Abstract
Soil contamination with petroleum hydrocarbon products such as diesel and engine oil is becoming one of the major environmental problems. This study describes hydrocarbons degrading bacteria (PHAD) isolated from long-standing petrol polluted soil from the eastern region, Dammam, Saudi Arabia. The isolated strains were firstly categorized by accessible shape detection, physiological and biochemistry tests. Thereafter, a technique established on the sequence analysis of a 16S rDNA gene was used. Isolation of DNA from the bacterial strains was performed, on which the PCR reaction was carried out. Strains were identified based on 16S rDNA sequence analysis, As follows amplified samples were spontaneously sequenced automatically and the attained results were matched to open databases. Among the isolated bacterial strains, S1 was identified as Staphylococcus aureus and strain S1 as Corynebacterium amycolatum.
Keywords: Hydrocarbons, Polluted soils, 16S rDNA, Molecular applications
1. Introduction
Environmental adulteration by gasoline derivatives has been a focus of study over the past periods. The outflow of these derivative oils, such as lubricant oils, is accomplished with harming the environment in several means (Atlas, 1995). The main anxiety for fuel hydrocarbon bioremediation is the existence of heavy compounds such as polycyclic aromatic hydrocarbons (PAHs), asphaltenes and many forked compounds with 20 or more carbon atoms (Winkelmann et al., 2009). These heavy hydrocarbon constituents are not simply metabolized by microbes and are well-thought-out as a probable health risks owing to their potential carcinogenicity and mutagenicity (Baheri and Meysami, 2002).
Crude lubricant is essentially has alkanes, cycloalkanes and aromatic alkanes, which establish nearly 50 to 80% of the oil composition. These ingredients are termed petrol hydrocarbons (Feng, 2004). Furthermore, above 230 hydrocarbons have been recognized in lubricant (Katsivela et al., 2005). As a significant source of energy, oil showed an essential role in manufacturing fabrication. Consequently, the 20th century was called the “oil century” (Sun, 2006).
It is well recognized that a lot of microorganisms are able to consume fuel hydrocarbons as a carbon source. Simultaneously, certain native microorganisms have progressively adjusted to the long-term oil polluted soil and established a greater municipal which able to use oil pollutants via distinctive substrate enrichment (Zhao et al., 2005; Mohammed et al., 2007). Consequently, biological cleanup of the polluted soil has a wide-ranging vision for the reason that its little budget, no secondary contamination, treating in situ (Alexander, 1994; Atlas and Cerniglia, 1995; Boonchan et al., 2000; Misshra and Jyot, 2001; Minai-Tehrani et al., 2009).
In recent times, bioremediation of soil contaminated with petroleum oil has been commonly described globally (Walworth et al., 2003; Daniel et al., 2007; Minai-Tehrani et al., 2009; Zhang et al., 2010), which offers an orientation and management for improvement and useful application of biological remediation technologies. In the previous period, owing to the extensive usage of PCR and DNA sequencing, 16S rDNA sequencing has showed a fundamental role in the perfect proof of identity of the isolated bacteria and the finding of new bacteria in microbiological laboratories. The aim of this study was to ascertain isolated microbial strains from oil contaminated soils through molecular techniques using 16S rDNA sequence examination in conjunction with morphological, physiological and biochemistry experiments. This was done by: isolation of the genomic DNA of the bacteria; amplifying the 16S rDNA gene using the polymerase chain reaction (PCR); automatic sequencing and identification of bacteria species examined based on the analysis of DNA sequences in the National Center for Biotechnology Information (NCBI) database.
2. Materials and methods
2.1. Sampling of soil
Oil polluted soil samples (approximately 5 g) were gathered with a sterile spatula from various locations in the eastern region, Dammam, Saudi Arabia in 2013. The samples were taken up to a distance of 20 cm after taking out nearly 3 cm of the soil superficial. The samples were retained in a sterile polyethylene bags, closed strongly and stored under −20 °C. One g of the soil samples was liquefied in 10 ml of water to make soil suspensions.
2.2. Isolation of bacteria from soil
The sampling soils were collected from the second industrial areas (Eastern region, Dammam, Saudi Arabia). In accordance to the methodology, which was presented by Krupa (2004), soil samples were shaken in sterile saline. Next, they were surface inoculated in Petri dishes with agar nutrient. The plates were incubated at a temperature of about 37 °C for 24 h. After which colonies were observed and were grouped according to morphology, growth and color and were recognized according to the method defined by Poole et al. (2001). Single representatives from each group were streaked to obtain pure cultures.
2.3. Sub culturing
Isolated bacteria were sub cultured into nutrient agar slants for short time conservation and to purify the isolated strains. The bacteria were inoculated in nutrient agar slant using a germ-free wire loop and inculcated at 37 °C for 24 h. The slant bottles containing the bacteria were reserved in a refrigerator at 4 °C for short time storing before biochemistry tests were ran on the isolates for identification.
2.4. Biochemical tests
2.4.1. Indole test
Tryptophan (1%) broth in a test tube was inoculated with bacteria colony. After an incubation period of 48 h at 37 °C, 1 ml chloroform was added to the broth. The test tube was shaken moderately, then 2.1 of Kovac’s reagent was added and this was also shaken slightly and allowed to stand for 20 min. The formation of red coloration at the upper layer specified positive and yellow coloration indicates negative.
2.4.2. Catalase test
A drop of hydrogen peroxide was added on a clean slide. With the edge of extra slide, a colony of the isolates was selected and permitted to be in contact with the hydrogen peroxide. Occurrence of bubbles designates positive reaction while nonappearance of bubbles indicates negative reaction.
2.4.3. Oxidase test
A filter paper soaked with the substrate tetramethyl-p-phenylenediamine dihydrochloride was taken and moisten the paper with sterile distilled water. After that, pick the colony to be tested with wooden or platinum loop and smear in the filter paper observe inoculated area of paper for a color change to deep blue or purple within 10–30 s.
2.5. Initial identification of bacteria
Cell morphology was assessed by Gram stain method, which allowed the bacteria of the respondents predict the cocci Gram-positive and Gram-negative bacteria typed as polycyclic hydrocarbons degrading bacteria (PHDB). Particular attention was paid to the genera Staphylococcus and Corynebacterium. The phenotypic classification of the bacteria according to the recommendations given by Burbianka and Pliszka (1997) includes features such as: cell morphology, mobility and color of the colony. Moreover biochemical tests were used to confirm the bacterial identification such as catalase and oxidase activities.
3. Molecular identification
Total DNA of the isolated strains was extracted using bacterial DNA extraction kit (166-0003EDU Bio-Rad Laboratories LTD). The universal 16S rDNA primers (Linderman, 1998) 27 F 5′-AGAGtttGAtcAtGGctcAG-3′ and 1492 R 5′-tAcGGttAccttGttAcGActt-3′ were used for the PCR reaction. Analysis of the PCR amplified products was carried out on a 2% Agarose gel containing ethidium bromide at 0.5 mg/ml. The obtained 16S rDNA fragments of strains were sequenced. The sequencing results were examined by Blast online comparison (http://www.ncbi.nlm.nih.gov) for identification of the strains (Stach et al., 2001).
4. Hydrocarbon degradation
4.1. Degradation in liquid medium
The two isolated strains were cultured in lysogeny broth (LB) liquid medium containing 1% crude oil at 37 °C. The degradation rate of crude oil hydrocarbons was measured by the weighting process every week for a month, and non-inoculated liquid medium was used as the control.
4.2. Degradation of contaminated soil
Ten grams of the polluted soil was dried after sieving and sterilized at 121 °C for 1 h. The sterilized soil was mixed with treated oil solution so as to obtain soil with a 1% concentration of crude oil. The soil was inoculated with 1% inoculum, and then cultured at 30 °C for a month. The oil degradation rate in the soil was determined by the weighting method each week, and germfree soil was considered as the control.
5. Results and discussion
5.1. Strain isolation and identification
In this study, 5 strains were isolated from polluted soil samples from the eastern region, Dammam; Saudi Arabia. Two hydrocarbons-degrading bacteria were carefully chosen along with their colony form and growth on solid LB medium with polluted oil. The strains were nominated as S1 and S2.
Morphology, physiology and biochemistry of the strains, combined with 16S rDNA sequence analysis were used for identification of the bacterial strains. The isolated strain S1 formed irregular colonies and strain S2 formed circular colonies. The cell shapes of both isolated stains S1 and S2 were found to be rounded-shaped. On solid LB medium, both S1 and S2, developed similarly and made greater colonies due to their fast growth and great colony size (Fig. 1).
Figure 1.

Morphology of the isolated stains S1 (A and B) and S2 (C and D) from polluted soil in the eastern region, Dammam; Saudi Arabia.
According to the assayed biochemical and physiological tests, both strains were Gram and indole positive, whereas only strain S2 was oxidase-positive. Both stains are catalase positive. The results are shown in Table 1.
Table 1.
Morphological features and physiological and biochemical characterization of the isolated strains S1 and S2 from polluted soil in the eastern region, Dammam; Saudi Arabia.
| Strain | Morphological features |
Physiological and biochemical tests |
||||
|---|---|---|---|---|---|---|
| Colony form | Cell shape | Gram stain | Indole | Catalase | Oxidase | |
| S1 | Irregular | Rounded | + | + | + | - |
| S2 | Circular | Rounded | + | + | + | ++ |
The 16S rDNA PCR magnification product of the strains is a fragment around 1 Kb in size (Fig. 2). All the fragments were significantly comparable to identified bacterial strains agreeing to the 16S rDNA sequence judgment. Based on these results, together with the physiological, morphological and biochemical features, strain S1 was well-known as Staphylococcus aureus; strain S2, as Corynebacterium amycolatum (Table 2).
Figure 2.
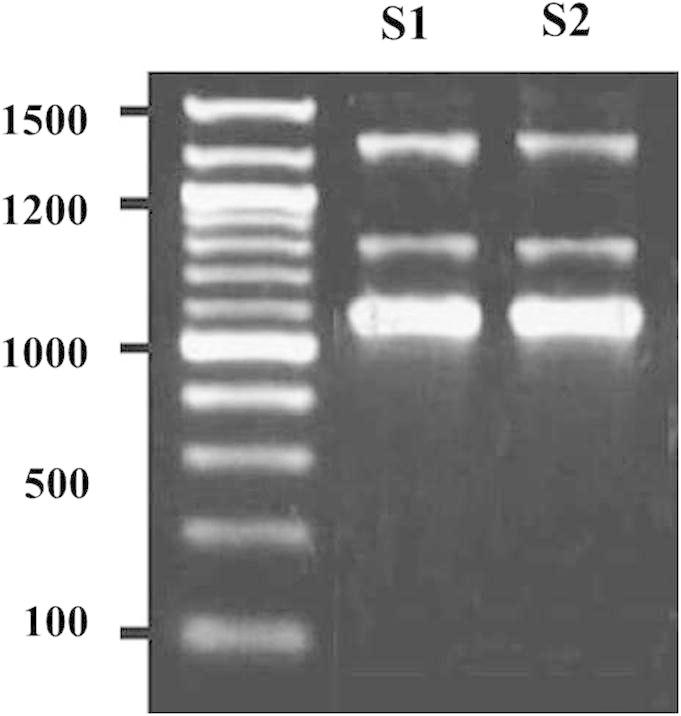
Agarose electrophoresis of 16S rDNA PCR products of the isolated strains Staphylococcus aureus and Corynebacterium amycolatum.
Table 2.
Molecular identification based on 16S rDNA sequencing data of the isolated strains S1 and S2 from polluted soils in the eastern region, Dammam; Saudi Arabia.
| No. of strain | Total length (bp) | Gene bank accession no. | Identification result | Similarity (%) |
|---|---|---|---|---|
| S1 | 1446 | KJ081495.1 | Staphylococcus aureus | 99 |
| S2 | 1454 | KJ081492.1 | Corynebacterium amycolatum | 99 |
Molecular method that was used for the identification of bacteria is based on the analysis of the 16S rDNA gene. Particles of 16S rDNA are characterized by highly conserved regions, which help in the analysis. They also have regions of high variability, making it possible to define the evolutionary distance of given organisms. To assign bacteria to the species, the differences in their sequences cannot exceed 3% (Więckowicz, 2009). The method based on the comparison of the available sequences of the 16S rDNA gene to gene libraries is very useful and widely used due to its simplicity and allows to pre-determine the structure of the bacterial population in the soil (Janssen, 2006). According to our results, a high degree of sequence conformity was achieved in each case, more than 98%. It can be concluded that the isolates were correctly identified.
5.2. Hydrocarbons degradation in liquid and solid media
A pronounced dissimilarity was recognized in the hydrocarbons’ degradation rate among the studied strains, particularly the S2 C. amycolatum strain (Fig. 3). The mean degradation rate of the strains in liquid medium gradually increased with time, and for S2 strain exceeded 55% for the 28 d processing period: 38% for strain S1. Moreover, hydrocarbon degradation in contaminated soil exhibited that the decomposition rate of strain S2 was also the highest in polluted soil and reached 70% for the 28 d period. The degradation rate of strain S1 ranged from 30% to 40%. (Fig. 3)
Figure 3.
Degradation of isolated strains petroleum hydrocarbons Staphylococcus aureus (S1) and Corynebacterium amycolatum S2 in liquid medium (A), and in polluted soil (B).
6. Conclusions
Two bacterial strains were isolated from polluted soil from the eastern region, Dammam; Saudi Arabia. The isolates were identified as S. aureus (S1) and C. amycolatum (S2). In the biodegradation experiment, the tested bacterial strains showed better degradation ability toward hydrocarbons.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RGP-VPP 297.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alexander M. Academic Press; San Diego, CA: 1994. Biodegradation and Bioremediation. [Google Scholar]
- Atlas R.M., Cerniglia C.E. Bioscience. 1995;45(5):332–338. [Google Scholar]
- Atlas R.M. Bioremediation of petroleum pollutants. Int. Biodeterior. Biodegradation. 1995;35(1–3):317–327. [Google Scholar]
- Baheri H., Meysami P. Feasibility of fungi bioaugmentation in composting a flare pit soil. J. Hazard. Mater. 2002;B89:279–286. doi: 10.1016/s0304-3894(01)00318-1. [DOI] [PubMed] [Google Scholar]
- Boonchan S., Britz M.L., Stanley G.A. Appl. Environ. Microbiol. 2000;66(3):1007–1019. doi: 10.1128/aem.66.3.1007-1019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbianka M, Pliszka A (1997). Mikrobiologia żywności. Mikrobiologiczne metody badańproduktów żywnościowych. PZWL.
- Daniel D., Emilien P., Frederic C. Cold Reg. Sci. Technol. 2007;48(2):74–84. [Google Scholar]
- Feng JY (2004). Isolation and Study of The Degradation Character of Petroleum Predominant Strains (M.Sc. thesis), Xi’an University of Architecture and Technology, pp. 1–2.
- Janssen P.H. Identifying the dominant soil bacteria taxa in libraries of 16S rRNA and 16S rNA genes. Appl. Environ. Microbiol. 2006;72(3):1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsivela E., Moore E.R.B., Maroukli D., Strompl C. Biodegradation. 2005;16(2):169–180. doi: 10.1007/s10532-004-4883-y. [DOI] [PubMed] [Google Scholar]
- Krupa P. Wydawnictwo Uniwersytetu Śląskiego; Katowice: 2004. Ektomikoryzyich znaczenie dla drzew rosnących na terenach zanieczyszczonych metalami ciężkimi. 2004. [Google Scholar]
- Linderman R.G. Mycorrhiza interactions with the rhizosphere microflora: the Mycorrhizosphere Effect. Phytopatology. 1998;78:366–371. [Google Scholar]
- Minai-Tehrani D., Minoui S., Herfatmanesh A. B. Environ. Contam. Tox. 2009;82(2):179–184. doi: 10.1007/s00128-008-9548-9. [DOI] [PubMed] [Google Scholar]
- Misshra S., Jyot J. Curr. Microbiol. 2001;43(5):328–335. doi: 10.1007/s002840010311. [DOI] [PubMed] [Google Scholar]
- Mohammed D., Ramsubhag A., Beckles D.M. Water Air Soil Pollut. 2007;182:349–356. [Google Scholar]
- Poole E.J., Bending G.D., Whipps J.M., Read D.J. Bacteria associated with Pinus sylvestris–Lactarius rufus ectomycorrhizas and their effects on mycorrhiza formation in vitro. New Phytol. 2001;151:743–751. doi: 10.1046/j.0028-646x.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- Stach J.E., Bathe S., Clapp J.P., Burns R.G. FEMS Microbiol. Ecol. 2001;36(2–3):139–151. doi: 10.1111/j.1574-6941.2001.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Sun YJ (2006). Screening of the Predominant Bacteria for Petroleum and Study of their Characters (M.Sc. thesis), Xi’an University Degradation of Architecture and Technology, pp. 1–3.
- Walworth J.L., Woolard C.R., Harris K.C. Cold Reg. Sci. Technol. 2003;37(2):81–88. [Google Scholar]
- Więckowicz M. Molekularne metody identyfikacji mikroorganizmów w złożonych ekosystemach. Postępy Mikrobiologii. 2009;48(1):67–73. [Google Scholar]
- Winkelmann M., Hunger N., Hüttl R., Wolf G. Calorimetric investigations on the degradation of water insoluble hydrocarbons by the bacterium Rhodococcus opacus 1CP. Thermochim. Acta. 2009;482:12–16. [Google Scholar]
- Zhang X.X., Li J.B., Thring W.R. J. Can. Petrol. Technol. 2010;49(5):34–39. [Google Scholar]
- Zhao G.H., Feng X.B., Sun J. Environ. Sci. 2005;31:38–69. [Google Scholar]



