Abstract
Evasion of extracellular matrix detachment-induced apoptosis (“anoikis”) is a defining characteristic of metastatic tumor cells. The ability of metastatic carcinoma cells to survive matrix detachment and escape anoikis enables them to disseminate as viable circulating tumor cells and seed distant organs. Here we report that αB-crystallin, an antiapoptotic molecular chaperone implicated in the pathogenesis of diverse poor-prognosis solid tumors, is induced by matrix detachment and confers anoikis-resistance. Specifically, we demonstrate that matrix detachment downregulates extracellular-signal regulated kinase (ERK) activity and increases αB-crystallin protein and mRNA levels. Moreover, we show that ERK inhibition in adherent cancer cells mimics matrix detachment by increasing αB-crystallin protein and mRNA levels, while constitutive ERK activation suppresses αB-crystallin induction during matrix detachment. These findings indicate that ERK inhibition is both necessary and sufficient for αB-crystallin induction by matrix detachment. To examine the functional consequences of αB-crystallin induction in anoikis, we stably silenced αB-crystallin in two different metastatic carcinoma cell lines. Strikingly, silencing αB-crystallin increased matrix detachment-induced caspase activation and apoptosis but did not affect cell viability of adherent cancer cells. In addition, silencing αB-crystallin in metastatic carcinoma cells reduced the number of viable circulating tumor cells and inhibited lung metastasis in two orthotopic models, but had little or no effect on primary tumor growth. Taken together, our findings point to αB-crystallin as a novel regulator of anoikis-resistance that is induced by matrix detachment-mediated suppression of ERK signaling and promotes lung metastasis. Our results also suggest that αB-crystallin represents a promising molecular target for antimetastatic therapies.
Keywords: Anoikis, Apoptosis, Extracellular matrix, αB-crystallin, Caspases
Introduction
The detachment of epithelial cells from the extracellular matrix (ECM) disrupts integrin-mediated cell survival signals to activate a caspase-dependent cell death mechanism known as “anoikis”.1 Anoikis plays a critical role in maintaining tissue architecture by eliminating epithelial cells that have become displaced from their normal ECM microenvironment.2 Matrix detachment initiates anoikis by engaging one or more components of the conserved apoptotic cell death machinery, namely, the intrinsic (mitochondrial) or the extrinsic (death receptor) apoptotic pathways. In the intrinsic pathway, matrix-detachment triggers the induction and mitochondrial translocation of the proapoptotic BH3-only proteins Bim and Bmf, which promote apoptosis at least in part by binding and inhibiting antiapoptotic Bcl-2 family members.3-5 Matrix detachment also leads to the mitochondrial localization of the proapoptotic Bcl-2 family member Bax, which plays an essential role in mitochondrial outer membrane permeabilization, cytochrome c release and subsequent caspase activation.6 In the extrinsic pathway, matrix-detachment increases expression of death receptors such as DR5/TRAIL-R2 and activates the initiator procaspases-8 and -10.7-9 These apoptotic pathways culminate in the proteolytic activation of executioner caspases, including caspase-3, which initiate apoptosis by cleaving key signaling and structural proteins.10
In contrast to normal epithelial cells, carcinoma cells must overcome anoikis and survive matrix detachment in order to disseminate from the primary tumor as circulating tumor cells en route to colonizing distant organs. Hence, anoikis is a critical barrier to metastasis, and the acquisition of anoikis-resistance is a defining hallmark of metastatic carcinoma cells.2,11 However, the molecular mechanisms by which tumor cells escape anoikis are poorly understood. Some tumor cells suppress anoikis by constitutively activating receptor tyrosine kinases, which inhibit matrix detachment-induced downregulation of extracellular-signal regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) activity, a critical step for the induction of the anoikis mediators Bim and Bmf.4,5 Additional signaling pathways downstream of receptor tyrosine kinases, including Src and Akt, have also been implicated in anoikis-resistance in some cancer cells.12,13 Moreover, diverse tumor types evade anoikis by overexpressing antiapoptotic Bcl-2 family members, including Bcl-xL and Mcl-1.14,15 Indeed, circulating tumor cells from patients with small-cell lung cancer express Bcl-2 and Mcl-1,16 confirming the likely clinical relevance of these observations. Nevertheless, overexpression of additional antiapoptotic proteins and/or inactivation of other proapoptotic molecules in metastatic tumor cells are likely to contribute to anoikis-resistance.
We postulated that the antiapoptotic molecular chaperone αB-crystallin, which is expressed in a variety of solid tumors,17 might contribute to anoikis-resistance in cancer. αB-crystallin negatively regulates apoptosis by inhibiting the proteolyic activation of procaspase-3, suppressing the translocation of Bax and Bcl-xs to the mitochondria, and attenuating the production of reactive oxygen species.18-22 αB-crystallin is expressed in a subset of breast cancers, renal cell carcinomas, glioblastomas, hepatocellular carcinomas and head and neck cancers.22-27 In breast cancer, αB-crystallin is preferentially expressed in poor-prognosis estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor receptor-2 (HER-2) triple-negative tumors, and its expression is associated with poor survival, lymph node metastasis and resistance to neoadjuvant chemotherapy.23,24,28-30 More recently, αB-crystallin has been shown to be expressed in brain metastases from breast cancer patients and to promote brain metastasis in murine models of triple-negative breast cancer (TNBC).31 Collectively, the well-established antiapoptotic function of αB-crystallin, together with its documented expression in metastatic breast cancer, make it an attractive candidate for regulating anoikis-resistance.
Here we report that matrix detachment downregulates ERK activity and leads to a robust increase in αB-crystallin protein and mRNA levels. Treatment of adherent cancer cells with pharmacologic MAPK/ERK kinase (MEK) inhibitors mimicked matrix detachment by downregulating ERK activity and inducing αB-crystallin protein and mRNA levels, while constitutive activation of the ERK pathway suppressed αB-crystallin induction during matrix detachment. Silencing αB-crystallin in metastatic carcinoma cells sensitized them to matrix detachment-induced caspase activation and apoptosis but did not affect their cell viability in adherent culture. Furthermore, silencing αB-crystallin in metastatic carcinoma cells reduced the number of viable circulating tumor cells and inhibited lung metastasis in two orthotopic xenograft models. Taken together, our findings point to αB-crystallin as a novel regulator of anoikis-resistance that promotes lung metastasis.
Results
αB-crystallin is induced by matrix detachment-mediated inhibition of ERK signaling in cancer cells
The ability of primary tumor cells to survive detachment from the ECM and evade anoikis is a critical early step in the metastatic cascade.2,11 We modeled matrix detachment in vitro by growing cancer cells in suspension on ultra-low attachment plates. αB-crystallin protein levels were robustly induced in 435-LvBr1-mCherry and GILM2-mCherry cells grown in suspension for 72 h compared to cells grown in adherent culture (Figure 1a, left). In contrast, the expression of the related small heat shock protein Hsp27 was not altered by matrix detachment. αB-crystallin mRNA levels were also greater in cells grown in suspension compared to those grown in adherent culture, although the magnitude of the difference in mRNA levels was more modest than the observed differences in protein levels (Figure 1a, right). Consistent with a prior report,4 matrix detachment inhibited ERK signaling as determined by a reduction in p-ERK levels in 435-LvBr1-mCherry and GILM2-mCherry cells grown in suspension (Figure 1b). This reduction in p-ERK levels in cancer cells grown in suspension was accompanied by a robust induction of αB-crystallin. To determine whether ERK inhibition was sufficient to induce αB-crystallin expression, adherent 435-LvBr1-mCherry cells were treated with a panel of kinase inhibitors, including the MEK inhibitors AZD6244 (Selumetinib) and CI-1040, the phosphatidylinositol-3 (PI-3) kinase inhibitor LY294002 or the Src family kinase inhibitor PP2. Both MEK inhibitors reduced p-ERK levels and induced αB-crystallin protein and mRNA levels (Figure 1c). In contrast, LY294002 and PP2 had little effect on αB-crystallin protein and mRNA levels in adherent 435-LvBr1-mCherry cells. Similar results were obtained in adherent GILM2-mCherry cells treated with these kinase inhibitors (Figure 1d).
Figure 1. αB-crystallin is induced by matrix detachment-mediated inhibition of ERK signaling in cancer cells.
(a) 435-LvBr1-mCherry and GILM2-mCherry cancer cells were grown for 72 h in adherent or suspension (susp.) culture. αB-crystallin and Hsp27 levels were analyzed by immunobloting (left), and αB-crystallin mRNA levels were determined by RT-PCR (right). mRNA levels were normalized to expression in adherent cells. (b) 435-LvBr1-mCherry and GILM2-mCherry cancer cells were grown for 72 h in adherent or suspension culture, and the expression of αB-crystallin, ERK and p-ERK was analyzed by immunobloting. (c) and (d) 435-LvBr1-mCherry (c) or GILM2-mCherry cancer cells (d) were treated with vehicle (cont.), 40 μM LY294002 (LY), 50 μM PP2, 10 μM CI-1040 (CI) or 10 μM Selumetinib (AZD) for 48 h. αB-crystallin, ERK, p-ERK, Akt, p-Akt, Src, and p-Src levels were determined by immunoblotting (left), and αB-crystallin expression was determined by RT-PCR (right). (e) Immunoblot of MCF10A-Vector or MCF-10A-RasV12 cells (left panel) or GILM2 cells expressing empty Vector or constitutively active MEK-DD (right panel) grown in adherent or suspension culture in the absence or presence of 10 μM CI-1040 or 10 μM Selumetinib for 72 h. In (a), (c) and (d), **P < 0.01 and ***P < 0.001.
To evaluate the functional role of ERK inhibition in the induction of αB-crystallin by matrix detachment, we examined the induction of αB-crystallin in MCF-10A breast epithelial cells stably expressing empty vector or the H-RasV12 oncogene. MCF-10A-Vector cells grown in suspension culture had reduced p-ERK levels and markedly elevated αB-crystallin levels compared to cells grown in adherent conditions (Figure 1e, left panel). Treatment of adherent MCF-10A-Vector cells with AZD6244 or CI-1040 decreased p-ERK levels and induced αB-crystallin expression. In contrast, MCF-10A-RasV12 cells grown in suspension had higher p-ERK levels than MCF-10A-Vector cells grown in suspension and failed to induce αB-crystallin expression. Treatment of MCF-10A-RasV12 cells grown in suspension with AZD6244 or CI-1040 decreased p-ERK levels and increased αB-crystallin levels. Moreover, ectopic expression of a constitutively active MEK-DD mutant resulted in enhanced p-ERK levels in GILM2 cells grown in suspension compared to Vector-expressing GILM2 cells grown in suspension and inhibited αB-crystallin induction by matrix detachment (Figure 1e, right panel). These effects mediated by constitutive activation of MEK were suppressed by AZD6244 and CI-1040. Collectively, these results indicate that matrix detachment induces αB-crystallin by downregulating ERK activity.
αB-crystallin inhibits anoikis in cancer cells
To examine the role of αB-crystallin in anoikis, we stably silenced αB-crystallin in 435-LvBr1-mCherry and GILM2-mCherry cells using two different shRNAs (sh-αB1 and sh-αB2). Both shRNAs reduced αB-crystallin levels in cancer cells compared to a non-silencing (NS) control (Figure 2a). We also coexpressed sh-αB1 with an RNAi-resistant αB-crystallin mutant (sh1-αBM) to control for possible off-target effects by rescuing expression of wild-type αB-crystallin. Stably silencing αB-crystallin in 435-LvBr1-mCherry cells or GILM2-mCherry cells did not affect cell viability when these cells were grown in adherent culture (Figure 2b and 2c, respectively). In contrast, silencing αB-crystallin in 435-LvBr1-mCherry cells or GILM2-mCherry cells resulted in decreased cell viability when these cells were grown in suspension compared to the corresponding cells stably expressing the non-silencing (NS) construct or the RNAi rescue construct (sh1-αBM). These results indicate that silencing αB-crystallin does not affect cell viability under normal growth conditions but sensitizes cancer cells to anoikis.
Figure 2. αB-crystallin inhibits anoikis in cancer cells.
(a) Immunoblot analysis of αB-crystallin expression in 435-LvBr1-mCherry and GILM2-mCherry cancer cells stably expressing a non-silencing (NS) construct, αB-crystallin shRNA (sh1-αB or sh2-αB), or sh1-αB and a mutant αB-crystallin that is resistant to gene silencing but retains its coding sequence (sh1-αBM). (b) and (c) MTS cell viability assay of 435-LvBr1-mCherry (b) and GILM2-mCherry cells (c) stably expressing NS, sh1-αB, sh2-αB or sh1-αBM grown in adherent or suspension culture. The number of viable cells is expressed as fold change normalized to day 1 (n = 3). **P < 0.01, ***P < 0.001.
αB-crystallin inhibits matrix detachment-induced caspase activation and apoptosis and promotes cell survival in response to combined MEK inhibition and matrix detachment
Because αB-crystallin inhibits caspase-3 activation,18,19,22 we examined whether αB-crystallin suppressed matrix detachment-induced cleavage of the caspase substrate PARP and proteolytic processing of procaspase-3. Silencing αB-crystallin in 435-LvBr1-mCherry and GILM2-mCherry cells resulted in enhanced PARP cleavage as detected by reduced intensity of the full-length protein in cells grown in suspension compared to cells stably expressing the NS and sh1-αBM constructs (Figure 3a). Moreover, αB-crystallin silencing resulted in more robust proteolytic processing of procaspase-3 to its active subunit in matrix-detached cells compared to cells expressing the NS or sh1-αBM constructs. To quantitate anoikis, 435-LvBr1-mCherry and GILM2-mCherry breast cancer cells stably expressing NS, sh1-αB, or sh1-αBM were grown in suspension for 24 h, and Annexin V-positive cells were scored by flow cytometry. Consistent with our cell viability and immunoblotting findings, silencing αB-crystallin resulted in enhanced Annexin V-positive cells upon matrix detachment compared to NS or sh1-αBM control cells (Figure 3b). Notably, the augmented anoikis observed in cancer cells with αB-crystallin silencing was suppressed by the caspase inhibitor zVAD-fmk. Collectively, these results indicate that αB-crystallin inhibits anoikis by negatively regulating matrix detachment-induced caspase activation.
Figure 3. αB-crystallin inhibits matrix detachment-induced caspase activation and apoptosis and promotes cell survival in response to combined MEK inhibition and matrix detachment.
(a) 435-LvBr1-mCherry and GILM2-mCherry cancer cells stably expressing NS, sh1-αB, sh2-αB or sh1-αBM were grown in suspension for 48 h. Full-length PARP and cleaved caspase-3 were detected by immunoblotting. (b) 435-LvBr1-mCherry and GILM2-mCherry breast cancer cells stably expressing NS, sh1-αB, or sh1-αBM were grown in suspension for 24 h. Cancer cells stably expressing sh1-αB were untreated or treated with 50 μM zVAD-fmk. Apoptosis was measured by Annexin V labeling using flow cytometry. (c) MTS cell viability assay of 435-LvBr1-mCherry and GILM2-mCherry cells stably expressing NS or sh1-αB treated with vehicle or 10 μM AZD6244 in adherent or suspension culture for 48 h (n = 3). *P < 0.05, **P < 0.01.
Based the observed induction of αB-crystallin expression by MEK inhibitors (Figure 1c and 1d), we postulated that MEK inhibitors would confer resistance to anoikis by an αB-crystallin-dependent mechanism. To this end, 435-LvBr1-mCherry and GILM2-mCherry cells stably expressing NS or sh1-αB were treated with vehicle or 10 μM AZD6244 in adherent or suspension culture for 48 h. Under these conditions, stably silencing αB-crystallin resulted in diminished cell viability in cancer cells treated with AZD6244 and grown in suspension, but not in cancer cells treated with this drug and grown in adherent culture (Figure 3c). Consistent with our prior observations (Figure 2b and 2c), silencing αB-crystallin in these cells did not alter their sensitivity to anoikis by itself at 48 h, but only to the combination of MEK inhibition and matrix detachment. These latter findings suggest that αB-crystallin promotes cell survival in response to the combination of MEK inhibition and matrix detachment, an observation with potential therapeutic implications given the clinical development of MEK inhibitors as targeted therapies for cancer.
αB-crystallin increases the number of viable circulating tumor cells and promotes lung metastasis in vivo
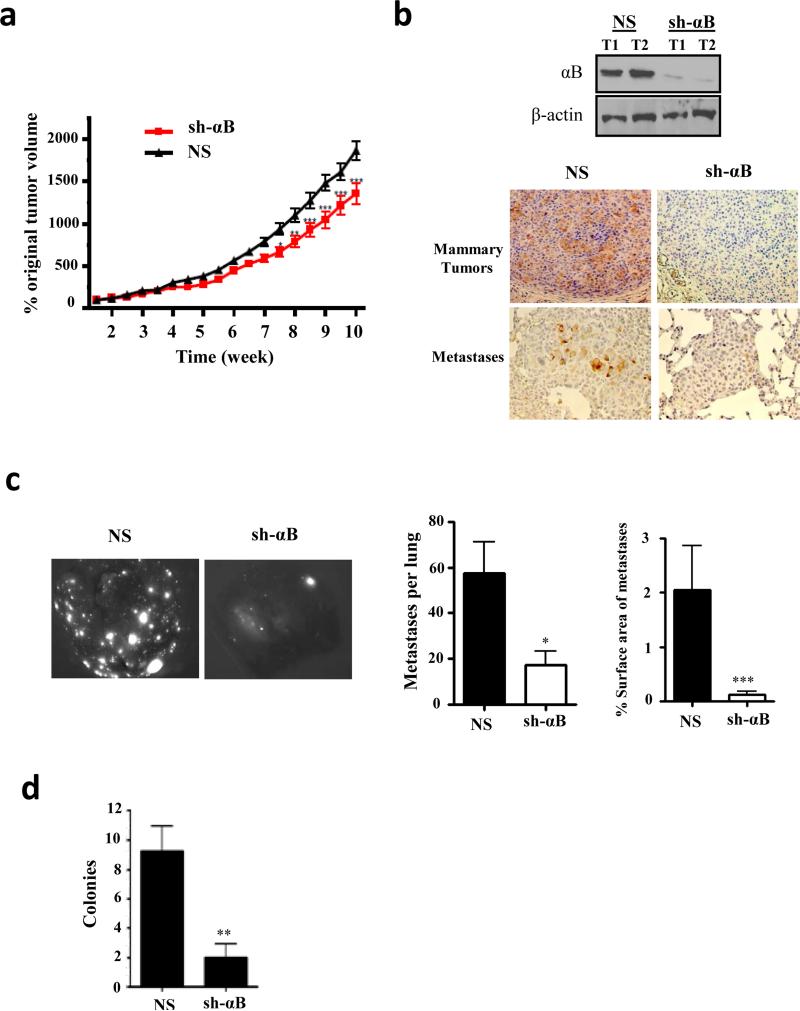
To explore the potential role of αB-crystallin in lung metastasis in vivo, we injected 435-LvBr1-mCherry-sh1-αB or 435-LvBr1-mCherry-NS cells intraductally into the 4th mammary glands of female athymic nude mice. Silencing αB-crystallin modestly inhibited mammary tumor growth in the final weeks of the experiment (Figure 4a). Importantly, αB-crystallin silencing resulted in a robust reduction in αB-crystallin levels in mammary tumors and lung metastases compared to NS controls (Figure 4b). Silencing αB-crystallin also resulted in fewer mCherry-positive lung metastases (number) and reduced lung metastatic tumor burden (percentage of the lung surface area with metastases) at autopsy at 10 weeks compared to mice with NS tumors (Figure 4c). αB-crystallin silencing did not affect apoptosis of mammary tumors or lung metastases analyzed at 10 weeks (Figure S1a). Given our observation that αB-crystallin inhibits anoikis, we postulated that αB-crystallin promotes survival of circulating tumors cells, an early step in metastasis. To this end, we obtained blood from mice bearing NS and sh-αB mammary tumors at 10 weeks and selected for viable circulating tumor cells, which express a puromycin-resistance gene, by culturing them in media supplemented with puromycin. Silencing αB-crystallin reduced the number of puromycin-resistant colonies compared to NS controls (Figure 4d).
Figure 4. αB-crystallin promotes circulating tumor cell survival and lung metastasis in an orthotopic 435-LvBr1-mCherry model.
(a) 435-LvBr1-mCherry cells stably expressing NS or sh-αB were injected intraductally into the 4th mammary glands of female athymic nude mice. Mammary tumor volume was measured weekly with calipers and expressed as the percentage original tumor volume at 2 weeks (n=10 mice per group). (b) 435-LvBr1-mCherry-NS and 435-LvBr1-mCherry-sh-αB mammary tumors were immunoblotted. αB-crystallin expression in mammary tumors and lung metastases was determined by immunohistochemistry in both groups (c) Representative whole lung images by fluorescence microscopy. The number of fluorescent metastases per lung and the percentage of the surface area occupied by lung metastases in NS and sh-αB groups (n = 10 mice per group) are indicated. (d) The number of colonies per mouse derived from the blood of mice bearing 435-LvBr1-mCherry-NS or 435-LvBr1-mCherry-sh-αB xenografts (n=5 mice per group). In (a), (c) and (d), *P < 0.05, **P < 0.01, and ***P < 0.001.
To confirm these finding in a second orthotopic model, we injected GILM2-mCherrysh1-αB and GILM2-mCherry-NS cells intraductally into the 4th mammary glands of female athymic nude mice. Silencing αB-crystallin did not significantly affect mammary tumor growth (Figure 5a) despite a sustained reduction of αB-crystallin levels in mammary tumors compared to NS control tumors (Figure 5b). Mammary tumors were resected at 6 weeks, and mCherry-positive lung metastases were identified at autopsy six weeks later. Silencing αB-crystallin resulted in fewer lung metastases and reduced lung metastatic burden compared to mice with NS tumors (Figure 5c). Interestingly, some sh-αB lung metastases had foci with αB-crystallin expression (Figure 5b, lower panels). αB-crystallin silencing did not affect apoptosis in mammary tumors or lung metastases analyzed at autopsy (Figure S1b). However, silencing αB-crystallin resulted in fewer puromycin-resistant colonies obtained from blood compared to NS controls (Figure 5d). Collectively, these findings indicate that αB-crystallin increases the number of viable circulating tumor cells and promotes lung metastasis in two orthotopic models.
Figure 5. αB-crystallin promotes circulating tumor cell survival and lung metastasis in an orthotopic TNBC model.
(a) GILM2-mCherry cells stably expressing NS or sh-αB were injected intraductally into the 4th mammary glands of female athymic nude mice. Mammary tumor volume was measured weekly and expressed as the percentage original tumor volume at 2 weeks (n=10 mice per group). (b) GILM2-mCherry-NS and GILM2-sh-αB mammary tumors were immunoblotted, and αB-crystallin expression was determined by immunohistochemistry of mammary tumors and lung metastases in both groups. (c) Representative whole lung images by fluorescence microscopy. The number of fluorescent metastases per lung and the percentage surface area of lung metastases in NS and sh-αB groups (n = 10 mice per group) are shown. (d) The number of colonies per mouse derived from the blood of mice bearing GILM2-mCherry-NS or GILM2-mCherry-sh-αB xenografts (n=5 mice per group). In (a), (c) and (d), **P < 0.01 and ***P < 0.001.
Discussion
Although detachment of epithelial cells from the ECM engages the apoptotic cell death machinery to promote anoikis,2 we have identified a novel prosurvival pathway that is activated by matrix detachment, namely, induction of the antiapoptotic chaperone αB-crystallin. Indeed, αB-crystallin mRNA and protein levels are robustly increased in immortalized human breast epithelial cells and metastatic carcinoma cells in response to matrix detachment by growth in suspension culture. We have also shown that downregulation of ERK signaling by matrix detachment is both necessary and sufficient for αB-crystallin induction. Specifically, constitutive ERK activation by oncogenic Ras or MEK-DD suppressed αB-crystallin induction during matrix detachment, while pharmacologic MEK inhibitors mimicked matrix detachment by inducing αB-crystallin in adherent cells. Hence, disruption of integrin and/or growth factor signaling by matrix detachment inhibits ERK signaling, which activates both proapoptotic pathways (induction and mitochondrial translocation of BH3-only proteins Bim and Bmf 3-5) and prosurvival pathways (αB-crystallin induction). αB-crystallin, then, can be added to a short list of cell survival proteins, including PERK, the transcription repressor TLE1 and others, that are activated by matrix detachment and counteract anoikis.32,33 On a cellular level, the relative balance of these diametrically opposed molecular events likely determines whether matrix-detachment results in anoikis or cell survival.
We have also demonstrated that metastatic carcinoma cells rely on this paradoxical detachment-induced cell survival pathway to overcome anoikis. Notably, silencing αB-crystallin in metastatic carcinoma cells attenuates the induction of αB-crystallin by matrix detachment and renders cancer cells more vulnerable to matrix detachment-induced caspase activation and anoikis. These observations are unlikely to reflect off-target effects of shRNAs because similar results were obtained with two different αB-crystallin shRNAs and the phenotype was rescued by an RNAi-resistant mutant αB-crystallin encoding the wild-type protein. Intriguingly, silencing αB-crystallin did not affect cell viability of cancer cells grown in adherent conditions, suggesting that the prosurvival function of αB-crystallin is unmasked by matrix detachment and subsequent engagement of the apoptotic cell death machinery. These findings are consistent with well-established antiapoptotic function of αB-crystallin, which inhibits procaspase-3 proteolytic activation and the mitochondrial translocation of Bax and Bcl-xs.18-20,22 Additional evidence for the essential role of caspase inhibition by αB-crystallin in regulating anoikis-resistance comes from our observation that the enhanced sensitivity of carcinoma cells to anoikis mediated by silencing αB-crystallin is abrogated by the caspase inhibitor zVAD-fmk. Furthermore, αB-crystallin confers protection to the combination of MEK inhibition and matrix detachment, suggesting that αB-crystallin may contribute to resistance to MEK inhibitors in the clinic. Taken together, these results point to a key role of αB-crystallin in anoikis-resistance by inhibiting caspase activation in response to matrix detachment and suggest that αB-crystallin may be a promising drug target to enhance anoikis sensitivity.
Consistent with of role of anoikis suppression in the early steps in the metastatic cascade, we observed that silencing αB-crystallin reduced the number of viable circulating tumor cells and suppressed lung metastasis in two orthotopic models. Strikingly, silencing αB-crystallin had modest or no effect on primary tumor growth and no effect on cell death in primary or metastatic tumors at the completion of the study. These findings strongly suggest that the prometastatic function of αB-crystallin is mediated at least in part by antagonizing anoikis in circulating tumor cells and thereby enhancing their metastatic dissemination to distant organs. However, αB-crystallin may also increase the intravasation of tumor cells into the circulation. Consistent with this idea, αB-crystallin has been reported to promote cell migration, invasion and angiogenesis.23,26,34,35 Moreover, other mechanisms are also likely to contribute to the prometastatic function of αB-crystallin. For example, αB-crystallin promotes adhesion to brain microvascular endothelial cells (HBMECs), transmigration through an HMEC/human astrocyte co-culture model of the blood-brain barrier, and brain metastasis in orthotopic TNBC models in female NOD scid IL2 receptor γ chain knockout (NSG) mice.31 In the latter brain metastasis models, αB-crystallin expression did not alter lung metastatic tumor burden at the conclusion of the study. However, female NSG mice had extensive lung metastatic burden, before developing brain metastasis as a late event; hence, it is unclear whether αB-crystallin might affect lung tumor burden at earlier stages in this model. In contrast, lung metastasis is less extensive in the orthotopic models described in this report in female athymic nude mice and no brain metastases were observed, pointing to fundamental differences in tumor progression in these different immunodeficient murine hosts. Collectively, these finding strongly suggest that αB-crystallin promotes metastasis by multiple mechanisms, including anoikis suppression. Importantly, the clinical relevance of these preclinical studies pointing to an important role of αB-crystallin in metastasis are supported by multiple studies linking αB-crystallin to invasion, lymph node metastases and poor clinical outcomes in diverse solid tumors.23,24,26,27
In summary, we have identified a novel prosurvival pathway activated by matrix detachment that plays a crucial role in suppressing anoikis in metastatic cancer cells. αB-crystallin mediates resistance to anoikis by specifically inhibiting caspase activation in response to matrix detachment. Moreover, our observation that silencing αB-crystallin enhances anoikis, reduces the number of viable circulating tumor cells and inhibits lung metastasis in murine models strongly suggests that αΒ-crystallin warrants further study as a potential drug target for antimetastatic therapies. Given its role in the early stages of the metastatic cascade, such αB-crystallin-targeted therapies might be particularly effective in reducing the number of circulating tumor cells, an emerging biomarker in cancer.36 Furthermore, our finding that MEK inhibitors increase αB-crystallin expression in cancer cells may have therapeutic implications for drug resistance to these agents given the antiapoptotic function of αB-crystallin.
Material and Methods
Cell culture and reagents
Human GILM2-mCherry TNBC cells stably expressing a non-silencing construct, αB-crystallin shRNAs, or αB-crystallin shRNA and a mutant αB-crystallin cDNA that is resistant to gene silencing but retains its coding sequence were described previously.31 GILM2-mCherry cells were cultured in DMEM/F12 supplemented with 10% FBS, 100 units/mL penicillin/streptomycin and Insulin/Transferrin/Sodium Selenite mix (Invitrogen, Grand Island, NY, USA). MDA-MB-435-LvBr1 (435-LvBr1) cells, a highly metastatic variant of triple-negative MDA-MB-435 cells, were graciously provided by Dr. Janet Price.24,37 435-LvBr1-mCherry cells stably expressing a non-silencing construct, αB-crystallin shRNAs, or αB-crystallin shRNA and a RNAi-resistant mutant αB-crystallin that retains its coding sequence were generated by retroviral transduction as described.31 435-LvBr1-mCherry cells were cultured in DMEM with 5% FBS, 1 mM sodium pyruvate, 100 units/mL penicillin/streptomycin and 1% MEM vitamin solution (Invitrogen). Human MCF-10A breast epithelial cells stably expressing the H-RasV12 oncogene or empty vector were described previously23 and were grown in DMEM/F12 media with 5% horse serum, 100 units/ml penicillin-streptomycin (Invitrogen), 20 ng/ml EGF, 0.5 mg/ml hydrocortisone, 100 ng/ml cholera toxin, and 10 μg/ml insulin (Sigma, St. Louis, MO, USA). LY294002, PP2, CI-1040 and Selumetinib were purchased from Selleckchem (Houston, TX, USA), and z-VAD-fmk was purchased from Sigma. pBabe-Puro-MEK-DD plasmid38 was kindly provided by Dr. William Hahn (plasmid # 15268, Addgene, Cambridge, MA, USA) and used to generate retroviruses for retroviral transduction as described.31
Real-time PCR
Total RNA was prepared using SpinSmart™ Total RNA Purification kit with DNaseI treatment according to the manufacturer's instructions (Denville Scientific, South Plainfield, NJ, USA). cDNA was synthesized using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Specific primers for αB-crystallin (5-GCACTTCTCCCCAGAGGAAC-3 and 5-CCATTCACAGTGAGGACCCC-3) and GAPDH (5-GAAGGTGAAGGTCGGAGTC-3 and 5-GAAGATGGTGATGGGATTTC-3) were purchased from Integrated DNA Technologies. PCR was performed with 100 ng cDNA using iQ™ SYBR Green supermix (Bio-Rad) and a CFX96 Real-Time PCR detection system (Bio-Rad). Cycling conditions were 50°C for 2 min, 95°C for 2 min followed by 40-cycle amplification at 95°C for 15 s, and 57°C for 45 s. Experiments were repeated two times and samples were analyzed in triplicate. Data were presented as Ct values, the threshold PCR cycle at which the product was first detected. A comparative Ct method was used to compare the RNA expression in samples to that of the control in each experiment.
Immunoblotting
Immunoblotting was performed as described23 with primary antibodies for αB-crystallin (Enzo Life Sciences/Stressgen, Farmingdale, NY, USA), PARP (BD Pharmingen, San Jose, CA, USA), caspase-3, Hsp27, p44/42 (ERK) and phospho-p44/42 (p-ERK), Akt and p-Akt, Src and p-Src (Cell Signaling, Danvers, MA, USA), and β-actin (Sigma).
Anoikis assay
435-LvBr1-mCherry and GILM2-mCherry cells were grown in complete growth medium containing 1% methylcellulose on Corning Costar Ultra-Low attachment plates (Fisher Scientific, Waltham, MA, USA) at a density of 1.0 × 104 cells/well (96-well plates) or 2.0 × 105 cells/well (6-well plates) for 0-96 h.
Cell viability and apoptosis assays
Cell viability was determined by MTS assay (Promega, Madison, WI, USA) and expressed as fold change relative to day 1, and apoptosis was detected by flow cytometry using the Annexin-FITC Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) as previously described.39
Orthotopic models of breast cancer lung metastasis
All animal experiments were approved by the institutional Animal Care and Use Committee. A Matrigel (BD Biosciences) suspension of 435-LvBr1-mCherry (2 × 106) or GILM2-mCherry (1 × 106) cells stably expressing a non-silencing construct or αB-crystallin shRNA1 was injected intraductally into the 4th mammary glands of 4- to 5-week old female athymic nu/nu mice (Harlan Laboratories, Madison, WI, USA). Tumors were measured weekly with calipers, and tumor volume was calculated as described.39 In the GILM2 model, mammary tumors were resected 6 weeks after tumor inoculation. Mice were euthanized at 10 weeks (435-LvBr1-mCherry model) or 12 weeks (GILM2-mCherry model). Fluorescent metastases were visualized in isolated whole lungs using a Leica MZ10F fluorescent stereomicroscope (Buffalo Grove, IL, USA), and images were analyzed with NIH ImageJ software as described.39
Immunohistochemistry
αB-crystallin immunohistochemistry was performed using an αB-crystallin mAb (1B6.1-3G4, Enzo Life Sciences/Stressgen) as previously described.31 Photomicrographs of stained sections were obtained using a Nikon Eclipse Ti microscope (Brighton, MI USA).
Circulating tumor cell colony assay
Blood (1 ml) was collected from the heart by terminal blood draw under deep anesthesia prior to euthanasia. Samples were diluted three-fold in PBS containing 1 mg/mL casein and 1 mM EDTA, subjected to Percol gradient centrifugation as described,40 and cultured in media containing 1 μg/ml puromycin (Sigma) for 10 days to select for puromycin-resistant colonies.
Statistical methods
Data are presented as mean ± SEM. Statistical significance was determined by ANOVAs with Bonferroni posttests or two-tailed unpaired t tests with Welch's correction (xenograft experiments) using GraphPad Prism Software (La Jolla, CA, USA).
Supplementary Material
Acknowledgments
We are indebted to Dr. Janet Price for providing the MDA-MB-435-LvBr1 cell line. We also thank Dr. Caroline Alexander and members of the Cryns lab for their critical reading of the manuscript.
Grant support: Breast Cancer Research Foundation (VLC and WJG), Susan G. Komen for the Cure Postdoctoral Fellowship Award (DM and VP), and P30CA014520 University of Wisconsin Comprehensive Cancer Center core facility support.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 2.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis--pathways to anchorage-independent growth in cancer. J Cell Sci. 2011;124:3189–3197. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 3.Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 4.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 5.Schmelzle T, Mailleux AA, Overholtzer M, Carroll JS, Solimini NL, Lightcap ES, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc Natl Acad Sci USA. 2007;104:3787–3792. doi: 10.1073/pnas.0700115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J Cell Biol. 2003;162:599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laguinge LM, Samara RN, Wang W, El-Deiry WS, Corner G, Augenlicht L, et al. DR5 receptor mediates anoikis in human colorectal carcinoma cell lines. Cancer Res. 2008;68:909–917. doi: 10.1158/0008-5472.CAN-06-1806. [DOI] [PubMed] [Google Scholar]
- 8.Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- 9.Marconi A, Atzei P, Panza C, Fila C, Tiberio R, Truzzi F, et al. FLICE/caspase-8 activation triggers anoikis induced by β1-integrin blockade in human keratinocytes. J Cell Sci. 2004;117:5815–5823. doi: 10.1242/jcs.01490. [DOI] [PubMed] [Google Scholar]
- 10.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 11.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 12.Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, et al. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 13.Haenssen KK, Caldwell SA, Shahriari KS, Jackson SR, Whelan KA, Klein-Szanto AJ, et al. ErbB2 requires integrin α5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells. J Cell Sci. 2010;123:1373–1382. doi: 10.1242/jcs.050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel A, Rosen K, Filmus J, Kerbel RS. Induction of anoikis and suppression of human ovarian tumor growth in vivo by down-regulation of Bcl-X(L). Cancer Res. 2001;61:4837–4841. [PubMed] [Google Scholar]
- 16.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 17.Arrigo AP, Gibert B. HspB1, HspB5 and HspB4 in human cancers: Potent oncogenic role of some of their client proteins. Cancers. 2014;6:333–365. doi: 10.3390/cancers6010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 19.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, et al. The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 20.Mao YW, Liu JP, Xiang H, Li DW. Human αA- and αB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 21.Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 22.Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, et al. αB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chelouche-Lev D, Kluger HM, Berger AJ, Rimm DL, Price JE. αB-crystallin as a marker of lymph node involvement in breast carcinoma. Cancer. 2004;100:2543–2548. doi: 10.1002/cncr.20304. [DOI] [PubMed] [Google Scholar]
- 25.Pinder SE, Balsitis M, Ellis IO, Landon M, Mayer RJ, Lowe J. The expression of αB-crystallin in epithelial tumours: a useful tumour marker? J Pathol. 1994;174:209–215. doi: 10.1002/path.1711740310. [DOI] [PubMed] [Google Scholar]
- 26.Huang XY, Ke AW, Shi GM, Zhang X, Zhang C, Shi YH, et al. αB-crystallin complexes with 14-3-3ζ to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57:2235–2247. doi: 10.1002/hep.26255. [DOI] [PubMed] [Google Scholar]
- 27.Mao Y, Zhang DW, Lin H, Xiong L, Liu Y, Li QD, et al. αB-crystallin is a new prognostic marker for laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2012;31:101. doi: 10.1186/1756-9966-31-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitterding SM, Wiseman WR, Schiller CL, Luan C, Chen F, Moyano JV, et al. αB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008;12:33–40. doi: 10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Lee Y, Lim YA, Kang HJ, Kim LS. αB-Crystallin is a novel oncoprotein associated with poor prognosis in breast cancer. J Breast Cancer. 2011;14:14–19. doi: 10.4048/jbc.2011.14.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov O, Chen F, Wiley EL, Keswani A, Diaz LK, Memmel HC, Rademaker A, et al. αB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008;111:411–4117. doi: 10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- 31.Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, et al. αB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res. 2014;20:56–67. doi: 10.1158/1078-0432.CCR-13-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avivar-Valderas A, Bobrovnikova-Marjon E, Alan Diehl J, Bardeesy N, Debnath J, Aguirre-Ghiso JA. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 2013;32:4932–4940. doi: 10.1038/onc.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunquell C, Biliran H, Jennings S, Ireland SK, Chen R, Ruoslahti E. TLE1 is an anoikis regulator and is downregulated by Bit1 in breast cancer cells. Mol Cancer Res. 2012;10:1482–1495. doi: 10.1158/1541-7786.MCR-12-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimberg A, Rylova S, Dieterich LC, Olsson AK, Schiller P, Wikner C, et al. αB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood. 2008;111:2015–2023. doi: 10.1182/blood-2007-04-087841. [DOI] [PubMed] [Google Scholar]
- 35.Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, et al. αB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunology Immunother. 2013;62:931–939. doi: 10.1007/s00262-012-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ, Lee MS, et al. Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res. 2008;14:4059–4066. doi: 10.1158/1078-0432.CCR-07-4039. [DOI] [PubMed] [Google Scholar]
- 38.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 39.Malin D, Chen F, Schiller C, Koblinski J, Cryns VL. Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. Clin Cancer Res. 2011;17:5005–5015. doi: 10.1158/1078-0432.CCR-11-0099. [DOI] [PubMed] [Google Scholar]
- 40.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.