Abstract
OBJECTIVE
The present study explored the role of self-referencing on false alarm rates among people with mild cognitive impairment suggestive of the early signs of the Alzheimer’s disease pathophysiologic process (MCI-AD). Given that people with MCI-AD demonstrate higher rates of false alarms and that false alarms have been shown to increase for self-relevant information, it was predicted that people with MCI-AD would experience a disproportionate increase in memory errors for highly self-related information.
METHODS
Twenty-three MCI-ADs with a diagnosis of MCI-AD and 27 healthy controls rated words for self-descriptiveness or commonness and completed a surprise recognition test.
RESULTS
Contrary to expectations, results indicated that people with MCI-AD were at no greater risk for false alarms than controls as a function of self-descriptiveness, relative to a control condition. Despite the MCI-ADs’ greater bias to say ‘yes’ in the self condition, increasing self-descriptiveness did not lead to higher false alarm rates and did not impair performance in the self condition relative to commonness judgments.
CONCLUSIONS
Therefore, while people with MCI-AD may be more susceptible to memory errors, they are at no greater risk of self-related errors than healthy controls.
Keywords: MCI-AD, Self-reference, False memory, Response bias
As we age, memory becomes more prone to errors and distortions. This is particularly true for people with mild cognitive impairment suggestive of the early signs of the Alzheimer’s disease pathophysiologic process (MCI-AD; Budson & Solomon, 2012). While episodic memory typically suffers greater loss than other types of memory in normal aging (Old & Naveh-Benjamin, 2008), disproportionate decline in episodic memory is considered to be the hallmark sign of AD (Salmon, Butters, & Chan, 1999). While recent work has shown that self-reference is one memory tool that may assist people with memory impairment (Kalenzaga, Bugaiska, & Clarys, 2013; Kalenzaga & Clarys, 2013; Rosa, Deason, Budson, & Gutchess, in press), in certain circumstances it is possible the same mechanisms that contribute to accurate memory may increase the risk of error.
Self-reference aids memory by connecting new information to the self (Symons & Johnson, 1997) allowing for deeper processing (Craik & Lockhart, 1972) and better organization of information (Klein & Kihlstrom, 1986). As a result, self-reference offers a spontaneous and efficient (Rogers, Kuiper, & Kirker, 1977; Yang, Truong, Fuss & Bislimovic, 2012) benefit to memory that may be different from other memory strategies. This benefit appears to persist in AD. Recent work by Kalenzaga and colleagues using a Remember/Know/Guess paradigm demonstrated that for participants with AD, a self-reference condition increased the number of ‘Remember’ responses compared to a control condition, even though this group had fewer ‘Remember’ responses overall compared to controls (Kalenzaga, et al., 2013; Kalenzaga & Clarys, 2013). In our previous work we have also found that self-referencing affects memory among people with AD-MCI with a benefit in item memory and a reduction in self-related source errors (Rosa, et al., in press). Based on these previous studies, it appears as though self-reference does continue to provide a benefit to accurate memory in people with MCI-AD despite losses associated with the disease.
The same mechanisms that allow us to receive a benefit from self-reference may, however, also contribute to self-related false memory. As we age, accurate recall decreases while false memory, remembering an event that never happened or misremembering the details of an event, increases and this pattern is even stronger in MCI-AD (Balota et al., 1999). Given this increased risk of false memory, the deeper processing believed to play a role in self-reference may trigger an increase in associations and increase false memory, particularly in MCI-AD. Self-reference may also increase false memory because the self is well known and information connected to the self may be processed more fluently. While this is typically seen as a mechanism that facilitates the use of the self in memory (Symons & Johnson, 1997), increased fluency of processing may contribute to false memory (Rogers, Rogers, & Kuiper, 1979), particularly with age (Rosa & Gutchess, 2013). When information is fluently processed due to self-relevance, it is likely to create a sense of familiarity, or feelings of having been previously encountered (Jacoby & Whitehouse, 1989), and these feelings of familiarity may serve to increase the intrusions that lead to false memory (Gallo, Cotel, Moore, & Schacter, 2007). Familiarity could also lead to an increase in false memory in recognition if there is a sense of experience with a word or object and there is not a more recognizable option. While familiarity may contribute to false memory in healthy older adults (Gallo, Cotel, et al., 2007), people with MCI-AD are less able to utilize recollection to oppose familiarity (Pierce, Sullivan, Schacter, & Budson, 2005). People with MCI-AD demonstrate a more liberal response bias, a greater tendency to respond ‘old’ on a memory test (Balota, Burgess, Cortese & Adams, 2002), and familiarity may increase this bias (Deason, Hussey, Ally, & Budson, 2012). Therefore familiarity may lead people to say new information is old and likely poses an increased risk of false memory in those with MCI-AD (Budson, Daffner, Desikan & Schacter, 2000).
The present study expands on previous work by exploring the role of self-reference on false memory among people with cognitive impairment due to MCI-AD. Previous research (Rogers, et al., 1979; Rosa & Gutchess, 2013) has shown an increase in false memories as information is more self-descriptive, however this effect has not been tested in people with cognitive impairment. Self-reference may increase the familiarity of information in such a way that the fluency, or ease of access, of the information increases as well (Jacoby & Whitehouse, 1989). This increase in familiarity and fluency may lead participants to believe new self-related information is old and result in increased false alarms. While older adults are already at risk for increased false memory (Schacter, Koutstaal, & Norman, 1997), older adults with MCI-AD are even more vulnerable (Balota, et al., 1999; Gallo, et al., 2006). Therefore, we expected that the increase in false alarms as a result of self-reference would be even greater for MCI-ADs than controls.
Methods
Participants
Twenty-three (12M/11F) participants with a clinical diagnosis of MCI due to the AD pathophysiologic process (Albert et al., 2011) and 27 (11M/16F) healthy older adults are included in all analyses. Participation took place in the participant’s home or at Brandeis University.
As shown in table 1, MCI-ADs performed worse than controls on all tests of cognitive functioning (Adjutant General’s Office, 1944; Folstein, Folstein, & McHugh, 1975; Mack, Freed, Williams, & Henderson, 1992; Monsch, et al., 1992; Morris, et al., 1989).
Table 1.
Demographics by Group
| Control | Patient | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 75.63 | 6.56 | 78.65 | 5.01 |
| Education | 15.39 | 3.04 | 14.04 | 2.84 |
| Shipley (out of 40) * | 36.48 | 3.16 | 31.73 | 6.47 |
| MMSE (out of 30) * | 28.89 | 1.53 | 24.91 | 3.32 |
| CERAD | ||||
| - Immediate (out of 30) * | 16.35 | 3.50 | 13.14 | 3.34 |
| - Delayed (out of 10) * | 5.77 | 1.63 | 2.75 | 1.52 |
| - Recognition (out of 10) * | 9.69 | .62 | 7.95 | 1.54 |
| Word Fluency | ||||
| - FAS * | 49.50 | 13.96 | 37.19 | 11.43 |
| - CAT * | 44.96 | 9.79 | 32.67 | 11.69 |
| Boston Naming (out of 15) * | 14.35 | 1.09 | 11.76 | 3.13 |
| Trails A * | 35.52 | 10.12 | 61.35 | 40.26 |
| Trails B * | 88.35 | 53.36 | 154.21 | 84.14 |
Sig. different at p<.01
Materials
Two lists were created using a list of adjectives rated for likability (Anderson, 1968). An equal number of positive and negative words were selected from the extreme ends of the ratings to create 2 lists (old and new) of 120 words each and were equated for likeability. Words contained an average of 9.15 letters and the mean frequency score was 17.04 (Kucera & Francis, 1967).
Procedure
Participants rated 60 adjectives for self-descriptiveness (“I am”) and 60 adjectives for commonness (“How commonly is the word used”) on a 6-point scale (“this word never describes me/this word is never used” [1]; “this word always describes me/this word is always used” [6]) either verbally with a key press made by the experimenter (MCI-ADs) or with a key press (controls). Adjectives were presented for six seconds, followed by a blank screen for one second. Conditions alternated, with blocks of 7–8 words per reference condition. A fixation cross appeared between each block to indicate a change in condition. Following a retention interval, participants completed a surprise, self-paced recognition test comprised of the previously seen 120 words as well as 120 new lures. Participants indicated either verbally (MCI-ADs) or using a key press (controls) whether each word was “old” or “new” to denote whether the word was seen during the encoding. Finally, participants rated the 120 new lure words for self-descriptiveness using the same scale used at encoding.
Results
Scoring
To minimize missing data in the event that some participants did not use all values on the scale, ratings were collapsed (1–2=“low”, 3–4=“middle”, 5–6= “high”). See table 2 for proportion of items in each category.1
Table 2.
Hits, false alarms, corrected recognition and response bias in each condition
| Control | MCI-AD | ||||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | ||
| Proportion of Items in each Category | |||||
| Self | Low | .37 | (.11) | .29 | (.11) |
| Middle | .29 | (.15) | .34 | (.18) | |
| High | .33 | (.13) | .25 | (.12) | |
| Common | Low | .17 | (.14) | .16 | (.15) |
| Middle | .33 | (.18) | .36 | (.23) | |
| High | .49 | (.25) | .32 | (.27) | |
| Hits | |||||
| Self | Low | 0.67 | (0.14) | 0.62 | (0.19) |
| Middle | 0.72 | (0.15) | 0.65 | (0.18) | |
| High | 0.81 | (0.14) | 0.74 | (0.23) | |
| Common | Low | 0.64 | (0.28) | 0.60 | (0.28) |
| Middle | 0.59 | (0.22) | 0.62 | (0.28) | |
| High | 0.69 | (0.21) | 0.75 | (0.21) | |
| False Alarms | |||||
| Self | Low | 0.25 | (0.15) | 0.41 | (0.23) |
| Middle | 0.25 | (0.20) | 0.47 | (0.22) | |
| High | 0.46 | (0.19) | 0.64 | (0.20) | |
| Common | Low | 0.32 | (0.27) | 0.35 | (0.27) |
| Middle | 0.27 | (0.16) | 0.46 | (0.21) | |
| High | 0.37 | (0.23) | 0.57 | (0.19) | |
| Corrected Recognition | |||||
| Self | Low | 0.43 | (0.14) | 0.25 | (0.17) |
| Middle | 0.48 | (0.25) | 0.20 | (0.17) | |
| High | 0.37 | (0.19) | 0.09 | (0.21) | |
| Common | Low | 0.28 | (0.30) | 0.17 | (0.25) |
| Middle | 0.31 | (0.26) | 0.19 | (0.18) | |
| High | 0.29 | (0.21) | 0.22 | (0.17) | |
| Response Bias | |||||
| Self | Low | 0.10 | (0.42) | −0.03 | (0.67) |
| Middle | 0.10 | (0.44) | −0.23 | (0.71) | |
| High | −0.55 | (0.51) | −0.89 | (1.00) | |
| Common | Low | 0.16 | (0.98) | 0.13 | (1.09) |
| Middle | 0.23 | (0.42) | −0.28 | (0.83) | |
| High | −0.12 | (0.84) | −0.69 | (0.82) | |
Hits
Hit rates were calculated as a proportion of correctly recognized old items within each reference condition at each rating level. Using a factorial ANOVA with group as the between subjects variable and reference (self/common) and rating (low/middle/high) as within subject variables, there was a main effect of reference (F(1,40)=4.95, p=.03,η2p=.11) with self (M=.70, SD=.15, 95% CI[.66, .75]) related words leading to higher hit rates than words rated for commonness (M=.65, SD=.22, 95% CI[.58, .71]). There was also a main effect of rating, F(2,98)=16.56, p<.001,η2p=.29. As shown in table 2, words in the high rating level led to higher hit rates (M=.75, SD=.18, 95% CI[.69,.80]) than those in the low (M=.63, SD=.20, 95% CI[.58, .69]; F(1,40)=22.59, p<.001,η2p=.36) and middle rating levels (M=.65, SD=.19, 95% CI[.59, .70]; F(1,40)=24.10, p<.001,η2p=.38). There was no significant difference between the low and middle levels, p>.10. There were no significant interactions involving rating, p’s>.10. There was no main effect of group and no interaction involving group (p’s>.10).
Based on previous work in which healthy older adults demonstrated a higher hit rate in the self condition relative to common (Rosa & Gutchess, 2013),, we ran separate analyses for each group to determine if the previous pattern could be replicated. As shown previously, controls exhibited a main effect of reference, F(1,23)=7.28, p=.01,η2p=.24, such that self led to higher hit rates (M=.74, SD=.01, 95% CI[.69, .78]) than common (M=.64, SD=.01, 95% CI[.56, .73]), but the same was not true for MCI-AD patients even when differences in cognitive ability were controlled for using MMSE as a covariate, p>.10. While the patients also did not show a main effect of rating when controlling for MMSE, p>.10, there was also a main effect of rating among controls, F(2,46)=6.31, p=.004,η2p=.22, with words in the high rating leading to more hits (M=.75, SD=.14, 95% CI[.69, .80]) than those in the low (M=.66, SD=.20, 95% CI[.58, .74]) or middle ratings (M=.66, SD=.16, 95% CI[.60, .72]). There were no interactions for either group, p’s>.10.
False Alarms
We hypothesized that false alarms would increase along with self-descriptiveness, particularly for MCI-ADs. To test this, false alarms were calculated as the proportion of new items incorrectly called ‘old’ within each condition at each rating level. A factorial ANOVA with group as the between subjects variable and reference (self/common) and rating (low/middle/high) as the within subject variables indicated a main effect of group (F(1,32)=10.79, p=.002,η2p=.25), with MCI-ADs exhibiting higher false alarm rates (M=.48, SD=.25, 95% CI[.41, .55]) than controls (M=.30, SD=.23, 95% CI[.24, .39]) as predicted (see table 2). There was also the predicted main effect of rating (F(2,64)=20.18, p<.001,η2p=.39), with words in the high rating level leading to more false alarms (M=.51, SD=.22, 95% CI[.44, .58]) than words in the low (M=.33, SD=.20, 95% CI[.27, .39]) or middle rating levels (M=.36, SD=.20, 95% CI[.30, .42]). Specific comparisons indicate that the high rating level was significantly different from the low (F(1,32)=25.82, p<.001,η2p=.45) and middle levels (F(1,32)=47.19, p <.001,η2p=.60), but there was no significant difference between the low and middle levels, p>.10. Despite the hypothesis that self reference would lead to higher false alarm rates than the common condition for words rated as highly self-relevant, especially for MCI-ADs, there was no main effect of reference and there were no significant interactions, p’s>.10.
We again used MMSE as a covariate for patients and looked at each group separately to assess convergence with prior studies. For both controls (F(2,30)=9.02, p=.001,η2p=.38) and patients (F(2,20)=3.47, p=.04,η2p=.19) there was a main effect of rating reflecting the same pattern described above. There was no main effect of reference and no interactions for either group, p’s>.10.
Corrected Recognition
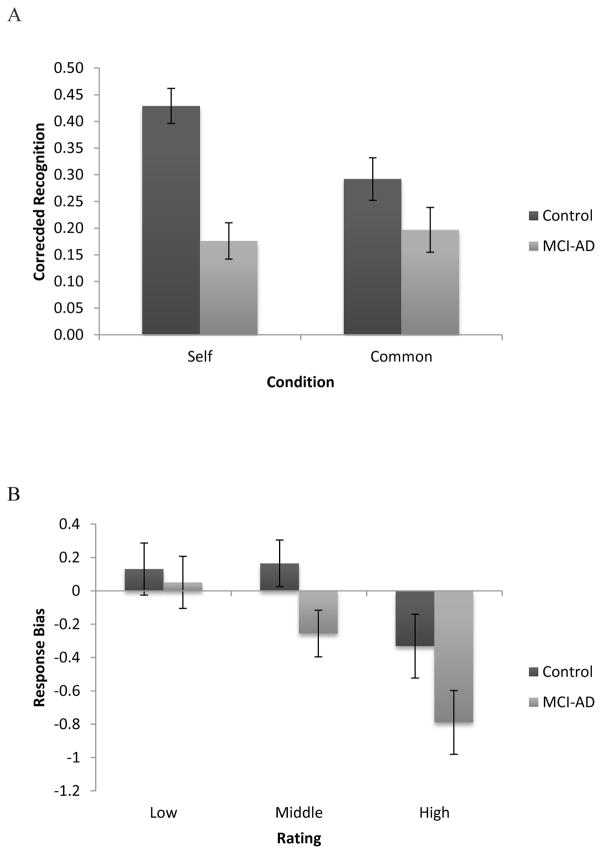
Corrected Recognition scores were calculated by subtracting the proportion of false alarms from the proportion of hits for each rating level, to test whether there was a difference in memory accuracy across conditions as an effect of self-reference. It was expected that increases in false alarms would diminish overall memory performance as rating increased, particularly in the self condition relative to common. To test this, a factorial ANOVA with group as the between subject variable and reference (self/common) and rating (low/middle/high) as the within subject variables indicated a main effect of group with controls demonstrating better memory (M=.36, SD=.20, 95% CI[.31, .42]) than MCI-ADs (M=.19, SD=.24, 95% CI[.13, .24]; F(1,29)=20.28, p<.001,η2p=.41). There was a significant interaction between group and condition, F(1,29)=4.62, p=.04,η2p=.14, that is driven by the differences between the self and common conditions for the controls. When the two groups are analyzed separately, as seen in figure 1, controls exhibited a main effect of reference, F(1,15)=5.57, p=.03,η2p=.27, such that self led to better memory (M=.43, SD=.20, 95% CI[.35, .51]) than common (M=.29, SD=.23, 95% CI[.20, .39]), but the same was not true for MCI-AD patients even when differences in cognitive ability were controlled for using MMSE as a covariate, p>.10. Neither group showed a main effect rating or any interactions when looking at controls and when controlling for MMSE among patients, p’s>. Lastly, while there was no main effect of rating (p>.10), there was a marginal interaction between rating and reference, F(2,58)=2.57, p=.09,η2p=.08. This interaction appears to be driven by differences within the self condition. As shown in table 2, in the common condition, performance is relatively flat across the three rating levels but in the self condition, as expected, memory for words in the low and middle rating levels was better than memory for words in the high rating. Looking just at the self condition, high significantly differs from the low (F(1,29)=6.21, p=.02,η2p=.1, 95% CI[.02, .20]) and middle rating conditions (F(1,29)=9.73, p=.004,η2p=.25, 95% CI[.04, .18]) while there is no difference between the low and middle rating conditions, p>.10. In the common condition, there are no significant differences between any of the rating levels, p’s>.10.
Figure 1.
Figure 1A: Pattern of corrected recognition showing the different pattern between reference and group with controls demonstrating better memory for the self condition than for the common condition while MCI-AD performance is relatively flat across both conditions. 1B: Pattern of response bias showing differences between MCI-ADs and controls at the middle and high rating levels. Error bars represent standard error.
Response bias
Based on previous work demonstrating that response bias can influence memory performance, particularly for MCI-ADs (Deason, Hussey, Ally, & Budson, 2012), c values were calculated to test for differences between MCI-ADs and controls. Negative c values indicate a bias to say ‘yes’, while positive values indicate a ‘no’ bias. A factorial ANOVA with group as the between subject variable and reference condition (self/common) and rating (low/middle/high) as the within subject variable indicated a main effect of reference, F(1,30)=3.21, p=.08,η2p=.10, such that while there was an overall bias to say ‘yes’ in both conditions, this was more pronounced in the self condition (M=−.25, SD=.67, 95% CI[−.46, −.04]) than in the common condition (M=−.09, SD=.83, 95% CI[−.34, .16]), as seen in table 2. There was also a main effect of rating, F(2,60)=29.18, p<.001,η2p=.49, with a stronger bias to say ‘yes’ in the middle (M= −.05, SD=.67, 95% CI[−.25, .16]) and high (M= −.56, SD=.90, 95% CI[−.84, −.28]) rating levels but to respond ‘no’ in the low rating level (M=.09, SD=.76, 95% CI[−.14, .32]). While the main effect of group was not significant (p<.10), as shown in figure 2, there was a marginal interaction between group and rating, F(2,60)=2.69, p=.08,η2p=.08, that appears to be driven by a marginal difference between the controls and MCI-ADs in the middle (F(1,44)=3.58, p=.07,η2p=.08, 95% CIs [−.09, .35] and [−.42, .06] respectively) and high (F(1,42)=3.88, p=.06,η2p=.09, 95% CIs [−.58, −.02] and [−1.04, −.40] respectively) rating levels.
Again exploring the two groups separately, we see differences between the patient and control groups in response bias. When we use MMSE as a covariate to control for differences in cognitive ability in the patient group, we find a marginal effect of rating (F(2,26)=2.75, p=.09,η2p=.17) that mirrors the pattern described above and a marginal interaction between reference and rating (F(2,26)=3.04, p=.07,η2p=.19) that appears to be driven by a stronger ‘yes; bias in the self condition, particularly at the low and high rating levels. There was no main effect of reference, p>.10. For controls, there is a main effect of reference (F(1,15)=3.47, p=.08,η2p=.19) and a main effect of rating (F(2,30)=11.90, p<.001,η2p=.44) that follow the above pattern. There was no interaction between the two, p>.10.
Discussion
Unlike previous studies (Kalenzaga, et al., 2013; Kalenzaga & Clarys, 2013), MCI-ADs in the present study did not show a self-reference benefit for hits. In fact, hit rates appear similar in the self and common conditions. MCI-ADs also do not exhibit an increase in hits across rating levels in the same way as controls. In contrast, controls show a self-reference benefit, with an increase in hits in the self condition relative to the common condition, as seen in previous work (Rosa & Gutchess, 2013). For false alarms, while MCI-ADs commit more false alarms as predicted, we also expected that false alarms would be higher in the self condition but there was no difference between the self and common condition indicating that self-reference does not necessarily lead to an increase in false alarms. Therefore, MCI-ADs, when compared to controls, do not seem to be at a greater risk of false alarms for self-related information.
For corrected recognition, the self-reference benefit to hits is maintained for controls in the self condition when controls are examined alone, with better memory performance in the self condition than in the common condition. Therefore, for controls, self-reference potentially provides the expected benefit to memory through an increase in hit rates without an associated increase in overall false alarms. For MCI-ADs, corrected recognition indicates that self-reference may not be benefitting memory, but it is also not hurting memory through an increase in false alarms. Given the lack of benefit to correct recognition, it is possible that MCI-ADs are not using the self in memory in the same way as seen in controls. Based on previous work (Kalenzaga, et al., 2013; Kalenzaga & Clarys, 2013; Rosa et al., in press), it was initially hypothesized that self-referencing may build upon intact memory for self-relevant information such that MCI-ADs would have better memory for self-related new information. However, self-reference may benefit people with MCI-AD in some situations more than others. In our previous work, we found that self-reference benefits people with MCI-AD in that they are less likely to falsely claim an item as being self-related (Rosa et al., in press). In a Remember/Know/Guess paradigm, Kalenzaga and colleagues (2012; 2013) found a self-reference benefit with no significant differences between patients and controls in correct Remember (R) responses but not for Know (K) responses. It is possible participants in the present study similarly felt more confident in their memory for some items than others and when these items are pooled together, the benefits seen in R responses are diminished by performance in the K responses.
Uncertainty in memory may lead to an increase in reliance on gist among people with MCI-AD. As shown in previous research, reliance on gist can hurt performance in people with AD (Budson, et al., 2006; Gallo, et al., 2006; Pierce, et al., 2005). Cognitively impaired individuals may have been swayed by a general memory of having seen self-related information. Lacking specific memory of the information, they may have utilized gist and a feeling of familiarity to make their decision. However, for controls, it is possible that reliance on gist helps to distinguish between old and new stimuli and contributes to accurate memory. Controls may be better able to utilize this general memory to differentiate stronger and weaker feelings of familiarity. People with MCI-AD may experience greater difficulty with this (Budson, et al., 2006) and as a result experience an increase in false memories. Because the increase in false alarms is seen in both the self and common condition for MCI-ADs, we conclude that MCI-AD does not make one particularly prone to an increase in false alarms as a result of self-referencing. Contrary to our predictions, self-referencing does not pose a greater threat for the overall memory performance of MCI-AD patients in the present task.
The group differences in memory performance in the present study may also be better understood by considering response bias. Results indicate that items in the self condition evoke a stronger ‘yes’, or ‘old’, bias for both controls and MCI-ADs. In fact, across all conditions, MCI-ADs follow a pattern that is similar to the controls, although somewhat more exaggerated at the middle and high rating levels. While people with MCI-AD demonstrated a stronger response bias to say ‘old’, particularly in the self condition, this was not reflected in higher false alarms to words connected to the self relative to other conditions. In fact, false alarm rates were similar across both the self and common condition for MCI-ADs. The difference between the two groups seems to manifest in how this response bias influences memory performance. For controls, this bias results in higher hit rates while for MCI-ADs it is more likely to result in false alarms. Given that people with AD are typically more prone to false alarms than healthy controls (Balota et al., 1999), this seems to indicate that controls are better at discriminating between old and new information than MCI-ADs.
The present study is limited in that the neuropsychological scores of the MCI-AD groups were relatively high, indicating the present findings may not be representative of patients with more significant impairment. However, neuropsychological scores were significantly different between the MCI-AD and control groups indicating that the two groups did differ in cognitive ability. While it is possible that people with more advanced AD may experience more memory errors and fewer benefits related to self-reference, the differences found in the present study do serve to shed some light on the role of cognitive impairment on the use of self-reference. This is important, as patients at the earliest stages of impairment, such as MCI, would be the most likely to benefit from memory strategies, such as self-referencing.
Previous work has suggested that self-referencing can be a beneficial strategy for people with AD, however this previous research has utilized a Remember/Know paradigm and benefits have been shown in Remember judgments (Kalenzaga, et al., 2013; Kalenzaga & Clarys, 2013). Our procedure also differed in that participants were required to frequently switch between the two encoding conditions. Although the control task of commonness judgments had been used successfully in prior work (Rosa & Gutchess, 2013), it is possible that the task invoked the self to some degree, particularly among those with MCI-AD. This potential overlap across conditions may have detracted from the self-related benefits found by others (Kalenzaga, et al., 2013; Kalenzaga & Clarys, 2013). Future work should explore the use of other control tasks that may better differentiate between the self and non-self in people with cognitive impairment.
Adding to previous work, the present study indicates that self-reference does not consistently aid memory for people with MCI-AD, as patients do not show an advantage for self-related information over the common condition. While cognitively healthy older adults receive an overall boost in memory when information is connected to the self, people with cognitive impairment do not appear to be experiencing the benefits associated with self-reference. However, an advantage to this lack of a self-reference effect in memory is that self-referencing also does not lead to a disproportionate increase in false alarms. This is striking because people with MCI-AD tend to experience higher rates of false alarms compared to controls on many memory tasks. Thus, connecting information to the self does not harm memory nor does it add to an already elevated risk of memory error for MCI-ADs.
Acknowledgments
This research was supported by National Institute on Aging grants R01 AG025815 (AEB) and P30 AG13846 (AEB), by Department of Veterans Affairs, Veterans Health Administration, VISN 1 Early Career Development Award (RGD), and by the Psychology Department at Brandeis University (NMR). This material is also the result of work supported with resources and the use of facilities at the VA Boston Healthcare System.
The authors thank Peter Millar and Sean Flannery for their research assistance, and acknowledge the helpful comments and suggestions of Dr. Leslie Zebrowitz, Dr. Joseph Cunningham, and Dr. Alice Cronin-Golomb.
Footnotes
We also examined the distribution of ratings across the groups to evaluate whether patients and controls differed in their ratings. Focusing on differences between the two groups, there was a main effect of group, F(1,48)=20.44, p<.001,η2p=.11, with controls rating more words in the high category (M=.33, SD=.04) than patients (M=.29, SD=.04). There was a significant interaction between condition and group, F(1,48)=9.48, p=.003,η2p=.17, with controls rating words in both conditions in a similar manner (Self M=.33, SD=.04 Vs. Common M=.33, SD=.05) while patients rated more words as high in the self condition (M=.29, SD=.04) than in the common condition (M=.28, SD=.05). There was also a marginal interaction between rating and status F(1,96)=2.84, p=.06,η2p=.06. It appears as though controls rated more words in the high rating level (M=.41, SD=.23) while patients used the middle category more (M=.35, SD=.23). There was no interaction between group, condition and rating, p>.10. Despite these slight differences in patterns, both groups distributed their responses rather evenly across the high, medium, and low levels, which allows us to compare memory performance for patients and controls using these bins of items.
References
- Adjutant General’s Office. Army individual test battery: Manual of directions and scoring. Washington, DC: 1944. [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Hotzman DM, Jagust WJ, Peterson RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging and Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011:1–10. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NH. Likableness Ratings of 555 Personality-Trait Words. Journal of Personality and Social Psychology. 1968;9(3):272–279. doi: 10.1037/h0025907. http://dx.doi.org/10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Balota DA, Burgess GC, Cortese MJ, Adams DR. The word-frequency mirror effect in young, old and early-stage Alzheimer’s disease: Evidence for two processes in episodic recognition performance. Journal of Memory and Language. 2002;46:199–226. doi: 10.1006/jmla.2001.2803. [DOI] [Google Scholar]
- Balota DA, Cortese MJ, Duchek D, Roediger HL, McDermott KB, Yerys BE. Verdical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology. 1999;16:361–384. doi: 10.1080/026432999380834. [DOI] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer’s disease. Neuropsychology. 2000;14(2):277–287. doi: 10.1037/0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Solomon PR. New diagnostic criteria for Alzheimer’s disease and mild cognitive impairment for the practical neurologist. Practical Neurology. 2012;12:88–96. doi: 10.1136/practneurol-2011-000145. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of Processing - Framework for Memory Research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. doi: 10.1016/S0022-5371(72)80001-X. [DOI] [Google Scholar]
- Deason RG, Hussey EP, Ally BA, Budson AE. Changes in response bias with different study-test delays: Evidence from young adults, older adults, and patients with Alzheimer’s disease. Neuropsychology. 2012;1:119–126. doi: 10.1037/a0026330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for graing the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Cotel SC, Moore CD, Schacter DL. Aging can spare recollection-based retrieval monitoring: the importance of event distinctiveness. Psychology and Aging. 2007;22:209–213. doi: 10.1037/0882-7974.22.1.209. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer’s disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: False recognition influenced by unconscious perception. Journal of Experimental Psychology: General. 1989;118:126–135. doi: 10.1037/0096-3445.118.2.126. [DOI] [Google Scholar]
- Kalenzaga S, Bugaiska A, Clarys D. Self-reference effect and autonoetic consciousness in Alzheimer’s disease: Evidence for a persistent affective self in dementia patients. Alzheimer’s Disease and Associated Disorders. 2013;27:116–122. doi: 10.1097/WAD.0b013e318257dc31. [DOI] [PubMed] [Google Scholar]
- Kalenzaga S, Clarys D. Self-referential processing in Alzheimer’s disease: Two different ways of processing self-knowledge? Journal of Clinical and Experimental Neuropsychology. 2013;35:455–471. doi: 10.1080/13803395.2013.789485. [DOI] [PubMed] [Google Scholar]
- Klein S, Kihlstrom J. Elaboration, organization, and the self-reference effect. Journal of Experimental Psychology. 1986;115:26–38. doi: 10.1037/0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown Unversity Press; 1967. [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Conparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part 1. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;49:1159–1165. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M, editors. Age related changes in memory: Experimental approaches. Thousand Oaks, CA: Sage Publications; 2008. [Google Scholar]
- Pierce BH, Sullivan AL, Schacter DL, Budson AE. Comparing source-based and gist-based false recognition in aging and Alzheimer’s disease. Neuropsychology. 2005;19:411–419. doi: 10.1037/0894-4105.19.4.411. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35:677–688. doi: 10.1037/0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Rogers PJ, Kuiper NA. Evidence for the self as a cognitive prototype: The “false alarms effect”. Personality and Social Psychology Bulletin. 1979;5:53–56. doi: 10.1177/014616727900500111. [DOI] [Google Scholar]
- Rosa NM, Deason RG, Budson AE, Gutchess AH. Source memory for self and other in patients with mild cognitive impairment due to Alzheimer’s Disease. Journal of Gerontology: Psychological Sciences. doi: 10.1093/geronb/gbu062. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa NM, Gutchess AH. False memory in aging resulting from self-referential processing. Journal of Gerontology: Psychological Sciences. 2013;68:882–892. doi: 10.1093/geronb/gbt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Butters N, Chan AS. The determination of semantic memory in Alzheimer’s disease. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie experimentale. 1999;53:108–117. doi: 10.1037/h0087303. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Norman KA. False memories and aging. Trends in Cognitive Sciences. 1997;1:229–236. doi: 10.1016/S1364-6613(97)01068-1. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychological Bulletin. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Yang L, Truong L, Fuss S, Bislimovic S. The effects of ageing and divided attention on the self-reference effect in emotional memory: Spontaneous or effortful mnemonic benefits? Memory. 2012;20(6):596–607. doi: 10.1080/09658211.2012.690040. [DOI] [PubMed] [Google Scholar]