Abstract
Outer medullary isolated descending vasa recta have proven to be experimentally tractable, and consequently much has been learned about outer medullary vasa recta endothelial transport, pericyte contractile mechanisms, and tubulovascular interactions. In contrast, inner medullary vasa recta have never been isolated from any species, and therefore isolated vasa recta function has never been subjected to in vitro quantitative evaluation. As we teased out inner medullary thin limbs of Henle's loops from the Munich-Wistar rat, we found that vasa recta could be isolated using similar protocols. We isolated ∼30 inner medullary vasa recta from 23 adult male Munich-Wistar rats and prepared them for brightfield or electron microscopy, gene expression analysis by RT-PCR, or isolated tubule microperfusion. Morphological characteristics include branching and nonbranching segments exhibiting a thin endothelium, axial surface filaments radiating outward giving vessels a hairy appearance, and attached interstitial cells. Electron microscopy shows multiple cells, tight junctions, and either continuous or fenestrated endothelia. Isolated vasa recta express genes encoding the urea transporter UT-B and/or the fenestral protein PV-1, genes expressed in descending or ascending vasa recta, respectively. The transepithelial NaCl permeability (383.3 ± 60.0 × 10−5 cm/s, mean ± SE, n = 4) was determined in isolated perfused vasa recta. Future quantitative analyses of isolated inner medullary vasa recta should provide structural and functional details important for more fully understanding fluid and solute flows through the inner medulla and their associated regulatory pathways.
Keywords: renal medulla, renal blood flow, renal hemodynamics
significant insights into renal vascular physiology and pathophysiology can be achieved by more fully understanding the basic physiology of the descending and ascending vasa recta (5, 16, 18). Regulation of medullary blood flow rates and blood distribution patterns involves a host of endocrine and paracrine systems as well as contractile cells associated with descending vasa recta (16, 22). Endothelins (ET-1, ET-2, and ET-3) are potent vasoconstrictors of outer medullary descending vasa recta (25), and the ETB receptor subtype plays an important role in regulation of medullary blood flow (9). Common to descending vasa recta in both outer and inner medulla are the water channel aquaporin-1 (AQP1) and the facilitative urea transporter UT-B, and common to ascending vasa recta and fenestrated capillaries is PV-1, a protein associated with the fenestral diaphragm (8, 13, 26, 29). While isolation of rat or human outer medullary vasa recta and tissue slice preparations have made it possible to study the roles of regulatory pathways and membrane proteins in endothelial transport, pericyte contraction, and endothelial interactions (7, 16, 24), individual inner medullary vasa recta have never been isolated from tissue parenchyma of any species for in vitro functional studies.
The peak osmolality of the rat outer medulla is about twice that of systemic plasma (∼600 mosmol/kgH2O) whereas the peak osmolality of the inner medulla reaches ∼10-fold that of plasma. This corticomedullary osmolality gradient is paralleled by a steep Po2 gradient, which is related in part to different degrees of active transport along the corticomedullary and lateral axes (6). The outer medullary architecture is significantly different from that of the inner medulla as are the inner medullary fluid and solute compartment-to-compartment flows and tubulovascular interactions (8, 11, 20, 21, 28–30). On this basis alone, it might be predicted that functional characteristics of outer and inner medullary vasa recta are significantly different from each other.
We discovered that, while teasing out thin limbs of Henle's loops for isolated tubule perfusion (12), vasa recta could also be isolated in a similar fashion. With subillumination on a stereomicroscope stage, vasa recta take on an appearance that is distinct from thin limbs, allowing us to identify and collect individual blood vessels and prepare them for brightfield and electron microscopy, RT-PCR, and isolated tubule microperfusion. Using these techniques, we establish the feasibility of more fully characterizing transport pathways, regulatory mechanisms, and structural proteins of isolated inner medullary vasa recta.
METHODS
Animals.
Male Munich-Wistar rats (average age ∼120 days; average wt ∼400 g) were reared in the University Animal Care facility at the University of Arizona, Tucson, AZ, and provided with chow (Teklad 7001) and water ad libitum. Animals were euthanized with CO2. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and were approved by the University of Arizona Institutional Animal Care and Use Committee.
Vasa recta dissection and identification of segments on the basis of structural features.
Vasa recta were teased from the isolated inner medulla in a solution consisting of 280 mM sucrose/10 mM HEPES, adjusted to pH 7.4 with Tris-base and gassed with 100% O2 before dissection. Dissection was carried out without the aid of enzymatic agents at 4°C under a stereomicroscope using reflected light below the dissection dish. Upper and lower vasa recta segments were teased from the upper and lower 50% of the inner medulla, respectively. Vasa recta were teased by first pinning down the medullary parenchyma with a dissecting needle (held in one hand) then peeling off successive layers of tissue with a pair of forceps (held in the other hand). Single vasa recta were then isolated from other tubular structures using fine needles.
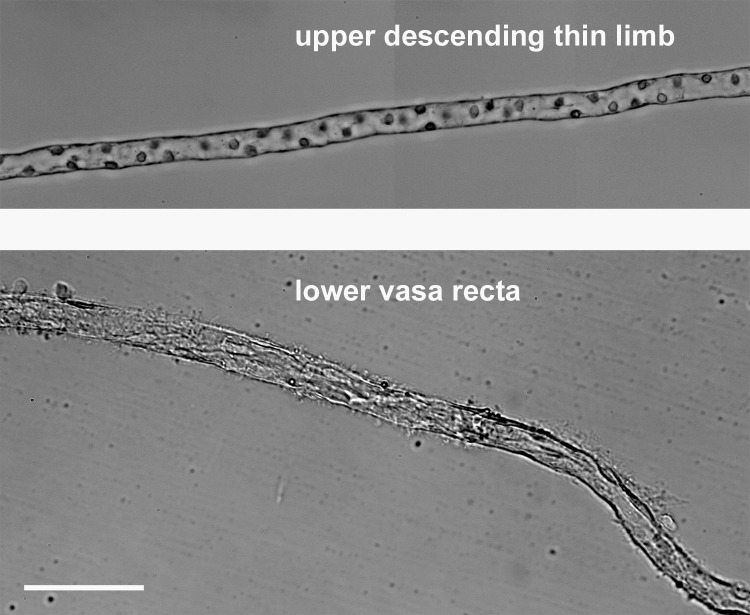
Vasa recta are distinguished from thin limbs of Henle's loops primarily by their hairlike surface projections and by their cell type (Fig. 1). Descending thin limbs have nuclei that protrude into the lumen, whereas ascending thin limbs have round flat nuclei (12). These nuclei were often not clearly recognizable in isolated vasa recta. These differences are shown most clearly when the tubule segments are viewed under a compound microscope with brightfield (Fig. 1) or differential interference contrast optics, but they can also be seen under a stereomicroscope.
Fig. 1.
Isolated descending thin limb from the upper inner medulla and isolated nonbranching vasa recta from the lower inner medulla. Scale bar = 100 μm.
Electron microscopy.
Isolated vasa recta or thin limbs were drawn into a glass Pasteur pipette and deposited in the cavity of a depression slide and fixed with 2.5% glutaraldehyde and 2% paraformaldehyde for at least 2 h at 4°C. An equal volume of 3% agar in 0.2 M PIPES was then added to the cavity and mixed with the fixative. After hardening, the agar with vessel or tubule was trimmed, postfixed with 1% osmium tetroxide, incubated in 2% aqueous uranyl acetate for 20 min, and embedded in Spurrs. Thin sections were stained with 3% lead citrate and imaged using a Tecnai Spirit.
Vasa recta gene expression.
Single vasa recta segments (length 500–1,000 μm) were characterized by detection of mRNA coding for UT-B, PV-1, ETB, and the water channels AQP1 and AQP2. Each segment was transferred with 5–10 μl of buffer into a 0.5-ml microcentrifuge tube and lysed with 10 mM l-arginine/1% Triton X-100, pH 2.5. Lysate cDNA was synthesized using Maxima H Minus Reverse Transcriptase (Thermo Scientific, Waltham, MA) and oligo (dT17) primer and amplified for 40 PCR cycles with Phire II polymerase (Thermo Scientific) on an Eppendorf Mastercycler (Eppendorf, Hauppage, NY). UT-B (accession no. NM019346) was amplified with 5′-gctttgtcgctgtaccttcc-3′ (sense), 5′-ggagctgcaagactgagacc-3′ (anti-sense), PV-1 (accession no. NM020086) was amplified with 5′-aggtggtcaaggagaaggc-3′ (sense), 5′-gacttggccttgacgcagg-3′ (anti-sense), ETB (accession no. XM006252431) was amplified with 5′-ctgtggggatcacagtgttg -3′ (sense), 5′-tgcatgaaggctgttttctg-3′ (anti-sense), AQP1 (accession no. NM012778) was amplified with 5′-ccgagacttaggtggctcag-3′ (sense), 5′-tcatgcggtctgtaaagtcg-3′ (anti-sense), and AQP2 (accession no. NM012909) was amplified with 5′-ggttcccagtgcagagtagc-3′ (sense), 5′-gcggagacgagcacttttac-3′ (anti-sense). Correct PCR products were verified by size on gels stained with ethidium bromide. Specificity of each primer set was validated by sequencing whole tissue products.
Perfusion of isolated tubule segments.
Tubule segments were perfused in vitro in a temperature-controlled chamber (37°C) (2, 12). The upstream portion of the tubule was drawn into a holding pipette, which contained a perfusion pipette and an exchange pipette. The downstream end of the tubule was drawn into a holding pipette that had a tip of appropriate diameter to form a complete seal between perfusate and bath solution. The perfusion rate was ∼15–30 nl/min, and the bath was superfused at ∼1 ml/min. Tubule dimensions were determined by ocular micrometer.
Transepithelial NaCl permeability measurements.
NaCl permeability (PNaCl) was determined from the unidirectional 22Na flux resulting from a 125 mM lumen-to-bath NaCl concentration gradient. The perfusion solution consisted of (in mmol/l) 125 NaCl, 25 N-methyl-d-glucamine, 2.5 K2HPO4, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, and 5 urea, adjusted to pH 7.4. The bath solution was identical except 236 mM mannitol replaced 125 mM NaCl, producing solutions of nearly equal osmolalities (∼ 290 mosmol/kgH2O) and preventing osmotic volume flux. Unidirectional lumen to bath Na flux (JNa; pmol·min−1·mm tubule length−1) was calculated using the following equation: JNa = C0Vo − CLVL, where Co and CL are the Na concentrations in the perfusate and collectate, respectively, Vo is the perfusion rate per unit tubule length, and VL is the collection rate per unit tubule length. As no volume marker was included, volume flux was not measured. Vo and VL were assumed equal.
PNaCl (cm/s) was calculated for lumen-to-bath fluxes from the following equation: PNa = JNa/(πDδC), where D is the tubule diameter and δC = δC1 − δC2/ln (δC1/δC2), where δC1 is the Na concentration gradient at the perfusion end of the tubule and δC2 is the Na concentration gradient at the collection end of the tubule (3, 12). Net volume flow is zero in the absence of a transepithelial osmolality gradient and tracer backflux was negligible as short tubule segments were used, and tracer concentration in the trans compartment was <5% of that in the cis compartment. We did not measure the transendothelial electrical potential difference and so cannot account for any possible effect of potential on Na flux. The transepithelial electrical potential of Sprague-Dawley isolated perfused outer medullary vasa recta was ∼0 mV (17).
Statistical analysis.
Data combined from three or more samples are reported as means ± SE; n is the number of replicates.
RESULTS
Inner medullary vasa recta dissection.
Thirty vasa recta were isolated from 23 adult male Munich-Wistar rats and were prepared for light level or electron microscopy, quantitative PCR to characterize expression of the urea transporter UT-B, the water channel AQP1, and/or the fenestral protein PV-1, and isolated tubule microperfusion to determine transepithelial NaCl permeabilities. Dissection techniques are comparable to those used for isolating inner and outer medullary thin limbs of Henle's loops (4, 12) and outer medullary vasa recta (17). No morphological characteristics were recorded with light level microscopy that distinguished descending vasa recta from ascending vasa recta; however, the presence of pericytes in descending vasa recta of the rat outer inner medulla and outer medulla of rat has been documented (22), and these serve as a key indicator for dissection and isolation of mouse and rat outer medullary descending vasa recta (15). Red blood cells were rarely seen in inner medullary blood vessels. Rat inner medullary descending and ascending vasa recta both have extensive nonbranching portions as well as one or more branching points that connect to a capillary plexus as shown previously with three-dimensional reconstruction (8, 20, 29). The diameters of isolated inner medullary vasa recta were variable, and while some were significantly <20 μm, the average diameter of perfused vasa recta was ∼24 μm.
Morphological characteristics of isolated vasa recta.
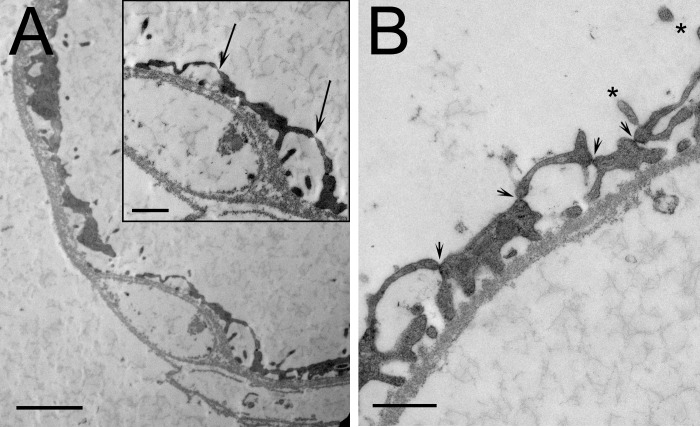
Isolated vasa recta observed with brightfield microscopy included branching segments (not shown) and nonbranching segments (Fig. 1) exhibiting a thin endothelium. The primary characteristics observed during dissection that distinguished vasa recta from thin limbs were the filaments that project out from the vessel surface giving them a hairy or bushy appearance that contrasted with the relatively smooth surface of thin limbs (Fig. 1). Interstitial cells were sparsely attached to most isolated vasa recta along their lengths. Electron microscopy of isolated vasa recta in transverse sections variably showed multiple cells and either continuous or fenestrated endothelia. The vas rectum in Fig. 2A shows few fenestrations (arrows in the Inset), suggesting that it may be a descending vas rectum transitioning into an ascending vas rectum. A type II descending thin limb that was identified during dissection on the basis of structural criteria (12) shows numerous tight junctions and microvilli (Fig. 2B), characteristics common in type II descending thin limbs of several species (19).
Fig. 2.
Electron micrographs of isolated inner medullary vas rectum (A) and type II descending thin limb of Henle's loop (B). A: vas rectum shows few fenestrations (arrows in inset), suggesting that it may be a descending vas rectum transitioning into an ascending vasa rectum. B: type II descending thin limb shows numerous tight junctions (arrowheads) and several microvilli (asterisks). Scale bars = 500 nm.
Vasa recta gene expression.
Transcripts for UT-B and PV-1 are expressed in single isolated vasa recta as determined with RT-PCR (Table 1) and gel analysis of PCR products (not shown). Future studies of gene expression in length-normalized segments can be conducted with quantitative PCR and deep sequencing of RNA species as has been done for isolated nephron segments (10, 12). As it stands, we show that RT-PCR can be useful for identification of UT-B- and PV-1-expressing segments, or descending vasa recta and ascending vasa recta/capillaries, respectively. Inner medullary descending vasa recta coexpress UT-B and PV-1 protein for variable lengths immediately before joining the fenestrated capillary plexus (20). Segments coexpressing UT-B and PV-1 mRNA likely are descending vasa recta; segments expressing PV-1 and not UT-B mRNA likely are ascending vasa recta (Table 1). Some, but not all UT-B-positive segments express AQP1 mRNA (Table 1) as has been shown for protein expression (20). The endothelin receptor ETB was expressed in both UT-B-positive and UT-B-negative segments, whereas AQP2 was not detected (Table 1).
Table 1.
Gene expression in isolated nonbranching vasa recta
| Gene Transcripts |
||||||
|---|---|---|---|---|---|---|
| Vessel ID | Vessel Location | Endothelin Receptor B | UT-B | PV-1 | Aquaporin-1 | Aquaporin-2 |
| 1 | Upper IM | − | − | + | + | − |
| 2 | Lower IM | + | − | + | − | − |
| 3 | Lower IM | − | + | + | − | − |
| 4 | Lower IM | + | + | + | − | − |
Gene expression was determined with RT-PCR and analyzed by gel electrophoresis. IM, inner medulla.
PNaCl of isolated perfused vasa recta.
Four isolated vasa recta were dissected from the lower 50% of the inner medulla (lower ∼2.5 mm) and were mounted on glass pipettes for microperfusion and determination of passive lumen-to-bath 22Na flux and PNaCl (Table 2). Both branching and nonbranching segments were perfused. The branching portions were either removed before transferring the unbranched portion to the perfusion chamber, or the branching portions were held within the collection pipette. Branching and nonbranching vasa recta exhibited a PNaCl of 383.3 ± 60.0 × 10−5 cm/s (mean ± SE).
Table 2.
PNaCl of isolated perfused rat inner medullary vasa recta
| Na Concentration |
||||||||
|---|---|---|---|---|---|---|---|---|
| Segment | Vasa Recta Length, μm | Vasa Recta Diameter, μm | Collection Rate, nl·min−1· mm−1 | Collectate 22Na, μM | Perfusate 22Na, μM | Collectate total Na, mM | JNaCl, pmol·min−1·mm−1 | PNaCl, 10−5 cm/s |
| a1 | 575 | 32 | 60.8 | 0.02 | 0.52 | 4.72 | 7,256.58 | 326.17 |
| a2 | 150 | 25 | 191.6 | 0.14 | 0.48 | 37.19 | 16,840.64 | 494.53 |
| b3 | 175 | 20 | 117.9 | 0.10 | 0.40 | 30.36 | 11,150.00 | 443.86 |
| b4 | 50 | 17.5 | 216.4 | 0.31 | 0.47 | 82.16 | 8,960.99 | 268.68 |
| Mean | 237.5 | 23.63 | 146.67 | 0.14 | 0.47 | 38.61 | 11,052.05 | 383.31 |
| SE | 133.59 | 3.69 | 40.93 | 0.07 | 0.03 | 18.60 | 2,410.52 | 60.04 |
| n | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
All segments were dissected from the lower 50% of the inner medulla. Branching portions of branching segments were either removed before perfusion, leaving a single unbranched segment, or were held along with the main branch within the collecting pipette. The total concentration of unlabeled Na in the flowing bath was 0 mM, and the total initial concentration of unlabeled Na in the perfusate was 125 mM.
Nonbranching segment.
Branching segment. See the text for definitions.
DISCUSSION
In this study, we show for the first time that it is feasible to isolate inner medullary vasa recta from tissue parenchyma in Munich-Wistar rats, making it possible to carry out physiological studies on a population of renal vasculature that has never been investigated using in vitro techniques. We found that with light level microscopy, the morphological characteristics of inner medullary vasa recta are well defined and segments can clearly be distinguished from thin limbs of Henle's loops. Isolated vasa recta can also be analyzed with electron microscopy. The mRNA from isolated vasa recta is sufficiently abundant and stable to permit analysis of gene expression in single segments by RT-PCR. Finally, we showed that isolated segments can be cannulated and microperfused in vitro for study of membrane transport properties.
The PNaCl that we measured in vasa recta in vitro from the lower 50% of the inner medulla was higher than values measured in vasa recta from the terminal ∼2 mm of the inner medulla of young female Munich-Wistar rats using in vivo microperfusion (17). In our study, it is possible that NaCl efflux from the lumen exceeds mannitol influx driven by the mannitol bath to lumen gradient, thereby creating an osmolar gradient (see methods). Depending on the water permeability of the endothelium and magnitude of reabsorptive fluid flux, PNaCl could be overestimated. In the in vivo study mentioned above, PNa (× 10−5 cm/s) of descending vasa recta was 75 and that of ascending vasa recta was 115; values for Purea were nearly identical to PNa (17). The differences in PNa in the two studies could reflect gender and/or age effects as well as functionally different populations of vasa recta. A significant accumulation of isotope in the perivascular interstitium with in vivo measurements could lead to underestimation of PNa; however, boundary effects were considered unlikely as PNa and Purea were significantly different from each other (17). It has been suggested on the basis of functional studies that Na and urea transendothelial fluxes are primarily paracellular in the papilla (17). Structural studies support this idea as UT-B protein, the only known urea transporter in this segment, is only weakly expressed in vasa recta in the terminal 2 mm of the Munich-Wistar rat, and most vasa recta are fenestrated (8, 29). In the outer inner medulla and outer medulla, while the transendothelial Na flux is largely paracellular, the urea flux is primarily carrier mediated, passing by way of the urea transporter UT-B (14, 17, 23).
Although we have established the feasibility of obtaining and perfusing isolated vasa recta segments from the inner medulla, a number of potential complications may be encountered in future studies. 1) It may be problematic to distinguish inner medullary descending and ascending vasa recta from each other based solely on morphological characteristics, making it difficult to subsequently identify genes that are selectively expressed within each of these segments. However, in future studies pericytes may prove to be a key marker for distinguishing descending vasa recta as they do for outer medullary descending vasa recta (15, 22). 2) A significant range in vasa recta diameter was observed, although not quantified, with the smallest being at most 50% the diameter of the largest. Thus a number of isolated vasa recta had a diameter too small to cannulate and perfuse with our current perfusion apparatus, yet it was still possible to collect them for gene analysis. A more rigorous characterization of vasa recta morphology using light level microscopy will be beneficial for more accurately categorizing different populations of vasa recta. 3) Collected samples of isolated vasa recta are unlikely to be completely pure populations of vascular endothelia and may include pericytes, interstitial cells, and cells that are derived from other tubular segments, such as collecting ducts. Fenestrated capillaries are anchored along the entire collecting duct length by endothelial projections (20). These projections interface with the plasma membrane of collecting duct cells as well as other structures (1, 20, 27). Tissue damage as a result of teasing and collecting duct cell carry-over during transfer of segments into PCR reaction tubes may be inevitable. To monitor collecting duct principal cell contamination, we measured AQP2 expression in RT-PCR analyses. Specific protocols will need to be further developed to identify, minimize, and account for other potential contaminants. Future studies should provide insights into expression of genes and proteins that are associated with membrane transport, cell growth and metabolism, and hormone regulation of cell processes.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK083338.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.K.E., C.M.N., and T.L.P. contributed conception and design of research; K.K.E., C.M.N., and T.L.P. performed experiments; K.K.E., C.M.N., and T.L.P. analyzed data; K.K.E., C.M.N., and T.L.P. interpreted results of experiments; K.K.E., C.M.N., and T.L.P. drafted manuscript; K.K.E., C.M.N., and T.L.P. edited and revised manuscript; K.K.E., C.M.N., and T.L.P. approved final version of manuscript; T.L.P. prepared figures.
REFERENCES
- 1.Bulger RE, Trump BF. Fine structure of the rat renal papilla. Am J Anat 118: 685–722, 1966. [DOI] [PubMed] [Google Scholar]
- 2.Burg MB, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210: 1293–1298, 1966. [DOI] [PubMed] [Google Scholar]
- 3.Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: urea and NaCl permeabilities. Am J Physiol Renal Fluid Electrolyte Physiol 264: F337–F343, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol Renal Fluid Electrolyte Physiol 263: F417–F426, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Fry BC, Edwards A, Sgouralis I, Layton AT. Impact of renal medullary three-dimensional architecture on oxygen transport. Am J Physiol Renal Physiol 307: F263–F272, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM. Renal pericytes: regulators of medullary blood flow. Acta Physiol (Oxf) 207: 212–225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol 299: F273–F279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemley KV, Kriz W. Cycles and separations: the histotopography of the urinary concentrating process. Kidney Int 31: 538–548, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Nawata CM, Evans KK, Dantzler WH, Pannabecker TL. Transepithelial water and urea permeabilities of isolated perfused Munich-Wistar rat inner medullary thin limbs of Henle's loop. Am J Physiol Renal Physiol 306: F123–F129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen S, Pallone T, Smith BL, Christensen EI, Agre P, Maunsbach AB. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1023–F1037, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Pallone TL. Characterization of the urea transporter in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol 267: R260–R267, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Pallone TL, Edwards A, Ma T, Silldorff EP, Verkman AS. Requirement of aquaporin-1 for NaCl-driven water transport across descending vasa recta. J Clin Invest 105: 215–222, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 93: 212–222, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannabecker T, Layton A. Targeted delivery of solutes and oxygen in the renal medulla: role of microvessel architecture. Am J Physiol Renal Physiol 307: F649–F655, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pannabecker TL. Structure and function of the thin limbs of the loops of Henle. In: Comprehensive Physiology, edited by Terjung RL. Bethesda, MD: Wiley, 2012, p. 2063–2086. [DOI] [PubMed] [Google Scholar]
- 20.Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park F, Mattson DL, Roberts LA, Cowley AW Jr. Evidence for the presence of smooth muscle a-actin within pericytes of the renal medulla. Am J Physiol Regul Integr Comp Physiol 273: R1742–R1748, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Sands JM, Layton HE, Fenton RA. Urine concentration and dilution. In: Brenner and Rector's The Kidney (9th ed), edited by Taal MW, Chertow GM, Marsden PA, Skorecki KL, Yu ASL, and Brenner BM. Philadelphia, PA: Elsevier, 2012, p. 326–352. [Google Scholar]
- 24.Sendeski MM, Liu ZZ, Perlewitz A, Busch JF, Ikromov O, Weikert S, Persson PB, Patzak A. Functional characterization of isolated, perfused outer medullary descending human vasa recta. Acta Physiol (Oxf) 208: 50–56, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Silldorff EP, Yang S, Pallone TL. Prostaglandin E2 abrogates endothelin-induced vasoconstriction in renal outer medullary descending vasa recta of the rat. J Clin Invest 95: 2734–2740, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA 96: 13203–13207, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi-Iwanaga H. The three-dimensional cytoarchitecture of the interstitial tissue in the rat kidney. Cell Tissue Res 264: 269–281, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Westrick KY, Serack BJ, Dantzler WH, Pannabecker TL. Axial compartmentation of descending and ascending thin limbs of Henle's loops. Am J Physiol Renal Physiol 304: F308–F316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J, Pannabecker TL. Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange. Am J Physiol Renal Physiol 299: F265–F272, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai XY, Thomsen JS, Birn H, Kristoffersen IB, Andreasen A, Christensen EI. Three-dimensional reconstruction of the mouse nephron. J Am Soc Nephrol 17: 77–88, 2006. [DOI] [PubMed] [Google Scholar]