Abstract
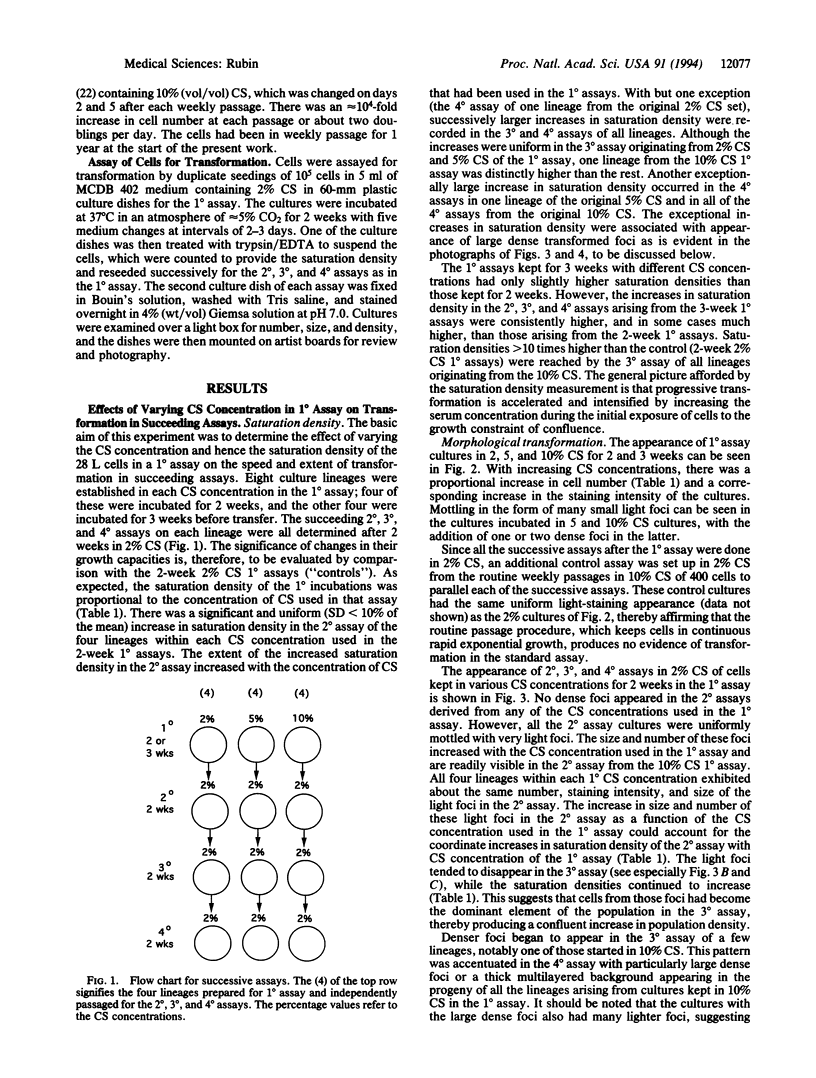
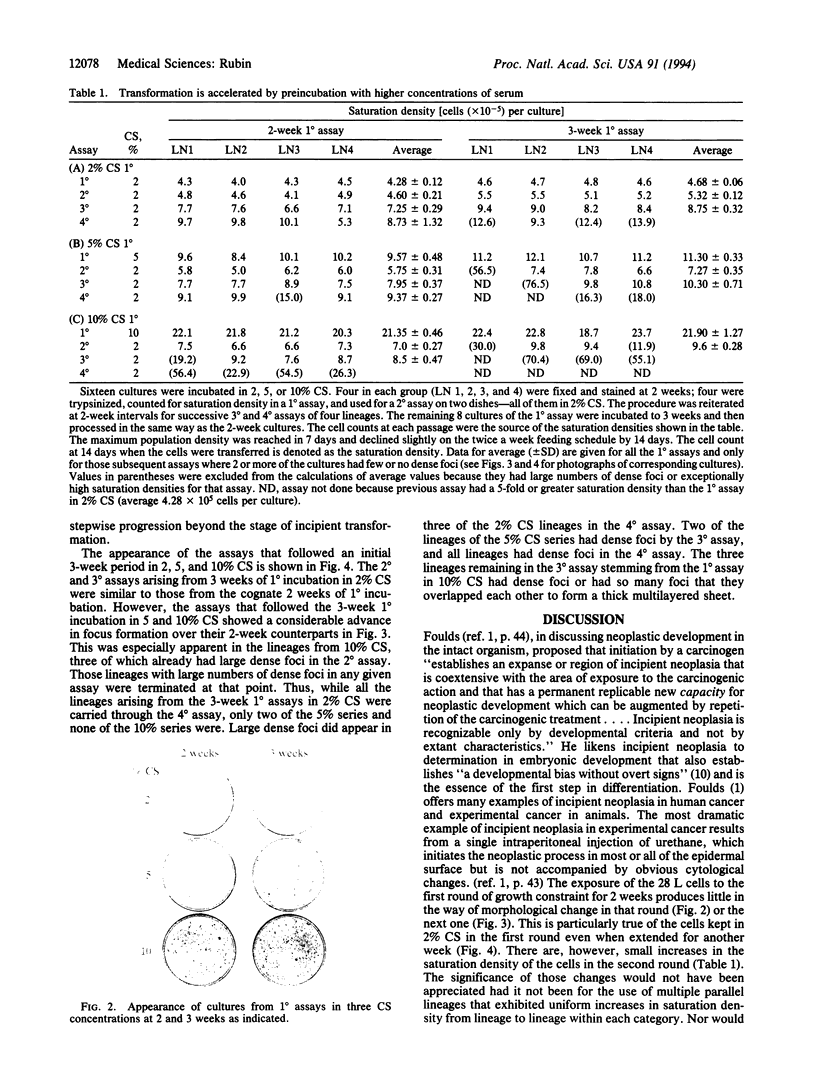
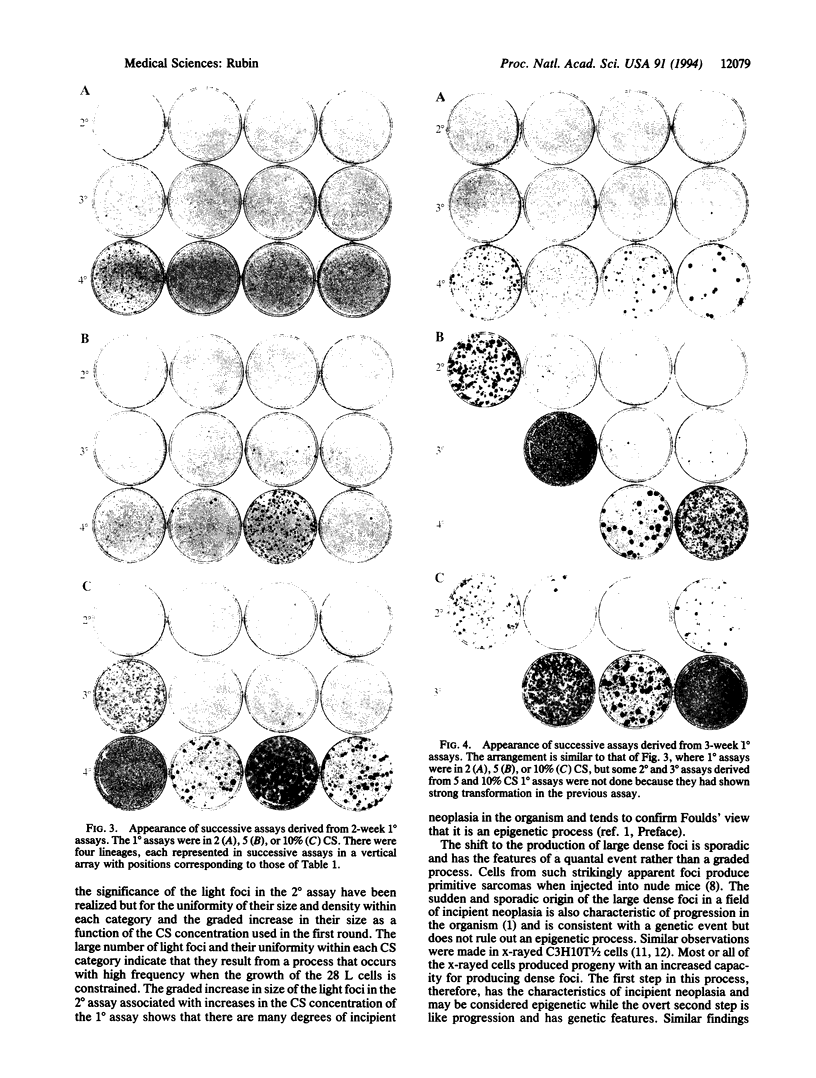
A subline of NIH 3T3 cells was developed that multiplies to low saturation density and produces no transformed foci in a standard assay at confluence if the cells had been kept in continuous exponential growth by passage at extremely low density. Repeated cycles of extended incubation at confluence result in the production of large dense foci with large increases in saturation density of the culture. I investigated the changes preceding overt transformation and the conditions that favor them by making a preliminary 2- or 3-week incubation of cells at high density in 2, 5, or 10% (vol/vol) calf serum (CS) followed by three consecutive assays at 2-week intervals, all in 2% CS. The cell counts on transfer of each consecutive assay constituted their saturation density, and staining of sister dishes exhibited transformed foci and other morphological evidence of transformation. To strengthen the significance of the observations, each CS category consisted of four separate but parallel lineages. The initial incubation in 2, 5, and 10% CS is referred to as the first-round (1 degree) assay. In the second-round (2 degrees) assay in 2% CS after the primary incubation for 2 weeks in different concentrations of CS, there were small but consistent increases in saturation density that were proportional to the CS concentration used in the first round. There were also large numbers of very light foci in the 2 degrees assay that increased in size as a function of the CS concentration used in the 1 degree assay but were uniform within each CS category. In the 3 degrees and 4 degrees successive assays, large dense foci appeared in a sporadic manner but these were also dependent on the CS concentration in the initial incubation. Cultures kept in the 1 degree assay in 5 and 10% CS for 3 weeks produced large dense foci at an earlier stage of the subsequent assays than did those kept for only 2 weeks in the 1 degree assay. The barely perceptible nature of the early morphological changes suggests an analogy to incipient neoplasia. Their graded nature, relatively high frequency, regularity of occurrence, and uniformity of appearance are characteristic of an epigenetic process, which Foulds [Foulds, L. (1969) Neoplastic Development, Vol. I (Academic, New York), preface, pp. 41-89] cited as the most plausible basis for incipient neoplasia [corrected]. In contrast, the low-frequency and sporadic occurrence of the large dense foci, which have the main features of overt neoplasia, are consistent with both genetic and epigenetic processes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I. L. Cell proliferation and renewal in aging mice. J Gerontol. 1972 Apr;27(2):162–172. doi: 10.1093/geronj/27.2.162. [DOI] [PubMed] [Google Scholar]
- Clark W. H. Tumour progression and the nature of cancer. Br J Cancer. 1991 Oct;64(4):631–644. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann F. E., Freitas M. A., Gould M. N., Clifton K. H. Quantifying the frequency of radiogenic thyroid cancer per clonogenic cell in vivo. Radiat Res. 1994 Mar;137(3):330–337. [PubMed] [Google Scholar]
- Kennedy A. R., Cairns J., Little J. B. Timing of the steps in transformation of C3H 10T 1/2 cells by X-irradiation. Nature. 1984 Jan 5;307(5946):85–86. doi: 10.1038/307085a0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M., Travis G. L., Ray F. A., Cram L. S. Spontaneous neoplastic evolution of Chinese hamster cells in culture: multistep progression of phenotype. Cancer Res. 1983 Oct;43(10):4822–4827. [PubMed] [Google Scholar]
- Lavrovsky V. A., Guvakova M. A., Lavrovsky Y. V. High frequency of tumour cell reversion to non-tumorigenic phenotype. Eur J Cancer. 1992;28(1):17–21. doi: 10.1016/0959-8049(92)90375-c. [DOI] [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. In vitro malignant transformation by methylcholanthrene of the progeny of single cells derived from C3H mouse prostate. Proc Natl Acad Sci U S A. 1970 Jan;65(1):219–225. doi: 10.1073/pnas.65.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J. Y., Hemstreet G. P., 3rd, Hurst R. E., Bonner R. B., Jones P. L., Min K. W., Fradet Y. Alterations in phenotypic biochemical markers in bladder epithelium during tumorigenesis. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8287–8291. doi: 10.1073/pnas.90.17.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. J., Haggitt R. C., Rubin C. E., Rabinovitch P. S. Barrett's esophagus. Correlation between flow cytometry and histology in detection of patients at risk for adenocarcinoma. Gastroenterology. 1987 Jul;93(1):1–11. [PubMed] [Google Scholar]
- Rubin A. L., Arnstein P., Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A. L., Rubin H. Selective nature of phorbol 12-myristate 13-acetate-induced neoplastic transformation in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2320–2323. doi: 10.1073/pnas.91.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. 'Spontaneous' transformation as aberrant epigenesis. Differentiation. 1993 Jun;53(2):123–137. doi: 10.1111/j.1432-0436.1993.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Rubin H. Experimental control of neoplastic progression in cell populations: Foulds' rules revisited. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6619–6623. doi: 10.1073/pnas.91.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Bell W. N., Grisham J. W. Clonal analysis of the expression of multiple transformation phenotypes and tumorigenicity by morphologically transformed 10T1/2 cells. Cancer Res. 1993 Feb 1;53(3):500–508. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Nelkin B. D., Celano P., Yen R. W., Falco J. P., Hamilton S. R., Baylin S. B. High expression of the DNA methyltransferase gene characterizes human neoplastic cells and progression stages of colon cancer. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3470–3474. doi: 10.1073/pnas.88.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]






