Abstract
We conducted a Phase I trial of allogeneic T-cells sensitized in vitro against a pool of 15-mer peptides spanning the sequence of CMVpp65 for adoptive therapy of 17 allogeneic hematopoietic cell transplant recipients with CMV viremia or clinical infection persisting despite prolonged treatment with antiviral drugs. All but three of the patients had received T-cell depleted transplants without GVHD prophylaxis with immunosuppressive drugs post transplant. The CMVpp65-specific T-cells (CMVpp65CTLs) generated were oligoclonal and specific for only 1–3 epitopes, presented by a limited set of HLA class I or II alleles. T-cell infusions were well tolerated without toxicity or GVHD. Of 17 patients treated with transplant donor (N=16) or third party (N=1) CMVpp65CTLs, 15 cleared viremia including 3 of 5 with overt disease. In responding patients, the CMVpp65CTLs infused consistently proliferated and could be detected by T-cell receptor (TCR) Vβ usage in CMVpp65/HLA tetramer+ populations for period of 120 days to up to 2 years post infusion. Thus, CMVpp65CTLs generated in response to synthetic 15-mer peptides of CMVpp65 are safe and can clear persistent CMV infections in the post transplant period.
Introduction
CMV infections remain a major cause of morbidity and mortality in allogeneic hematopoietic cell transplant (HCT) recipients.1,2 Although prophylactic or preemptive treatment with ganciclovir or foscarnet has reduced the incidence and mortality of early CMV infections, prolonged antiviral treatment may delay recovery of virus-specific immune responses, and predispose patients to late onset disease.2–5 Furthermore, treatment with antiviral drugs often cannot be sustained due to complicating myelosuppression or nephrotoxicity.2
Reconstitution of CMV-specific CD8+ cytotoxic T-cells (CMVCTLs) post HCT is correlated with control of CMV infections 2,6–14 Riddell et al.15,16 first demonstrated that adoptive transfer of donor-derived CD8+ CMVCTL clones sensitized with autologous CMV-infected fibroblasts could protect allogeneic marrow recipients from infection. Subsequent studies employing CMV-specific, predominantly CD8+, T-cell lines sensitized with autologous dendritic cells (DCs) or peripheral blood mononuclear cells (PBMCs) loaded with lysates of CMV-infected cells 17,18 or single peptides of immunodominant antigens such as CMVpp65 19, or DCs transduced to express immunogenic CMV proteins 20 have further documented the potential of such cells to prevent or treat CMV disease. However, regulatory concerns persist regarding the use of infected cell lysates or virus transduced cells. Similarly, sensitization with single peptides presented by specific HLA alleles, however prevalent, may limit their broad application.
We previously reported a method for generating CMVCTL by sensitization with autologous DCs loaded with a pool of 138 synthetic pentadecapeptides (15-mers), with 11 amino acid overlaps spanning the amino acid sequence of CMVpp65.21 With this approach, we were able to generate CMVpp65 peptide-specific T-cell lines (CMVpp65CTLs) from each CMV seropositive donor tested, irrespective of HLA-type, and to characterize these lines as to their epitope specificities and HLA restrictions.21 We now report results of a phase I trial reassessing the safety and antiviral activity of escalating doses of transplant donor-derived CMVpp65CTLs generated by this technique in allogeneic HCT recipients with CMV infections or persistent CMV viremia. By defining the epitope specificity, HLA restriction and TCR Vβ usage of the T-cells infused, we were also able to sequentially follow their growth and persistence in vivo and correlate their expansion with clearance of infection.
Materials and Methods
Design of clinical trial
This single institution phase I trial was designed to assess the toxicity and activity of escalating doses of CMVpp65CTLs derived from T-cell lines generated from CMV-seropositive healthy marrow transplant donors by sensitization in vitro with autologous, cytokine-activated monocytes (CAMS) loaded with a pool of synthetic 15-mer peptides spanning the sequence of CMV protein pp65.21 The trial was approved by the Institutional Review/Privacy Board at Memorial Sloan-Kettering Cancer Center, the National Marrow Donor Program and the Food and Drug Administration. Eligible pts were allogeneic HCT recipients who either had clinical CMV infection or CMV viremia that was persistent despite at least two weeks of treatment with antiviral drugs or could not be maintained on antiviral drugs because of associated toxicities.
Four dose levels of transplant donor-derived CMVpp65CTLs were sequentially evaluated: Group 1 (n=3) received 5×105 T-cells/Kg; Group 2 (n=4), 1×106 T-cells/Kgx1; Group 3 (n=3), 2×106 T-cells/Kgx1; Group 4 (n=6), 1×106 T-cells/Kgx3 weekly doses. Endpoints included incidence and severity of toxicities and acute GVHD as well as the clinical and virological responses observed and their correlation with alterations in CMV-specific T-cells detected post infusion.
Patient and Donor characteristics
Characteristics of the 16 patients who received transplant donor-derived CMVpp65 CTLs including diagnoses, disease status at time of transplantation, conditioning regimen and type of transplant are summarized in Table 1. All recipients were CMV-seropositive prior to transplantation.
Table 1.
Patient/Donor Characteristics
| UPN# | Age | Diagnosis | Disease status at HSCT |
Graft | Donor type | CMV IgG donor/recipient |
Weight at SCT (kg) |
Conditioning | GvHD Proph |
GvHD status prior to CMV CTLs |
GvHD proph/treatment at CMV CTL administration |
GvHD status at CMV CTLs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60.8 | SAA | Stable disease | BM unmodified | MUD | +/+ | 56.1 | Flu/TBI | CSA/MTX | - | CSA/MMF | - |
| 2 | 62.6 | NHL | Partial remission | PBSC unmodified | MRD (bro) | +/+ | 67.9 | Flu/Cyclo/TBI | CSA/MMF | Skin/Gut III | CSA/MMF/Methviored | Skin/Gut III |
| 3 | 44.5 | SAA | Refractory | TCD PBSC (Isolex)/E* | MUD | +/+ | 73.5 | Flu/Cyclo/Thio | TCD | - | - | - |
| 4 | 38.5 | MM | 2nd partial remission | TCD PBSC (Isolex)/E | MRD (sis) | +/+ | 98.4 | Bu/Mel/Flu | TCD | - | - | - |
| 5 | 45.8 | MDS/RAEB | Refractor cytopenia | TCD PBSC (Isolex)/E | MRD (sis) | +/+ | 85 | Bu/Mel/Flu | TCD | - | - | - |
| 6 | 59.5 | AML | 1st remission | TCD PBSC (Isolex)/E | MRD (sis) | +/+ | 104.7 | Bu/Mel/Flu | TCD | - | - | - |
| 7 | 69.6 | MM | 2nd partial response | TCD PBSC (Isolex)/E | MRD (bro) | +/+ | 82.7 | Bu/Mel/Flu | TCD | - | - | - |
| 8 | 65.1 | AML | 3rd remission | TCD PBSC (Isolex)/E | MRD (sis) | +/+ | 82 | Bu/Mel/Flu | TCD | - | - | - |
| 9 | 7.9 | ALL | 1st remission | TCD PBSC (Isolex)/E | MRD (bro) | +/+ | 37 | TBI/Thio/Cy | TCD | - | - | - |
| 10 | 60.7 | AML | 1st remission | TCD PBSC (Isolex)/E | MUD | +/+ | 92 | Bu/Mel/Flu | TCD | - | - | - |
| 11 | 60.9 | MPD Myelofibrosis | 1st chronic phase | TCD PBSC (CliniMACS) | MRD (bro) | +/+ | 48.9 | Bu/Mel/Flu | TCD | - | - | - |
| 12 | 44.2 | MDS/RAEB | Refractory anemia | TCD PBSC (CliniMACS) | MRD (sis) | +/+ | 98.2 | Bu/Mel/Flu | TCD | - | - | - |
| 13 | 57 | ALL | 1st remission | TCD PBSC (CliniMACS) | MRD (sis) | +/+ | 63.6 | Bu/Mel/Flu | TCD | - | - | - |
| 14 | 63.5 | MM | 2nd partial remission | TCD PBSC (CliniMACS) | MRD (sis) | +/+ | 103.1 | Bu/Mel/Flu | TCD | - | - | - |
| 15 | 47.1 | NHL | 1st remission | TCD PBSC (CliniMACS) | MRD (bro) | +/+ | 45.3 | TBI/Thio/Cy | TCD | - | - | - |
| 16 | 52.5 | MM | 2nd partial response | TCD PBSC (CliniMACS) | MUD | +/+ | 53.7 | Bu/Mel/Flu | TCD | - | - | - |
E = E-rosetting
All patients had been previously treated with antiviral drugs according to standard of care prior to administration of CMVpp65CTLs. Antiviral therapy was maintained following CMVpp65CTL infusion in 13 patients but had been discontinued in 4 patients (UPN 4, 5, 8 and 11) due to intolerable toxicities at time of CMVpp65CTL infusion.
Pt #17 was referred from an outside center with reactivation of drug-resistant CMV following a 9/10 HLA matched (HLA-A mismatch) HCT from a seronegative unrelated donor. This patient was treated with partially matched 3rd party CMV CTLs under an IRB and FDA approved SPU-IND.
Generation of Antigen Presenting Cells
Autologous transplant donor derived cytokine-activated monocytes (CAMS) and EBVBLCLs were generated as previously described.21–24 To identify HLA restrictions of CMVpp65CTL, a panel of EBV-BLCLs of defined HLA types were generated as previously described.21,22
Generation of clinical grade CMVpp65CTLs
Cultures of CMVpp65-specific T-cells from seropositive transplant donors were initiated at first detection of CMV viremia or prior to reactivation for seropositive transplant recipients at risk. CD3+ enriched T-cell fractions, isolated from PBMC by depletion of adherent monocytes and immunoadsorption of NK cells, were initially stimulated at an effector: stimulator ratio of 20:1 with irradiated (6000cGy) autologous CAMS loaded with the pool of overlapping pentadecapeptides of CMVpp65 (Invitrogen, Boston, MA) and propagated in vitro with weekly restimulation at an E:S ratio of 4:1, and supplementation with IL-2 beginning at day 10–16 as previously described and detailed in the supplement.21,22 After 28 days, T-cells were harvested, counted and tested for antigen specific cytotoxicity and lack of alloreactivity,21–23 as well as microbiological sterility, and endotoxin levels. Aliquots of CMVpp65CTLs meeting release criteria were cryopreserved in calculated doses for subsequent administration if and when indicated.
Characterization of CMVpp65CTL Lines and monitoring of CMV-specific T-cell responses
Yields of CD4+ and CD8+ T-cells and content of CD3− CD56+ NK cells and CD20+ B cells were quantitated in each culture. IFN-γ+ CD8+ and/or CD4+ CMVpp65-specific T cells were quantitated using a modification of the techniques of Waldrop et al25 as previously described.21–23 Quantitation of tetramer+ T-cells was performed using CMVpp65 MHC-peptide tetramers for HLA A*0201, A*2402 and B*0702 bearing peptide sequences NLVPMVATV, QYDPVAALF, RPHERNGFTV and TPRVTGGGAM respectively (Beckman Coulter, Inc Fullerton, CA) as previously described.22
Functional Characterization and Epitope Mapping by Intracellular IFN-gamma Assay
Epitope identification was performed using a mapping grid of CMVpp65 peptide subpools as previously described.21 T-cell responses to specific peptides or sub-pools of CMVpp65 were quantitated by measuring the number of IFN-γ+ T-cells generated upon secondary stimulation with autologous peptide-loaded APCs.21–23
Cytotoxicity of CMVpp65CTLs In-vitro
CMVpp65CTLs were assessed for their capacity to lyse CMVpp65 loaded targets using a standard 51chromium release assay.23,24 Targets used in all experiments included peptide-loaded and unloaded autologous and fully allogeneic PHA blasts. To define the HLA restriction of epitope-specific CMVpp65CTLs, they cytotoxic activity was measured against a panel of EBV-BLCL or PHA blasts loaded with the peptide, each sharing with T-cells of a given donor a single HLA allele, as previously described.21,24
Analysis of TCR Vβ Repertoire within CMVpp65 Specific T-cells
The CMVpp65 peptide-HLA tetramer+ T-cells contained within each CMVpp65CTL line were analyzed for TCR Vβ repertoire by FACS as previously described22, using a commercially available kit (IO Test® Beta Mark, Beckman Coulter, Inc, France) according to procedures provided by the manufacturer.26
Monitoring of patients and follow up after CMV CTL infusion
Patients were sequentially monitored for toxicities using the Common Terminology Criteria for Adverse Events (CTCAE) [Version 4, 2009] and for acute GvHD as graded by the CIBMTR Consensus. 27
CMV in the blood was measured by quantitation of CMV antigenemia in the initial 8 patients and by CMV polymerase chain reaction thereafter, using methods previously described.28–31 CMV levels were monitored prior to the T-cell infusion, at weekly intervals for 6 weeks thereafter and monthly until clearing or clinical progression.
T-cell responses were measured by quantitating IFNγ+ T-cells in response to the total pool of CMVpp65 peptides as previously described.21,22 The CMVpp65 epitope specifications and HLA restrictions of the T-cells in the blood were identified as discussed above. Following infusion of T-cells known to contain CMV peptide/HLA tetramer+ T-cells, the tetramer+ T-cells were also monitored by FACS.21,22 In addition, the T-cell receptor (TCR) Vβ repertoires of the tetramer+ cells or IFNγ+ CMVpp65CTLs were characterized both in the CMVpp65CTLs infused and sequentially in the patient.
Results
Patient status prior to infusion of CMVpp65CTLs
Of the 16 patients treated with transplant donor-derived CMVpp65CTLs following allogeneic HLA-matched related (n=12) or unrelated (n=4) HCT, two (UPN 1 + 2) received unmodified allo HSCT with post transplant immunosuppressive drugs as GVHD prophylaxis. UPN 1 was on standard doses of CSA and mycophenolate mofetil at time of treatment. UPN 2 was being treated for active GvHD, grade III, of skin and gut with CSA 200mg q 12 hrs, MMF 1gm q 12 hrs and methylprednisolone 0.5mg/kg daily. Patients 3–16 received TCD HSCT without immunosuppressive drugs to prevent GvHD. UPN 13 had been treated with parenteral steroids for grade II acute GvHD of the gut prior to CTL infusion but by the time of CTL infusion was off all immune suppression.
As detailed in Table 2, each patient had received extensive treatment with antiviral drugs prior to adoptive transfer of CMVpp65CTLs and had either not responded or was intolerant to further drug therapy. The median time to CMV reactivation post allo HSCT was 36 days (range 0 – 94 days). The median time to first treatment with CMVpp65CTLs post allo HSCT was 120 days (range 78 – 164 days). All patients had persistent CMV viremia at time of treatment with CMVpp65CTLs. Three patients (UPN 1, 6, 7) had concurrent interstitial pneumonia but biopsy or BAL was refused prior to treatment. Two patients (UPN 5 + 9) were diagnosed with CMV retinitis, including one with meningoencephalitis (UPN19).
Table 2.
Status at time of adoptiv transfer of pp65-specific T cells
| UPN# | Day of first CMV detection post transplant | Antiviral drugs before T cell transfer | Duration of antiviral treatment prior to CMV CTL infusion/outcome on antivirals | Site and symptoms of CMV Infection before T cell Transfer | Day of CTL infusion post SCT/(dose given) | Time post infusion to first detection of vivo expansion of transferred CMV-specific T cells |
|---|---|---|---|---|---|---|
| 1 | 71 | Foscarnet, Ganciclovir, Cytogam | Antivirals: 18 weeks/non responsive, rising titer | Viremia, Pneumonia | 164/(5×105/kg) | N/D* |
| 2 | 94 | Valcyte, Foscarnet, Cytogam | Antivirals: 8 weeks/non responsive, rising titer | Viremia | 156/(5×105/kg) | 1 week |
| 3 | 59 | Valcyte, Foscarnet, Cytogam | Antivirals: 13 weeks/non responsive, secondary reactivation + rising titer | Viremia | 116/(5×105/kg) | 4 weeks |
| 4 | 26 | Valcyte, Ganciclovir, Foscarnet, Cytogam | Antivirals: 12 weeks/non responsive, secondary reactivation + rising titer | Viremia, low grade fever | 126/(1×106/kg) | 1 week |
| 5 | 53 | Valcyte, Foscarnet, Cytogam | Antivirals: 12 weeks/non responsive, refractory, rising titer | Viremia, Retinitis | 140/(1×106/kg) | 2 weeks |
| 6 | 32 | Cytogam, Valcyte | Antivirals: 16 weeks/non responsive, rising titer | Viremia, Pneumonia | 138/(1×106/kg) | 2 weeks |
| 7 | 21 | Cytogam, Valcyte | Antivirals: 10 weeks/non responsive, rising titer | Viremia, Pneumonia | 98/(2×106/kg) | N/D |
| 8 | 0 | Valcyte, Foscarnet, Cytogam | Antivirals: 12 weeks/non responsive, rising titer | Viremia | 112/(2×106/kg) | 4 weeks |
| 9 | 0 | Foscarnet, Ganciclovir, Cytogam | Antivirals: 12 weeks/non responsive, resistant | Viremia, Retinitis, Asymptomatic Encephalitis | 99/(2×106/kg) | 4 weeks |
| 10 | 38 | Foscarnet | Antivirals: 4 weeks/drug toxicity | Viremia | 150/(2×106/kg) | 1 week |
| 11 | 29 | Foscarnet, Cytogam | Antivirals: 7 weeks/non responsive, secondary reactivation + rising titer | Viremia | 78/(2×106/kg) | 1 week |
| 12 | 26 | CMX, Valcyte, Cytogam | Antivirals: 12 weeks/non responsive, refractory, rising titer | Viremia | 104/(2×106/kg × 3 doses) | 1 week |
| 13 | 27 | Foscarnet, Valcyte, Cytogam | Antivirals: 8 weeks/non responsive, secondary reactivation + rising titer | Viremia | 88/(2×106/kg × 3 doses) | 4 weeks |
| 14 | 28 | Valcyte, Ganciclovir, Foscarnet, Cytogam | Antivirals: 13 weeks/non responsive, rising titer | Viremia | 145/(2×106/kg × 3 doses | 1 week |
| 15 | 42 | Valcyte, Foscarnet, Cytogam | Antivirals: 10 weeks/non responsive, secondary reactivation + rising titer | Viremia | 99/(2×106/kg × 3 doses) | 1 week |
| 16 | 29 | Valcyte, Ganciclovir, Foscarnet | Antivirals: 11 weeks/non responsive, rising titer | Viremia | 105/(2×106/kg × 3 doses) | 1 week |
N/D = Not determinable
Characterization of T-cells Infused
CMVpp65CTLs were predominantly CD8+ in 14/15 lines tested (Table 3). Tetramer+ CMVpp65CTLs were primarily (≥ 97%) of the CD62L−, CCR7−, CD45RO effector memory phenotype (TEM). However, small populations of tetramer+ CD62L+, CCR7+, CD45RO TCM were detected in 6/8 lines tested.
Table 3.
Characterization of CMV pp65-specific CTLs administered
| UPN # |
Total T cells /kg/dose |
CD8 (%) |
CD4 (%) |
Epitope | TCR Vβ | Tetramer positive CD8 | Interferon γ positive CD3 | Cytotoxicity dominant/ subdominant |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Abs# infused /kg/dose |
%CD62L, CCR7(+)/ %CD62L, CCR7(−) |
% | Abs# infused /kg/dose |
% | HLA restriction | ||||||
| 1 | 5 × 105 | 78 | 12 | QYDPVAALF | 5.1 | 4 | 2 × 104 | 0/100 | 3.2 | 1.6 × 104 | 38 | A2402 |
|
| ||||||||||||
| 2 | 5 × 105 | 91 | 9 | NLVPMVATV | 13.1 | 71 | 35 × 104 | 2/98 | 20 | 10 × 104 | 42 | A0201 |
|
| ||||||||||||
| 3 | 5 × 105 | 85 | 15 | RPHERNGFTV | 7.1, 13.2 | 7 | 3.5 × 104 | 0/100 | 5 | 2.5 × 104 | 47 | B0702 |
|
| ||||||||||||
| 4 | 1 × 106 | 92 | 8 | TPRVTGGGAM | 7.1, 17.1 | 12 | 12 × 104 | 3/97 | 15 | 15 × 104 | 61 | B0702 |
|
| ||||||||||||
| 5 | 1 × 106 | 68 | 32 | TPRVTGGGAM RPHERNGFTV |
7.1, 17.1 8, 21.3, 14 |
5 2 |
5 × 104 2 × 104 |
2/98 | 3 | 3 × 104 | 54 | B0702 |
|
| ||||||||||||
| 6 | 1 × 106 | 86 | 14 | QYDPVAALF | 5.2, 8 | 3 | 3 × 104 | 1/99 | 2 | 2 × 104 | 18 12 |
A2402/DRB1 0404 |
|
| ||||||||||||
| 7 | 2 × 106 | 64 | 36 | QPFMRPHER | N/D* | N/A | - | - | 4 | 80 × 104 | 21 14 11 |
DRB1 0301/C0601, A0301 |
|
| ||||||||||||
| 8 | 2 × 106 | 87 | 13 | RPHERNGFTV TPRVTGGGAM |
5.1, 1 7.1, 17, 1 |
11 5 |
22 × 104 10 × 104 |
4/96 3/97 |
9 - |
18 × 104 - |
51 - |
B0702 - |
|
| ||||||||||||
| 9 | 2 × 106 | 42 | 58 | QIFLEVQAIRET | N/D | N/A | - | - | 3 | 6 × 104 | 35 15 |
DRB1 1501/B3508 |
|
| ||||||||||||
| 10 | 2 × 106 | Not tested | - | - | - | - | - | - | - | - | - | |
|
| ||||||||||||
| 11 | 2 × 106 | 82 | 18 | NVSVNVHNPTG | N/D | N/A | - | - | 10.3 | 20.6 × 104 | 17 15 |
B2704/DRB1 1202 |
|
| ||||||||||||
| 12 | 1 × 106 ×3 | 88 | 12 | PTFTSQYRIQG | N/D | N/A | - | - | 9 | 9 × 104 × 3 | 71 25 33 |
A0101 B0801/DRB1 0301 |
|
| ||||||||||||
| 13 | 1 × 106 ×3 | 78 | 22 | FVFPTKDVAL | N/D | N/A | - | - | 13 | 13 × 104 × 3 | 18 | B3502 |
|
| ||||||||||||
| 14 | 1 × 106 ×3 | 97 | 3 | NLVPMVATV/QARLTVSGLA | 13.6, 21 | 6 | 6 × 104 × 3 | - | 2.4 | 2.4 × 104 × 3 | 42 18 |
A0201/B 5201 |
|
| ||||||||||||
| 15 | 1 × 106 ×3 | 92 | 8 | GVMTRGRLKA | N/D | N/A | - | - | 1.2 | 1.2 × 104 × 3 | 29 | B 0705 |
|
| ||||||||||||
| 16 | 1 × 106 ×3 | 91 | 9 | NLVPMVATV | 13.1, 14 | 42 | 42 × 104 × 3 | 3/97 | 9 | 9 × 104 × 3 | 48 | A0201 |
N/D = Not determinable
Responding T-cells were specific for a single pentadecapeptide derived from CMVpp65 in 9/15 cases adequately tested (Table 3). The peptide epitopes identified in these 9 lines and their presenting HLA alleles are described in Table 3. Six lines (UPN 6, 7, 9, 11, 12, 14) contained IFNγ+ T-cells specific for more than one CMVpp65 epitope. One line contained CD8+ T-cells specific for two epitopes; the NLV peptide presented by HLA-A*0201 elicited the dominant response while the QARLTVSGLA peptide presented by HLA-B*5201 induced a lesser or subdominant response. In 5 lines, a single CMVpp65 pentadecapeptide contained a nonamer presented by a class I and an overlapping 11-mer presented by a class II HLA allele shared by the donor that elicited CD8+ and CD4+ T-cell responses respectively. The proportion of IFNγ+ T-cells contained in the CTL lines specific for each epitope varied, ranging from 1.2% to 20%. As a result, doses of CMVpp65 peptide specific T-cells ranged from 1–15×104 IFNγ + T-cells/kg, and were not well correlated with the total T-cell doses administered from each CMVpp65CTL line.
The TCR Vβ phenotype of tetramer + CMVpp65CTLs was also analyzed in CTL lines responding to epitopes for which HLA peptide tetramers were available. These tetramer+ CMVpp65CTLs demonstrated an oligoclonal TCR Vβ repertoire, with the majority of epitope specific T-cells bearing Vβ segments from 1–3 TCR Vβ families (Table 3).
Clinical Outcomes and Toxicities
Infusions at each dose level were well tolerated. No patient experienced fever, alterations in vital signs or other toxicities over the course of the first 48 hours of observation. No patient developed manifestations of de novo acute GVHD. Furthermore, neither of the two patients who had GVHD (UPN 2 and 13) prior to infusion exhibited an exacerbation or worsening of their GVHD.
As summarized in Table 4, clearance of CMV was observed in 14/16 patients. Five patients had presumed or documented CMV disease prior to adoptive therapy. Two with documented CMV retinitis cleared their disease. Of the three patients with interstitial pneumonia, UPN7 failed to respond over 31 days follow-up and died of pneumonia without clearing CMV. UPN1 cleared CMV viremia within 14 days of infusion, but died of pneumonia 36 days post CTL infusion. Bronchoalveolar lavage obtained in the week prior to her death was positive for Mycobacterium avium but negative for CMV. UPN6 exhibited delayed clearance of CMV antigenemia, with persisting interstitial pneumonia. She cleared her CMV viremia by 47 days post infusion but died of persistent pneumonia 12 days later. BAL 7 days earlier was CMV negative.
Table 4.
Responses to adoptive transfer of pp65-specific CTLs
| UPN # | Course of CMV Infection after T cell transfer | Clearance from time of CTL infusion and (transplant) Cause of Death until last observation | Additional observation | |||
|---|---|---|---|---|---|---|
| Pre-infusion | 2 weeks post | 4 weeks post | 8 weeks post | |||
| 1 | >100 cells/slide | 13 cells/slide | Negative | NE | Death of Interstitial pneumonia 36 days post infusion (d 200 post SCT) | BAL culture prior to death + for M. avium: CMV negative Succumbed day 36 |
|
| ||||||
| 2 | 5 cells/slide | 17 cells/slide | Negative | Negative | Clearance of CMV viremia 4 weeks post infusion (d 180 post SCT) | Death of complication of GVHD 464 days post SCT |
|
| ||||||
| 3 | 19 cells/slide | 20 cells/slide | 2 cells/slide | Negative | Clearance of CMV viremia 8 weeks post infusions (d 172 post SCT) | Death of pulm failure due to hepato-pulm syndrom: 2nd to VOD, 225 days post SCT |
|
| ||||||
| 4 | 33 cells/slide | Negative | Negative | Negative | Clearance of CMV viremia 2 weeks post infusion (d 140 post SCT) | Completed follow-up on protocol – remains in long term follow-up > 4 years post SCT |
|
| ||||||
| 5 | 24 cells/slide | 14 cells/slide | 19 cells/slide | Negative | Clearance of CMV viremia 7 weeks post infusion (d 187 post SCT) | Completed follow-up on protocol – remains in long term follow-up > 4 years post SCT |
|
| ||||||
| 6 | >100 cells/slide | >100 cells/slide | >100 cells/slide | Negative | Clearance of viremia 47 days post CTL infusion (d 181 post SCT) | Death of pulmonary failure: BAL d 182 culture positive for panresistent Enterococcus; CMV negative; died 193 days post SCT |
|
| ||||||
| 7 | >100 cells/slide | >100 cells/slide | >100 cells/slide | NE | Death of pneumonia/CMV 31 days post CTL infusion (d 129 post SCT) | Death of pneumonia; unclear etiology/CMV probable 129 days post SCT |
|
| ||||||
| 8 | 35 cells/slide | 65 cells/slide | 65 cells/slide | 1 cells/slide | Clearance of CMV viremia By 9 weeks post CTL infusion (d 175 post SCT) |
Relapse of AML; 9 months post SCT; died 588 days post SCT |
|
| ||||||
| 9 | PCR: 100 copies/mL (87,900 in CSF) | PCR: 100 copies/mL (18,900 in CSF) | Negative | Negative | Clearance of CMV viremia By 4 weeks post SCT (d 127 post SCT) |
Completed follow-up on protocol – remains in long term follow-up > 3 years post SCT |
|
| ||||||
| 10 | PCR: 100 copies/mL | Negative | Negative | Negative | Clearance of CMV viremia 2 weeks post CTL infusion (d 164 post SCT) | Relapse of AML; 9 months post SCT; died 342 days post SCT |
|
| ||||||
| 11 | PCR: 1323 copies/mL | Negative | Negative | Negative | Clearance of CMV viremia 2 weeks post CTL infusion (d 92 post SCT) | Completed follow-up on protocol – remains in long term follow-up < 2 years post SCT |
|
| ||||||
| 12 | PCR: 1427 copies/mL | PCR: 1342 copies/mL | PCR: 1191 copies/mL | Negative | Clearance of CMV viremia 8 weeks post SCT (d 160 post SCT) | Relapse of Mantle Cell Lymphoma; 19 months post SCT; died 610 days post SCT |
|
| ||||||
| 13 | PCR: 3469 copies/mL | PCR: 720 copies/mL | Detected, but < 500 copies/mL | PCR: 1150 copies/mL | Reduction of CMV viremia 21 days post CTL infusion (d 109 post SCT) | Death by fungal and mycobacterial infections 220 days post SCT; still CMV viremic |
|
| ||||||
| 14 | PCR: 53,320 copies/mL | PCR: 9394 copies/mL | PCR: 13,710 copies/mL | Negative | Clearance of CMV viremia 8 weeks post CTL intusion (d 201 post SCT) | Developed hemolytic anemia day 270 post SCT Treated with high-dose steroids; CMV reactivated with CMV encephalitis, from which he died 342 days post SCT |
|
| ||||||
| 15 | PCR: 637 copies/mL | Negative | Negative | Negative | Transient clearance of CMV infection by 2 weeks post CTL infusion. Patient reactivated approximately 8 weeks post initial CTL infusion has received 2 add’l cycles of CMV CTLs. Clearance of CMV viremia 4 weeks post 3rd cycle of CTL infusion (d 259 post SCT) |
Patient remains CMV PCR negative 18 months post 3rd cycle of CMV CTLs |
|
| ||||||
| 16 | PCR: 3300 copies/mL | PCR: 76,900 copies/mL | PCR: 40,100 copies/mL | Negative | Clearance of CMV viremia 8 weeks post CTL infusion (d 161 post SCT) | Patient remains CMV PCR negative > 20 months post CMV CTLs |
NE = Not examined
Of 11 patients who received CMVpp65CTL as treatment for viremia persisting despite antiviral treatment, 10 cleared their viremia. In one patient (UPN13), CMV viremia was not cleared but reduced from 3649 to <500 copies CMVDNA/ml by 21 days post infusion (day 109 post HSCT). This patient subsequently received steroids for preexisting grade 2 intestinal GVHD. CMV viremia recurred; the patient subsequently died of sepsis 132 days post infusion.
Of the 16 patients, 12 were still maintained on antiviral drugs for periods of 15 – ≥ 36 days post T-cell infusion. However, for 4 patients (UPN 4, 5, 8 and 11), antiviral drugs were discontinued within one (UPN 4, 5 and 11) or 3 weeks (UPN 8) of receiving CMVpp65CTL, due to intolerable drug induced toxicities. Despite this, all cleared their viremia, including UPN5 who had advanced retinitis prior to initiation of T-cell infusions.
Alterations of circulating CMV specific T-cells following adoptive T-cell transfer and their correlation with CMV viremia
Results of sequential quantitation of IFNγ+ T-cells and/or CMVpp65-HLA tetramers are summarized in Table 5. Of 16 patients 5 pts. (UPN 1, 2, 11, 15, 16), had low but detectable CMVpp65-specific IFNγ+ T-cells, prior to T-cell infusion. In comparison, 6/9 patients (UPN 1, 2, 3, 5, 14, 16) expressing HLA-A*0201, A*2402 or B*0702 had quantifiable tetramer+ CMVCTL prior to CMVpp65CTL infusion including 2/10 patients without detectable IFNγ+ CMVCTL.
Table 5.
Characterizatioin of T-cell Responses to CMV pp65 after Infusion of CMT CTLs
| UPN# | Analysis (Tet = 1st row IFN = 2nd row) | Absolute number of CMV-Specific CTL/106 PBMC after T-cell transfer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-infusion | 1 week post | 2 weeks post | 4 weeks post | 6 weeks post | 8 weeks post | 16 weeks post | 32 weeks post | ||
| 1 | A24 | 60 | 510 | 80 | D | D | D | D | D |
| IFN | 60 | 280 | 10 | D | D | D | D | D | |
|
| |||||||||
| 2 | A2 | 200 | 900 | 1800 | 2200 | 3900 | 4200 | 1400 | 900 |
| IFN | 120 | 400 | 500 | 1400 | 1100 | 1800 | 900 | 500 | |
|
| |||||||||
| 3 | A2 | 50 | 600 | 170 | 840 | 760 | 2500 | 2000 | 1200 |
| B7 | 120 | 530 | 80 | 430 | 140 | 1000 | 720 | 400 | |
| IFN | 0 | 360 | 44 | 30 | 60 | 800 | 2700 | 800 | |
|
| |||||||||
| 4 | A2 | 0 | 50 | 100 | 70 | 0 | 200 | 50 | 100 |
| B7 | 0 | 1764 | 2200 | 950 | 533 | 6100 | 8400 | 11800 | |
| IFN | 0 | 172 | 240 | 100 | 400 | 2900 | 3300 | 4700 | |
|
| |||||||||
| 5 | A2 | 0 | 0 | 0 | 100 | 310 | 700 | 250 | 50 |
| B7 | 30 | 50 | 700 | 1700 | 5100 | 3200 | 800 | 300 | |
| IFN | 0 | 20 | 120 | 200 | 500 | 612 | 196 | 330 | |
|
| |||||||||
| 6 | A24 | 0 | 0 | 0 | 0 | NP | D | D | D |
| IFN | 0 | 60 | 190 | 52 | NP | D | D | D | |
|
| |||||||||
| 7 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 0 | 0 | 60 | 0 | D | D | D | D | |
|
| |||||||||
| 8 | B7 | 0 | 0 | 0 | 900 | 2100 | 600 | 1522 | D |
| IFN | 0 | 0 | 0 | 200 | 460 | 340 | 600 | D | |
|
| |||||||||
| 9 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 0 | 0 | 30 | 133 | 110 | 82 | 0 | D | |
|
| |||||||||
| 10 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 0 | 70 | 40 | 200 | 120 | 192 | D | D | |
|
| |||||||||
| 11 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 10 | 256 | 384 | 480 | 512 | 352 | 160 | 320 | |
|
| |||||||||
| 12 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 0 | 2200 | 830 | 732 | 300 | 400 | 3050 | 1100 | |
|
| |||||||||
| 13 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 0 | 5 | 3 | 15 | 40 | 80 | 290 | 140 | |
|
| |||||||||
| 14 | A2 | 860 | 2246 | 435 | 2423 | 8629 | 5707 | 2912 | NP |
| IFN | 0 | 270 | 140 | 822 | 2308 | 1711 | 1240 | NP | |
|
| |||||||||
| 15 | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| IFN | 92 | 2369 | 1130 | 1560 | 498 | 325 | 246 | 269 | |
|
| |||||||||
| 16 | A2 | 45 | 180 | 1485.96 | 1824 | 2600 | 9250 | NP | NP |
| IFN | 30 | 162 | 748.2 | 775.2 | 1170 | 5365 | NP | NP | |
Tet = HLA Peptide Tetramer, IFN = T-cell Interferon γ Analysis; NT = No Tetramer Available; NP= Not Performed; D = Deceased
Following infusion of the CMVpp65CTLs, 15/16 patients adequately tested had detectable increases in IFNγ+ CMVCTL to levels ≥ 100/106 PBMC in the blood. Such increments were detected within 1 week post infusion in 9/16 patients, but were not detected until 2–4 weeks in 4 patients and 16 weeks in 1 (UPN 13). IFNγ+ T-cells did not increase to ≥ 100/106 post infusion in 1 patient (UPN 7). UPN 7 and 13 did not clear CMV viremia. UPN 7 ultimately died of CMV infection; UPN 13 had a late T-cell response. She died of a secondary infection, but still had CMV viremia.
Of 9 patients inheriting HLA-A*0201, A*2402 or B*0702 tested, 8 exhibited increments in CMVpp65 tetramer+-T-cells following infusions of CMVpp65CTLs in which T-cells restricted by one of these alleles were immunodominant. These increments were detected by 1 week post infusion in 7 patients (including all 7 with detectable tetramer+ cells pre-infusion) and by 2–4 weeks in 2 of the patients. UPN 6, who received CMVpp65CTLs co-dominantly restricted by an overlapping peptide presented by HLA-A*2402 and DRB1*0401 did not generate HLA-A*2402/CMVpp65 tetramer+ T-cells post infusion but did develop IFNγ+ CMVCTL. Three patients (UPN 3, 4, 5) who received CMVpp65CTLs that selectively exhibited reactivity against CMVpp65 epitopes presented by HLA-B*0702 (Table 1), developed two CMVCTL populations, one specific for the epitope targeted by the infused T-cells presented by HLA-B*0702 allele, and the other specific for the NLV epitope presented by HLA A*0201. While responses to the epitope presented by HLA-A*0201 were markedly lower than those to the epitopes presented by HLA-B*0702 in patients UPN 4 and 5, the HLA A*0201 restricted response was dominant post CMVpp65CTL infusion, in patient UPN 3, potentially reflecting co-expansion of T-cells specific for the epitope presented by HLA-A*0201 detected in the patient prior to CMVpp65CTL infusion.
The kinetics of expansion of the CMVCTL post infusion and their correlation with alterations in CMV viremia are illustrated by three representative patients, UPN2, UPN5 and UPN8 (Figures 1 – 3). As shown in Fig 1, of the T-cells administered to UPN2. 71% bound HLA-A*0201/NLV tetramers (Fig 1A). Infusion of these T-cells was initially followed by a peak of CMV antigenemia followed by an abrupt fall to undetectable levels that was contemporaneous with the detection of expanding populations of CMVpp65/HLA-A*0201 tetramer+ cells in the blood (Fig 1D, 1G, 1E).
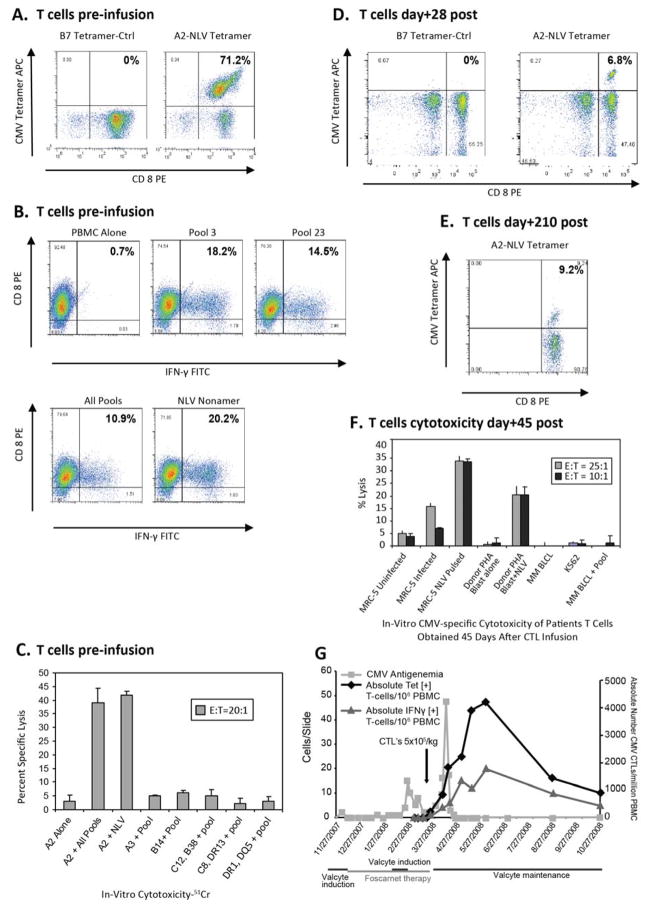
Figure 1. Induction of Clinical Response after infusion of Donor-Derived HLA-A*0201 Restricted NLV Epitope-Specific CMV CTLs in a Viremic Patient.
A–C. CMV CTL Characterization prior to infusion for UPN#2 is shown.
A. Aliquots of 105 T-cells labeled with anti-CD3 FITC and anti-CD8 PE as well as APC conjugated HLA-A*0201-NLV or HLA-B*0702-TPR (control) tetramers were analyzed via FACS.
B. Functional characterization and epitope mapping was performed for the CTLs prior to infusion by quantitating the proportion of CD8+ T-cells generating IFN-γ upon overnight stimulation with aliquots of autologous PBMC, each loaded with one of 24 individual subpools containing specific CMVpp65 pentadecapeptides. An overlapping grid of the peptide subpools permitted epitope identification. As shown, IFN-γ+ CD8+ T-cells were seen in response to targets loaded with pool 3 and 23 corresponding to the NLV peptide.
C. The in-vitro cytotoxicity and the HLA allele restriction of the T-cell cytotoxic activity are shown. A panel of peptide loaded EBV BLCLs sharing a single HLA allele with the T-cell donor were used in a 4 hour 51chromium release assay to define the HLA restriction. The T-cells shown were exclusively cytotoxic against HLA-A*0201 sharing targets loaded with CMVpp65 peptide pool or the NLV peptide.
D–F. UPN#2. Analysis of CMVpp65 specific T-cells post CMV CTL infusion.
D. Tetramer analysis is shown for T-cells directly obtained from patient’s peripheral blood at day 28 and 210 post infusion. Distinct populations of HLA-A*0201-NLV tetramer binding T-cells are demonstrated in comparison to a control HLA-B*0702-TPR tetramer.
E. Cytokine release assay is shown at day 45 post CTL infusion demonstrating CD8+ IFN-γ+ T-cells in response to overnight stimulation with autologous NLV peptide loaded PBMC.
F. The cytotoxic activity of T-cells recovered from patient’s blood 45 days after CTL infusion was tested in-vitro in a chromium release assay against an HLA-A*0201 [+] human fibroblast cell line (MRC5) either uninfected, or CMV AD169 infected or loaded with the NLV peptide. PHA blasts from the T-cell donor as well as HLA mismatched BLCLs either alone or loaded with the NLV peptide were used as controls.
G. Clinical response and in-vivo kinetics of CMV CTLs after infusion is shown for UPN#2. The arrow indicates the time of infusion of the CMV CTLs. The clinical response was followed by CMV antigenemia assay (■) performed twice a week. The number of CMV specific T-cells detected after infusion is plotted as the absolute number of HLA A*0201-NLV tetramer [+] T-cells/106 PBMC of blood (◆) and IFNγ+ CD3+ T-cells/106 PBMC of blood (Δ)detected at day 0, 1, 7 and weekly thereafter.
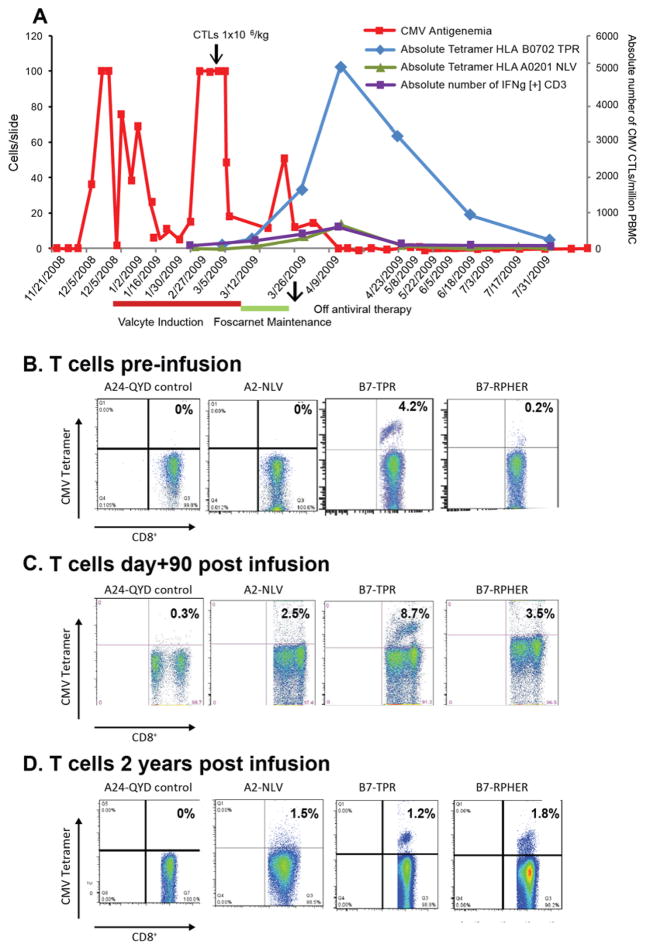
Figure 3. Sequential Evaluation of T-cell Responses and CMV Antigenemia post Adoptive Therapy in a Patient with CMV Chorioretinitis.
A. The clinical response and in-vivo kinetics of CMV CTLs after infusion is shown for UPN#5 inheriting HLA-A*0201 and HLA-B*0702. The arrow indicates the infusion of CMV CTLs. The clinical response was followed by CMV antigenemia assay (■) performed twice a week. The CMV CTLs detected after infusion are plotted as the absolute number of for HLA-B* 0702-TPR (◆) and HLA-A*0201-NLV tetramers (Δ) epitope specific tetramer[+] Tcells/106 PBMC of blood and of IFNγ+ CD3+ (■) T-cells/106 PBMC detected at day 0, 1, 7 and weekly thereafter.
B. Tetramer analysis of the CMV CTLs infused to patient UPN#5 co-inheriting HLA-A*0201 and HLA-B*0702 is shown. The CTLs predominantly bound to tetramers for HLA-B*0702-TPR, and also for HLA-B*0702-RPHER, but did not bind to tetramers for HLA-A*2402-QYD (control) or HLA-A*0201-NLV.
Tetramer analysis of patient’s T-cells performed at day 90 (C.), and at 2 years (D.) post infusion of CMV CTLs is shown, which demonstrates T-cells binding to tetramers for HLA-B* 0702-TPR, HLA-B*0702-RPHER, and emergence of T-cells binding to tetramers for HLA-A* 0201-NLV.
UPN 8 received a single infusion of 2×106 CMVpp65CTLs/Kg after failing to respond to ganciclovir or foscarnet. The IFNγ+ T-cells in this line selectively responded to the RPHER and TPR peptides of CMVpp65 presented by HLA B*0702 and could be tracked by their binding of RPHER/HLA B*0702 tetramers post infusion. As shown in Figure 2, following T-cell infusion, CMV antigenemia transiently increased, but then cleared as the concentration of tetramer+ cells increased in the blood.
Figure 2.
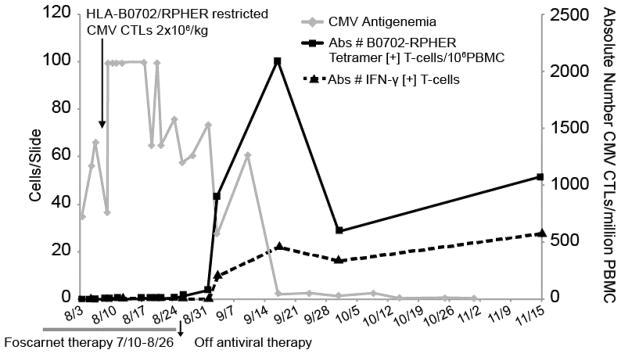
HLA-B*0701 Restricted T cells Induce Disease Clearance
The CMVpp65CTLs infused into UPN5 (Fig. 3) were also specific for these two epitopes presented by HLA-B*0702; 4.2% of the T-cells bound HLA B*0702 tetramers bearing the TPR peptide and 0.2% bearing the RPHER peptide (Fig. 3B). CMVpp65CTLs were administered following failed treatment with Valcyte, and initiation of Foscarnet, at maintenance dosing only, because of associated toxicity. Again, reduction of CMV antigenemia was observed only with the emergence of TPR/HLA-B*0702 tetramer-binding CMVCTL, and cleared 2 weeks post infusion, at which time Foscarnet maintenance was stopped. Thereafter, the patient remained free of CMV antigenemia (Fig. 3A). Levels of TPR/HLA-B*0702 tetramer+ cells decreased by 16 weeks post infusion but have remained detectable throughout follow-up. In addition, by day 90 post infusion (Fig. 3C), limited numbers of HLA-A*0201 restricted T-cells specific for the NLV peptide were also detectable. At 2 years post infusion (Fig. 3D) T-cells specific for TPR and RPHER are still prominent, as are the NLV specific T-cells first detectable at day 90.
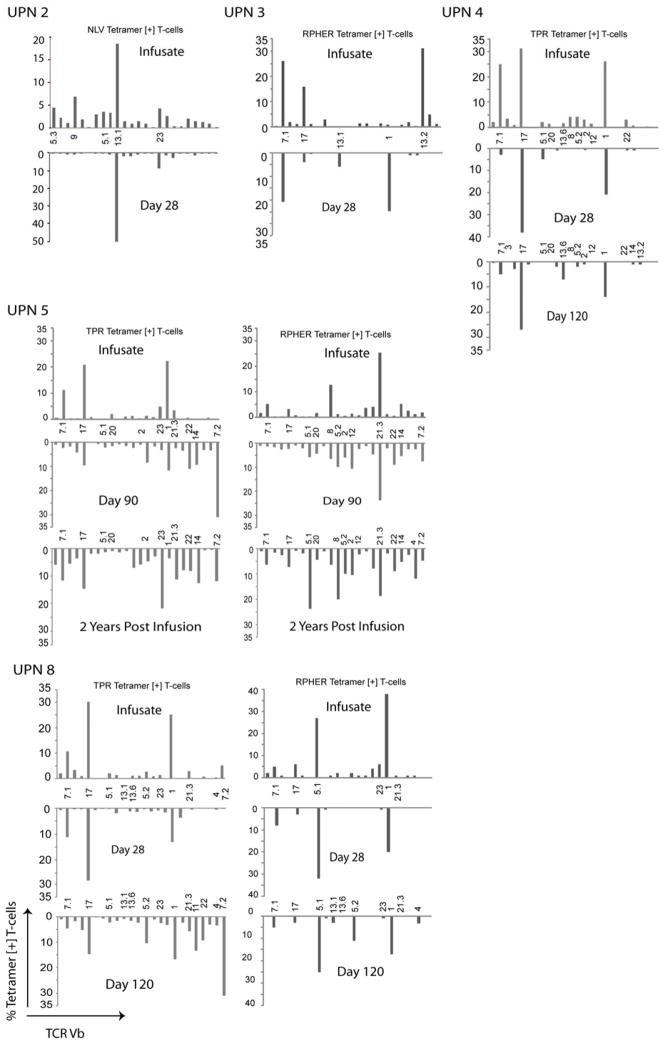
To further delineate the contribution of the adoptively transferred CMVpp65CTLs to the CMVCTL detected following CMVpp65CTL infusions, we examined the TCRs of isolated MHC-peptide tetramer+ T-cells for their Vβ usage as shown in Figure 4. The TCR Vβ characteristics of the tetramer+ CMVCTL isolated from the blood of patients 2, 3, 4, 5 and 8, 28–35 days post infusion closely matched those of the tetramer+ CMVpp65CTLs infused. For example, the CMVpp65CTLs infused into patient 2 were specific for the NLV peptide presented by HLA-A*0201. The NLV/HLA-A*0201 tetramer+ CMVpp65CTLs predominantly expressed TCRs bearing Vβ13.1. At day 28 post transfusion, the NLV/HLA-A*0201 tetramer+ CMVCTL detected in the blood were almost exclusively Vβ13.1+ T-cells. Similarly, the Vβ repertoires of the HLA-B*0702 restricted CMVCTL specific for the immunodominant TPR and RPHER epitopes isolated from the blood of patients 4 and 8 matched those of the CMVpp65CTLs infused. While RPHER/HLA B*0702 tetramer+ CMVCTL isolated from the blood of UPN 5 and 8, and TPR/HLA B*0702 tetramer+ CMVCTL in UPN 4, still contained a predominance of CMVCTL bearing TCRs with the same Vβs 90–120 days post infusion, CMVCTL bearing other Vβs are also in evidence. In UPN 5, T-cells binding NLV/HLA-A*0201 tetramers that were not detected in the CMVpp65CTLs infused, were also present. Taken together, these data support the hypothesis that the T-cells responding to and clearing CMV viremia early after adoptive transfer are the CMVpp65CTLs infused, while CMVCTL detected late after infusion are a mix of the CMVpp65CTLs transfused and other CMVCTL generated from expansion of undetected small populations of CMVCTL in the transferred cells, expansion of T-cells from residual mature CMVCTL in the patient’s transplant or new CMVCTL developing from precursors in the graft that mature in the host thymus.
Fig. 4. Infused CMV CTLs Demonstrate an Oligoclonal TCR Vb Phenotype Represented in Specific Vb Families which are also Detected after Infusion.
The TCR Vb phenotype of the infused tetramer positive CMV CTLs (top bar graph) was compared to the TCR Vb of the tetramer + CMV CTLs recovered from the patient’s blood after CTL infusion (bottom mirror image bar graph) at early and late time points.
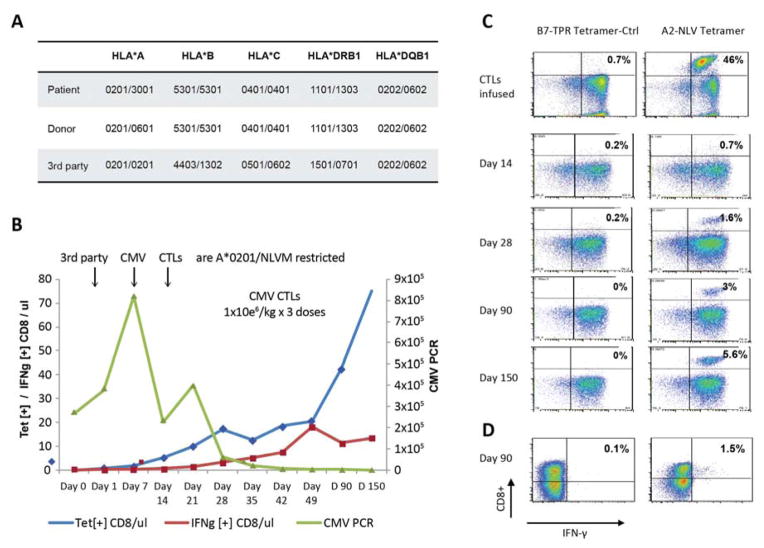
We similarly assessed the activity of third-party donor-derived CMVpp65CTLs in one patient. UPN 17 received an unmodified (HLA-A) mismatched unrelated transplant from a CMV seronegative donor at another center. CMV viremia developed post transplant but failed to respond to antiviral therapy. The patient’s CMV was found to be resistant to Foscarnet, Ganciclovir and Cidofovir by mutational analyses. As shown in Figure 5, this patient received 3 weekly doses of 1×106 third party CMVpp65CTLs/Kg that were matched for 2 HLA allelles, and specific for the NLV peptide presented by the HLA-A*0201 allele shared by patient, transplant donor and third-party CMVpp65CTL donor. Following the first infusion CMV DNA rose to 800K copies/μl blood, but then declined following the 2nd dose and subsequently cleared 21 days following the third dose of HLA-A*0201/NLV-restricted 3rd party CMVpp65CTLs. Figure 5C illustrates the corresponding incremental increase in HLA-A*0201/NLV tetramer+ CMVCTL frequencies in the patient’s blood from 0.7% on day +14 to 5.6% on day +150 post infusion. We also documented an increase in the IFN-γ+ T-cell frequencies responding to the total pool of CMVpp65 peptides and specifically the NLV peptide to 1.5% by day +49.
Fig. 5. Clinical Response after Infusion of Epitope Specific CMV CTLs from HLA Partially Matched Third Party Donors.
A. The HLA typing of the patient, the HSCT donor as well as the third party CMV CTL donor are shown. The third party donor was matched at HLA-A*0201 and HLA-DQB1*0202 and HLA-DQB1*0602 with the patient and donor.
B. The clinical response and in-vivo kinetics of CMV CTLs at various time points after infusion of third party donor derived CMV CTLs is shown. The black arrows indicate the infusion of CTLs. The CMV copies/μl blood (■) was followed as marker for clinical response, and the CMV CTLs were detected in patient’s blood using HLA A*0201-NLV tetramers specific for the epitope to which the infused third party T-cells were responsive. The absolute number of A*0201-NLV tetramer+ T-cells/μl (◆) and of IFNγ+CD8+ T-cells/μl (Δ) detected post infusion at day 0, 1, 7 and weekly thereafter are plotted.
C. Tetramer analysis of T-cells is shown from the patient prior to, days +14, +28, +90 and +150 after infusion of third party CMV CTLs. HLA-A*0201-NLV tetramer+ T-cells were not detected prior to CTL infusion, but were detectable thereafter with maximal response of 5.6% at day 150 post CMV CTL infusion.
D. Intracellular IFN-γ production of CD8+ cells is shown from the patient prior to and on day +90 (1.5%) after infusion of third party CMV CTLs.
Discussion
In 2005, our group described a technique for generating CMVpp65CTLs based on sensitization of HCT donor-derived T-cells with autologous CAMS loaded with a pool of 138 synthetic 15-mer peptides spanning the sequence of CMVpp65.21 We selected CMVpp65 because of the many proteins generated by CMV, CMVpp65 induces T-cell responses of the greatest magnitude and in the highest proportion of individuals, the second most immunogenic being the Immediate Early antigen 1 (I-E-1).32–34 More recent studies also indicate that presentation of CMVpp65 peptides, which does not require new protein synthesis, may be less susceptible to inhibition by subsequently generated evasins, the non-coding RNAs generated by CMV that can interfere with antigen processing and TAP mediated transport of antigen peptides to HLA alleles for presentation.35,36
In this Phase I trial, we tested the activity of transplant donor-derived T-cells sensitized with the pool of overlapping 15-mer peptides of CMVpp65 in a series of allogeneic HCT recipients who had either clinically overt infection or CMV viremia persisting despite prolonged treatment with antiviral drugs. As shown in Table 2, only 1 patient had received less than 2 antiviral drugs. All had been treated for periods of 7–18 weeks and at time of adoptive transfer had stable or increasing levels of CMV antigenemia or CMV DNA in the blood, despite antiviral therapy. Such patients have been reported to have a markedly increased mortality due to CMV disease and associated infections.37–39
The CMVpp65CTL infusions, at all doses, were well tolerated without clinical toxicities. Furthermore, no patient developed de novo acute or chronic GVHD or a flare of existing GVHD following adoptive transfer. This finding is similar to our own and other reported experiences with adoptive transfer of EBV-specific T-cells sensitized with autologous EBV BLCL over 3–5 weeks in vitro 40–43 to deplete allo responsive T-cells.
Of the total of 17 patients treated, all but two (UPN 7, 13) achieved durable clearance of CMV viremia. Of the three patients treated who had interstitial pneumonia at time of first infusion, two cleared CMV (UPN 1, 6) but all three ultimately died of complications of interstitial pneumonia, one with a concurrent MAI infection (UPN 1). Feuchtinger et al 44 have also reported continued pulmonary deterioration despite clearance of CMV. These findings raise concerns that treatment with CMVCTL may initially augment inflammatory responses in infected tissues resulting in additional tissue damage. On the other hand, UPN 5 and UPN 9 each had documented CMV retinitis that cleared without residual retinal damage.
Although groups of patients received escalating total doses of CMVpp65CTLs ranging from .05 to 2.0 × 106/Kg, the actual doses of CMVCTL provided at each dose level, as quantitated by peptide pool responsive IFNγ+ and tetramer+ T-cells, varied considerably (Table 3). However, we did not discern a relationship between clinical and/or viremic response and doses of CMVpp65CTLs/Kg administered or the absolute doses of CMVCTL provided. On the other hand, clinical response was consistently correlated with expansion of CMVpp65 reactive T-cell populations in vivo. These increments in the frequencies of CMVCTL were detected as early as day 7, and usually peaked by day 28. After clearance of CMV, CMVCTL levels fell to steady states that were maintained through 5–24 months of observation. In contrast, the two patients that failed to clear CMV had no significant increments in the frequencies of circulating CMVCTL.
Failure of in-vitro selected CMVpp65CTLs to proliferate after adoptive transfer has also been correlated with treatment failure by other groups 17,20,45, providing evidence both for the need to expand effector T-cell populations to achieve viral clearance, and the therapeutic potential of even small numbers of effector cells if they replicate sufficiently in vivo. 44 However, the factors contributing to a lack of proliferation are still poorly defined. In recipients of unmodified HSCT treated for CMV, ongoing treatment of GVHD with immunosuppressive drugs, particularly glucocorticosteroids, has been implicated.2,46 However, in our series, all but two patients received T-cell depleted transplants administered without immunosuppressive drug prophylaxis. In trials exploring adoptive T-cell therapy for EBV-associated lymphomas complicating allogeneic HSCT, failure of T-cells to expand in vivo has also been correlated with treatment failure, and has been ascribed to the inability of transferred T-cells to recognize the EBV+ lymphoma cells either because the EBV epitope targeted by the T-cells is deleted or mutated40,47 or, in HLA disparate patients, because the T-cells transferred are restricted by an HLA allele not shared by the tumor. Thus far, mutations in immunogenic peptides of CMVpp65 that would affect their recognition by HLA restricted CMVpp65 specific T-cells have been found to be infrequent among clinical isolates.48 However, CMV has developed a multi-tiered array of microRNAs, termed evasins, that can prevent the recognition or killing of infected cells by T-cells. For example, evasins such as US2,3,6 and 11 can disrupt the membrane localization and stability of specific MHC class I alleles, thereby impairing their expression.35,49,50 US3 and US6 can also prevent the transport and loading of processed peptides on HLA class I alleles for presentation to T-cells.36 In addition, Kim et al51 have recently shown that a CMV micro RNA US4-1 can downregulate the expression of an aminopeptidase essential to the editing of antigenic peptides during their processing within the endoplasmic reticulum. Unfortunately, we did not have infected cells or the viral isolates from the two patients who failed to clear CMV to examine whether one or more of these mechanisms may have contributed to the apparent failure of the transferred T-cells to recognize and expand in response to infected cells in the host.
Recently, other groups have also employed CMVpp65 15-mer peptide pools to stimulate the propagation of CMV-specific T-cells. Thus, Bao et al52 employed overlapping peptides of CMVpp65 and IE-1 to generate transplant donor-derived CMV specific T-cells for treatment of CMV viremia persisting despite antiviral treatment for >2 weeks in 7 patients, including 5 who had received T-cell depleted haploidentical grafts. Of these, 3 cleared CMV viremia and 3 had significant reduction of viral load, each associated with increments in circulating levels of CMV-specific T-cells. More recently, Peggs et al53 and Feuchtinger et al44 have evaluated donor-derived CMV-specific T-cells rapidly generated from the blood by sensitization for 16 hours with a pool of overlapping CMVpp65 peptides, followed by immunoselection of interferon γ producing T-cells. The median doses of CMVpp65 specific T-cells obtained and administered by this approach are low, (2–10×103/kg), which limits analysis of their specificities. Nevertheless, as shown by Feuchtinger et al,44 such T-cell doses can induce durable clearance of CMV in 50–60% of allogeneic HCT recipients with CMV infection or persistent viremia that have failed to respond to antiviral drugs. Furthermore, Peggs et al,53 have reported that over 90% of patients treated preemptively for CMV viremia with such T-cells clear infection after no or only 2–3 weeks treatment with a single antiviral drug. In both studies, in vivo expansion of CMVpp65-specific CD4+ and/or CD8+ T-cells was also correlated with response.
The group at Baylor College of Medicine has explored T-cells simultaneously sensitized to antigens from multiple viruses, including CMVpp65 and IE-1 to elicit CMV-specific T-cell responses. Initially, they used autologous dendritic cells or EBVBLCLs transduced with an adenoviral vector directing the expression of CMVpp65, and adenovirus proteins54 and, more recently, PBMC loaded with pools of overlapping 15-mer peptides from five viruses (Adv, EBV, CMV, BKV and HHV-6)55 to sensitize donor T-cells. In initial trials, both approaches have shown promise in limiting viremia55,56 and clearing CMV infection or drug refractory viremia54,55 as well as concurrent infections due to other targeted viruses.55 In the trial of T-cells sensitized with pooled peptides from five viruses, all of the T-cell cultures from seropositive donors generated T-cells specific for CMVpp65 or IE-1. This approach has the advantage of addressing infections from each of the viruses most often associated with disease in HCT recipients. However, T-cells responding to immunogeneic peptides from different viruses in the pool may also recognize epitopes presented by different HLA alleles on the antigen presenting cells. While such donor-derived T-cells would be expected to consistently include donor T-cells specific for viral peptides that are restricted by HLA alleles expressed by infected cells from an HLA matched recipient, they would not be active in an HLA-disparate patient unless the virus-specific T-cells are restricted by an HLA allele shared by infected cells of the host. Thus in HLA disparate hosts, the utility of such cells may be limited unless the HLA restrictions of the T-cells specific for each virus are identified.
For our study, we identified both the epitope specificity and HLA restriction of the CMVpp65 specific T-cells so as to gain information regarding the relative immunogenicity of the peptides and to be able to track the responding T-cells post infusion and correlate their growth in vivo with clinical activity. Despite the fact that the T-cells were sensitized with a pool containing broad array of CMVpp65 epitopes, of 138 15-mers, the CMVpp65CTLs generated over the 4–5 week course of in vitro culture consistently responded to only 1–3 peptide epitopes presented by one or more class I or II HLA alleles expressed by the donor (Table 3, Fig 1B). Analysis of the TCR Vβ usage of tetramer+ T-cells specific for these epitopes also revealed them to be oligoclonal (Fig. 4). The immunodominance of specific epitopes may have shaped the repertoire of CMVpp65CTLs generated in vitro. However, Kern et al, have observed a similar degree of this immunodominance in vivo in the blood of seropositive donors late after primary infection.57 As expected from prior reports, epitopes presented by HLA B*0702 were dominant in all 4 donors inheriting this allele, including 3 who co-inherited HLA A*0201.58 Similarly, the NLV peptide presented by HLA A*0201 was dominant in 3 donors inheriting HLA A*0201 that did not co-inherit HLA B*0702. All 8 recipients of CMVpp65CTLs restricted by HLA A*0201 or B*0702 cleared viremia including 3/3 with retinitis or pneumonia. In contrast, of 8 patients who received CMVpp65CTLs restricted by other alleles, 2 failed to clear viremia and ultimately died of infection and 1 patient (PT #15) uniquely required three cycles of CMVpp65CTLs to clear viremia.
Multicenter trials that accrue large and genetically diverse patient populations will be required to determine if this, efficacy of CMVpp65CTLs generated against specific immunodominant epitopes such as those presented by HLA A*0201, or B*0702 differs significantly from that of CMVpp65 CTL specific for to epitopes presented by other HLA alleles. However, even now the preferential expansion of T-cells responding to such immunodominant peptides should be considered also relevant to assessments of different approaches to T-cell generation for adoptive therapy. For example, we initially expected that the use of a pool of synthetic overlapping peptides of CMVpp65 for in vitro generation of virus-specific T-cells would have the advantage of generating T-cells against multiple immunogenic epitopes presented by many different HLA alleles, thus providing a broader repertoire of CMVpp65-specific T-cells to combat viral infection than that produced by sensitization with single viral peptides19,59 or by isolation of CMVpp65 specific T-cells directly from the blood with peptide/HLA tetramers or streptamers.60–62 However, our data indicates that the repertoire generated is actually more limited than anticipated, and support the hypothesis that small numbers of T-cells specific for single highly immunogenic immunodominant epitopes may be adequate to achieve control of viremia and resolution of infection. On the other hand, our data also raise the possibility that CMVpp65CTLs generated against certain less immunogenic epitopes of CMV presented by other alleles may be less effective, a possibility further supported by recent observations of Giest et al.63 Since tetramer and streptamer-based isolation of T-cells has thus far been limited to T-cells specific for the highly immunogenic and immunodominant epitopes presented by no more than 5 prevalent HLA alleles, particularly A*0201 and HLA B*0702, the excellent responses to such T-cells may not reflect these potential limitations.
The technique employed for generating CMVpp65-specific T-cells in this study advantages particularly the safety of employing synthetic peptides rather than viral products for T-cell sensitization and the consistency with which the technique induces large populations of cytotoxic CD8 and/or CD4 T-cells of required CMVpp65-specific and depletions of allo responsive T-cells from fresh or, shipped blood samples from every seropositive donor in our series. However, the 4–5 weeks required for in vitro generation of these CMVpp65 specific T-cells remains an impediments to timely treatment unless the transplant donor-derived T-cells are generated prior to or at the time of initial CMV reactivation in patients at risk. We adapted this approach for the present study, generating CMVpp65 specific T-cells from 30 transplant donors, but only treating 16 patients with clinical infection or CMV viremia that failed to respond to antiviral drugs. With the specific consent of the donors the unused T-cells lines were banked for potential use in transplant patients other than the individual from whom the donor provided an HCT. One of those banked CMVpp65-specific T-cells lines was used to clear CMV in patient 17. Current single center and planned multicenter Phase II trials are comparing transplant donor-derived versus HLA partially-matched, appropriately HLA-restricted third party donor-derived CMVpp65 specific T-cells, again in the treatment of patients with clinical disease or viremia persisting disparate extended therapy with anti viral drugs. Initial results from two centers are promising and suggest that virus-specific third party T-cells which are immediately accessible and broadly applicable but have a shorter survival in vivo, may nevertheless induce clearance of infection in a majority of care.45,64
In conclusion, adoptive transfer of transplant donor or third party derived CMVpp65CTLs sensitized in vitro with a pool of synthetic 15-mers peptides spanning CMVpp65 is safe, does not cause GVHD and can clear CMV infections in high risk patients who have failed prolonged therapy with antiviral drugs. Strikingly, the CMVpp65CTLs generated consistently exhibited specificity for 1–3 immunodominant epitopes presented by a limited number of Class I or II HLA alleles. In responding patients, transplant donor-derived CMVpp65CTLs proliferate and can be detected for periods of 120 days to up to 2 years post infusion, thereby providing sustained resistance to this pathogen.
Highlights.
Adoptive transfer of donor-derived CMV CTL for persisting CMV viremia
CMVpp65CTLs sensitized with 15-mers peptides spanning CMVpp65 are safe and effective.
CMV CTLs exhibit specificity for 1–3 epitopes presented Class I and II HLA alleles.
Acknowledgments
The authors gratefully acknowledge the efforts of Ramzi Khalaf and the Marrow Transplantation Services in Medicine and Pediatrics at Memorial Sloan Kettering Cancer Center in the support of these studies. Partial funding for this work was provided by NIH grants NCI CA23766 and NCI R21CA162002 and by The Major Family Fund for Cancer Research, The Max Cure Fund for Pediatric Cancer Research, The Aubrey Fund for Pediatric Cancer Research and The Claire Tow Chair in Pediatric Oncology Research. G.K. was supported by the ASCO Foundation with a Young Investigator Award.
Footnotes
Authorship Statement
GK performed the study, provided clinical care, supervised experiments, data collection and analyses and wrote the manuscript. AH supervised experiments, data collection and analyses and wrote the manuscript. ED supervised the generation of the CMV CTLs, data collection and analyses. SP provided clinical care, supervised experiments, data collection and analyses and commented on the manuscript. EMT processed samples, performed experiments, analyzed data. GW coordinated CMV CTL infusions and specimen collection and analyses. RJO supervised the study, provided clinical care, supervised experiments, data collection and analyses and wrote the manuscript
Conflicts of Interest
The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 2.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 3.Zaia JA, Gallez-Hawkins GM, Tegtmeier BR, et al. Late cytomegalovirus disease in marrow transplantation is predicted by virus load in plasma. J Infect Dis. 1997;176(3):782–785. doi: 10.1086/517301. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P. Immune reconstitution and viral infections after stem cell transplantation. Bone Marrow Transplant. 1998;21 (Suppl 2):S72–74. [PubMed] [Google Scholar]
- 5.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 6.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78(5):1373–1380. [PubMed] [Google Scholar]
- 7.Quinnan GV, Jr, Kirmani N, Rook AH, et al. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 8.Ohnishi M, Sakurai T, Heike Y, et al. Evaluation of cytomegalovirus-specific T-cell reconstitution in patients after various allogeneic haematopoietic stem cell transplantation using interferon-gamma-enzyme-linked immunospot and human leucocyte antigen tetramer assays with an immunodominant T-cell epitope. Br J Haematol. 2005;131(4):472–479. doi: 10.1111/j.1365-2141.2005.05800.x. [DOI] [PubMed] [Google Scholar]
- 9.Moins-Teisserenc H, Busson M, Scieux C, et al. Patterns of cytomegalovirus reactivation are associated with distinct evolutive profiles of immune reconstitution after allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2008;198(6):818–826. doi: 10.1086/591185. [DOI] [PubMed] [Google Scholar]
- 10.Luo XH, Huang XJ, Liu KY, Xu LP, Liu DH. Protective immunity transferred by infusion of cytomegalovirus-specific CD8(+) T cells within donor grafts: its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(7):994–1004. doi: 10.1016/j.bbmt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Avetisyan G, Larsson K, Aschan J, Nilsson C, Hassan M, Ljungman P. Impact on the cytomegalovirus (CMV) viral load by CMV-specific T-cell immunity in recipients of allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38(10):687–692. doi: 10.1038/sj.bmt.1705507. [DOI] [PubMed] [Google Scholar]
- 12.Pourgheysari B, Piper KP, McLarnon A, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43(11):853–861. doi: 10.1038/bmt.2008.403. [DOI] [PubMed] [Google Scholar]
- 13.Widmann T, Sester U, Gartner BC, et al. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS One. 2008;3(11):e3634. doi: 10.1371/journal.pone.0003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacey SF, Diamond DJ, Zaia JA. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol Blood Marrow Transplant. 2004;10(7):433–447. doi: 10.1016/j.bbmt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 16.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 17.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362(9393):1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 18.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 19.Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(6):707–714. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Micklethwaite KP, Clancy L, Sandher U, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112(10):3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi D, Williams RY, O’Reilly RJ, Koehne G. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood. 2005;105(7):2793–2801. doi: 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 22.Hasan AN, Kollen WJ, Trivedi D, et al. A panel of artificial APCs expressing prevalent HLA alleles permits generation of cytotoxic T cells specific for both dominant and subdominant viral epitopes for adoptive therapy. J Immunol. 2009;183(4):2837–2850. doi: 10.4049/jimmunol.0804178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehne G, Smith KM, Ferguson TL, et al. Quantitation, selection, and functional characterization of Epstein-Barr virus-specific and alloreactive T cells detected by intracellular interferon-gamma production and growth of cytotoxic precursors. Blood. 2002;99(5):1730–1740. doi: 10.1182/blood.v99.5.1730. [DOI] [PubMed] [Google Scholar]
- 24.Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O’Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012;120(8):1633–1646. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99(7):1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, Charmley P, Robinson MA, Concannon P. The extent of the human germline T-cell receptor V beta gene segment repertoire. Immunogenetics. 1994;40(1):27–36. doi: 10.1007/BF00163961. [DOI] [PubMed] [Google Scholar]
- 27.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 28.Landry ML, Ferguson D, Stevens-Ayers T, de Jonge MW, Boeckh M. Evaluation of CMV Brite kit for detection of cytomegalovirus pp65 antigenemia in peripheral blood leukocytes by immunofluorescence. J Clin Microbiol. 1996;34(5):1337–1339. doi: 10.1128/jcm.34.5.1337-1339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrnst A, Barkholt L, Brattstrom C, et al. Detection of CMV-matrix pp65 antigen in leucocytes by immunofluorescence as a marker of CMV disease. J Med Virol. 1993;39(2):118–124. doi: 10.1002/jmv.1890390207. [DOI] [PubMed] [Google Scholar]
- 30.Kearns AM, Turner AJ, Eltringham GJ, Freeman R. Rapid detection and quantification of CMV DNA in urine using LightCycler-based real-time PCR. J Clin Virol. 2002;24(1–2):131–134. doi: 10.1016/s1386-6532(01)00240-2. [DOI] [PubMed] [Google Scholar]
- 31.Mhiri L, Kaabi B, Houimel M, Arrouji Z, Slim A. Comparison of pp65 antigenemia, quantitative PCR and DNA hybrid capture for detection of cytomegalovirus in transplant recipients and AIDS patients. J Virol Methods. 2007;143(1):23–28. doi: 10.1016/j.jviromet.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70(11):7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boppana SB, Britt WJ. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology. 1996;222(1):293–296. doi: 10.1006/viro.1996.0424. [DOI] [PubMed] [Google Scholar]
- 34.Khan N, Best D, Bruton R, Nayak L, Rickinson AB, Moss PA. T cell recognition patterns of immunodominant cytomegalovirus antigens in primary and persistent infection. J Immunol. 2007;178(7):4455–4465. doi: 10.4049/jimmunol.178.7.4455. [DOI] [PubMed] [Google Scholar]
- 35.Ameres S, Besold K, Plachter B, Moosmann A. CD8 T cell-evasive functions of human cytomegalovirus display pervasive MHC allele specificity, complementarity, and cooperativity. J Immunol. 2014;192(12):5894–5905. doi: 10.4049/jimmunol.1302281. [DOI] [PubMed] [Google Scholar]
- 36.Lin A, Xu H, Yan W. Modulation of HLA expression in human cytomegalovirus immune evasion. Cell Mol Immunol. 2007;4(2):91–98. [PubMed] [Google Scholar]
- 37.Yanada M, Yamamoto K, Emi N, et al. Cytomegalovirus antigenemia and outcome of patients treated with pre-emptive ganciclovir: retrospective analysis of 241 consecutive patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32(8):801–807. doi: 10.1038/sj.bmt.1704232. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura R, Cortez K, Solomon S, et al. High-dose acyclovir and pre-emptive ganciclovir to prevent cytomegalovirus disease in myeloablative and non-myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;30(4):235–242. doi: 10.1038/sj.bmt.1703648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063–4071. [PubMed] [Google Scholar]
- 40.Doubrovina E, Oflaz-Sozmen B, Kernan N, Young J, Abramson S, Barker J, Boulad F, Castro-Malaspina H, Teruya-Feldstein J, Papdapoulos EB, Sacaradavou A, Small T, O’Reilly RJ. Adoptive transfer of EBV specific T-cells for treatment of primary and Rituxan resistant EBV lymphomas following allogeneic stem cell transplants (HSCT): clinical, viral and immunologic correlates. BBSMT. 2010;16(Supplement 2 S220) [Google Scholar]
- 41.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2(5):551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 43.Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360(9331):436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- 44.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 45.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95(7):2240–2245. [PubMed] [Google Scholar]
- 47.Gottschalk S, Ng CY, Perez M, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97(4):835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 48.Zaia JA, Gallez-Hawkins G, Li X, et al. Infrequent occurrence of natural mutations in the pp65(495–503) epitope sequence presented by the HLA A*0201 allele among human cytomegalovirus isolates. J Virol. 2001;75(5):2472–2474. doi: 10.1128/JVI.75.5.2472-2474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones TR, Hanson LK, Sun L, Slater JS, Stenberg RM, Campbell AE. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J Virol. 1995;69(8):4830–4841. doi: 10.1128/jvi.69.8.4830-4841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn K, Angulo A, Ghazal P, Peterson PA, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci U S A. 1996;93(20):10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Lee S, Shin J, et al. Human cytomegalovirus microRNA miR-US4–1 inhibits CD8(+) T cell responses by targeting the aminopeptidase ERAP1. Nat Immunol. 2011;12(10):984–991. doi: 10.1038/ni.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao L, Cowan MJ, Dunham K, et al. Adoptive immunotherapy with CMV-specific cytotoxic T lymphocytes for stem cell transplant patients with refractory CMV infections. J Immunother. 2012;35(3):293–298. doi: 10.1097/CJI.0b013e31824300a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peggs KS, Thomson K, Samuel E, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 54.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 55.Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6(242):242ra283. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heslop HE. Pharmacotherapy versus T lymphocytes for CMV. Blood. 2013;121(18):3544–3545. doi: 10.1182/blood-2013-03-487249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185(12):1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 58.Lacey SF, Villacres MC, La Rosa C, et al. Relative dominance of HLA-B*07 restricted CD8+ T-lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B* 07 alleles. Hum Immunol. 2003;64(4):440–452. doi: 10.1016/s0198-8859(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 59.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 60.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitt A, Tonn T, Busch DH, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51(3):591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 62.Uhlin M, Gertow J, Uzunel M, et al. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis. 2012;55(8):1064–1073. doi: 10.1093/cid/cis625. [DOI] [PubMed] [Google Scholar]
- 63.Giest S, Grace S, Senegaglia AC, et al. Cytomegalovirus-specific CD8(+) T cells targeting different HLA/peptide combinations correlate with protection but at different threshold frequencies. Br J Haematol. 2010;148(2):311–322. [PubMed] [Google Scholar]
- 64.Prockop SE, Hasan A, Koehne G, et al. Third Party Donor Derived CMV Specific T Cells for the Treatment of Refractory CMV Viremia and Disease after Hematopoietic Stem Cell Transplant. Blood. 2014;124(21) [Google Scholar]