Abstract
This study was carried out to determine the LC50 of zinc oxide nanoparticles (ZnONPs) on Oreochromis niloticus and to investigate the effect of vitamin E and C on hematological and biochemical alterations induced by two sublethal concentrations (1 and 2 mg/L) of ZnONPs. One hundred and eighty fish were used for studying the lethal concentrations of ZnONPs. For sublethal study two hundred and twenty-five males of O. niloticus were equally divided into five groups, control, the second and the third were treated with 1 and 2 mg/L ZnONPs respectively. The fourth and fifth were exposed to the same concentrations of ZnONPs plus vitamins E and C. The results revealed that the 96 h LC50 of ZnONPs was 3.1 ± 0.4 mg/L. The sublethal study revealed the presence of normocytic normochromic anemia in groups (2, 3 and 5) along the experiment period. The 4th group showed normocytic normochromic anemia at the 7th day and microcytic hypochromic anemia at the 15th day. Leukocytosis, heterophilia, lymphopenia and monocytopenia were recorded at the 7th day in all treated groups compared with the normal control. At the 15th day heteropenia, lymphopenia and monocytopenia were reported in all treated groups. A significant increase in the serum levels of alkaline phosphatase, aminotransferases, urea, creatinine and erythrocytic nuclear and morphological abnormalities along the experimental periods in all treated groups compared with the normal control. Serum total protein and albumin levels were significantly decreased at the same period in the same groups. Addition of vitamin E and C to the diet (groups 4 and 5) significantly improved all measured parameters compared with groups (2 and 3) which treated with ZnONPs only.
Keywords: ZnONPs, Vitamin E, Vitamin C, Hematology, Biochemistry, Oreochromis niloticus
1. Introduction
Nanoscience is the study of materials on the nanoscale level approximately between 1 and 100 nm (Rotello, 2003) and deals with the manipulation of formation of two and three-dimensional assemblies of molecular scale building blocks into well-defined nanostructures or nanomaterials (Rosi and Mirkin, 2005). Metal oxide nanoparticles are extensively used in a considerable number of applications in food, material, chemical and biological sciences (Aitken et al., 2006). It is well known that bulk materials based on TiO2, SiO2, aluminium and iron oxides have been massively produced for many years. More recently, nanoparticulate versions of these metal oxides have been manufactured and introduced in commercial products such as cosmetics and sunscreens (TiO2, Fe2O3 and ZnO) (Nowack and Bucheli, 2007), fillers in dental fillings (SiO2) in catalysis (TiO2) (Aitken et al., 2006), and as fuel additives (CeO2) (Laosiripojana et al., 2005). Metal oxide NPs may leak into natural bodies of water in their life cycles (production, storage, transportation, consumption, disposal, or reproduction), but relatively little is known about the magnitude of NPs released and exposed to organisms living in impacted aquatic environments, as well as the potential for toxic effects to aquatic species (Paterson et al., 2011). Therefore, there is an urgent need for information on the ecological risks of metal oxide NPs.
Zinc oxide Nanoparticles (ZnONPs), one of the most widely used nanomaterials, are already produced in industrial scale (Peralta-Videa et al., 2011). It will certainly be used more widely in different industries as a result of their unique advantages in industrial and medicinal products. Direct and indirect releases of nanoparticles (NPs) into aquatic environments via engineering applications and sewage effluent will also increase the exposure of humans and ecosystems to NPs. The potential effects of ZnONPs on aquatic ecosystems have attracted special attentions (Collins et al., 2012). ZnONPs have been investigated in various aspects, including biochemistry and toxicology (Li et al., 2011), and were confirmed to be one of the most harmful NPs in aquatic environment (Kahru and Dubourguier, 2010). Although some disagreements exist, the generally accepted toxicity mechanisms of ZnONPs include particle effects and dissolved free-ion effects (Ma et al., 2013). The solubility of ZnONPs (in the milligram-per-liter range) was similar to that of bulk ZnO, and at concentration of 60 mg Zn/L, the 72 h LC50 to a freshwater alga (Pseudokirchneriella subcapitata) was similar with the bulk ZnO (Franklin et al., 2007). Heinlaan et al. (2008) found that both nCuO and nZnO were toxic to bacteria and crustaceans. Also, García et al. (2011) obtained a 48 h median lethal concentration of 12 mg/L for nCeO2 treated on Daphnia magna. Furthermore, 50 and 100 mg/L of nZnO killed zebrafish embryos after 96 h of exposure and caused malformation at concentrations ranging from 1 to 25 mg/L (Bai et al., 2010). nCuO at 1.5 mg/L caused acute mortality on zebrafish (Danio rerio) after 48 h of exposure (Griffitt et al., 2007). In an aquatic environment, the exposure of living organisms to NPs leads to interactions between the chemical and the biological system and may give rise to biochemical disturbances or/and adaptive responses (Zhu et al., 2008; Lu et al., 2011). The biological responses, termed biomarkers, can be used to assess the health status of organisms and to obtain the earliest signs of environmental disturbances (Binelli et al., 2009).
This study was carried out to determine LC10, LC50 and LC90 of zinc oxide nanoparticles (ZnONPs) on Oreochromis niloticus and to investigate the effect of vitamin E and C on hematological and biochemical alterations induced by two sublethal concentrations (1 and 2 mg/L) of ZnONPs.
2. Material and methods
2.1. Preparation and characterization of ZnONPs suspension
ZnONPs was obtained in the form of dispersion from Sigma–Aldrich, Steinheim, Germany (CAS Number 1314-13-2) of concentration 50 wt.% in H2O, average particle size (APS) was <35 nm. The particle size distribution (hydrodynamic diameter) was <100 nm using dynamic light scattering (DLS) technique, pH 7 ± 0.1 (for aqueous systems) and density 1.7 ± 0.1 g/ml at 25 °C. Suspensions of ZnONPs in a concentration of 1 and 2 mg/L were daily prepared (JL-360, Shanghai, USA) for 20 min. To characterize the ZnONPs shape and size, a small drop of aqueous ZnONPs solution was air dried by directly placing onto a mesh of carbon-coated copper grid then examined under transmission electron microscope, TEM, (JEM-1011, JEOL, Japan)..
2.2. Experimental setup and acclimation of fish
The experiment had the approval of the Ethics Committee of the University of King Abdualaziz. Two hundred and fifty male O. niloticus (Nile tilapia, Perciformes, Cichlidae; weight 90 ± 5 g, length 15 ± 3 cm) were held in twenty glass aquaria (n = 10 individuals/aquarium), with 100 L of water (pH 7.16 ± 0.3, 0.52 mM Ca2+, and 0.24 mM Mg2+) that was changed daily. A continuous system of water aeration (Eheim Liberty 150 Bio-Espumador cartridges), and a 12:12-h light:dark photoperiod. Temperature was maintained at 28 ± 2 °C and dissolved oxygen, at 7.0 ± 0.5 mg/L. Fish were fed with commercial fish food, containing 6% lipids, 31% proteins, 37% carbohydrates, 2.5% fiber, 1.5% total phosphorus,12% ash, 200 mg α-tocopherol/kg, 1700 IU vitamin D3/kg feed, and 10,000 IU vitamin A/kg feed. The amount of feed (on dry matter basis) given daily to fish was 10% of body weight and the fish were fed 3 times daily. Fish were acclimatized for 15 days before the beginning of the experiments.
2.3. Acute toxicity
In median lethal toxicity study, lethality was the endpoints. Test concentrations for lethality were 0, 1, 2, 4, 8 and 16 mg/L for ZnONPs. Test suspensions were prepared and dispersed using bath sonicator for 1 h immediately prior to use without the addition of any stabilizing agents. Ten fish were randomly exposed to each concentration for 24, 48 and 96 h in a 2.5 L container with 1.5 L of the test solution. All the test solutions were changed every 24 h (semi-static method) to ensure a constant concentration. The control group was provided with distilled water without nanoparticles. Each treatment was run in triplicate and placed under the same conditions. Fish were not fed on the day before or during the experimental period to minimize the absorption of the nanoparticles in food and the production of faeces. The 24, 48 and 96 h sublethal concentrations were calculated according to the Environmental Protection Agency (EPA) probit analysis.
2.4. Experimental setup
Sublethal concentration (1 and 2 mg/L) of ZnONPs was applied as toxicants. Vitamin E and C were applied as 400 mg/kg diet.
2.5. Experimental design
After two weeks of acclimatization, fish were randomly classified into 5 groups: control and fourth treated groups. Each group consisted of fifty fish (15 fish/tank). The first group was served as a control. The second group was treated with 1 mg/L ZnONPs only. The third group was treated with 2 mg/L alone. The fourth group was treated with 1 mg/L ZnONPs plus combined of vitamins E and C, and the fifth group was treated with 2 mg/L ZnONPs plus combined vitamins E and C. Exposure duration was 7 and 15 days. Moreover, the ZnONPs water was completely replenished each day.
2.6. Blood sampling
Twenty-five fish were used for obtaining 5 blood samples from each group by pooling at the 7th and 15th days of the experiment. Two blood samples were collected from caudal vertebral vessels according to. The 1st sample of blood (0.5 ml) was collected in dipotassium salt of ethylene-diamine tetraacetic acid (EDTA) tubes for hematological analysis. The 2nd sample of blood (2 ml) was taken without anticoagulant in a sterile centrifuge test tube. The blood samples were left to clot and then centrifuged at 3000 rpm for separation of serum for biochemical analysis.
2.7. Hematological studies
The erythrocytic count was carried out by using hemocytometer and special diluting fluid (Natt–Herrick) for fish blood. Hemoglobin (Hb) was determined by using the cyanmethemoglobin colorimetric method after centrifugation according to the method of Stoskopf (1993). Packed cell volume (PCV) was measured by using microhematocrit tubes method as described by Dacie and Lewis (1991). The erythrocytic indices including mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated according to the method of Stoskopf (1993). Peripheral blood was used immediately for the analysis of nuclear and morphological abnormalities of erythrocytes as described by Çavaş and Ergene-Gözükara (2005). Three slides were examined for each fish.
2.8. Biochemical analysis
The serum total protein was measured according to the method of Grant et al. (1987). The serum albumin level was estimated according to the method of Doumas et al. (1981). The serum globulin was calculated by subtracting the albumin from the obtained total protein as described by Doumas and Biggs (1972). The serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined according to the method of Reitman and Frankel (1957). The serum alkaline phosphatase (ALP) activity was estimated according to the method of Kind and King (1954). The serum urea level was measured according to the method of Patton and Crouch (1977). The serum creatinine level was also determined colorimetrically according to the method of Henry (1974).
2.9. Statistical analysis
Data were processed using the statistical package for social science (SPSS Inc., Chicago, IL, version 20, USA). All results were expressed as mean ± SD. Comparison among groups was done using a one-way analysis of variance (ANOVA) according to Tamhane and Dunlop (2000). Duncan’s test was used for testing the inter-grouping homogeneity. Statistical significance was set at P < 0.05.
3. Results
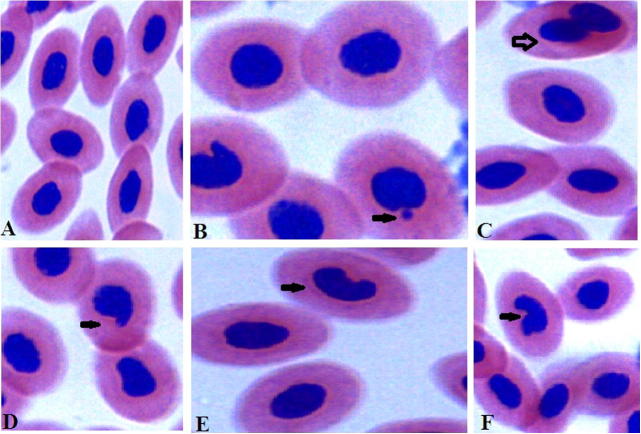
For the control group no mortality was observed. In the presence of ZnONPs, some mortality was recorded and it increased with the ZnONPs concentration. The values of LC10 were 3.23 ± 0.4, 2.81 ± 0.3 and 2.3 ± 0.5 mg/L after 24, 48 and 96 h respectively (Table 1). Although ZnONPs at a concentration of 1 mg/L produced no mortality in fish, as in the control group, the 16 mg/L ZnONPs suspension caused 100% mortality with a calculated 96 h LC90 of 4.4 ± 0.2 mg/L. Table 2 showed a significant decrease in the red blood cells (RBCs) count, PCV and Hb concentration in groups 2, 3 and 5 at the 7th and 15th days of the experiment, while the MCV, MCH and MCHC showed non significant changes. The erythrogram revealed the presence of normocytic normochromic anemia in the same groups at the same periods. The 4th group showed normocytic normochromic anemia at the 7th day and microcytic hypochromic anemia at the 15th day. While data in Table 3 revealed the presence of normal erythrocytes in the blood films of normal O. niloticus (Fig. 1A). A significant increase in the number of cells contain micronuclei (Fig. 1B), binuclei (Fig. 1C), kidney-shaped nuclei (Fig. 1D), blebbed nuclei (Fig. 1E) and hook-shaped nuclei (Fig. 1F) was recorded in all treated groups at the 7th and 15th days of the experiment compared with the normal control. Addition of vitamin E and C to the diet in groups 4 and 5 significantly decreases the frequencies of these cells to become less than those recorded in groups 2 and 3 which treated with metals only. The result in Table 4 showed the frequencies of morphological abnormalities which were recorded in the erythrocytes of the normal control and treated fish at the 7th and 15th days of the experiment. Morphologically, the erythrocytes showed different patterns of altered cell morphology including poikilocytosis and anisocytosis. These patterns include acanthocytes (Fig. 2A), shistocytes (Fig. 2B) and microcytes (Fig. 2C). It was noted that groups 2 and 3 which treated with metals only, the frequencies of acanthocytes, tear drop-like erythrocytes and shistocytes were increased significantly with the increase of exposure time. On the other hand, addition of vitamin E and C to the diet significantly decreases the frequencies of acanthocytes, tear drop-like erythrocytes and shistocytes than that recorded in fish treated with ZnONPs only. Groups 4 and 5 which treated with metals plus vitamin E and C showed a significant increase in the frequencies of microcytes compared with groups 2 and 3. Data in Table 5 showed a significant increase in the serum levels of ALP, AST, ALT, urea and creatinine at the 7th and 15th days of the experiment in all treated groups. Serum total protein and albumin levels were significantly decreased at the same periods in the same groups (Table 4).
Table 1.
Lethal concentrations (mg/L) of ZnONPs on O. niloticus after 24, 48 and 96 h of exposure.
| Time (hours) | Toxicity (mg/L) |
||
|---|---|---|---|
| LC10 | LC50 | LC90 | |
| 24 | 3.23 ± 0.4 | 4.2 ± 0.7 | 6.1 ± 0.3 |
| 48 | 2.81 ± 0.3 | 3.9 ± 0.5 | 5.1 ± 0.4 |
| 96 | 2.3 ± 0.5 | 3.1 ± 0.4 | 4.4 ± 0.2 |
Table 2.
Effects of ZnONPs, vitamin E and vitamin C on hematological parameters.
| Parameters | Time (days) |
Control | ZnONPs (1 mg/L) |
ZnONPs (2 mg/L) |
ZnONPs (1 mg/L) and vitamin E + C |
ZnONPs (2 mg/L) and vitamin E + C |
|---|---|---|---|---|---|---|
| RBCs (×106/μl) | 7 | 1.99 ± 0.07a | 1.44 ± 0.06c | 1.12 ± 0.04d | 1. 68 ± 0.02b | 1.52 ± 0.04c |
| 15 | 2.06 ± 0.07 a | 1.34 ± 0.03 d | 0.95 ± 0.08e | 1. 91 ± 0.08b | 1.63 ± 0.04c | |
| HB (g/dl) | 7 | 6.43 ± 0.22 a | 4.68 ± 0.06 c | 3.78 ± 0.13d | 5.42 ± 0.16b | 4.97 ± 0.09c |
| 15 | 6.83 ± 0.09 a | 4.23 ± 0.12 d | 3.07 ± 0.07e | 5.57 ± 0.18b | 5.13 ± 0.07c | |
| PCV (%) | 7 | 27.00 ± 0.58a | 19.00 ± 0.57c | 15.00 ± 0.56d | 22.00 ± 0.58 b | 20.00 ± 0.55c |
| 15 | 28.00 ± 0.56a | 18.00 ± 0.58d | 13.00 ± 0.55e | 25.00 ± 0.54b | 22.00 ± 0.58c | |
| MCV (Fl) | 7 | 135.68 ± 1.91a | 131.94 ± 2.54a | 133.92 ± 1.01a | 130.95 ± 2.40a | 131.58 ± 0.73a |
| 15 | 135.92 ± 0.35a | 134.32 ± 1.58ab | 135.41 ± 1.11ab | 130.89 ± 0.26b | 134.96 ± 2.26ab | |
| MCH (Pg) | 7 | 32.31 ± 2.09a | 32.50 ± 0.34a | 33.75 ± 2.08a | 32.26 ± 0.63a | 32.70 ± 0.45a |
| 15 | 33.81 ± 0.55a | 31.56 ± 0.41a | 31.98 ± 0.93a | 29.16 ± 0.23b | 31.47 ± 0.50a | |
| MCH C (%) | 7 | 23.81 ± 1.24a | 24.63 ± 1.06a | 25.20 ± 1.64a | 24.62 ± 0.42a | 24.85 ± 0.29a |
| 15 | 25.30 ± 0.34a | 23.49 ± 0.18ab | 23.62 ± 0.68ab | 22.26 ± 0.22b | 23.31 ± 0.37ab |
Means within the same row carrying different superscripts are significant at p < 0.05.
Table 3.
The effects of ZnONPs, vitamin E and vitamin C on The frequency of nuclear abnormalities in erythrocytes.
| Parameters | Time (days) |
Control | ZnONPs (1 mg/L) |
ZnONPs (2 mg/L) |
ZnONPs (1 mg/L) and vitamin E + C |
ZnONPs (2 mg/L) and vitamin E + C |
|---|---|---|---|---|---|---|
| Micronuclei | 7 | 1.80 ± 0.37e | 15.40 ± 0.81b | 18.40 ± 0.51a | 6.20 ± 0.37d | 9.80 ± 0.58c |
| 15 | 2.20 ± 0.36e | 30.00 ± 1.14b | 48.20 ± 0.37a | 5.20 ± 0.37d | 8.00 ± 0.55c | |
| Binuclei | 7 | 0.80 ± 0.37e | 6.40 ± 0.25b | 7.80 ± 0.58a | 3.60 ± 0.24d | 5.00 ± 0.45c |
| 15 | 1.40 ± 0.24e | 6.00 ± 0.32b | 7.20 ± 0.36a | 3.60 ± 0.40d | 4.80 ± 0.36c | |
| Blebbed | 7 | 10.80 ± 1.02e | 39.60 ± 1.50b | 50.80 ± 1.32a | 18.20 ± 1.07d | 28.80 ± 1.24c |
| 15 | 10.40 ± 1.36e | 42.00 ± 1.41b | 54.20 ± 1.74a | 15.20 ± 0.36d | 27.00 ± 1.41c | |
| Kidney-shaped | 7 | 5.40 ± 0.93e | 20.40 ± 0.87b | 28.80 ± 0.86a | 13.80 ± 0.58d | 17.20 ± 0.37c |
| 15 | 4.40 ± 0.51e | 23.80 ± 1.28b | 29.80 ± 0.97a | 10.20 ± 0.57d | 15.20 ± 0.73c | |
| Hook-shaped nuclei | 7 | 3.20 ± 0.58d | 10.00 ± 0.32b | 12.00 ± 1.14a | 5.60 ± 0.51c | 8.20 ± 0.37b |
| 15 | 3.00 ± 0.71e | 10.60 ± 0.50b | 13.00 ± 0.84a | 4.80 ± 0.37d | 7.80 ± 0.36c |
Means within the same row carrying different superscripts are significant at p < 0.05.
The values in the table are representing the mean/1000 cells ± SD.
Figure 1.
The different types of RBCs nuclear abnormalities. Normal erythrocytes (A), micronucleus (B), binucleated nucleus (C), blebbed nucleus (D), Kidney shape nucleus (E), and Hook shape nucleus (F).
Table 4.
The effects of ZnONPs, vitamin E and vitamin C on The frequency of morphological abnormalities in erythrocytes.
| Parameters | Time (days) |
Control | ZnONPs (1 mg/L) |
ZnONPs (2 mg/L) |
ZnONPs (1 mg/L) and vitamin E + C |
ZnONPs (2 mg/L) and vitamin E + C |
|---|---|---|---|---|---|---|
| Acanthocyte | 7 | 12.80 ± 1.39e | 58.00 ± 1.64b | 40.00 ± 1.70a | 20.40 ± 1.44d | 29.40 ± 1.29c |
| 15 | 13.20 ± 1.16e | 67.00 ± 2.68b | 47.60 ± 2.06a | 18.80 ± 1.24d | 27.40 ± 0.87c | |
| Shistocyte | 7 | 10.80 ± 1.02e | 39.60 ± 1.50b | 50.80 ± 1.32a | 18.20 ± 1.07d | 28.80 ± 1.24c |
| 15 | 10.40 ± 1.36e | 42.00 ± 1.41b | 54.20 ± 1.74a | 15.20 ± 0.36d | 27.00 ± 1.41c | |
| Microcyte | 7 | 9.00 ± 0.71a | 3.80 ± 0.37d | 2.40 ± 0.50e | 6.80 ± 0.20b | 5.40 ± 0.25c |
| 15 | 8.20 ± 0.37c | 5.80 ± 0.73d | 3.20 ± 0.58e | 18.40 ± 0.93a | 11.80 ± 0.66b |
Means within the same row carrying different superscripts are significant at p < 0.05. The values in the table are representing the mean/1000cells ± SD.
Figure 2.
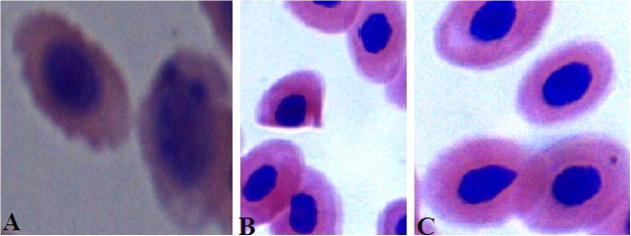
Showing the different types of poikilocytosis and anisocytosis. Acanthocyte (A), Shistocyte (B), and Microcyte (C).
Table 5.
The effects of ZnONP, vitamin E and vitamin C on blood chemistry.
| Parameters | Time (days) |
Control | ZnONPs (1 mg/L) |
ZnONPs (2 mg/L) |
ZnONPs (1 mg/L) and vitamin E + C |
ZnONPs (2 mg/L) and vitamin E + C |
|---|---|---|---|---|---|---|
| ALP (U/L) | 7 | 8.31 ± 0.48e | 27.37 ± 0.75b | 37.70 ± 0.90a | 13.33 ± 0.24d | 16.40 ± 0.31c |
| 15 | 8.37 ± 0.41e | 33.20 ± 0.53b | 45.80 ± 0.76a | 13.87 ± 0.18d | 19.83 ± 0.44c | |
| ALT (U/L) | 7 | 12.43 ± 0.34e | 32.66 ± 0.41b | 43.73 ± 0.71a | 14.26 ± 0.37d | 20.13 ± 0.47c |
| 15 | 12.20 ± 0.23e | 47.66 ± 0.88b | 58.17 ± 1.20a | 23.66 ± 0.37d | 28.56 ± 0.43c | |
| AST (U/L) | 7 | 62.66±.88e | 122.0 ± 1.16b | 140 ± 1.16a | 92.66 ± 0.88d | 102.67 ± 1.45c |
| 15 | 61.67±.88e | 135.0 ± 1.15b | 158 ± 1.20a | 94.33 ± 0.88d | 104.67 ± 1.45c | |
| Total protein (g/dl) | 7 | 4.65 ± 0.03a | 3.72 ± 0.05d | 3.41 ± 0.04e | 4.23 ± 0.02b | 3.94 ± 0.03c |
| 15 | 4.66 ± 0.04a | 3.57 ± 0.05d | 3.29 ± 0.02e | 4.18 ± 0.02b | 3.80 ± 0.06c | |
| Albumin (g/dl) | 7 | 2.23 ± 0.03a | 1.42 ± 0.06d | 1.18 ± 0.02e | 1.80 ± 0.05b | 1.64 ± 0.03c |
| 15 | 2.24 ± 0.03a | 1.27 ± 0.07d | 1.01 ± 0.06e | 1.77 ± 0.04b | 1.48 ± 0.09c | |
| Globulin (g/dl) | 7 | 2.42 ± 0.01a | 2.31 ± 0.06a | 2.28 ± 0.05a | 2.43 ± 0.04a | 2.37 ± 0.07a |
| 15 | 2.42 ± 0.02a | 2.30 ± 0.06a | 2.29 ± 0.04a | 2.43 ± 0.04a | 2.32 ± 0.04a | |
| Creatinine (mg/dl) | 7 | 0.91 ± 0.02e | 1.51 ± 0.02b | 1.67 ± 0.01a | 1.15 ± 0.03d | 1.29 ± 0.02c |
| 15 | 0.89 ± 0.02e | 1.65 ± 0.04b | 1.80 ± 0.01a | 1.18 ± 0.03d | 1.31 ± 0.01c | |
| Urea (mg/dl) | 7 | 5.55 ± 0.08e | 11.63 ± 0.13b | 12.63 ± 0.06a | 7.47 ± 0.09d | 9.8 ± 0.06c |
| 15 | 5.36 ± 0.08e | 12.87 ± 0.35b | 15.72 ± 0.16a | 8.08 ± 0.09d | 11.00 ± 0.23c |
Means within the same row carrying different superscripts are significant at p < 0.05.
4. Discussion
The erythrogram revealed a significant decrease in the RBCs count, PCV and hemoglobin concentration, beside non significant change in MCV, MCH and MCHC at the 7th day of the experiment in all treated groups. This normocytic normochromic anemia may be due to decrease of the life span of RBCs or suppression of the bone marrow stem cell activity. The increase in the frequencies of shistocytes indicates the presence of hemolysis. The main toxic mechanism of nano ZnO was possibly by increasing cellular oxidative stress (Hao et al., 2013). The 4th group revealed signs of regeneration and this was clarified by the appearance of microcytic hypochromic anemia at the 15th day of exposure. The microcytosis appeared in the 4th and 5th groups indicate the presence of immature RBCs in the blood (signs of regeneration). As fish immature RBCs are smaller than mature cells, its increase indicates the presence of hemolytic anemia. From our opinion this improvement may be due to the effect of vitamin E and C which decreased the oxidative stress. The leukocytosis, heterophilia, lymphopenia, monocytopenia and eosinopenia observed at the 7th day in all treated groups indicate the presence of stress. Leukopenia recorded in all treated groups at the 15th day is due to heteropenia, lymphopenia and monocytopenia. This may be due to the negative impact on bone marrow and lymphoid tissues.
The use of biomarkers for pollution in fish is an effective strategy for monitoring the aquatic environment and diagnosing of negative impact (Lasheen et al., 2012). Genotoxicity and cytotoxicity of nano ZnO on erythrocytes of O. niloticus were evaluated in this study. The genotoxic alterations include formation of micronuclei, binuclei, blebbed nuclei, kidney-shaped nuclei and hook-shaped nuclei; while the morphological abnormalities include the formation of canthocytes and shistocytes in groups 2–5. Microcytes significantly increased at the 15th day of exposure in groups 4 and 5. The microcytosis appeared in these groups is a sign of regeneration. A significant increase in the frequencies of canthocytes clarified the negative impact of nano ZnO on liver. Micronuclei are chromatin masses appearing as small nuclei outside the nucleus which can arise from either the breakage of chromosomes which leads to the formation of chromosome fragments or dysfunction of mitotic spindle apparatus that leads to entire chromosomes lagging behind in the anaphase stage and fail to become incorporated into daughter cell nuclei during cell division (Okonkwo et al., 2011). The genetic damages such as fragmentation of chromosomes or changes in their number may lead to alteration of gene number and their orders (Okonkwo et al., 2011), and consequently may cause disturbance in gene expressions and overall activities of cells and daughter cells (Harabawy and Mosleh, 2014). The binuclei and blebbed-nucleated cells exhibit a similar origin as micronuclei and are considered as genotoxic analogs of micronuclei (Serrano-Garcia and Montero-Montoya, 2001). The formation of binuclear cell is an indicator for abnormal cell division due to blocking of cytokinesis (Mahboob et al., 2014). Crott and Fenech (2001) suggested that the blebbed nuclei may be a precursor of micronuclei. Accordingly, the other forms of abnormal nuclei recorded in the present work such as kidney-shaped nuclei and hook shaped nuclei may represent different precursors of micronuclei or binuclei phenomena (Harabawy and Mosleh, 2014). So the formation of nuclear abnormalities may represent a process by which the cell eliminates any amplified genetic material from the nucleus (Shimizu et al., 1998). The abnormalities of erythrocytes may be due to the liberation of reactive oxygen species (ROS) which cause lipid peroxidation for unsaturated fatty acids in the cell membrane and subsequently damage the cell membrane (Ahmad et al., 2006). Our results revealed a significant increase in the frequencies of nuclear abnormalities (micronuclei, binuclei, blebbed nuclei, kidney-shaped nuclei and hook shaped nuclei) in groups 2–5. These findings support the previously recorded results by Parveen and Shadab (2012) as they reported that the frequencies of nuclear abnormalities were elevated in fish inhabiting the polluted places or exposed to toxicants. Also, Udroiu et al. (2006) mentioned that the micronuclei in fish erythrocytes occur from one to five days following exposure to cytotoxic and/or genotoxic agents. It was noted that the frequencies of nuclear abnormalities recorded at the 7th day of exposure to metal only (groups 1 and 2) were higher than those recorded at the 15th day. Vitamins are organic compounds necessary for normal growth and health; often are not synthesized by fish, and must be supplied in the diet. In the present study, addition of vitamin E and C to the diet in groups 4 and 5 significantly decreased the frequencies of nuclear abnormalities to become less than fish treated with ZnONPs only. Moreover, the frequencies of nuclear abnormalities recorded at the 7th day in groups 4 and 5 were lower than those recorded at 15th day. This indicates that vitamin E and C could reduce the level of ROS and hence decrease the oxidative stress caused by the toxicity of ZnONPs.
The serum activities of ALP, AST and ALT revealed a significant increase in all treated groups along the experimental periods. The previous observations may be due to the hepatic damage caused by ZnONPs which leads to extensive liberation of these enzymes to the blood circulation. On the same ground, Hao et al. (2013) mentioned that the liver and gill might be the target tissues with exposure to ZnONPs. The observed hypoproteinemia in all treated groups is due to hypoalbuminemia. From our opinion the decreased serum albumin level may be due to decreased feed intake, loss through the intestine and kidney, and disturbed metabolism of the liver. Most urea in fish is produced by the liver and execrated mainly by the gills. The present elevation of serum urea level in all treated groups may be due to the gill dysfunction which leads to disturbance in the diffusion of urea between the blood and the water. An elevated elevation of blood urea nitrogen (BUN) is not indicative of renal disease but it mostly associated with gill and liver diseases (Stoskopf, 1993). The present elevation of serum creatinine level may be due to the renal damage. From the aforementioned result of groups 4 and 5 clarified an improvement in liver and kidney function tests compared to the 2nd and 3rd groups. This indicates that vitamin E and C could be decreased but not prevent completely the toxic effect of ZnONPs.
5. Conclusion
The 96 h LC10, LC50 and LC90 of ZnONPs on O. niloticus were 2.3 ± 0.5, 3.1 ± 0.4 and 4.4 ± 0.2 mg/L. ZnONPs in exposure concentration of 1 and 2 mg/L induced a deleterious hematological and biochemical effects on O. niloticus. A combination of vitamins E and C produced a protective effect against the severe effects of ZnONPs.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (1-965-35-RG). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad I., Maria V.L., Oliveira M., Pacheco M., Santos M.A. Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to beta-naphthoflavone. Mutat. Res. 2006;608:16–28. doi: 10.1016/j.mrgentox.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., Chaudhry M.Q., Boxall A.B.A., Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup. Med. 2006;56:300–306. doi: 10.1093/occmed/kql051. [DOI] [PubMed] [Google Scholar]
- Bai W., Zhang Z., Tian W. Toxicity of zinc oxide nanoparticles to zebrafish embryo: a physicochemical study of toxicity mechanism. J. Nanopart. Res. 2010;12:1645–1654. [Google Scholar]
- Binelli A., Parolini M., Cogni D. A multi-biomarker assessment of the impact of the antibacterial trimethoprim on the non-target organism Zebra mussel (Dreissena polymorpha) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009;150:329–336. doi: 10.1016/j.cbpc.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Çavaş T., Ergene-Gözükara S. Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquat. Toxicol. 2005;74:264–271. doi: 10.1016/j.aquatox.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Collins D., Luxton T., Kumar N., Shah S., Walker V.K. Assessing the impact of copper and zinc oxide nanoparticles on soil: a field study. PLoS ONE. 2012;7(8):1–11. doi: 10.1371/journal.pone.0042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crott J., Fenech M. Preliminary study of the genotoxic potential of homocysteine in human lymphocytes in vitro. Mutagen. 2001;16:213–217. doi: 10.1093/mutage/16.3.213. [DOI] [PubMed] [Google Scholar]
- Dacie V., Lewis S.M. 7th ed. Churchill Livingstone; London: 1991. Practical Hematology. pp 556. [Google Scholar]
- Doumas B.T., Biggs H.G. Determination of serum globulin. In: Cooper, editor. Vol. 7. Academic Press; New York: 1972. (Standard Methods of Clinical Chemistry). [Google Scholar]
- Doumas B.T., Bayso D.D., Carter R.J., Peter T., Schaffer R. Determination of serum albumin. Clin. Chem. 1981;27:1642. [PubMed] [Google Scholar]
- Franklin N.M., Rogers N.J., Apte S.C., Batley G.E., Gadd G.E. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ. Sci. Technol. 2007;41(24):8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- García A., Espinosaa R., Delgadoa L. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination. 2011;269:136–141. [Google Scholar]
- Grant G.H., Silverman L.M., Christenson R.H. 3th ed. WB Saunders Company; Philadelphia: 1987. Aminoacids and proteins. (Fundamental of Clinical Chemistry). [Google Scholar]
- Griffitt R.J., Weil R., Hyndman K.A. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio) Environ. Sci. Technol. 2007;41:8178–8186. doi: 10.1021/es071235e. [DOI] [PubMed] [Google Scholar]
- Hao L., Chen L., Hao J., Zhong N. Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): a comparative study with its bulk counterparts. Ecotoxicol. Environ. Saf. 2013;91:52–60. doi: 10.1016/j.ecoenv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Harabawy A.A., Mosleh Y. The role of vitamins A, C, E and selenium as antioxidants against genotoxicity and cytotoxicity of cadmium, copper, lead and zinc on erythrocytes of Niletilapia, Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2014;104:28–35. doi: 10.1016/j.ecoenv.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Heinlaan M., Ivask A., Blinova I. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Henry T.J. 2th ed. Harper and Row Publishers; New York: 1974. Clinical Chemistry Principles and Techniques. [Google Scholar]
- Kahru A., Dubourguier H.C. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269(2):105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Kind P.R., King E.G. Colorimetric determination of alkaline phosphatase activity. J. Clin. Pathol. 1954;7:322. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laosiripojana N., Sutthisripok W., Assabumrungrat S. Synthesis gas production from dry reforming of methane over CeO2 doped Ni/Al2O3: influence of the doping ceria on the resistance toward carbon formation. Chem. Eng. J. 2005;112(1–3):13–22. [Google Scholar]
- Lasheen M.R., AbdelGawad F.Kh., Alaneny A.A., AbdElbary H.M.H. Fish as Bio indicators in aquatic environmental pollution assessment: a case study in Abu-Rawash Area, Egypt. World Appl. Sci. J. 2012;19:265–275. [Google Scholar]
- Li L.Z., Zhou D.M., Peijnenburg W.J.G.M., Gested C.A.M., Jin S.Y. Toxicity of zinc oxide nanoparticles in the earthworm, Eisenia fetida and subcellular fractionation of Zn. Environ. Int. 2011;37(6):1098–1104. doi: 10.1016/j.envint.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Lu G.H., Chen W., Li Y. Effects of PAHs on biotransformation enzymatic activities in fish. Chem. Res. Chin. U. 2011;27:413–416. [Google Scholar]
- Ma H., Williams P.L., Diamond S.A. Ecotoxicity of manufactured ZnO nanoparticles-A review. Environ. Pollut. 2013;172:76–85. doi: 10.1016/j.envpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Mahboob S., Al-Balwai H.F.A., Al-Misned F., Ahmad Z. Investigation on the genotoxicity of mercuric chloride to fresh water Clarias gariepinus. Pak. Vet. J. 2014;34(1):100–103. [Google Scholar]
- Nowack B., Bucheli T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007;150:5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Okonkwo J.C., Obiakor M.O., Nnabude P.C. Micronuclei profile: an index of chromosomal aberrations in fresh water fishes (Synodontis clarias and Tilapia nilotica) Online J. Anim. Feed Res. 2011;1:40–45. [Google Scholar]
- Parveen N., Shadab G.G. Cytogenetic evaluation of cadmium chloride on Channa punctatus. J. Environ. Biol. 2012;33(3):663–666. [PubMed] [Google Scholar]
- Paterson G., Ataria J.M., Hoque M.E. The toxicity of titanium dioxide nanopowder to early life stages of the Japanese medaka (Oryzias latipes) Chemosphere. 2011;82:1002–1009. doi: 10.1016/j.chemosphere.2010.10.068. [DOI] [PubMed] [Google Scholar]
- Patton C.J., Crouch S.R. Enzymatic determination of urea. Anal. Chem. 1977;49:464–469. [Google Scholar]
- Peralta-Videa J.R., Zhao L., Lopez-Moreno M.L., de la Rosa G., Hong J. Nanomaterials and the environment: a review for the biennium 2008–2010. J. Hazard. Mater. 2011;186(1):1–15. doi: 10.1016/j.jhazmat.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Reitman S., Frankel S. Colorimetric method for determination of serum transaminases activities. Am. J. Clin. Path. 1957;28:56–68. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Rosi N.L., Mirkin C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- Rotello V.M. 1st ed. Springer; New York: 2003. Nanoparticles: Building Blocks for Nanotechnology. [Google Scholar]
- Serrano-Garcia L., Montero-Montoya R. Micronuclei and chromatid buds are the result of related genotoxic events. Environ. Mol. Mutagen. 2001;38:38–45. doi: 10.1002/em.1048. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Itoh N., Utiyama H., Wahl G.M. Selective entrapment of extra chromosomally amplified DNA by nuclear budding and micronucleation during S phase. J. Cell Biol. 1998;140:1307–1320. doi: 10.1083/jcb.140.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoskopf M.K. WB Saunders Company; Philadelphia, London: 1993. Fish Medicine. [Google Scholar]
- Tamhane A.C., Dunlop D.D. Upper Saddle River; USA: 2000. Statistics and Data Analysis from Elementary to Intermediate. [Google Scholar]
- Udroiu I., Ieradi A., Cristaldi M., Tanzarella C. Detection of clastogenic and aneugenic damage in newborn rats. Environ. Mol. Mutagen. 2006;47(5):320–324. doi: 10.1002/em.20209. [DOI] [PubMed] [Google Scholar]
- Zhu X.S., Zhu L., Duan Z.H. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2008;43:278–284. doi: 10.1080/10934520701792779. [DOI] [PubMed] [Google Scholar]