIR2DL3 are specific for the C1 ligand group, and inhibitory KIR2DL1 is specific for the C2 ligand group. The inhibitory KIR3DL1 receptor is specific for HLA molecules with the HLA-Bw4 epitope6.
When inhibitory KIR encounter self-HLA class I ligands on target cells, they signal inhibition and establish tolerance4, 7, 8. In contrast, lack of HLA class I ligand engagement of inhibitory KIR in the context of simultaneous activation signaling permits NK activation and target cell cytotoxicity. NK alloreactivity due to lack of self-class I ligand (“missing self”) is evident in HLA-mismatched allogeneic HCT, the clinical setting in which potent anti-leukemic effects of donor NK cells first became recognized3. Similarly, in HLA-matched HCT, lack of class I ligand in the recipient for donor inhibitory KIR (“missing ligand”) can also result in lower AML relapse following HCT 9, 10.
Stimulation of specific activating KIR can result in NK cell killing4, 11. Donor activating KIR genotype has been reported to influence post-transplant outcomes including grade II-IV acute GVHD, transplant-related mortality, relapse-free survival and overall survival 12–15. Donor KIR2DS1 has been associated with a lower relapse of AML after allogeneic HCT in an HLA-C1-dependent manner15. KIR group B haplotypes16, 17, enriched for stimulatory KIR genes, have been reported to be associated with less relapse and improved survival compared to KIR A-haplotypes, enriched for inhibitory genes, in AML patients undergoing unrelated donor HCT 18. This association was strongest for activating genes located in the centromeric (cen) region of the KIR gene complex (i.e., cen-B homozygosity). Interestingly, effects of the activating KIR are strongest in patients with HLA-C1 ligand15, 19.
Donor HLA ligands are also important in NK cell licensing. Lack of donor HLA ligand for cognate inhibitory KIR has been associated with adverse survival due to disease progression after URD HCT20.
KIR-HLA interactions have been reported to influence outcomes of myeloablative haploidentical 3, 21, HLA-matched related 10, 22 and unrelated donor 9, 23, 24 allogeneic HCT, particularly for AML patients. KIR-mediated effects may also influence outcomes after RIC for haploidentical and umbilical cord blood transplants, although studied cohorts were small25, 26. In RIC allogeneic HCT, where both donor and recipient hematopoiesis may coexist, the effect of KIR-HLA interactions on outcomes is not clear. We therefore examined the various models of NK cell alloreactivity and their associations with post-transplant outcomes among a large cohort of 909 AML and MDS patients receiving an allograft from a 7/8 or 8/8 HLA-matched URD following RIC. We find that specific donor-recipient KIR-HLA combinations are associated with post-transplant outcomes, including some but not all previously observed in myeloablative HCT.
Patients and Methods
Study Design
This retrospective study was designed to test the hypothesis that donor-recipient KIR-HLA interactions are associated with improved post-transplant outcomes following RIC-URD allogeneic HCT for AML and MDS. Clinical data was provided by the Center for International Blood and Marrow Transplant Research (CIBMTR).
Patients and Donors
The study population included all AML and MDS patients reported to the CIBMTR who received RIC allogeneic HCT from 1999 to 2007 from unrelated donors matched at 7 or 8 of the possible 8 alleles at HLA-A, -B, -C, and -DR loci and who had samples stored in the National Marrow Donor Program (NMDP) Research Repository. Transplant conditioning regimen intensity was determined according to the CIBMTR RIC Regimen Workshop criteria27. Some patients had in vivo T cell depletion with either anti-thymocyte globulin or alemtuzumab.
HLA and KIR Genotyping
Human leukocyte antigen genotyping for patients and donors was performed through the ongoing NMDP retrospective typing program as previously described28. KIR genotyping was performed retrospectively for donors through the NMDP contract laboratory network using commercially available KIR genotyping kits (Invitrogen, Canoga Park, CA and One Lambda, Grand Island, NY) and/or DNA sequencing29.
Data Sources
The CIBMTR is a combined research program of the Medical College of Wisconsin and the NMDP. CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a centralized statistical center. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule. Additional details regarding the data source are as previously described30.
Study Endpoints
The study endpoints included acute and chronic graft-vs.-host disease (GvHD), relapse, treatment-related mortality (TRM), disease-free survival (DFS) and overall survival. TRM was defined as death in continuous remission of primary disease. This event was summarized by the cumulative incidence estimate with relapse as a competing risk and second transplant as a censoring event. Grades II–IV and III – IV acute GvHD were defined according to the Glucksberg scale31. Chronic GvHD included the development of symptoms in any organ system fulfilling the criteria of limited or extensive chronic GvHD32. These events were summarized by the cumulative incidence estimate with death as a competing risk and second transplant as a censoring event. Disease relapse consisted of a clinical relapse of the primary disease as defined by the CIBMTR. The event was summarized by the cumulative incidence estimate with death as a competing risk and second transplant as a censoring event. Overall survival was defined as time to death from any cause, and DFS was determined from time to disease relapse or death.
Statistical Analysis
Cox proportional hazards models were used to examine the association of donor KIR genotype with the hazard of failure for various time-to-event outcomes of HCT (death from any cause, disease relapse, DFS and death without relapse). The assumption of proportional hazards for adjusted clinical factors in the Cox model was tested using time-dependent covariates. Factors that violated the proportional hazard assumption were adjusted via stratification. The multivariate models were built using a stepwise forward/backward model selection approach, with the adjusted clinical covariates being selected at the 0.05 significance level. The following variables were analyzed for their prognostic value on each of the outcomes: patient characteristics (age, gender, Karnofsky performance score, recipient race/ethnicity), disease characteristics (disease status at transplantation), and transplant-related factors (time from diagnosis to HCT, recipient cytomegalovirus [CMV] status, hematopoietic graft type, transplant conditioning regimen, GvHD prophylaxis, number of high resolution HLA matches, in vivo T-cell depletion, and donor race/ethnicity). Adjustments for multiple variables were performed for outcomes analysis for each model of NK alloreactivity. Patient-, disease- and transplant- related factors were compared between groups using the Chi-square test for categorical variables and the Wilcoxon two sample test for continuous variables. Cumulative incidence estimates to account for competing risks were calculated for TRM, acute and chronic GvHD, and disease relapse. DFS and survival curves were estimated by the Kaplan-Meier method 33. Cox proportional hazards models were used to examine the association of donor KIR genotype with the hazard of failure for various time-to-event outcomes of HCT (death from any cause, disease relapse, DFS and death without relapse). The assumption of proportional hazards for adjusted clinical factors in the Cox model was tested using time-dependent covariates. Factors that violated the proportional hazard assumption were adjusted via stratification. The multivariate models were built using a stepwise forward/backward model selection approach, with the adjusted clinical covariates being selected at the 0.05 significance level. Each model of NK alloreactivity was tested for all the seven outcomes with adjustments for the selected clinical covariates. To adjust for multiple testing, the KIR variables with p-values less than 0.01 were considered statistically significant.
The main effects were assessed separately for patients with AML and for those with MDS. The following mechanisms of NK alloreactivity were evaluated:
Missing KIR Ligand Model: This model assessed the presence or absence of recipient HLA ligands for cognate donor inhibitory KIR. The presence/absence of HLA-Bw4, HLA-C1, and HLA-C2 KIR ligands were segregated into the following groups: all ligands present vs. missing C1 ligand only vs. missing C2 ligand only vs. missing Bw4 ligand only vs. missing both Bw4 and C1 or C2. A two-group comparison between the presence of any missing ligand in the recipient vs. the presence of all ligands was also evaluated. This model was also used to assess the presence or absence of specific HLA-Bw4, HLA-C1, and HLA-C2 ligands in the recipient irrespective of any other KIR ligand. Each ligand was evaluated separately from the others, with pairwise comparisons between the presence and absence of the KIR ligand. Donor KIR ligand absence was also assessed to evaluate its potential impact on achievement of donor T cell chimerism on a subset of patients who had this data available. Cases were only included in this analysis if the chimerism studies were performed with short tandem repeat or variable copy numbers of tandem repeats (VNTR) methods.
Assessment of individual donor activating KIRs: Each activating KIR (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, and KIR3DS1) was evaluated separately from the others, with pairwise comparisons between the presence and absence of the KIR gene in the donor.
Effects of KIR2DS1 and HLA-C background: KIR2DS1 presence vs. absence in the donor was assessed. KIR2DS1+ donors were then further segregated based on presence or absence of HLA-C1 in the donor, as previously described15.
Donor centromeric KIR content: This model assessed the presence of specific centromeric KIR combinations which have been previously reported to increase survival. Donors were classified as: (1) homozygous for centromeric B haplotype (KIR2DS2+ and/or KIR2DL2+, KIR2DL3-; (2) homozygous for the centromeric A haplotype KIR2DL3+, KIR2DL1+, KIR2DL2-, KIR2DS2-) or (3) heterozygous for centromeric haplotypes A and B. These groupings were assessed for a cumulative dose effect of centromeric B (centromeric AA vs. centromeric AB vs. centromeric BB).
Results
Patient and Donor Characteristics
Among the 909 patients in this study population, there were 612 AML and 297 MDS subjects (Table 1). These included 478 AML and 234 MDS donor-recipient pairs that were 8/8 HLA-matched and 134 AML and 63 MDS that were 7/8 HLA-matched. Among the AML and MDS patients, 29% and 32%, respectively, were Bw6/Bw6 (therefore missing Bw4 ligand); 41% and 40%, respectively, were C1C1 (missing C2 ligand); 14% and 9%, respectively, were C2C2 (missing C1 ligand). One hundred and eighty-one (30%) of the AML patients had an antecedent hematologic disorder, including 107 (17%) MDS. The median age was 56 years (range, 20–74 years), and the hematopoietic graft sources included 169 (19%) bone marrow and 740 (81%) peripheral blood progenitor cells. RIC consisted of 210 (23%) total body irradiation-based and 699 (77%) chemotherapy-based regimens. Anti-thymocyte globulin was administered to 317 (35%) patients, and alemtuzumab was given to 73 (8%) subjects.
Table 1.
Characteristics of Study Subjects by Recipient C2 Ligand Status
| Variable | C2 Negative N (%) | C2 Positive N (%) | p-value |
|---|---|---|---|
| Number of Patients | 368 | 541 | |
| Number of centers | 82 | 86 | |
| Age, median (range), years | 56 (20–74) | 57 (20–74) | 0.54 |
| Age at transplant | 0.13 | ||
| 20–29 | 13 ( 4) | 32 ( 6) | |
| 30–39 | 32 ( 9) | 31 ( 6) | |
| 40–49 | 63 (17) | 86 (16) | |
| 50+ | 260 (71) | 392 (72) | |
| Recipient race/ethnicity | 0.15 | ||
| Caucasian race, non-Hispanic | 336 (95) | 492 (93) | |
| African American race, non-Hispanic | 3 ( 1) | 17 ( 3) | |
| Asian race, non-Hispanic | 1 (<1) | 3 ( 1) | |
| Native American race, non-Hispanic | 0 | 1 (<1) | |
| Hispanic, Caucasian race | 13 ( 4) | 16 ( 3) | |
| Other/Multiple | 1 (<1) | 0 | |
| Missing | 14 | 12 | |
| Male Sex | 214 (58) | 305 (56) | 0.60 |
| Karnofsky score prior to transplant | |||
| < 90 | 129 (35) | 173 (32) | 0.38 |
| >= 90 | 196 (53) | 313 (58) | |
| Missing | 43 (12) | 55 (10) | |
| HLA matching | 0.25 | ||
| 8-Aug | 294 (80) | 418(77) | |
| 7/8 MM at HLA-A | 23 ( 6) | 39 ( 7) | |
| 7/8 MM at HLA-B | 8 ( 2) | 26 ( 5) | |
| 7/8 MM at HLA-C | 41 (11) | 53 (10) | |
| 7/8 MM at HLA-DRB1 | 2 ( 1) | 5 ( 1) | |
| Disease at transplant | 0.86 | ||
| AML | 249 (68) | 363 (67) | |
| MDS | 119 (32) | 178 (33) | |
| ATG Use - Yes | 116 (32) | 201 (37) | 0.08 |
| Alemtuzumab Use - Yes | 26 ( 7) | 47 ( 9) | 0.40 |
| Disease status at transplant | 0.49 | ||
| Early | 160 (43) | 217 (40) | |
| Intermediate | 57 (15) | 77 (14) | |
| Advanced | 125 (34) | 197 (36) | |
| Missing | 26 ( 7) | 50 ( 9) | |
| Conditioning regimen | 0.60 | ||
| TBI 200 cGy | 7 ( 2) | 4 ( 1) | |
| Fludarabine + TBI | 72 (20) | 98 (18) | |
| Fludarabine + Cy | 32 ( 9) | 51 ( 9) | |
| Fludarabine + Melphalan | 100 (27) | 139 (26) | |
| Fludarabine + Busulfan + ATG | 112 (30) | 195 (36) | |
| Fludarabine + Busulfan (No ATG) | 41 (12) | 49 ( 9) | |
| Thiotepa + Cy + ATG | 4 ( 1) | 5 ( 1) | |
| GvHD prophylaxis | 0.94 | ||
| CsA + MTX | 16 ( 4) | 25 ( 5) | |
| CsA + MMF | 63 (17) | 96 (18) | |
| CsA ± other (No MTX and no MMF) | 13 ( 4) | 13 ( 2) | |
| FK506 + MTX | 121 (33) | 177 (33) | |
| FK506 + MMF | 92 (25) | 139 (26) | |
| FK506 ± other (No MTX and no MMF) | 25 ( 7) | 45 ( 9) | |
| T-cell depletion | 38 (10) | 46 ( 9) | |
| Stem cell source | 0.78 | ||
| Bone marrow | 70 (19) | 99 (18) | |
| Peripheral Blood Progenitor Cells | 298 (81) | 442 (82) | |
| Donor/recipient sex match | 0.10 | ||
| Male -> Male | 165 (45) | 214 (40) | |
| Male -> Female | 99 (27) | 131 (24) | |
| Female -> Male | 48 (13) | 91 (17) | |
| Female -> Female | 56 (15) | 105 (19) | |
| Donor/recipient CMV match | 0.14 | ||
| Negative/Negative | 91 (25) | 150 (28) | |
| Negative/Positive | 156 (42) | 184 (34) | |
| Positive/Negative | 37 (10) | 57 (11) | |
| Positive/Positive | 77 (21) | 136 (25) | |
| Unknown | 7 ( 2) | 14 ( 3) | |
| TBI Use - Yes | 92 (25) | 118 (22) | 0.26 |
| Donor age, median (range), years | 34 (18–59) | 35 (18–61) | 0.20 |
| Donor age | 0.32 | ||
| 18–19 | 9 ( 2) | 9 ( 2) | |
| 20–29 | 112 (30) | 158 (29) | |
| 30–39 | 136 (37) | 197 (36) | |
| 40–49 | 91 (25) | 128 (24) | |
| 50+ | 20 ( 5) | 49 ( 9) | |
| Interval from Dx to Tx, months, AML, Median, Range | 6 (1.0–90) | 7 (0.5–69) | 0.22 |
| Interval from Dx to Tx, months, MDS, Median, Range | 10 (0.6–186) | 10 (1–270) | 0.44 |
| Year of transplant | 0.15 | ||
| 1999 | 4 ( 1) | 4 ( 1) | |
| 2000 | 14 ( 4) | 11 ( 2) | |
| 2001 | 28 ( 8) | 27 ( 5) | |
| 2002 | 17 ( 5) | 33 ( 6) | |
| 2003 | 36 (10) | 50 ( 9) | |
| 2004 | 64 (17) | 70 (13) | |
| 2005 | 70 (19) | 116 (21) | |
| 2006 | 92 (25) | 150 (28) | |
| 2007 | 43 (12) | 80 (15) | |
| Median follow-up of survivors, months | 63 (5–133) | 58 (4–121) | 0.009a |
Abbreviations: Dx – Diagnosis, Tx - Transplant
– Log-rank p-value.
Missing KIR Ligand Model
For AML patients, there were no significant findings in any of the post-transplant outcomes when comparing recipients with all KIR ligands (n=217) vs. those lacking C1 ligand only (n=136) vs. those lacking C2 ligand only (n=59) vs. those lacking Bw4 ligand only (n=80) vs. those lacking both Bw4 and C1 or C2 ligands (n=120). Similarly, no associations with outcomes were observed using this approach for patients with MDS. Risk for acute GvHD was higher among AML patients lacking KIR ligands after adjusting for other clinical factors. In particular, patients lacking one or more KIR ligands (n=393) experienced higher grades III–IV acute GvHD (HR 1.6, 95% CI, 1.16–2.28, p=0.005) compared to those with all KIR ligands present (n=217) (Table 2). The 100 day and 1-year adjusted cumulative incidence rates were 29% (25–34%) vs. 20% (16–26%) (p=0.013), and 31% (27–36%) vs. 21% (16–27%) (p=0.004), respectively. No other outcomes were significant for AML or MDS when comparing transplant recipients with all inhibitory KIR ligands present to those lacking at least one.
Table 2.
Multivariate analysis of recipient KIR Ligands for AML only
| Recipient KIR Ligands | All present | ≥ 1 absent | |||
|---|---|---|---|---|---|
| N | Reference | N | HR (95% CI) | P-value | |
| Overall survivala | 217 | 1.00 | 395 | 1.04 (0.85–1.28) | 0.69 |
| DFSb | 217 | 1.00 | 395 | 0.93 (0.75–1.15) | 0.49 |
| TRMc | 213 | 1.00 | 390 | 1.43 (1.01–2.01) | 0.04 |
| Relapsed | 215 | 1.00 | 394 | 0.81 (0.62–1.05) | 0.11 |
| aGVHD II–IVe | 217 | 1.00 | 392 | 1.22 (0.97–1.53) | 0.09 |
| aGVHD III–IVf | 217 | 1.00 | 393 | 1.63 (1.16–2.28) | 0.005 |
| cGVHDg | 208 | 1.00 | 387 | 1.07 (0.84–1.37) | 0.59 |
Adjusted for disease stage, donor/recipient CMV matching, GVHD prophylaxis, recipient race/ethnicity and stratified by graft type.
Adjusted for disease stage and stratified by graft type, donor/recipient CMV matching and Karnofsky score.
Adjusted for recipient race/ethnicity, donor/recipient sex matching, conditioning regimen, GVHD prophylaxis, time from diagnosis to transplant and stratified by graft type.
Adjusted for disease stage and time from diagnosis to transplant and stratified by conditioning regimen and Karnofsky score.
Adjusted for graft type, GVHD prophylaxis, recipient race and stratified by use of CAMPATH.
Adjusted for HLA matching, recipient race/ethnicity, GVHD prophylaxis and Karnofsky score.
Adjusted for in-vivo T-cell depletion, conditioning regimen, GVHD prophylaxis and stratified by recipient CMV status.
Pairwise comparisons between absence and presence of specific inhibitory HLA-KIR ligands were next considered. In a multivariate analysis, AML patients lacking HLA-C2 for donor KIR2DL1 (n=248) experienced higher grade II–IV (HR 1.4, 95% CI, 1.1–1.7, p=0.002) and III–IV acute GvHD (HR 1.5, 95% CI 1.1–2.0, p=0.01) compared to those in whom the ligand was present (n=361) (Table 3). The respective 100 day and 1-year adjusted cumulative incidence rates for grade II–IV acute GvHD were 59% (53–65%) vs. 48% (43–52%) (p=0.004), and 63% (57–68%) vs. 51% (46–56%) (p=0.002); and for grade III–IV acute GvHD were 31% (25–36%) vs. 23% (19–28%) (p=0.04), and 33% (27–39%) vs. 24% (20–29%) (p=0.02). When patients who had received alemtuzumab (N=53) were excluded absence of HLA-C2 continued to be associated with higher grade II–IV (HR 1.4, 95% CI, 1.13–1.76, p=0.003) and III–IV acute GvHD (HR 1.5, 95% CI, 1.12–2.11, p=0.007). There were no differences in baseline characteristics between patients with or without HLA-C2 (see Table 1). For the combined AML and MDS patient cohort lack of HLA-C2 (n= 367) was associated with increased grade II–IV (HR 1.3, 95% CI, 1.1–1.6, p=0.005) and III–IV acute GvHD (HR 1.5, 95% CI 1.2–1.9, p=0.002). However, no significant association of lack of HLA-C2 with grades II–IV or III–IV acute GvHD was observed in MDS patients alone (HR 1.1, 95% CI, 0.77–1.5, p=0.71; HR 1.5, 95% CI, 0.94–2.9, p=0.09, respectively). There were no differences in chronic GvHD, relapse, TRM, DFS and overall survival between those with or without HLA-C2 for AML or MDS. Furthermore, there were no differences in any of the outcomes for AML or MDS when comparing the absence and presence of HLA-C1 ligand for KIR2DL2/KIR2DL3. No associations with outcomes were observed when considering absence of HLA-Bw4 ligand for patients with AML (Table 4) or MDS.
Table 3.
Multivariate analysis of recipient C2 Ligand for AML only
| Recipient C2 Ligand status | Present | Absent | |||
|---|---|---|---|---|---|
| N | Reference | N | HR (95% CI) | P-value | |
| Overall survivala | 363 | 1.00 | 249 | 1.08 (0.88–1.32) | 0.48 |
| DFSb | 363 | 1.00 | 249 | 1.00 (0.82–1.23) | 0.97 |
| TRMc | 358 | 1.00 | 245 | 1.22 (0.90–1.67) | 0.20 |
| Relapsed | 361 | 1.00 | 248 | 0.94 (0.72–1.23) | 0.69 |
| aGVHD II–IVe | 361 | 1.00 | 248 | 1.41 (1.12–1.72) | 0.002 |
| aGVHD III–IVf | 362 | 1.00 | 248 | 1.47 (1.09–2.00) | 0.01 |
| cGVHDg | 353 | 1.00 | 242 | 0.95 (0.74–1.22) | 0.68 |
Adjusted for disease stage, donor/recipient CMV matching, GVHD prophylaxis, recipient race/ethnicity and stratified by graft type.
Adjusted for disease stage and stratified by graft type, donor/recipient CMV matching and Karnofsky score.
Adjusted for recipient race/ethnicity, donor/recipient sex matching, conditioning regimen, GVHD prophylaxis, time from diagnosis to transplant and stratified by graft type.
Adjusted for disease stage and time from diagnosis to transplant and stratified by conditioning regimen and Karnofsky score.
Adjusted for graft type, GVHD prophylaxis, recipient race and stratified by use of CAMPATH.
Adjusted for HLA matching, recipient race/ethnicity, GVHD prophylaxis and Karnofsky score.
Adjusted for in-vivo T-cell depletion, conditioning regimen, GVHD prophylaxis and stratified by recipient CMV status.
Table 4.
Multivariate analysis of recipient Bw4 Ligand for AML only
| Recipient Bw4 Ligand status | Present | Absent | |||
|---|---|---|---|---|---|
| N | Reference | N | HR (95% CI) | P-value | |
| Overall survivala | 433 | 1.00 | 179 | 0.96 (0.78–1.19) | 0.69 |
| DFSb | 433 | 1.00 | 179 | 0.87 (0.69–1.09) | 0.21 |
| TRMc | 427 | 1.00 | 176 | 1.15 (0.83–1.59) | 0.41 |
| Relapsed | 430 | 1.00 | 179 | 0.76 (0.56–1.03) | 0.07 |
| aGVHD II–IVe | 433 | 1.00 | 176 | 0.96 (0.76–1.22) | 0.75 |
| aGVHD III–IVf | 433 | 1.00 | 177 | 1.25 (0.90–1.72) | 0.18 |
| cGVHDg | 420 | 1.00 | 175 | 0.94 (0.73–1.22) | 0.68 |
Adjusted for disease stage, donor/recipient CMV matching, GVHD prophylaxis, recipient race/ethnicity and stratified by graft type.
Adjusted for disease stage and stratified by graft type, donor/recipient CMV matching and Karnofsky score.
Adjusted for recipient race/ethnicity, donor/recipient sex matching, conditioning regimen, GVHD prophylaxis, time from diagnosis to transplant and stratified by graft type.
Adjusted for disease stage and time from diagnosis to transplant and stratified by conditioning regimen and Karnofsky score.
Adjusted for graft type, GVHD prophylaxis, recipient race and stratified by use of CAMPATH.
Adjusted for HLA matching, recipient race/ethnicity, GVHD prophylaxis and Karnofsky score.
Adjusted for in-vivo T-cell depletion, conditioning regimen, GVHD prophylaxis and stratified by recipient CMV status.
Donor T cell chimerism was assessed for 608 patients (403 AML and 205 MDS) at days +30, +100, +183, + 365. No association was observed for achievement of complete donor T cell chimerism when comparing donors with all ligands present vs. those missing one or more KIR ligands.
Assessment of individual donor activating KIRs
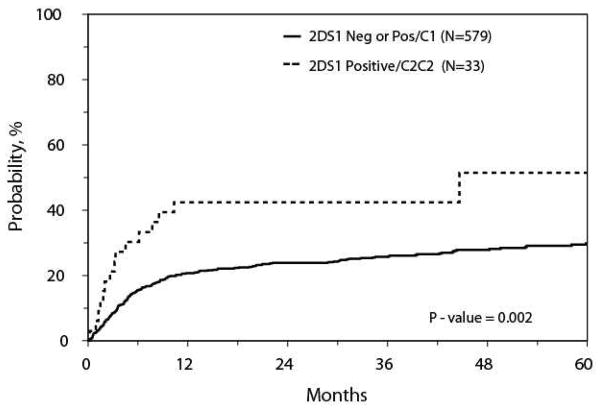
Pairwise comparisons between the absence and presence of each donor activating KIR (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, and KIR3DS1) independent of the others did not identify differences in any of the study endpoints. However, when assessing AML patients with KIR2DS1-positive donors, those with C2/C2 backgrounds (n=33) were associated with greater TRM compared to those with C1 backgrounds (n=189) or with KIR2DS1-negative donors (n=390) (HR 2.4, 95% CI, 1.4–4.2, p=0.002; Figure). The 100 day and 1-year adjusted cumulative incidence rates of TRM were 26% (12–40%) vs. 9% (6–11%) (p=0.017), and 40% (25–56%) vs. 20% (17–23%) (p=0.011), respectively. There were no significant differences in characteristics or in the causes of TRM between the groups. The causes of TRM for those with KIR2DS1-positive donors and C2/C2 backgrounds included 9 AML, 4 idiopathic pneumonia syndrome, 4 other organ failure, 4 infection, and 3 GvHD. This association of increased TRM was not observed for those with MDS. No other study endpoints were found to be significant for AML or MDS when segregating KIR2DS1+ donors by their C1 vs. C2/C2 backgrounds.
Figure.
TRM for AML Patients with Donors KIR2DS1 Positive with a C2C2 background vs. KIR2DS1 Negative or KIR2DS1 Positive with any C1.
Donor centromeric KIR content
Donor activating KIRs were assessed and categorized as homozygous for the centromeric B -haplotype (cenBB; n=57 for AML and 22 for MDS), homozygous for the centromeric A-haplotype (cenAA; n=298 for AML and 145 for MDS), or heterozygous centromeric AB (n=255 for AML and 128 for MDS). There were no significant differences in acute or chronic GvHD, relapse, TRM, DFS and overall survival between the donor cenBB, cenAA and cenAB haplotypes for AML or MDS. In addition, there were no significant associations between donor overall B-haplotype KIR content and RIC HCT outcomes.
Discussion
In this large series, specific donor-recipient KIR-HLA combinations are associated with post-transplant outcomes following RIC HCT for AML. Interestingly, combinations associated with reduced relapse in myeloablative HCT were not associated with protection from relapse in this RIC HCT cohort. The absence of HLA ligands for donor inhibitory KIR and the presence of activating KIR are associated with a lower risk of relapse in myeloablative HCT 9, 10, 24, but the effect of these genetic conditions on relapse in RIC HCT did not reach statistical significance in our analysis for AML or MDS alone.
Several factors may have contributed to the evident lack of KIR effect on relapse in this cohort. Compared to previously published studies in myeloablative HCT 15, 19, this more contemporary RIC HCT cohort contained more peripheral blood progenitor cells than bone marrow as a graft source. Although the mechanism is unclear, the missing ligand effect has been more reported in HCT with a bone marrow stem cell source 9. However, missing KIR ligand has had no significant impact on transplantation outcome after nonmyeloablative HCT 34. Characteristics associated with RIC regimen and the patients treated with the regimen may also obscure possible KIR-HLA effects. While better tolerated, the lower intensity of chemotherapy in RIC HCT may also be less effective than myeloablative conditioning at leukemic clearance, leaving a higher leukemic burden for immune-mediated leukemic clearance and a less successful outcome 35, 36. The vast majority of patients in this cohort were older than 50; and older age is associated with higher risk leukemia, as evidenced by the high percentage of patients transplanted with advanced disease in this cohort. Furthermore, older patients may have had unrecognized or underreported antecedent hematologic diseases which inherently have higher risk for relapse post-transplant. Since AML and MDS represent different disease populations and NK effects against MDS are unclear, the analyses for KIR-HLA combinations were performed separately for each disease. However, any NK cell-mediated effect on GVL may have been mitigated in the AML study population, which included 30% of patients with a prior MDS or other antecedent hematologic disorder. Combined, these factors may explain the apparent lack of KIR-HLA effect in this RIC HCT cohort.
Lack of data regarding cytogenetic risk groups in the registry is a limitation of this study. If differences in the proportion with poor risk cytogenetics existed between the KIR-HLA combinations that we analyzed this may also have confounded a NK cell-mediated GVL effect. Furthermore, although our analysis was adjusted for GvHD prophylaxis, differences between RIC approaches regarding duration of immunosuppressant therapy or antibody dosing for in vivo T-cell depletion may have also impacted relapse and other outcomes.
We observed that the combination of donor KIR2DS1 with HLA-C2/C2 in the donor was associated with higher TRM in AML patients. In vitro evidence suggests that NK cell reactivity is decreased for KIR2DS1 in the presence of high amounts of HLA-C2 ligand 37–40. This combination has been associated with a diminished capacity to control AML relapse after myeloablative transplant conditioning15. We were unable to appreciate such an effect on relapse in the current series of RIC transplants, possibly related to the small number of patients with such donors. Furthermore, no survival benefit or reduction in acute GvHD was observed for those with donors positive for KIR3DS1 in this RIC study population in contrast to this reported association for myeloablative HCT 14, 15.
Donor centromeric B-KIR haplotypes have been reported to contribute to relapse protection and improved survival after T-cell replete myeloablative HCT for AML, where homozygosity for the centromeric-B partial haplotype had the strongest independent effect18. Contrasting results, however, have been found in T-cell depleted HCT41. Haplotype B/x donors have also been associated with a higher incidence of chronic GvHD13. In the RIC HLA-haploidentical transplant setting a KIR haplotype B donor has been associated with lower risk of relapse for patients with hematologic malignancies42. We found that in RIC HCT for AML and MDS, donor homozygosity for the centromeric B-haplotype does not have a significant impact in leukemia relapse, overall survival, TRM or GvHD.
We observed that recipients lacking one or more KIR ligands, in particular those lacking HLA-C2 ligand for donor inhibitory KIR2DL1, were associated with increased acute GvHD. Although acute GvHD has previously been reported to be associated with missing KIR ligand, including HLA-C2 ligand 9, 43–45, we did not observe increased acute GvHD for RIC HCT with regard to AA haplotypes as previously reported 44, 46, 47. For those patients with HLA-C disparate donors increased expression levels of the mismatched HLA-C allotype may also have increased the risks of grades III to IV acute GvHD, as recently reported48. In contrast to prior myeloablative HCT studies, patients treated with RIC in this series were older and more commonly received peripheral blood progenitor cells and T-cell replete grafts, factors that may have influenced the development of acute GvHD.
The precise mechanism underlying these KIR associations with GvHD remains elusive. Increased NK cell interferon-γ production has been correlated with acute GvHD49; however, donor NK lysis of recipient antigen-presenting cells abrogates GvHD3. Clearly, more studies are needed to delineate how NK and T-cell interaction, either direct or indirect through cytokine-mediated changes, affects GvHD.
KIR/HLA ligand matching has been reported to influence achievement of T-cell complete donor chimerism after non-myeloablative related donor HCT performed for many different diseases50. The current report restricted to AML and MDS patients undergoing unrelated donor RIC HCT is, to our knowledge, the largest experience of T-cell chimerism in this transplant setting. Donor KIR ligand absence was not associated with the development of T-cell complete donor chimerism.
Our results suggest that specific donor-recipient KIR-HLA interactions are associated with post-transplant outcomes following RIC-URD HCT for AML. The unfavorable association of lack of HLA-C2 for donor KIR2DL1 and GvHD are useful for discussions of prognosis. The finding that donor HLA-C2 homozygosity and KIR2DS1 is associated with higher transplant-related mortality suggests that this should also be considered when selecting donors. In light of previous findings that HLA-C2 homozygosity is associated with higher risk for relapse15, 22 selection of a HLA-C mismatched donor in preference to other mismatched donors at other HLA loci may be justified. Donor selection based on activating KIR profile otherwise was not supported by this study, which did not demonstrate salutary effects of donor activating KIR, particularly those located in the centromeric portion of the KIR haplotype.
Investigation of larger cohorts of more homogeneous patient populations as well as functional studies is warranted to help further assess the influence of KIR-HLA combinations on outcomes after RIC HCT. In addition, the vast majority of patients in this series were Caucasian. Future prospective studies of KIR-mediated effects in RIC HCT should ideally be performed with ethnically diverse populations to better assess for differences in outcomes among specific ethnic subsets. Further elucidation of the biology of NK cell alloreactivity in the RIC setting may also provide guidance for future approaches to help optimize conditions for generating GVL activity without GvHD, and for minimizing TRM in this transplant population.
Highlights.
We assessed models of NK cell reactivity in AML/MDS patients undergoing RIC URD HCT
AML patients lacking ≥1 KIR ligands experienced higher grade III–IV GvHD
Absence of HLA-C2 was associated with higher grade II–IV & III–IV acute GvHD in AML
AML patients with KIR2DS1+, HLA-C2 homozygous donors had greater TRM
No associations with outcomes for donor activating KIRs or centromeric KIR content
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Actinium Pharmaceuticals; Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; University of Minnesota; University of Utah; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Authorship Contributions:
RMS, KCH, MA, SJL, SRS, TW, MH drafted the research plan; MFV, KJM, CM, MB, JG, MRV, OR, SRM, SD, JD, MB, YI, AW, PS, MP, DW, JM, CKH critically revised research plan; TW and MH performed statistics; RMS, KCH, MA, SJL, SRS, TW analyzed and interpreted data; RMS, KCH, SJL, SRS, TW, MH drafted the paper; RMS, KCH, MA, SJL, SRS, TW, MH, MFV, KJM, CM, MB, JG, MRV, OR, SRM, SD, JD, MB, YI, AW, PS, MP, DW, JM, CKH critically revised the paper.
Disclosure of Conflicts of Interest: The authors have no relevant conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nature medicine. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 2.Hercend T, Takvorian T, Nowill A, et al. Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood. 1986;67:722–728. [PubMed] [Google Scholar]
- 3.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 4.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 5.Boyington JC, Sun PD. A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Molecular immunology. 2002;38:1007–1021. doi: 10.1016/s0161-5890(02)00030-5. [DOI] [PubMed] [Google Scholar]
- 6.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. The Journal of experimental medicine. 1995;181:1133–1144. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Current opinion in immunology. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 8.Vales-Gomez M, Reyburn HT, Mandelboim M, Strominger JL. Kinetics of interaction of HLA-C ligands with natural killer cell inhibitory receptors. Immunity. 1998;9:337–344. doi: 10.1016/s1074-7613(00)80616-0. [DOI] [PubMed] [Google Scholar]
- 9.Miller JS, Cooley S, Parham P, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long EO. Signal sequences stop killer cells. Nature. 1998;391:740–741. 743. doi: 10.1038/35739. [DOI] [PubMed] [Google Scholar]
- 12.Pende D, Marcenaro S, Falco M, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119–3129. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 13.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venstrom JM, Gooley TA, Spellman S, et al. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2010;115:3162–3165. doi: 10.1182/blood-2009-08-236943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. The New England journal of medicine. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yawata M, Yawata N, Abi-Rached L, Parham P. Variation within the human killer cell immunoglobulin-like receptor (KIR) gene family. Critical reviews in immunology. 2002;22:463–482. [PubMed] [Google Scholar]
- 17.Martin AM, Kulski JK, Gaudieri S, et al. Comparative genomic analysis, diversity and evolution of two KIR haplotypes A and B. Gene. 2004;335:121–131. doi: 10.1016/j.gene.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. Journal of immunology. 2014;192:4592–4600. doi: 10.4049/jimmunol.1302517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak J, Koscinska K, Mika-Witkowska R, et al. Donor NK cell licensing in control of malignancy in hematopoietic stem cell transplant recipients. American journal of hematology. 2014 doi: 10.1002/ajh.23802. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 22.Cook MA, Milligan DW, Fegan CD, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103:1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 23.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 24.Hsu KC, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Brunstein CG, Wagner JE, Weisdorf DJ, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113:5628–5634. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen DF, Prasad VK, Broadwater G, et al. Differential impact of inhibitory and activating Killer Ig-Like Receptors (KIR) on high-risk patients with myeloid and lymphoid malignancies undergoing reduced intensity transplantation from haploidentical related donors. Bone marrow transplantation. 2012;47:817–823. doi: 10.1038/bmt.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spellman S, Setterholm M, Maiers M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Vierra-Green C, Roe D, Hou L, et al. Allele-level haplotype frequencies and pairwise linkage disequilibrium for 14 KIR loci in 506 European-American individuals. PloS one. 2012;7:e47491. doi: 10.1371/journal.pone.0047491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone marrow transplantation. 2008;42 (Suppl 1):S1–S2. doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 31.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. The American journal of medicine. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 33.MPKE Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958:457–481. [Google Scholar]
- 34.Baron F, Petersdorf EW, Gooley T, et al. What is the role for donor natural killer cells after nonmyeloablative conditioning? Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:580–588. doi: 10.1016/j.bbmt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martino R, de Wreede L, Fiocco M, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone marrow transplantation. 2013;48:761–770. doi: 10.1038/bmt.2012.236. [DOI] [PubMed] [Google Scholar]
- 36.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. Journal of immunology. 2007;179:854–868. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- 38.Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaelsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115:1166–1174. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 39.Sivori S, Carlomagno S, Falco M, Romeo E, Moretta L, Moretta A. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood. 2011;117:4284–4292. doi: 10.1182/blood-2010-10-316125. [DOI] [PubMed] [Google Scholar]
- 40.Pittari G, Liu XR, Selvakumar A, et al. NK cell tolerance of self-specific activating receptor KIR2DS1 in individuals with cognate HLA-C2 ligand. Journal of immunology. 2013;190:4650–4660. doi: 10.4049/jimmunol.1202120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schellekens JSB, Mayor NP, et al. The influence of donor KIR repertoire on the clinical outcome after unrelated, T-cell depleted HSCT for patients with AML. Bone marrow transplantation. 2013;48:abstr# O315. [Google Scholar]
- 42.Michaelis SU, Mezger M, Bornhauser M, et al. KIR haplotype B donors but not KIR-ligand mismatch result in a reduced incidence of relapse after haploidentical transplantation using reduced intensity conditioning and CD3/CD19-depleted grafts. Annals of hematology. 2014;93:1579–1586. doi: 10.1007/s00277-014-2084-2. [DOI] [PubMed] [Google Scholar]
- 43.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19:1446–1451. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 44.Ludajic K, Balavarca Y, Bickeboller H, et al. KIR genes and KIR ligands affect occurrence of acute GVHD after unrelated, 12/12 HLA matched, hematopoietic stem cell transplantation. Bone marrow transplantation. 2009;44:97–103. doi: 10.1038/bmt.2008.432. [DOI] [PubMed] [Google Scholar]
- 45.Bjorklund AT, Schaffer M, Fauriat C, et al. NK cells expressing inhibitory KIR for non-self-ligands remain tolerant in HLA-matched sibling stem cell transplantation. Blood. 2010;115:2686–2694. doi: 10.1182/blood-2009-07-229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagne K, Busson M, Bignon JD, et al. Donor KIR3DL1/3DS1 gene and recipient Bw4 KIR ligand as prognostic markers for outcome in unrelated hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1366–1375. doi: 10.1016/j.bbmt.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Kim SY, Choi HB, Yoon HY, et al. Influence of killer cell immunoglobulin-like receptor genotypes on acute graft-vs-host disease after unrelated hematopoietic stem cell transplantation in Koreans. Tissue antigens. 2007;69 (Suppl 1):114–117. doi: 10.1111/j.1399-0039.2006.762_9.x. [DOI] [PubMed] [Google Scholar]
- 48.Petersdorf EW, Gooley TA, Malkki M, et al. HLA-C expression levels define permissible mismatches in hematopoietic cell transplantation. Blood. 2014;124:3996–4003. doi: 10.1182/blood-2014-09-599969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobecks RM, Ball EJ, Askar M, et al. Influence of killer immunoglobulin-like receptor/HLA ligand matching on achievement of T-cell complete donor chimerism in related donor nonmyeloablative allogeneic hematopoietic stem cell transplantation. Bone marrow transplantation. 2008;41:709–714. doi: 10.1038/sj.bmt.1705954. [DOI] [PubMed] [Google Scholar]