Abstract
Elevated plasma branched-chain amino acids (BCAA) in the setting of insulin resistance have been relevant in predicting type 2 diabetes mellitus (T2DM) onset, but their role in the etiology of hepatic insulin resistance remains uncertain. We determined the link between BCAA and dysfunctional hepatic tricarboxylic acid (TCA) cycle, which is a central feature of hepatic insulin resistance and nonalcoholic fatty liver disease (NAFLD). Plasma metabolites under basal fasting and euglycemic hyperinsulinemic clamps (insulin stimulation) were measured in 94 human subjects with varying degrees of insulin sensitivity to identify their relationships with insulin resistance. Furthermore, the impact of elevated BCAA on hepatic TCA cycle was determined in a diet-induced mouse model of NAFLD, utilizing targeted metabolomics and nuclear magnetic resonance (NMR)-based metabolic flux analysis. Insulin stimulation revealed robust relationships between human plasma BCAA and indices of insulin resistance, indicating chronic metabolic overload from BCAA. Human plasma BCAA and long-chain acylcarnitines also showed a positive correlation, suggesting modulation of mitochondrial metabolism by BCAA. Concurrently, mice with NAFLD failed to optimally induce hepatic mTORC1, plasma ketones, and hepatic long-chain acylcarnitines, following acute elevation of plasma BCAA. Furthermore, elevated BCAA failed to induce multiple fluxes through hepatic TCA cycle in mice with NAFLD. Our data suggest that BCAA are essential to mediate efficient channeling of carbon substrates for oxidation through mitochondrial TCA cycle. Impairment of BCAA-mediated upregulation of the TCA cycle could be a significant contributor to mitochondrial dysfunction in NAFLD.
Keywords: branched chain amino acids, nonalcoholic fatty liver disease, insulin resistance, mitochondrial metabolism
of the circulating amino acids, branched-chain amino acids (BCAA) comprised of leucine, isoleucine, and valine are among the most responsive to insulin (4, 34). Consequently, disturbances in BCAA metabolism have been described in several insulin-resistant states, including obesity, type 2 diabetes mellitus (T2DM), kidney, and liver dysfunction, and associated with future onset of T2DM (16, 18, 33, 39, 45, 47). Considering that nonalcoholic fatty liver disease (NAFLD) is a frequent comorbidity of insulin resistance, obesity, and T2DM (7, 29) and that BCAA can interact with mitochondria through substrate-mediated and molecular mechanisms(11, 33, 38), we sought to determine whether elevated BCAA may contribute to hepatic mitochondrial dysfunction in NAFLD.
Key pathways in the liver that synthesize glucose, lipids, and ketones are fueled by mitochondria, which house the tricarboxylic acid (TCA) cycle, which is closely coupled to mitochondrial respiration and ATP synthesis. These mitochondrial redox reactions are also responsible for generation of reactive oxygen species and normal inflammatory responses, which is critical to cell survival. During obesity and hepatic insulin resistance, the altered nutrient and hormonal milieu favor increased triglyceride accumulation promoting development of NAFLD (14, 29). Hepatic insulin resistance and inflammation in NAFLD closely mirror alterations in TCA cycle flux in both rodents and humans (36, 41). Furthermore, impaired mitochondrial energetics occur concurrent to incomplete fat oxidation, resulting in higher levels of lipotoxic metabolites that mediate inflammation and impair insulin signaling (2, 10, 17, 25). Taken together, dysfunctional mitochondrial TCA cycle in the setting of hepatic insulin resistance is a key feature of NAFLD.
The byproducts of BCAA transamination in the muscle and adipose tissue, which include alanine and glutamine and also the branched-chain keto acids (BCKA), can be catabolized in the liver to acetyl-CoA, ketones, and/or intermediates of TCA cycle, all of which can be metabolized through hepatic TCA cycle (6, 20). In addition, BCAA can signal hepatic mitochondria through molecular targets, including mammalian target of rapamycin complex 1 (mTORC1), AMP-activated protein kinase, peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α), and PPARα (11, 33). Thus elevated systemic BCAA, which could be a consequence of whole body insulin resistance (1, 30), have the potential to interfere with hepatic insulin signaling (32, 33). Furthermore, inefficient catabolism of anaplerotic substrates, including BCAA, can result in “anaplerotic stress” and suboptimal TCA cycle activity (1, 2, 16). We hypothesized that impaired cross-talk between BCAA and hepatic TCA cycle is a contributing mechanism to hepatic insulin resistance and NAFLD.
Utilizing targeted metabolomics of human plasma under euglycemic hyperinsulinemic clamp and a mouse model of NAFLD, we demonstrate that chronic overload from BCAA on the liver can contribute to dysfunctional TCA cycle activity.
RESEARCH DESIGN AND METHODS
Human subjects.
Human studies were approved by the University of Texas Health Science Center at San Antonio (UTHSCSA; San Antonio, TX), and informed written consent was obtained from each patient. Ninety-four subjects were recruited from responses to local newspaper advertisements or referrals from hepatology clinics at the UTHSCSA. Subjects with evidence of any chronic disease [other than NAFLD/nonalcoholic steatohepatitis (NASH), T2DM, or components of metabolic syndrome], as determined by history, physical exam, routine blood and urine chemistries, and electrocardiography, were excluded. Subjects with T2DM who were on thiazolidinediones, incretin-mimetics, or dipeptidyl peptidase-IV inhibitors were also excluded. Other exclusion criteria were history of alcohol abuse (≥20 g/day), liver disease other than NASH (i.e., hepatitis B or C, autoimmune hepatitis, hemochromatosis, Wilson's disease, drug-induced diseases, or others), type 1 diabetes, or a history of clinically significant renal, pulmonary, or heart disease (New York Heart Classification greater than grade II) (28).
Human study design.
Subjects arrived at the Frederic C. Bartter Clinical Research Unit, Audie L. Murphy Veterans Administration Medical Center (San Antonio, TX), after an overnight fast. The following clinical and metabolic assessments were conducted (5, 28): 1) fasting plasma glucose, hemoglobin A1c (Hb A1c), lipid profile, liver function tests, fasting plasma insulin (FPI), and fasting free fatty acids (FFA); 2) total body fat by dual energy X-ray absorptiometry (DEXA); 3) liver fat content by 1H-magnetic resonance spectroscopy; and 4) euglycemic hyperinsulinemic clamps with 3-[3H]glucose tracer to measure endogenous glucose production (EGP) and tissue-specific and total body insulin sensitivity.
Measurements of insulin sensitivity in human subjects.
Following a 12-h overnight fast, two-step euglycemic hyperinsulinemic clamps were performed after a basal equilibration period (120–180 min) (5, 24, 28). At the start of the basal equilibration period, a primed (25 μCi × [fasting glucose/100]) infusion of 3-[3H]glucose (DuPont-NEN, Boston, MA) was initiated, followed by continuous (0.25 mCi/min) infusion until the end of the study. During the last 30 min of the basal period, plasma samples were taken at 5- to 10-min intervals for determination of plasma glucose, insulin concentrations, and 3-[3H]glucose-specific activity. This was followed by a primed continuous infusion of insulin at 10 mIU·m2·min−1 for 2 h (to assess suppression of EGP), followed by another 2 h of insulin at an infusion rate of 80 mIU·m2·min−1 to assess whole body insulin-stimulated glucose disposal (Rd). During the insulin-stimulated stages, plasma glucose was measured every 5 min, and a variable infusion of 20% glucose was adjusted based on the negative feedback principle to maintain plasma glucose concentration at ∼90–100 mg/dl. Plasma samples were collected every 5–10 min for determination of glucose, insulin, and FFA concentrations and 3-[3H]glucose-specific activity.
Measurements of insulin resistance were determined as reported previously (29). Briefly, hepatic insulin resistance index (HIRI) was calculated as the product of fasting EGP × FPI. Adipose tissue insulin resistance index (Adipo-IR) was calculated as the product of fasting plasma FFA × FPI. Suppression of EGP and suppression of FFA were determined during the low-dose insulin infusion phase of the insulin clamp. Skeletal muscle insulin sensitivity was estimated as Rd during the second 2 h of the insulin clamp with a high-dose insulin infusion.
Targeted metabolomics of human plasma and mouse tissues.
Overnight fasting (basal) and insulin-stimulated human plasma amino acid and acylcarnitine levels were determined by gas chromatography (GC)- and liquid chromatography (LC)-based mass spectrometry (MS), respectively, as detailed below. Relationships between indices of insulin resistance vs. basal and insulin-stimulated plasma metabolite levels were evaluated.
Animal studies.
Mouse studies were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Mice (C57BL/6) were purchased from Jackson Laboratories (Bar Harbor, ME) at 6–8 wk of age and started on either a control diet (C; 10% fat calories, no. D09100304; Research Diets) or a high-fructose-high trans-fat diet (TFD; 40% fat calories, no. D09100301; Research Diets) (44). Metabolic experiments were conducted following 8 wk of dietary treatment, at which point TFD-fed mice had already developed NAFLD. Five days prior to the metabolic experiments, a jugular vein catheter was implanted into the mice for infusion of stable isotope tracers and/or unlabeled BCAA. Every 10 ml of the unlabeled BCAA mixture consisted of 0.138 g of leucine, 0.138 g of isoleucine, and 0.124 g of valine, resulting in a 4% BCAA stock. On the day of metabolic experiments, C and TFD mice were randomly divided to receive either an acute infusion (4 h) of saline or a mixture of BCAA (0.3 μmol/min). This resulted in four treatment groups (C + saline, C + BCAA, TFD + saline, and TFD + BCAA). Following the 4-h infusion, blood was collected by exsanguination, and tissues were collected and frozen at −80°C for further analysis.
Study 1: [13C6]leucine metabolism in the liver.
A study was conducted to follow the metabolic fates of [13C6]leucine. For this, the four treatment groups received trace amounts of [13C6]leucine (0.5 μmol/h) in their infusion mixture for 4 h. Plasma and intrahepatic [13C6]leucine enrichment was determined by GC-MS, as detailed below. Incorporation of [13C6]leucine into its downstream catabolic intermediates was determined from the enrichment of [13C5]isovaleryl carnitine in the liver, as detailed below. The impact of acute BCAA challenge on various acylcarnitines was also evaluated.
Plasma and tissue amino acids.
Amino acid concentrations were determined by isotope dilution with GC-MS (9). To 50 μl of plasma, a known concentration of internal standard (hydrolyzed [U-13C,15N]algae protein powder) was added. Samples were deproteinized with cold acetone (250 μl), and the supernatant was collected after centrifugation and dried under N2 before conversion to the t-butyldimethylsilyl derivative. Amino acid derivatives were separated on a HP-5MS column (30 m × 0.25 mm × 0.25 μm; Agilent) and fragmented under electrical ionization (HP 5973N Mass Selective Detector; Agilent). The unlabeled amino acids were compared with their respective internal standards to calculate concentrations.
Plasma and tissue samples were processed similarly as above without any internal standard for the measurement of [13C6]leucine enrichment. Abundance of ion fragments of leucine 302 (unlabeled, M) and 308 ([13C6]leucine, M + 6) were used to calculate the tracer/tracee ratio.
Tissue acylcarnitines.
Acylcarnitines were extracted and quantified as described previously (3, 31). Detection was performed on a Thermo TSQ Access triple-quadrupole MS with an Accela 1200 LC pump and HESI source. Acylcarnitines were separated on an ACE PFP-C18 column (100 × 2.1 mm, 2 μm pore size) at 40°C. Mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient began at 0.5% B, rose to 90% B over 10 min, remained isocratic for 4 min, went back to starting in 0.1 min, and equilibrated for 7 min. Data were collected using SRM mode by monitoring reactions fragmenting to m/z 85.3. The MS was operated in positive mode with a spray voltage of 3 kV, capillary temperature of 300°C, and vaporizer temperature of 300°C. Quantification of tissue acylcarnitines was done by comparison of individual ion peak areas with their respective internal 13C standard (Cambridge Isotope Laboratories).
For determination of intrahepatic isovalerylcarnitine enrichment, liver samples were deproteinized with cold acetonitrile, and the supernatant was dried down under N2. The dried sample was then derivatized with butanol-HCl (50 μl) at 60°C for 30 min and dried at 40°C before reconstitution in methanol-water (90:10) for analysis, as described above.
Study 2: in vivo hepatic mitochondrial TCA cycle activity.
Functional activity of the hepatic TCA cycle in response to a BCAA challenge was determined by NMR in all four groups. For this, a mixture of [13C3]propionate and [3,4-13C2]glucose was infused during the last 90 min of the 4-h saline or BCAA infusion. Blood was collected by exsanguination under anesthesia and processed for NMR-based isotopomer analysis of monoacetone glucose (8, 21, 36, 41, 42). Briefly, the isotopomer analysis of the multiplets arising from 13C labeling of carbon 2 of glucose provided us direct functional activity of hepatic TCA cycle metabolism (35, 36), including 1) gluconeogenesis, 2) mitochondrial anaplerotic flux that drives gluconeogenesis, and 3) pyruvate cycling, all of which are expressed relative to citrate synthase (22). Absolute rates of TCA cycle flux were calculated by dividing EGP with gluconeogenesis measured relative to citrate synthase (22).
Western blot analysis.
Approximately 50 mg of frozen livers were homogenized in lysis buffer containing protease inhibitors (Roche Diagnostics, Indianapolis, IN). Following SDS-PAGE, proteins were transferred on to a nitrocellulose membrane (Protran; Whatman/GE Healthcare, Piscataway, NJ) and incubated with primary antibodies (Cell Signaling Technology, Danvers, MA).
Metabolites and hormone measurements.
Plasma total ketone and FFA concentrations were determined using an analytical kit (Wako chemicals). Plasma insulin was measured by enzyme-linked immunoassay using the Mouse Insulin ELISA kit (Crystal Chem). Triglyceride concentrations were determined using an analytical kit from Sigma (St. Louis, MO).
Statistical analysis.
Data were expressed as means ± SE for continuous variables and percentages for categorical variables. Comparisons between two groups were performed by unpaired Student's t-test or Chi2 (Fisher's exact test when needed), depending on variable type. Continuous variables among three groups of human subjects were compared using ANOVA, with Bonferroni's as the post hoc test. Analyses between measurements of insulin sensitivity and metabolites were conducted using Pearson's correlation in R 2.15.3 (43). Areas and the color intensity of the circles in the correlation plot indicate the absolute value of the corresponding correlation coefficients. All other tests were performed using Stata 11.1 (StataCorp, College Station, TX). A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Subject characteristics.
To assess the interplay between plasma BCAA and insulin resistance, patients were divided according to the degree of insulin resistance. Because skeletal muscle is believed to be the primary organ responsible for BCAA oxidation, insulin-stimulated muscle uptake (Rd) was used to divide the groups. Groups were divided based on the following arbitrary cutoff points to simplify interpretation of patients' characteristics in Table 1: 1) Rd ≥ 10 (normal), 2) Rd (5–10) (mild to moderate insulin resistance), and 3) Rd ≤ 5 (severe insulin resistance). Of note is that overall results were unchanged when groups were selected on the basis of tertiles (cutoff points of ≤5.4, 5.4–8.0, and ≥8.0) or even when four groups were selected on the basis of quartiles (cutoff points of ≤4.4, 4.4–6.4, 6.4–9.8, and ≥9.8) (data not shown). Human subjects, when grouped based on Rd, allowed us to maximize and clearly demonstrate the waning action of insulin on BCAA (Fig. 1B). In fact, all of the other indices of insulin sensitivity also showed a progressive decline similar to Rd (Table 1). Furthermore, these groups were well matched for their total body fat measured by DEXA, thus allowing us to examine the relationships between BCAA and insulin sensitivity independent of obesity. Fasting plasma glucose and FFA remained similar between groups. Liver fat content and liver aminotransferases were higher between subjects with Rd ≥ 10 and Rd ≤ 5, a reflection of worsening hepatic insulin resistance (Table 1). In summary, this patient population provided us with three groups of subjects with worsening insulin resistance to test the relationship between amino acids and insulin resistance.
Table 1.
Clinical characteristics of human subjects
| Rd ≥ 10 (n = 21) | Rd (5–10) (n = 45) | Rd ≤ 5 (n = 28) | P Value | |
|---|---|---|---|---|
| Age, yr | 50 ± 2 | 50 ± 2 | 53 ± 2 | 0.49 |
| Sex (male), % | 52 | 67 | 86† | 0.04 |
| Body mass index, kg/m2 | 28.3 ± 0.8 | 31.2 ± 0.3* | 31.7 ± 0.4† | <0.0001 |
| Total body fat, % | 30 ± 2 | 31 ± 1 | 30 ± 1 | 0.86 |
| Type 2 diabetes mellitus, % | 33 | 38 | 61 | 0.09 |
| Hb A1c, % | 6.0 ± 0.2 | 5.9 ± 0.1 | 6.7 ± 0.2†# | 0.002 |
| Fasting plasma glucose, mg/dl | 104 ± 3 | 115 ± 4 | 122 ± 5† | 0.03 |
| Fasting plasma insulin, μU/ml | 5 ± 1 | 10 ± 1* | 18 ± 2†# | <0.0001 |
| Fasting FFA, mmol/l | 0.58 ± 0.05 | 0.55 ± 0.04 | 0.56 ± 0.04 | 0.93 |
| Liver fat, % | 15 ± 3 | 24 ± 2 | 28 ± 2† | 0.008 |
| AST, U/l | 32 ± 4 | 35 ± 3 | 51 ± 4†# | <0.001 |
| ALT, U/l | 38 ± 5 | 51 ± 4 | 76 ± 8†# | <0.001 |
| Measures of insulin sensitivity | ||||
| HOMA-IR | 1.2 ± 0.1 | 2.9 ± 0.3* | 5.2 ± 0.6†# | <0.0001 |
| Adipo-IR, mmol·l−1·μU−1·ml−1 | 1.9 ± 0.3 | 4.2 ± 0.5* | 7.5 ± 0.9†# | <0.0001 |
| HIRI, mg·kg−1·min−1 per μU/ml | 8.5 ± 0.6 | 15.5 ± 1.4 | 29.7 ± 3.4†# | <0.0001 |
| Muscle insulin sensitivity (Rd), mg·kg LBM−1·min−1 | 12.6 ± 0.5 | 7.0 ± 0.2* | 3.6 ± 0.2†# | <0.0001 |
| Suppression of EGP, % | 54 ± 4 | 41 ± 3 | 30 ± 4†# | <0.001 |
| Suppression of FFA, % | 70 ± 4 | 54 ± 2* | 35 ± 3†# | <0.0001 |
| Plasma BCAA | ||||
| Valine (basal) | 307 ± 19 | 343 ± 7 | 344 ± 8 | 0.056 |
| Valine (insulin stimulated) | 194 ± 12 | 240 ± 6* | 277 ± 8†# | <0.001 |
| Leucine (basal) | 174 ± 13 | 193 ± 5 | 193 ± 6 | 0.162 |
| Leucine (insulin stimulated) | 72 ± 6 | 102 ± 3* | 130 ± 5†# | <0.001 |
| Isoleucine (basal) | 137 ± 10 | 153 ± 4 | 157 ± 5 | 0.083 |
| Isoleucine (insulin stimulated) | 50 ± 4 | 75 ± 3* | 99 ± 4†# | <0.001 |
Values are represented as means ± SE.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA-IR, homeostatic model assessment of insulin resistance; Adipo-IR, adipose tissue insulin resistance index; Rd, glucose disposal; EGP, endogenous glucose production; FFA, free fatty acids; HIRI, hepatic insulin resistance index; LBM, lean body mass; BCAA, branched-chain amino acids.
Pairwise comparisons were performed using Bonferroni's multiple-comparison tests.
P < 0.05 between Rd ≥ 10 and Rd (5–10);
P < 0.05 between Rd ≥ 10 and Rd ≤ 5;
P < 0.05 between Rd (5–10) and Rd ≤ 5.
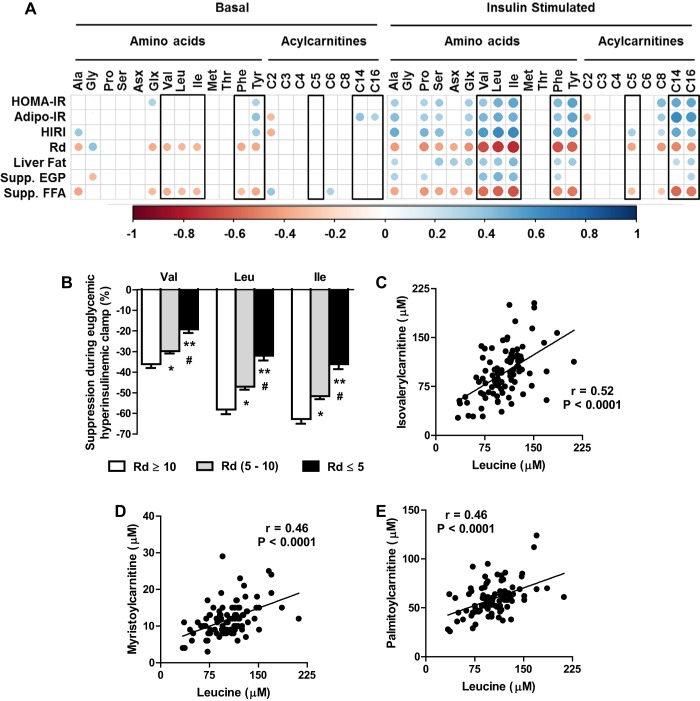
Fig. 1.
Targeted metabolomics coupled to hyperinsulinemic euglycemic clamps in human subjects. A: correlation between indices of insulin sensitivity and metabolites profiled after an overnight fast (basal) and during insulin stimulation (euglycemic hyperinsulinemic clamp). Only correlations that are significant at P < 0.05 are represented with circles in the matrix. Areas and the color intensity of the circles in the correlation plot indicate the absolute value of the corresponding correlation coefficients. B: impaired suppression of BCAA during insulin stimulation in human subjects with increasing severity of muscle insulin resistance [glucose disposal (Rd)]. C–E: relationships between leucine (C), isovalerylcarnitine myristoylcarnitine (D), and palmitoylcarnitine (E) during euglycemic hyperinsulinemic clamp. Values in the bar graph are represented as means ± SE. *P < 0.05 between “Rd ≥ 10” and “Rd (5–10)”; **P < 0.05 between Rd ≥ 10 and “Rd ≤ 5”; #P < 0.05 between Rd (5–10) and Rd ≤ 5. Ala, Alanine; Gly, Glycine; Pro, Proline; Ser, Serine; Asx, aspartate/asparagine; Glx, glutamate/glutamine; Val, valine; Leu, leucine; Ile, isoleucine; Met, methionine; Thr, threonine; Phe, phenylalanine; Tyr, tyrosine; C2, acetylcarnitine; C3, propionylcarnitine; C4, butyrylcarnitine; C5, isovalerylcarnitine; C6, hexanoylcarnitine; C8, octanoylcarnitine; C14, myristoylcarnitine; C16, palmitoylcarnitine.
Relationships between BCAA and insulin resistance were amplified under insulin stimulation.
Basal fasting levels of all the three BCAAs and the aromatic amino acids showed subtle but statistically significant relationships to indices of insulin resistance (Fig. 1A). More importantly, for the first time, we compared insulin-stimulated levels of metabolites to indices of the liver (HIRI and suppression of EGP), muscle (Rd), and adipose (Adipo-IR and suppression of FFA) tissue insulin resistance along with HOMA-IR and liver fat content (Fig. 1A). Interestingly, these indices of insulin resistance had a much stronger correlation with insulin-stimulated levels of BCAA and aromatic amino acids compared with basal fasting levels (Fig. 1A). Furthermore, insulin-stimulated levels of plasma leucine and plasma isovalerylcarnitine showed a robust positive correlation (Fig. 1C). Because isovalerylcarnitine is a degradation product of leucine, this positive correlation suggests that attenuation of complete oxidative BCAA catabolism is a potential mechanism contributing to elevated plasma BCAA, which may be restricted to specific tissues (32). However, we acknowledge the possibility that insulin stimulation could have resulted in higher rates of oxidation of BCAA, contributing to higher levels of isovalerylcarnitine in plasma. Taken together, metabolomics of human plasma under insulin stimulation helped us demonstrate the chronic systemic overload from BCAA (Fig. 1B) despite only an ∼15–20% increase in their basal levels. These data further highlight the utility of euglycemic hyperinsulinemic clamps to amplify subtle relationships between metabolites and insulin sensitivity, which may not be apparent under basal conditions.
Robust correlations between plasma BCAA and long-chain acylcarnitines in humans suggested an interaction between BCAA and mitochondrial metabolism.
As part of targeted metabolomics, we profiled basal and insulin-stimulated plasma acylcarnitines. A correlation analysis between all of the metabolites revealed an interesting relationship between BCAA and long-chain acylcarnitines (C14, C16). A subtle but significant correlation was evident under basal fasting between all BCAA and long-chain acylcarnitines. Interestingly, this correlation was amplified with insulin stimulation. Thus, as represented in Fig. 1, D and E, under insulin stimulation, levels of leucine correlated significantly with C14 and C16 acylcarnitines. Similarly, C14 and C16 acylcarnitines also correlated significantly with isoleucine (C14: r = 0.47, P < 0.001; C16: r = 0.41, P < 0.001) and valine (C14: r = 0.48, P < 0.001; C16: r = 0.48, P < 0.001). Considering that long-chain acylcarnitines are fuels for mitochondrial fat oxidation and the TCA cycle, we interpret our results as an interaction between BCAA and mitochondrial fatty acid metabolism, as reported previously (2, 32). We tested whether this interaction is evident in the liver using a diet-induced rodent model that develops NAFLD by artificially increasing systemic BCAA levels.
Hepatic mitochondrial metabolism was blunted following acute BCCA infusion in mice with NAFLD.
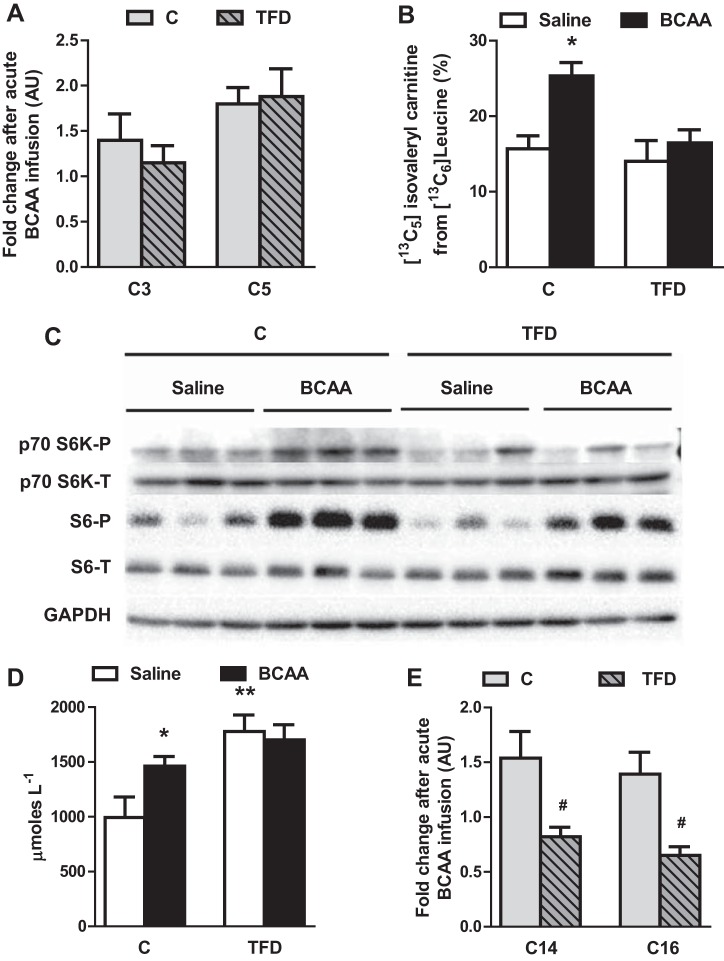
Following a 4-h infusion of a mixture of BCAA, plasma BCAA were elevated approximately two- to 2.5-fold [valine: 2.2 ± 0.1 (C) vs. 2.4 ± 0.2 (TFD); leucine: 1.8 ± 0.1 (C) vs. 1.9 ± 0.1 (TFD); isoleucine: 2.4 ± 0.1 (C) vs. 2.7 ± 0.2 (TFD)] compared with the saline-infused mice. Liver triglycerides were significantly higher in mice fed TFD for 8 wk (44 ± 15 vs. 159 ± 20 mg/g). However, the BCAA challenge did not alter fasting glucose, FPI, FFA, or liver triglycerides in either control of TFD-fed mice (Table 2). The levels of short-chain acylcarnitines (C3 and C5), which are intermediates of BCAA degradation, responded similarly to acute BCAA in control and TFD-fed mice (Fig. 2A). We then tracked specifically the incorporation of [13C6]leucine in the liver into hepatic [13C5]isovalerylcarnitine. Because hepatocytes are known to have low levels of branched-chain aminotransferase (BCATm) activity, our tracer protocol relies on the assumption that BCKA arising from muscle and adipose tissue are 13C precursors for the liver (20). The control mice responded to a BCAA challenge by increasing the incorporation of [13C6]leucine into [13C5]isovalerylcarnitine from 17 to 26% (P = 0.007). However, in the mice with NAFLD, the incorporation rates remained similar between the saline and BCAA infusions, suggesting a blunted response to BCAA (Fig. 2B). Furthermore, the phosphorylation status of p70 S6K and S6, both indicators of mTORC1 activity, were robustly induced by BCAA in control mice. The BCAA-mediated activation of p70 S6K and S6 was blunted in TFD mice (Fig. 2C). A similar response was observed with BCAA inducing ketone production in control mice (994 vs. 1,462 μM; P < 0.05) but failing to elevate plasma ketones in TFD mice challenged with BCAA (1,779 vs. 1,709 μM) (Fig. 2D). More importantly, both long-chain acylcarnitines (C14 and C16), which are intermediates of mitochondrial fat metabolism, failed to respond to BCAA infusion in mice with NAFLD (Fig. 2E). In summary, these results suggest that BCAA modulate mitochondrial metabolism, and this action is impaired in mice with NAFLD.
Table 2.
Biochemical profile of C57Bl/6J mice fed either a C or a high fructose/high TFD for 8 wk and then challenged with an acute 4-h infusion of BCAA mixture
| C |
TFD |
|||
|---|---|---|---|---|
| Saline | BCAA | Saline | BCAA | |
| Body weight, g | 25 ± 1 | 23 ± 1 | 28 ± 1* | 28 ± 1* |
| Fasting glucose, mg/dl | 112 ± 12 | 112 ± 5 | 120 ± 7 | 120 ± 10 |
| Fasting plasma insulin, ng/ml | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.17 ± 0.02 |
| Fasting FFA, mM | 0.41 ± 0.05 | 0.47 ± 0.03 | 0.47 ± 0.04 | 0.43 ± 0.03 |
| Liver triglyceride, mg/g | 44 ± 15 | 53 ± 10 | 159 ± 20* | 160 ± 24* |
| Plasma BCAA, μM | ||||
| Leucine | 215 ± 24 | 378 ± 25# | 168 ± 8 | 329 ± 24# |
| Isoleucine | 153 ± 13 | 369 ± 19# | 129 ± 6 | 346 ± 23# |
| Valine | 229 ± 28 | 514 ± 28# | 183 ± 7 | 449 ± 38# |
Values are means ± SE (n = 6–7/group).
C, control diet; TFD, trans-fat diet.
P ≤ 0.05 between C and TFD;
P ≤ 0.05 between saline and BCAA.
Fig. 2.
Hepatic metabolism failed to respond optimally to an acute branched-chain amino acid (BCAA) challenge in mice with nonalcoholic fatty liver disease (NAFLD). A: hepatic short-chain acylcarnitines (C3, propionyl; C5, isovaleryl) responded similarly to BCAA challenge in both control (C) and high-trans-fat diet (TFD) groups. B: incorporation of 13C from intracellular [13C6]leucine into its degradation product [13C5]isovalerylcarnitine was impaired in TFD-fed mice, suggesting an impairment in BCAA degradation. C: BCAA induced phosphorylation of p70S6K and S6, both indices of mammalian target of rapamycin complex 1 activity in control mice, but this induction was blunted in mice with NAFLD. D: BCAA challenge increased plasma ketones in control mice, but this response was blunted in mice with NAFLD. E: the response of long-chain acylcarnitines (C14, myristoyl; C16, palmitoyl) to BCAA challenge was impaired in mice with NAFLD. Values in the bar graph (n = 6–7) are represented as means ± SE. *P < 0.05 between C + saline and C + BCAA; **P < 0.05 between C + saline and TFD + saline; #P < 0.05 between C and TFD.
Hepatic TCA cycle failed to respond to BCAA challenge in mice with NAFLD.
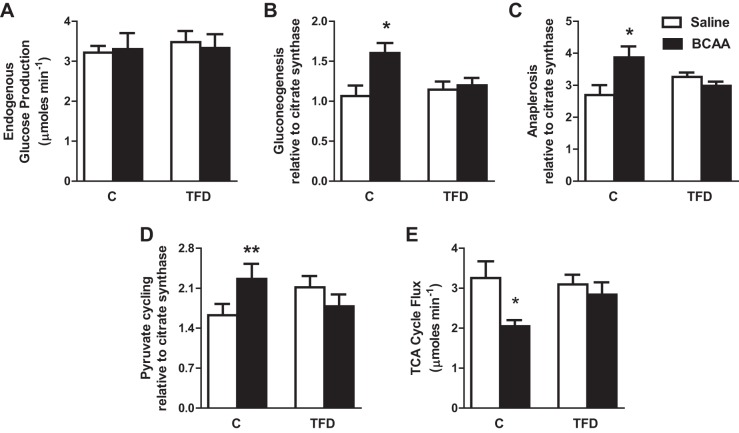
We determined whether BCAA can directly regulate the activity of hepatic TCA cycle following a 4-h BCAA infusion. Multiple fluxes through the hepatic TCA cycle were determined using NMR-based isotopomer analysis (22, 36). Acute infusion of BCAA did not increase EGP in either control of mice with NAFLD (Fig. 3A). However, BCAA infusion resulted in elevated rates of gluconeogenesis (P = 0.01; Fig. 3B), mitochondrial anaplerosis (P = 0.03; Fig. 3C), and pyruvate cycling (P = 0.08; Fig. 3D), all of which were expressed relative to citrate synthase activity. Because these relative fluxes provide us an index of mitochondrial TCA cycle activity (22, 36), we concluded that BCAA induced the hepatic TCA cycle in control mice. Interestingly, all these effects of acute BCAA infusion on the hepatic mitochondrial TCA cycle were blunted in mice with NAFLD. We can calculate the absolute rate of hepatic TCA cycle by dividing EGP with gluconeogenesis relative to citrate synthase (22). Absolute flux through mitochondrial TCA cycle was lower in control mice infused with BCAA, whereas it remained unchanged in mice with NAFLD (Fig. 3E). Taken together, our results demonstrate that BCAA directly modulate hepatic the TCA cycle and that impairment in BCAA-mediated upregulation of the hepatic TCA cycle may be a critical component in the progression of NAFLD.
Fig. 3.
BCAA failed to regulate tricarboxylic acid (TCA) cycle activity in mice with NAFLD. A–D: endogenous glucose production remained similar in both the C and high-TFD groups following BCAA challenge. Acute 4-h BCAA challenge resulted in the induction of TCA cycle metabolism in control mice (A); however, mice with NAFLD failed to respond to BCAA, as evidenced by impaired mitochondrial TCA cycle fluxes of gluconeogenesis (B), anaplerosis (C), and pyruvate cycling (D) determined relative to citrate synthase. E: absolute TCA cycle flux was suppressed by BCAA in control mice, whereas TCA cycle failed to respond to BCAA infusion in mice with NAFLD. Values in the bar graph (n = 6–7) are represented as means ± SE. *P < 0.05 between C + saline and C + BCAA; **P < 0.1 between C + saline and C + BCAA.
DISCUSSION
Although elevated plasma BCAA and defects in their tissue-specific metabolism are evident during obesity and insulin resistance (15, 16, 33, 39, 45, 47), their role, if any, in the progression of hepatic insulin resistance and NAFLD has not been investigated. In this study, we combined targeted metabolomics, metabolic flux analysis, and the gold standard measurement of insulin action (euglycemic hyperinsulinemic clamp) in human subjects and a diet-induced mouse model of NAFLD. This approach led us to two major findings. First, worsening insulin resistance in the setting of NAFLD is associated with the progressive inability of insulin to suppress plasma BCAA, which in turn results in their elevated plasma levels. Second, because BCAA and/or their degradation products are involved in the optimal upregulation of hepatic TCA cycle, dysregulated BCAA metabolism in the setting of insulin resistance could impair the normal physiological response of the hepatic TCA cycle. Taken together, this impaired cross-talk between elevated BCAA and the hepatic TCA cycle could be a contributing factor to dysfunctional mitochondrial metabolism during NAFLD.
The relationship between plasma BCAA and whole body insulin resistance is evidenced largely on the basis of relatively crude indices of insulin sensitivity, including plasma insulin, HOMA-IR, and Hb A1c (16, 33, 45, 47). We conducted state-of-the-art measurements in patients with a broad spectrum of insulin resistance to fully unravel the relationships between BCAA and insulin action, especially at the level of the liver. This included comparing basal and insulin-stimulated levels of plasma BCAA to indices of liver (HIRI and suppression of EGP), muscle (Rd), and adipose (Adipo-IR and suppression of FFA) tissue insulin resistance along with HOMA-IR and liver fat content (Fig. 1A). Interestingly, insulin-stimulated levels of plasma BCAA provided significantly better correlations with all the indices of insulin resistance compared with basal plasma BCAA, as depicted in Fig. 1A. Thus metabolic perturbation by insulin can help amplify subtle defects that may not be apparent under basal fasting conditions. More importantly, worsening insulin-mediated suppression of BCAA with insulin resistance also explains the gradual and subtle (10–20%) increase in basal plasma BCAA. This small but chronic increase in plasma BCAA with insulin resistance may be sufficient enough to disrupt signaling events in the mitochondria of the muscle and liver (e.g., through mTORC1) (33, 38), in turn contributing to mitochondrial dysfunction.
Consistent with previous reports (32, 33), targeted metabolomics of human plasma after insulin stimulation revealed relationships between BCAA and acylcarnitines. The gradual increase in plasma BCAA with severity of insulin resistance was very well correlated (P < 0.0001) with a rise in plasma long-chain acylcarnitines (C14, C16), which was determined during the euglycemic hyperinsulinemic clamp (Fig. 1, D and E). We consider this a significant observation, as long-chain acylcarnitines are metabolic fuels for mitochondrial β-oxidation and TCA cycle, and thus we interpreted these results as an interaction between BCAA and mitochondrial fat metabolism. Here, we acknowledge the limitation that plasma acylcarnitines may not provide a true reflection of hepatic mitochondrial metabolism (37, 40), and extension of this correlation to liver metabolism should be taken with caution. Despite this, because hepatic mitochondria are primed for fat oxidation through the TCA cycle, and furthermore, a robust correlation also existed between plasma BCAA and hepatic insulin resistance (HIRI; Fig. 1A), we postulated that chronic elevation of BCAA will alter hepatic mitochondrial metabolism. This may then provide a link between hepatic insulin resistance and NAFLD, which are widespread comorbidities of obesity and T2DM (7, 29), and dysfunctional mitochondrial TCA cycle metabolism (36, 41).
To test the impact of BCAA on hepatic mitochondria, we artificially elevated plasma BCAA two- to 2.5-fold in control mice and mice with NAFLD. Following BCAA challenge in mice with NAFLD, we observed a blunted response in the incorporation of 13C from [U13C]leucine into hepatic isovalerylcarnitine (Fig. 2B), reduced phosphorylation of p70 S6K and S6 (Fig. 2C), and a failure to increase plasma total ketones (Fig. 2D) and long-chain acylcarnitines (Fig. 2E). Blunted branched-chain keto acid dehydrogenase (BCKDH) activity, observed in various models of obesity and T2DM (23, 26, 30), may be a contributing factor. However, we did not observe any impairment in BCKDH activity, which could manifest with more severe hepatic insulin resistance. Of note is that in both hepatocytes and muscle, changes in BCKA catabolism accompanied a mostly FFA-rich environment (12, 19, 46). Furthermore, a high-fat diet background or a FFA-rich environment may manifest defects in mitochondrial fuel oxidation following BCAA supplementation (1, 19, 33). On the contrary, plasma BCAA has been shown to upregulate mitochondrial biogenesis through the sirtuin 1/PGC-1α/mTORC1 axis (13, 27), alleviate oxidative damage (13), and upregulate genes involved in hepatic fat oxidation (27). Taken together with our results, these reports underline the mitochondrial mode of BCAA action. More importantly, a “lipotoxic” environment may be a prerequisite for manifesting impairments in BCAA action on the hepatic mitochondria during NAFLD.
Dysfunctional mitochondrial TCA cycle metabolism is intrinsic to both mice (36) and humans (41) with hepatic insulin resistance and NAFLD. Thus, we decided to test whether BCAA act by directly modulating hepatic TCA cycle metabolism. Indeed, relative TCA cycle fluxes through gluconeogenesis, anaplerosis, and pyruvate cycling increased with BCAA challenge in normal mice. In fact, absolute rates of the hepatic TCA cycle were lower in normal mice infused with BCAA (Fig. 3E). This suggests that biochemical products of BCAA catabolism could sustain the activity of certain pathways of mitochondrial metabolism (e.g., ketogenesis, gluconeogenesis, and pyruvate cycling) in healthy mice, albeit with lower absolute TCA cycle flux through citrate synthase. However, this BCAA-mediated induction of relative hepatic TCA cycle fluxes was blunted in mice with NAFLD (Fig. 3, B–D), suggesting that elevated BCAA coupled to a lipotoxic environment could impair the physiological induction of the mitochondrial TCA cycle. Consistent with our observation is the proposed mechanism of “anaplerotic stress” (1, 2), wherein attenuation of BCAA degradation during insulin resistance results in diminished availability of anaplerotic substrates for the hepatic TCA cycle (16). Thus considering the low activity of BCATm in the liver, the blunted response of the hepatic TCA cycle to BCAA infusion in mice with NAFLD may have resulted from reduced flux of BCKA from adipose tissue. Furthermore, because BCAA have potent signaling functions (11, 38), it is plausible that impaired molecular interaction between BCAA and molecular mediators of mitochondrial fat metabolism could also result in impaired TCA cycle activity. Although the mechanisms responsible for the interaction between BCAA and the hepatic TCA cycle remain to be investigated, our results clearly illustrate for the first time that the cross-talk between BCAA and the hepatic TCA cycle is impaired during NAFLD.
In summary, dysregulated catabolism of BCAA during hepatic insulin resistance and the resultant “anaplerotic stress” fail to optimally induce TCA cycle metabolism, potentially contributing to mitochondrial dysfunction in NAFLD. Because TCA cycle activity is at a crossroads with substrate oxidation, mitochondrial respiration, and free radical generation, our results open up a new avenue in our understanding of the interaction between BCAA and the hepatic mitochondrial TCA cycle. Because we know that hepatic insulin resistance and inflammation in NAFLD closely mirror impaired mitochondrial TCA cycle energetics in both rodent models (36) and humans (41), identifying the metabolic and molecular mechanisms through which BCAA contribute to this process will be a future goal.
GRANTS
This work was supported by a Burroughs Wellcome Fund Translational Research Award (1006762; to K. Cusi), a University of Florida Research Opportunity Seed Fund Award (00089467; to N. E. Sunny), a Southeast Center for Integrated Metabolomics NIDDK Grant U24 DK-097209, and a National Institutes of Health and National Center for Research Resources Clinical and Translational Science Award to the University of Florida (UL1-TR-000064).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.E.S. conception and design of research; N.E.S., S.K., T.J.G., M.N., and J.T.M. performed experiments; N.E.S., S.K., F.B., T.J.G., M.N., J.T.M., C.M.W., and K.C. analyzed data; N.E.S., F.B., C.M.W., and K.C. interpreted results of experiments; N.E.S., S.K., F.B., M.N., and C.M.W. prepared figures; N.E.S., S.K., F.B., T.J.G., and K.C. drafted manuscript; N.E.S., S.K., F.B., M.N., C.M.W., and K.C. edited and revised manuscript; N.E.S. and K.C. approved final version of manuscript.
REFERENCES
- 1.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2: 445–456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10: 268–274, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi G, Marchesini G, Brunetti N, Manicardi E, Montuschi F, Chianese R, Zoli M. Impaired insulin-mediated amino acid plasma disappearance in non-alcoholic fatty liver disease: a feature of insulin resistance. Dig Liver Dis 35: 722–727, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bril F, Lomonaco R, Orsak B, Ortiz-Lopez C, Webb A, Tio F, Hecht J, Cusi K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 59: 2178–2187, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr 136: 207S–211S, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, Mulder H, Holm C, Sherry AD, Malloy CR. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab 289: E53–E61, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Calder AG, Garden KE, Anderson SE, Lobley GE. Quantitation of blood and plasma amino acids using isotope dilution electron impact gas chromatography/mass spectrometry with U-(13)C amino acids as internal standards. Rapid Commun Mass Spectrom 13: 2080–2083, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem 280: 20148–20153, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausserès N, Steiler T, Gaudichon C, Tomé D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab 297: E1313–E1323, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Corkey BE, Martin-Requero A, Walajtys-Rode E, Williams RJ, Williamson JR. Regulation of the branched chain alpha-ketoacid pathway in liver. J Biol Chem 257: 9668–9676, 1982. [PubMed] [Google Scholar]
- 13.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 12: 362–372, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 281: 811–816, 1969. [DOI] [PubMed] [Google Scholar]
- 16.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 5: e15234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 285: 11348–11356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Jaskiewicz JA, Harris RA. Ethanol and oleate inhibition of alpha-ketoisovalerate and 3-hydroxyisobutyrate metabolism by isolated hepatocytes. Arch Biochem Biophys 299: 57–62, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr 135: 1557S–1564S, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem 327: 149–155, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Jones JG, Naidoo R, Sherry AD, Jeffrey FM, Cottam GL, Malloy CR. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-(13)C3]propionate. FEBS Lett 412: 131–137, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Kadota Y, Toyoda T, Kitaura Y, Adams SH, Shimomura Y. Regulation of hepatic branched-chain alpha-ketoacid dehydrogenase complex in rats fed a high-fat diet. Obes Res Clin Pract 7: e439–e444, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52: 2461–2474, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, Goto H, Fujita Y, Shikano R, Muramatsu Y, Bajotto G, Tamura T, Tamura N, Shimomura Y. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun 373: 94–98, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Xu M, Lee J, He C, Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab 303: E1234–E1244, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomonaco R, Ortiz-Lopez C, Orsak B, Finch J, Webb A, Bril F, Louden C, Tio F, Cusi K. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology 54: 837–845, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10: 723–736, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis 13: 321–324, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15: 606–614, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest 101: 1519–1529, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes 57: 2012–2021, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 53: 1080–1092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schooneman MG, Achterkamp N, Argmann CA, Soeters MR, Houten SM. Plasma acylcarnitines inadequately reflect tissue acylcarnitine metabolism. Biochim Biophys Acta 1841: 987–994, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468: 1100–1104, 2010. [DOI] [PubMed] [Google Scholar]
- 39.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 293: E1552–E1563, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soeters MR, Sauerwein HP, Duran M, Wanders RJ, Ackermans MT, Fliers E, Houten SM, Serlie MJ. Muscle acylcarnitines during short-term fasting in lean healthy men. Clin Sci 116: 585–592, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunny NE, Satapati S, Fu X, He T, Mehdibeigi R, Spring-Robinson C, Duarte J, Potthoff MJ, Browning JD, Burgess SC. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 298: E1226–E1235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Team R Core. R: A language and environment for statistical computing [Online]. Vienna, Austria: R Foundation for Statistical Computing, 2013. [The software is available at http://www.r-project.org/]. [Google Scholar]
- 44.Trevaskis JL, Griffin PS, Wittmer C, Neuschwander-Tetri BA, Brunt EM, Dolman CS, Erickson MR, Napora J, Parkes DG, Roth JD. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 302: G762–G772, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448–453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson JR, Walajtys-Rode E, Coll KE. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. I. Regulation of branched chain alpha-ketoacid metabolism. J Biol Chem 254: 11511–11520, 1979. [PubMed] [Google Scholar]
- 47.Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, Viikari JS, Raitakari OT, Ala-Korpela M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36: 648–655, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]