Abstract
Aim and objective
Hippophae rhamnoides is an edible, nutrient rich plant found in the northern regions of India. It belongs to the family Elaeagnaceae and is well known for its traditional pharmacological activities. The present study was aimed to investigate the antioxidant and neuroprotective activities of H. rhamnoides.
Methodology
The hydroalcoholic extract of H. rhamnoides was evaluated for free radical scavenging activity using DPPH, hydroxyl radical scavenging and ferric thiocyanate assays. In vitro neuroprotective activity was assessed on human neuroblastoma cell line-IMR32 against hydrogen peroxide (H2O2) induced cytotoxicity. The neuroprotective effect was determined by measuring the cell viability through tetrazolium dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reducing assay and propidium iodide (PI) staining. Also the intracellular reactive oxygen species (ROS) activity was assessed using dichloro-dihydro-fluorescein diacetate (DCFDA) assay by flowcytometer.
Results
The results of the study demonstrated that H. rhamnoides extract possesses potential free radical scavenging activity. The IC50 value for DPPH and OH radical scavenging assay was 70.92 μg/ml and 0.463 mg/ml, also the extract was also found to have considerable level of lipid peroxidation activity. The neuroprotective effect of H. rhamnoides was confirmed by its cell viability enhancing capacity against hydrogen peroxide induced cell cytotoxicity. The extract acted on IMR32 cells in a dose dependent manner as observed through PI and MTT assays. The percentage intracellular ROS activity was reduced by 60–70% in treated cells compared to H2O2 control.
Conclusion
Thus the outcome of the study suggests that H. rhamnoides acts as a neuroprotectant against oxidative stress induced neurodegeneration.
Keywords: Hippophae rhamnoides, Neuroprotective, Oxidative stress, Human neural cell line, Cell viability, Cytotoxicity
1. Introduction
Hippophae rhamnoides (L.) is a unique, valuable plant known for its medicinal value and belongs to the Elaeagnaceae family. It is a thorny nitrogen-fixing deciduous shrub grown in cold arid regions, native to Europe and Asia and commonly known as Sea buckthorn. It is currently domesticated in several parts of the world due to its nutritional and medicinal properties (Rousi, 1971; Li, 2003). Recent researches aims to understand and support the traditional uses of H. rhamnoides. The known pharmacological effects of H. rhamnoides include antioxidant, immunomodulatory, anti-atherogenic, anti-stress, hepatoprotective, radioprotective and tissue regeneration property (Suleyman et al., 2001; Geetha et al., 2002a,b; Goel et al., 2002; Xing et al., 2002; Gao et al., 2003; Gupta et al., 2005; Basu et al., 2007; Chawla et al., 2007; Saggu et al., 2007; Upadhyay et al., 2009, 2011). However, very few researchers have focused to study the neuroprotective activity of H. rhamnoides. Literature has mentioned H. rhamnoides as a potent antioxidant; keeping this as a key factor, we have chosen the plant for studying the in vitro neuroprotective activity.
Overproduction and accumulation of free radicals lead to chronic diseases such as atherosclerosis, cancer, diabetes, rheumatoid arthritis, cardiovascular diseases, inflammation, aging and other degenerative diseases (Freidovich, 1999; Yun-Zhong et al., 2002; Emerit and Edeas, 2004). Oxidative stress is one of the prime causes for cell death and damage especially in the brain cells. Neural cells are highly susceptible to oxidative stress induced injury because of its high metabolic rate; insufficient level of antioxidants and oxidative damage to DNA and biomolecules has been noted during the pathogenesis of aging and neurodegenerative diseases (Rao, 2007; Rodesh et al., 2008; Ramakrishna and Setty, 2009). Though, the biological systems have an internal defense mechanism to fight against intracellular ROS, at a certain point this fails due to the over expression of ROS (Hallliwell, 2009). Hence, identification of alternate source of antioxidants is required for conferring protection to cells and to slow down the process of aging and neurodegeneration.
Utilization of cell line was initiated due to the difficulties in working with live human tissue sample and several studies have demonstrated that cell line is one of the best models to study stress induced cell damage and neurodegeneration (Piga et al., 2005; Guan et al., 2006; Mandraju et al., 2008). IMR32 is a human neuronal cell line which may be best suited to study the mechanism of drugs effective in treating stress induced neurodegeneration in humans. Thus, we have hypothesized the present study to evaluate the in vitro antioxidant and neuroprotective activity of H. rhamnoides using IMR32 human neuroblastoma cell line.
2. Methodology
2.1. Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH), l-ascorbic acid, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, propidium iodide (PI) and dichlorofluorescein diacetate (DCFDA) were obtained from Sigma Chemical Co. and Lonza, India. All other chemicals used were of analytical grade.
2.2. Extract preparation
Fruits and leaves of H. rhamnoides were collected from northern India in the month of September and authenticated by Professor N.K. Dubey, Botanist, Dept. of Botany, Banaras Hindu University, Varanasi. The voucher specimen (HR: 0706, SH 18) is retained in the department for future reference. The fruits and leaves were shade dried and the dried material was milled to obtain coarse powder and then cold extracted using hydroalcohol in the ratio of 70:30 (ethanol:water). The extract was dried in a rotary evaporator under reduced pressure. The extract was stored at −20 °C in a dark bottle.
2.3. DPPH radical scavenging activity
DPPH is a stable free-radical molecule widely used assay to measure the free radical scavenging capacity of plant extracts. Assay was initiated by adding 200 μl of 0.004% DPPH methanolic solution into 96-well plate, followed by addition of 20 μl of H. rhamnoides extract, or solvent or the blank. The mixture was incubated at 30 °C for 1 h and the absorbance was measured at 515 nm in a microplate reader (Multiskan, Thermo Scientific) (Kim et al., 2002; Bertrand et al., 2005). The percentage of inhibition was calculated as:
where Ao is the absorbance of the blank and As is the absorbance of the sample at 515 nm.
2.4. Hydroxyl radical scavenging assay
Hydroxyl radical scavenging assay was carried following the method of Su et al. (1998) with slight modification. 2 ml of 6 mM ferrous sulfate was added to different concentrations of 2 ml of H. rhamnoides extract (0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 mg/ml), to which 2 ml of 6 mM hydrogen peroxide was added. The above mixture was incubated for 10 min, after which 2 ml of 6 mM sodium salicylate was added and incubated at 37 °C for 30 min. On completion of the incubation period the samples were read for absorbance at 510 nm using spectrophotometer (Lab India) and the percentage of inhibition was calculated as:
where As is the absorbance of the sample with sodium salicylate. Aw is the absorbance of the sample without sodium salicylate and Ao is the absorbance of the reagent.
2.5. FTC assay
The antioxidant activity of H. rhamnoides on inhibition of lipid peroxidation was determined using ferric thiocyanate assay (Kikuzaki and Nakatani, 1993). A mixture containing the plant extract 4 ml in absolute ethanol, 2.51% linoleic acid in absolute ethanol (4.1 ml), 0.05 M phosphate buffer pH 7 (8 ml) and distilled water (3.9 ml) was placed in a vial with a screw cap. The vial was placed in an oven at 40 °C in the dark. To 0.1 ml of the above solution 9.7 ml of 75% ethanol and 0.1 ml of 30% ammonium thiocyanate were added. Three minutes after adding 0.1 ml of 0.02 M ferrous chloride in 3.5% hydrochloric acid to the reaction mixture, the absorbance was measured at 500 nm, every 24 h until the absorbance of the control (without sample) reached maximum. α-tocopherol was used as standard.
2.6. Cell line maintenance
Human neural cell line-IMR32 was procured from National Center for Cell Sciences, Pune, India. The IMR32 cells were cultured in DMEM supplemented with 10% FBS, penicillin (100 units/ml) and streptomycin (100 units/ml) under standard culture condition at 37 °C and 5% CO2 incubator (Thermo Scientific).
2.7. Analysis of the direct effect of H. rhamnoides on IMR32
The direct effect of H. rhamnoides extract was assessed prior to the oxidative stress challenge in order to study the toxicity range of the extract. IMR32 cells were incubated with different concentrations of H. rhamnoides extract and assessed for viability using MTT assay. MTT is a colorimetric assay for assessing cell cytotoxicity. IMR32 cells in the exponential phase were seeded onto 96-well plate (1 * 104 cells/well), incubated overnight and pretreated with various concentrations of H. rhamnoides extracts (0–1000 μg/ml) for 24 h. Then, the medium was removed and cells were washed with phosphate buffer solution (PBS). 100 μl of MTT at a concentration of 5 mg/ml was added to each well and incubated for 2 h. The solution was then removed and 100 μl of DMSO was added to each well, after 10 min, the samples were read at 570 nm (Mosmann, 1983) in microplate reader (Multiskan, Thermo Scientific).
2.8. Analysis of neuroprotective of H. rhamnoides on oxidative stress induced cytotoxicity using propidium iodide staining
Propidium iodide (PI) is a fluorescent compound, used to stain cellular DNA in order to evaluate cell viability, DNA content, cell cycle analysis and DNA damage (Behl et al., 1995). Cells of 1 × 106 cells/well density were seeded in a 12-well plate and incubated for 24 h. The cells were then treated with different concentrations of H. rhamnoides extract (3.2–100 μg/ml) for 24 h, after which they were exposed to the toxic stress inducer H2O2 for 24 h. The cells were stained with PI and analyzed along with appropriate controls using flowcytometer (FACSCalibur, BD Bioscience) with emission and excitation range being ex 535/em 617.
2.9. Measurement of intracellular ROS
Oxidation-sensitive dye, DCFDA was employed to measure the intracellular ROS activity induced by hydrogen peroxide in human neural cell line (Wang and Joseph, 1999). On entering the cell, DCFDA is hydrolyzed by cellular esterase enzyme to a non-fluorescent dichloro-dihydro-fluorescein (DCFH) which is oxidized to highly fluorescent dichlorofluorescein (DCF) by the intracellular ROS. The amount of fluorescence is equal to the amount of intracellular ROS generated inside the cells (Lebel et al., 1992). Human neural cells were plated at a density of 1 × 106 cells/well in 12-well plate; after 24 h, cells were treated with different concentrations of H. rhamnoides extract and exposed to 250 μM of H2O2 for 6 h. At the end of treatment 10 μM DCFDA was added to the cells and incubated for 30 min. The fluorescence was detected in flowcytometer at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
2.10. Statistical analysis
All assays were carried out in triplicates and the data were represented as mean ± SD value. The significant differences among various groups were compared by ANOVA followed by Dunnett’s test using Graphpad prism software version 6. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Free radical scavenging assay
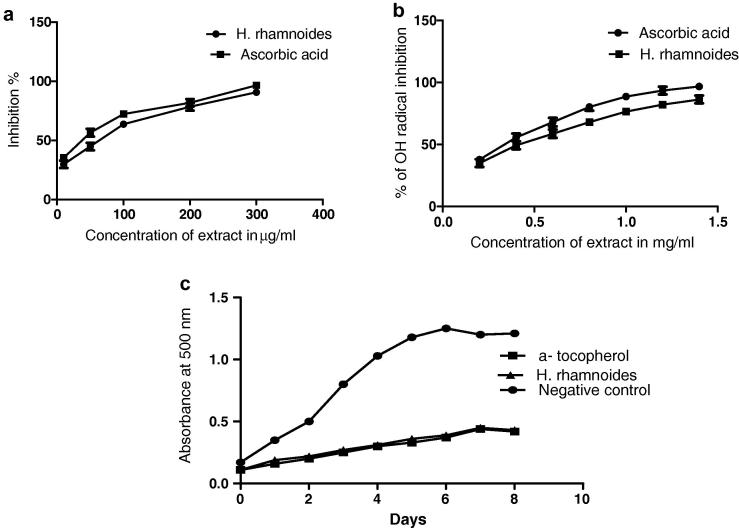
DPPH, OH radical scavenging and FTC assays were performed to evaluate the free radical scavenging activity of H. rhamnoides using ascorbic acid and α-tocopherol as standards. The results confirmed that H. rhamnoides extract showed a concentration dependent radical scavenging activity. The IC50 value for DPPH assay was found to be 70.91 μg/ml as seen in Fig. 1a. The extract was also found to show considerable OH radical scavenging activity, the highest scavenging effect was found to be 86.24% at 1.4 mg/ml, with IC50 value being 0.463 mg/ml (Fig. 1b). As OH radical scavenging activity is an important factor for evaluating antioxidant property the present study shows that the H. rhamnoides is an efficient antioxidant. FTC results portrayed that the extract possesses comparable lipid peroxidation activity with that of standard.
Figure 1.
Free radical scavenging activity of H. rhamnoides: (a) DPPH assay, (b) OH radical scavenging activity, (c) Lipid peroxidation activity.
3.2. Effect of H. rhamnoides extract on IMR32 cell line
Cytotoxic effect of H. rhamnoides extract on human neural cell line-IMR32 was evaluated by incubating it with various concentrations of H. rhamnoides extract (1–1000 μg/ml) as seen in Fig. 2 (1000 μg/ml data not shown). The toxicity results revealed a decrease in percentage of viability at higher concentrations of extract and the IC50 value was found to be 312.63 ± 2.6 μg/ml. The viability of cells was least affected at 100 μg/ml hence, chosen as the highest safe dose for evaluating efficacy of the extract.
Figure 2.
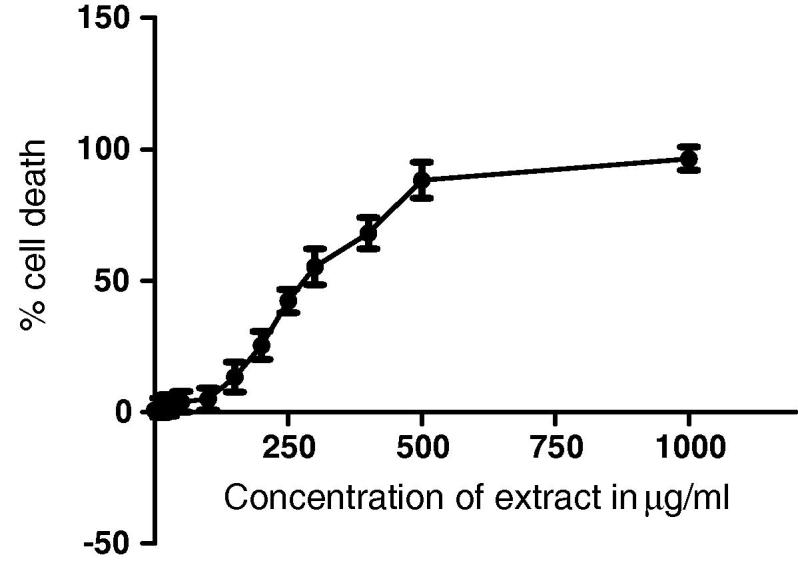
Concentration dependent effect of H. rhamnoides on Human neural cell line-IMR32. Note – Values represented as mean ± SD. Toxicity results for IMR32 cell line incubated with different concentrations of H. rhamnoides extract.
3.3. Neuroprotective effect of H. rhamnoides on H2O2 induced cytotoxicity
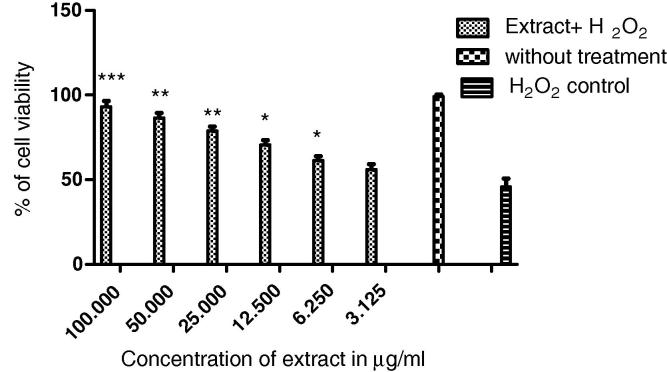
Neuroprotective activity of H. rhamnoides was assessed by subjecting IMR32 cells to 24 h H2O2 (250 μM) challenge, which were pretreated with different concentrations of H. rhamnoides extract for 24 h (3.2–100 μg/ml). The cell death was determined using propidium iodide staining assay. An increase in cell viability was observed in treated cells compared to H2O2 controls in a dose dependent manner as illustrated in Fig. 3. 100 μg/ml was found to be the effective concentration; similar results were obtained from MTT assay (results not shown). A significant increase in cell viability was observed at 100 μg/ml of the plant extract.
Figure 3.
Neuroprotective effect of H. rhamnoides on H2O2 induced cell cytotoxicity: PI staining assay. Note – Values represented as mean ± SD. ANOVA followed by dunnett’s test was carried out to determine the level of significance. Comparison was made between the treated and H2O2 control group. Toxicity results of IMR32 cell line induced with 250 μM of H2O2 and pre incubated with different concentrations of H. rhamnoides extract.
3.4. Effect of H. rhamnoides extract on intracellular ROS activity
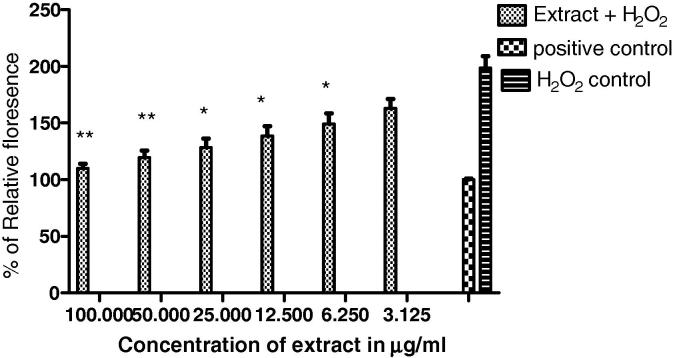
DCFDA assay was used to measure the amount of ROS generated in IMR32 cells during oxidative stress injury. The amount of fluorescence liberated was directly proportional to the ROS generated in the cells. Incubation of IMR32 cells with 250 μM of H2O2 induced intracellular ROS accumulation along with other peroxides which were detected with DCF stain. ROS activity was found to be decreased in pretreated cells as seen in Fig. 4. It was observed that H. rhamnoides extract significantly reduced the relative fluorescence by 60–70% when compared to un-treated cells, the level of significance in treated cells at 50 and 100 μg/ml concentration was P < 0.05 and P < 0.01 respectively.
Figure 4.
Effect of H. rhamnoides extract on intracellular ROS production. Note – Values represented as mean ± SD. ANOVA followed by dunnett’s test was carried out to determine the level of significance. Comparison was made between the treated and H2O2 control group. Intracellular ROS activity results for IMR32 cell line induced with 250 μM of H2O2 and pre incubated with different concentrations of H. rhamnoides extract.
4. Discussion
The present study provides information on the antioxidant and neuroprotective activities of H. rhamnoides against exogenous oxidative stress induced cytotoxicity.
Excess accumulation of ROS leads to cellular damage and inflammation of the tissues (Murphy et al., 2011). ROS plays a major role in cellular senescence paving way to neural cell death; therefore there is an urgent need for potential therapeutics that may prevent oxidative stress induced neurodegeneration. With the above context and the concerns side effects of conventional drugs have evoked researcher’s focus on natural antioxidants especially from plants, as they possess strong free radical scavenging activity, and prevent oxidant–induced cellular damage (Hussain et al., 2007; Kim et al., 2009; Miranda et al., 2008; Naval et al., 2007; Hake et al., 2009). Literature quotes H. rhamnoides as a potent antioxidant, which provoked us to evaluate its neuroprotective activity and thus we have conducted the study by screening the extract for its antioxidant property.
The antioxidant activity of H. rhamnoides was studied using DPPH, hydroxyl radical scavenging and FTC assays, as these assays are the best and accurate method for screening natural antioxidants. Our results confirmed that the hydroalcoholic extract of H. rhamnoides effectively scavenged the free radicals liberated.
The most common method applied for studying the in vitro neuroprotective activity of antioxidants is H2O2 induced cytotoxicity (Chow et al., 2005; Fallarero et al., 2006; Garcia-Alonso et al., 2006) hence; this method was employed to study the neuroprotective effect of H. rhamnoides. It was observed that human neural cell line (IMR32) was protected against H2O2 challenge by pretreating the cells with different concentrations of H. rhamnoides extract. A significant increase in cell viability was found in H. rhamnoides treated cells compared to H2O2 control and the increase in cell viability was dose dependent. This increase could be because of the ability of compounds present in plant extract to respond to cellular stress and protect the macromolecules from toxicity (Vichneveshaia and Roy, 2001).
H. rhamnoides extract was found to act on peroxides and other free radicals which were evident through the DCFDA results. A decrease in the level of fluorescence was observed in cells treated with H. rhamnoides extracts. The antioxidant and neuroprotective activity of H. rhamnoides would be the result of its rich bioactive contents like vitamins A, C, E, and F, riboflavin, folic acid, carotenoids, phytosterols, organic acids, polyunsaturated fatty acids and essential amino acids (Beveridge et al., 1999; Yang and Kallio, 2001; Pintea et al., 2005). Studies have also revealed H. rhamnoides as a neuroprotectant using aging brain and scopolamine induced models in rats (Shivapriya et al., 2013; 2014). Thus, the present study confirms that the plant acts as a potent antioxidant and protects the human neural cells IMR32 from hydrogen peroxide induced cell cytotoxicity.
5. Conclusion
The present study may be one of its kinds reporting the neuroprotective effect of H. rhamnoides on human neural cell line. We have found that H. rhamnoides extract protects the neural cells from oxidative stress induced damage through its antioxidant property. Hence, the plant may be advocated for neurodegenerative disorder induced by oxidative stress. It has to be further validated with advanced molecular mechanism and gene regulation studies.
Acknowledgement
Would like to thank Mr. Ganesh and other staffs for their support during the work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Basu M., Prasad R., Jayamurthy P., Pal K., Arumughan C., Sawhney R.C. Anti atherogenic effects of Sea buckthorn (Hippophae rhamnoides) seed oil. Phytomedicine. 2007;14:770–777. doi: 10.1016/j.phymed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Behl C., Widmann M., Trapp T., Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem. Biophy. Res. Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- Bertrand P., Alain S.C.S., Jacqueline S. Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J. Agric. Food Chem. 2005;54:7270–7276. doi: 10.1021/jf060808o. [DOI] [PubMed] [Google Scholar]
- Beveridge T., Li T.S.C., Oomah B.D. Sea buckthorn products: manufacture and composition. J. Agric. Food Chem. 1999;47:3480–3488. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- Chawla R., Arora R., Singh S., Sagar R.K., Sharma R.K., Kumar R., Sharma A., Gupta M.L., Singh S., Prasad J., Khan H.A., Swaroop A., Sinha A.K., Gupta A.K., Tripathi R.P., Ahuja P.S. Radioprotective and antioxidant activity of fractionated extracts of berries of Hippophae rhamnoides. J. Med. Food. 2007;10:101–109. doi: 10.1089/jmf.2006.007. [DOI] [PubMed] [Google Scholar]
- Chow J.M., Shen S.C., Huan S.K., Lin H.Y., Chen Y.C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharma. 2005;69(12):1839–1851. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Emerit J., Edeas M.F.B. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Fallarero A., Peltoketo A., Loikkanen J., Tammela P., Vidal A., Vuorela P. Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine. 2006;13(4):240–245. doi: 10.1016/j.phymed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Freidovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann. N.Y. Acad. Sci. 1999;893:13. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Gao Z.L., Gu X.H., Cheng F.T., Jiang F.H. Effect of Sea buckthorn on liver fibrosis: a clinical study. World J. Gastroenterol. 2003;9:1615–1617. doi: 10.3748/wjg.v9.i7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso M., Jacobs E., Raybould A., Nickson T.E., Sowig P., Willekens H. A tiered system for assessing the risk of genetically modified plants to non target organisms. Environ. Biosaf. Res. 2006;5:57–65. doi: 10.1051/ebr:2006018. [DOI] [PubMed] [Google Scholar]
- Geetha S., Sai Ram M., Singh V., Ilavazhagan G., Sawhney R.C. Antioxidant and immunomodulatory properties of Sea buckthorn (Hippophae rhamnoides) – an in vitro study. J. Ethnopharmacol. 2002;79:373–378. doi: 10.1016/s0378-8741(01)00406-8. [DOI] [PubMed] [Google Scholar]
- Geetha S., Sai Ram M., Singh V., Ilavazhagan G., Sawhney R.C. Effect of Sea buckthorn against sodium nitroprusside induced oxidative stress in murine macrophages. Biomed. Pharmacother. 2002;56:463–467. doi: 10.1016/s0753-3322(02)00290-1. [DOI] [PubMed] [Google Scholar]
- Goel H.C., Prasad J., Singh S., Sagar R., Prem Kumar I., Sinha A.K. Radioprotection by a herbal preparation of Hippophae rhamnoides RH-3, against whole body lethal irradiation in mice. Phytomedicine. 2002;9:135–143. doi: 10.1078/0944-7113-00077. [DOI] [PubMed] [Google Scholar]
- Guan S., Bao Y.M., Jiang B., An L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative Pc12 cell death. Eur. J. Pharm. 2006;538:73–79. doi: 10.1016/j.ejphar.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Gupta A., Kumar R., Pal K., Banerjee P.K., Sawhney R.C. A preclinical study of the effects of Sea buckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. Int. J. Low. Extrem. Wounds. 2005;4:88–92. doi: 10.1177/1534734605277401. [DOI] [PubMed] [Google Scholar]
- Hake I., Schonenberger S., Neumann J., Franke K., Paulsen-Merker K. Neuroprotection and enhanced neurogenesis of extract from the tropical plant Knema Laurina after inflammatory damage in living brain tissue. J. Neuroimmunol. 2009;206:91–99. doi: 10.1016/j.jneuroim.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Hallliwell B. The wanderings of free radicals. Free Radic. Bio. Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Hussain G.M., Mishra D., Singh P.N., Rao Ch.V., Kumar V. Ethnopharmacological review of native traditional medicinal plants for brain disorders. Pharm. Rev. 2007;119:513–537. [Google Scholar]
- Kikuzaki H., Nakatani N. Antioxidant effects of some ginger constituents. J. Food Sci. 1993;58:1407–1410. [Google Scholar]
- Kim I.S., Koppula S., Park P.J., Kim C.G. Chrysanthemum morifolium ramat (CM) extract protects human neuroblastoma SH-SY%Y cells against MPP+ induced cytotoxicity. J. Ethnopharmcol. 2009;126:447–454. doi: 10.1016/j.jep.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Kim J.K., Noh J.H., Lee S., Choi J.S., Suh H., Chung H.Y. The first total synthesis of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether (TDB) and its antioxidant activity. Bull. Korean Chem. Soc. 2002;23(5):661–662. [Google Scholar]
- Lebel C.P., Ischiropoulos H., Bondy S.C. Evaluation of the probe 20,70 dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Li T.S.C. Taxonomy, natural distribution and botany. In: Li T.S.C., Beveridge T., editors. Sea Buckthorn (Hippophae rhamnoides L.): Production and Utilization. NRC Research Press; Ottawa, ON: 2003. pp. 7–11. [Google Scholar]
- Mandraju R., Kannapiran P., Anand K.K. Distinct roles of topoisomerase II isoforms: DNA damage accelerating alpha, double strand break repair promoting beta. Arch. Biochem. Biophys. 2008;470:27–34. doi: 10.1016/j.abb.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Miranda D.D., Aracri D.P., Pedrazzoli J., Jr., Carvahhlo Pde O., Cerutti S.M. Protective effects of mate tea (llex paraguariensis) on H2O2 – induced DNA damage and DNA repair in mice. Mutagenesis. 2008;23:261–265. doi: 10.1093/mutage/gen011. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murphy M.P., Holmgren A., Larsson N.G., Halliwell B., Chang C.J. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naval M.V., Gomez-Serranillos M.P., Carretero M.E., Vilar A.M. Neuroprotective effect of ginseng (Panax ginseng) root extract on astrocytes primary culture. J Ethnopharmacol. 2007;112:262–270. doi: 10.1016/j.jep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Piga R., Saito Y., Chen Z., Yoshida Y., Niki E. Characterization of monochloramine toxicity on PC12 cells and protective effect of tocopherol via antioxidative function. Arch. Biochem. Biophys. 2005;436:101–109. doi: 10.1016/j.abb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Pintea A., Varga A., Stepnowski P., Socaciu C., Culea M., Diehl H.A. Chromatographic analysis of carotenol fatty acid esters in Physalis alkekengi and Hippophae rhamnoides. Phytochem. Anal. 2005;16:188–195. doi: 10.1002/pca.844. [DOI] [PubMed] [Google Scholar]
- Ramakrishna, V., Setty, O.H., 2009. Safety, efficacy and preclinical evaluation of plant products: Comp. Bio. Nat. Pro, (Studium press, USA).
- Rao K.S. DNA repair in aging rat neurons. Neurosciences. 2007;145:1330–1340. doi: 10.1016/j.neuroscience.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Rodesh V., Rundein-Pran E., Luna L., Mcmurray C., Bjoras M. Widespread distribution of DNA glycosylases removing oxidative DNA lesions in human and rodent brains. DNA Repair. 2008;7:1578–1588. doi: 10.1016/j.dnarep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousi A. The genus Hippophae L., a taxonomic study. Ann. Bot. Fennici. 1971;8:177–227. [Google Scholar]
- Saggu S., Divekar H.M., Gupta V., Sawhney R.C., Banerjee P.K., Kumar R. Adaptogenic and safety evaluation of Sea buckthorn (Hippophae rhamnoides) leaf extract: a dose dependent study. Food. Chem. Toxicol. 2007;45:609–617. doi: 10.1016/j.fct.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Shivapriya S., Ilango K., Agrawal A., Dubey G.P. In vivo antioxidant and neuroprotective effect of Hippophae rhamnoides. Int. J. Pharm. Pharm. Sci. 2013;5(2) doi: 10.1016/j.sjbs.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivapriya S., Ilango K., Agrawal A., Dubey G.P. Effect of Hippophae rhamnoides on cognitive enhancement via neurochemical modulation in scopolamine induced Sprague Dawley rats. Int. J. Pharm. Sci. Res. 2014;5(10) [Google Scholar]
- Su J.D., Osawa T., Kawakishi S., Namili M. Tannin antioxidants from Osbeckia chinensis. Phytochemistry. 1998;27:1315–1319. [Google Scholar]
- Suleyman H., Demirezer L.O., Buyukokuroglu M.E., Akcay M.F., Gepdiremen A., Banoglu Z.N., Gocer F. Antiulcerogenic effect of Hippophae rhamnoides. Phytother. Res. 2001;33:77–81. doi: 10.1002/ptr.831. [DOI] [PubMed] [Google Scholar]
- Upadhyay N.K., Kumar R., Mandotra S.K., Meena R.N., Siddiqui M.S., Sawhney R.C., Gupta A. Safety and wound healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil in experimental rats. Food Chem. Toxicol. 2009;47:1146–1153. doi: 10.1016/j.fct.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Upadhyay N.K., Kumar R., Siddiqui M.S., Gupta A. Mechanism of wound healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid. Based Complement. Alternat. Med. 2011 doi: 10.1093/ecam/nep189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichneveshaia K.D., Roy D.N. Oxidative stress and antioxidative defense with an emphasis on plants antioxidants. Environ. Rev. 2001;7:31–35. [Google Scholar]
- Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Bio. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Xing J., Yang B., Dong Y., Wang B., Wang J., Kallio P.H. Effects of sea buckthorn (Hippophae rhamnoides L.) seed and pulp oils on experimental models of gastric ulcer in rats. Fitoterapia. 2002;73:644–650. doi: 10.1016/s0367-326x(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Yang B., Kallio H.P. Fatty acid composition of lipids in Sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J. Agric. Food Chem. 2001;49:1939–1947. doi: 10.1021/jf001059s. [DOI] [PubMed] [Google Scholar]
- Yun-Zhong F., Sheng Y., Guoyao W.u. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]