Abstract
Blood vessels are subjected to numerous biomechanical forces that work harmoniously but, when unbalanced because of vascular smooth muscle cell (vSMC) dysfunction, can trigger a wide range of ailments such as cerebrovascular, peripheral artery, and coronary artery diseases. Human pluripotent stem cells (hPSCs) serve as useful therapeutic tools that may help provide insight on the effect that such biomechanical stimuli have on vSMC function and differentiation. In this study, we aimed to examine the effect of biomechanical strain on vSMCs derived from hPSCs. The effects of two types of tensile strain on hPSC-vSMC derivatives at different stages of differentiation were examined. The derivatives included smooth muscle-like cells (SMLCs), mature SMLCs, and contractile vSMCs. All vSMC derivatives aligned perpendicularly to the direction of cyclic uniaxial strain. Serum deprivation and short-term uniaxial strain had a synergistic effect in enhancing collagen type I, fibronectin, and elastin gene expression. Furthermore, long-term uniaxial strain deterred collagen type III gene expression, whereas long-term circumferential strain upregulated both collagen type III and elastin gene expression. Finally, long-term uniaxial strain downregulated extracellular matrix (ECM) expression in more mature vSMC derivatives while upregulating elastin in less mature vSMC derivatives. Overall, our findings suggest that in vitro application of both cyclic uniaxial and circumferential tensile strain on hPSC-vSMC derivatives induces cell alignment and affects ECM gene expression. Therefore, mechanical stimulation of hPSC-vSMC derivatives using tensile strain may be important in modulating the phenotype and thus the function of vSMCs in tissue-engineered vessels.
Keywords: extracellular matrix, human pluripotent stem cells, smooth muscle cells, strain
vascular diseases such as coronary artery, cerebrovascular, and peripheral artery disease affect various organs primarily because they are all attributable to the dysfunction blood vessels, which are ubiquitously located throughout the body (30). Cell-based tissue-engineered blood vessels promise to be a viable treatment for such vascular diseases and offer to address the functional limitations of synthetic and autologous grafts (22, 40). Vascular smooth muscle cells (vSMCs) are a crucial component of the cell-based engineered vessels, as they provide structural support by circumferentially surrounding the inner endothelial lining. Additionally, these vSMCs should provide enough vessel stability when transplanted and integrated with host vasculature to be able to maintain blood pressure.
Healthy contractile vSMCs (Con-vSMCs) are characterized by their deposition of the elastin extracellular matrix (ECM) protein and their contraction of the vessel wall to counteract the pulsatile force generated by the beating heart. vSMCs deposit elastin along with other ECM proteins such as collagen type I, collagen type III, collagen type IV, and fibronectin, which play integral roles in maintaining the mechanical integrity of blood vessels. Although elastin contributes to vessel elasticity, both collagen type I and collagen type III bear vessel tensile forces and set the limit of vessel elasticity (8). Additionally, changes in ECM protein expression often provide insight into the pathological state of cells. For instance, decreased elastin expression in vSMCs is indicative of a phenotypic switch from the contractile state to a synthetic phenotype. This switching is often triggered by vessel remodeling, injury, or disease in vivo although it generally occurs when vSMCs are cultured in vitro (27a). The switch from the desired contractile phenotype to the synthetic phenotype presents a challenge when engineering vessels, as the inadequate elastin and collagen deposition by synthetic vSMCs (Syn-vSMCs) often compromises the mechanical integrity of vessel constructs (3).
The use of human pluripotent stem cells (hPSCs) is advantageous in generating vSMCs because of the ability to direct the differentiation of these cells toward the desired vSMC phenotype in vitro using various stimuli (38). In vivo mechanical stimuli play a major role in the embryonic differentiation and later in the adult, as blood vessels exist in extremely dynamic tissue environments where cells are exposed to various mechanical forces (9, 41). Although adult vSMCs are exposed to interstitial shear stress from blood flow, they primarily experience a circumferential tensile stretch or strain as a result of the radial distention of vessels (1, 28, 39).
Because vSMCs are constantly exposed to tensile strain, they have the ability to sense biomechanical signals and respond by modulating intracellular pathways in a process called mechanotransduction (28). This mechanotransduction process may, additionally, be altered depending on cell exposure to the various subtypes of tensile strain including uniaxial, equibiaxial, and circumferential tensile strain. Although all the subtypes characteristically cause cell elongation, where there is an extension of the original length of a cell to a larger cell length, they all have distinctive directions of stress. It is therefore important to incorporate relevant biomechanical strain as a part of engineering vessels from hPSCs in vitro. Accordingly, there have been various studies that investigate the effect of the various tensile strains on adult vSMCs; however, much remains unknown regarding how tensile strain affects vSMCs at different stages of differentiation (20, 25, 26, 31).
In previous studies, we have established a stepwise differentiation protocol for the adherent and biochemically controlled derivation of vSMCs through different stages of maturation including smooth muscle-like cells (SMLCs), mature SMLCs (mSMLCs), and Con-vSMCs (38). In this study, we examined the ability of the hPSC derivatives to sense and respond to external tensile strain through changes in cell orientation and ECM gene expression. We exposed the vSMC derivatives to uniaxial strain to mimic the mechanical strain that early vSMCs and mature vSMCs experience in vivo. In the body, normal vSMCs express and deposit ECM in environments constantly stimulated by mechanical strain, and thus the exposure to in vitro cyclic uniaxial strain may similarly influence the differentiating vSMC ECM gene expression. Although all hPSC-vSMC derivatives aligned perpendicularly to the direction of cyclic uniaxial strain in adherent cultures, SMLCs tended to orient in a parallel direction to that of cyclic uniaxial strain when encapsulated in 3D collagen matrices. Moreover, short-term cyclic uniaxial strain and long-term cyclic circumferential biomechanical strain of hPSC mSMLCs induced elastin and collagen deposition. These data suggest that exposing hPSC-vSMC derivatives to biomechanical strain may improve mechanical integrity of implantable engineered vessels by increasing ECM protein production.
METHODS
Cell culture.
All cells were cultured in humidified incubators, with atmospheres at 37°C and 5% CO2.
Human PSCs.
Human induced PSC (hiPSC) line MR31 and BC1 (4, 5) (kindly provided by Dr. Cheng, SOM JHU) and human embryonic stem cell (hESC) line H9 (passages 15 to 40; WiCell Research Institute, Madison, WI) were grown on inactivated mouse embryonic fibroblast feeder layers (GlobalStem, Rockville, MD) in growth medium composed of 80% ES-DMEM/F12 (GlobalStem), 20% knockout serum replacement (Invitrogen, Carlsbad, CA), and 4 ng/ml basic fibroblast growth factor (bFGF; Invitrogen) for hESCs of 10 ng/ml bFGF for hiPSCs, as previously reported (38). hiPSCs were passaged every 4–6 days using 1 mg/ml of type IV collagenase (Invitrogen). Media were changed daily.
Human vSMCs.
The control cell type used was human aorta vSMCs (passages 4–7; ATCC, Manassas, VA). The cells were cultured in the specified ATCC complete SMC growth medium, composed of Kaighn's modification of Ham's F-12 medium (ATCC), 10.0% or 0.5% (Hyclone, Logan, UT), 0.01 mg/ml transferrin (Sigma, St. Louis, MO), 0.01 mg/ml insulin (Sigma), 10 mM HEPES buffer (Sigma), 10 mM 2-[Tris(hydroxymethyl)methylamino]ethane-1-sulphonic acid (Sigma), 0.05 mg/ml ascorbic acid (Sigma), 10.00 ng/ml sodium selenite (Sigma), and 0.03 mg/ml endothelial cell growth supplement (Sigma). Human vSMCs were passaged every 3–4 days using 0.25% trypsin (Invitrogen). Media were changed every 2–3 days.
vSMC differentiation protocol.
vSMCs were derived as previously described (38). Briefly, hPSCs were collected through digestion with TrypLE (Invitrogen) and were seeded at a concentration of 5 × 104 cells/cm2 onto plates previously coated with collagen type IV (R&D Systems, Minneapolis, MN). The hPSCs were cultured for 6 days in a differentiation medium, composed of α-MEM (Invitrogen), 10% FBS (Hyclone), and 0.1 mM β-mercaptoethanol (Invitrogen). Media were changed daily. On day 6, the mesodermal-like cells were collected through digestion with TrypLE (Invitrogen), separated with a 40-μm mesh strainer, and seeded at a concentration of 1.25 × 104 cells/cm2 on collagen type IV-coated plates. The differentiating hPSCs were then cultured in differentiation medium, with the addition of 10 ng/ml PDGF-BB (R&D Systems) and 1 ng/ml transforming growth factor (TGF)-β1 (R&D Systems) for an additional 6 days (total of 12 days) for SMLCs. Media were changed every second day. Serum-starved cells were passaged every 6–8 days with TrypLE, using α-MEM (Invitrogen), 10% FBS (Hyclone), and 0.1 mM β-mercaptoethanol (Invitrogen) to neutralize TrypLE but then seeded with 0.5% serum media.
Cyclic uniaxial strain.
hPSC-vSMC derivatives were seeded on collagen IV-coated polydimethylsiloxane chambers (B bridge), which were inserted between two metal frames inside of a cyclic strain-loading STREX instrument (ST-140; Strex, Osaka, Japan). Uniaxial strain was achieved as the STREX instrument utilized a computer-controlled step motor to drive two metal frames closer or further apart. For the 3D uniaxial strain experiments, derivatives were embedded in 2.5-mg/ml collagen gels and placed in a STREX 3D chamber (B-Bridge) where the gel solidified in a sponge for attachment to the chamber. The amplitude and frequency were controlled by the programmable microcomputer. In the present study, we used a cyclic strain in the 5–10% range with a frequency of 1 Hz. All strain experiments were performed with the supplementation of 1 ng/ml TGF-β1 and were performed in humidified incubators, with atmospheres at 37°C and 5% CO2.
Pulsatile flow-loading bioreactor design.
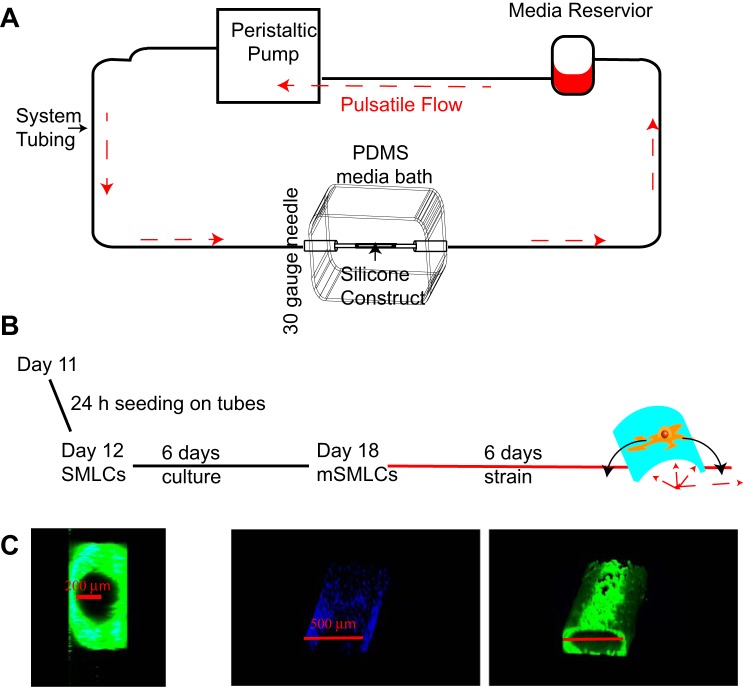
To examine the effect of circumferential strain on hPSC-vSMC derivatives, a bioreactor was designed to allow pressurized flow into tubular silicone constructs (silastic laboratory tubing; Dow Corning, Corning, NY) and allow the radial distention of the constructs (see Fig. 4 below). The system was used inside a standard humidified incubator, with atmospheres at 37°C and 5% CO2 and consisted of a media reservoir, a programmable peristaltic pump (Ismatec, Wertheim, Germany), and a polydimethylsiloxane (PDMS) media bath. The peristaltic pump was operated at speeds between 0.11 and 11.25 revolutions/min and propelled culture medium in a cyclic flow pattern through a Kel-F hub 30-gauge needle (Hamilton, Reno, NV) through the silicone construct secured on both ends using 6-0 nylon sutures (Henry Schein, Melville, NY). The path of fluid flow continued through the silicone construct and out of a second Kel-F hub 30-gauge needle (Hamilton) and finally into a media reservoir where media collects and is recycled back into the pump. The two needles were pierced through the PDMS media bath at opposite ends to hold tubular samples 300 μm in radial diameter and 3 cm in axial length. A PDMS lid was used to ensure sterility of the media bath.
Fig. 4.
Experimental setup to study the effect of circumferential strain using a pulsatile flow bioreactor. A: schematic representation of the experimental setup of pulsatile flow bioreactor where media is propelled inside system tubing by a peristaltic pump and travels into a 30-gauge needle then into a silicone construct, where circumferential strain is generated. The medium then travels from the silicone construct and out of a secondary 30-gauge needle and is finally recycled in a media reservoir. PDMS, polydimethylsiloxane. B: schematic representation of long-term uniaxial strain experiments. C: confocal images of a cross section and 3D reconstruction of a flexible silicone tube seeded with hPSC mSMLCs before pulsatile flow: phalloidin (green), nuclei (blue).
Cell seeding around the silicone tubes.
A 3-cm silicone tube segment was attached to the two Kel-F hub 30-gauge needles inside the media bath and secured by sutures. After the assembly was autoclaved, the silicone tubing was coated with a 2.5-mg/ml collagen gel solution to facilitate cell seeding. After a 30-min incubation of the coated silicone tube in the incubator, 1 × 106 SMLCs in 2 ml of media were added into the media bath. The media bath was then placed on an orbital shaker (Sigma) for 24 h to allow the full coverage of cells around the tube. After 6 days of serum starvation and supplementation with 1 ng/ml TGF-β1, the media bath was connected to the pump for circumferential strain studies.
Radial distention measurement.
Radial distention of the silicone tubes was quantified optically by flowing 2-μm fluorescent blue latex polystyrene beads (1:100; Sigma) using a fluorescent stereoscope (AxioZoom V16; Zeiss, Jena, Germany) and taking time-lapse streaming videos (Axiocam MRm, Zeiss) of silicone tube expansion. Radial distention was quantified as the ratio of the instantaneous outer diameter to original unpressurized outer diameter of the silicone tubes.
Immunohistochemistry.
Cells were prepared for immunofluorescence as previously described (38). Cells were fixed using 3.7% formaldehyde fixative for 15 min, washed with PBS, blocked with 1% BSA in PBS for 1 h minimum, permeabilized with a solution of 0.1% Triton X-100 (Sigma) for 10 min, washed with PBS, and incubated overnight with anti-human calponin (1:100; Dako, Glostrup, Denmark). For ECM staining, cells were incubated with anti-human fibronectin (1:200; Sigma), anti-human collagen type I (1:200; Abcam, Cambridge, MA), anti-human collagen type IV (1:100; Abcam), anti-human collagen type III (1:200; Abcam), or anti-human elastin (3:100; Abcam) overnight. Cells were rinsed twice with PBS and incubated with Alexa 488-conjugated phalloidin (1:100; Molecular Probes, Eugene, OR), anti-mouse IgG Cy3 conjugate (1:50; Sigma), or anti-rabbit IgG Alexa Fluor 546 conjugate (1:1,000; Molecular Probes) for 2 h, rinsed with PBS, and incubated with DAPI (1:1,000; Roche Diagnostics, Indianapolis, IN) for 10 min. Silicone tubes and stretch chambers were rinsed once more with PBS and imaged immediately. The immunolabeled cells were examined using fluorescence microscopy (Olympus BX60; Olympus, Center Valley, PA) and confocal microscopy (LSM 510 Meta; Carl Zeiss).
Cell orientation analysis.
Cell orientation was analyzed using three randomized images using ImageJ (NIH, Bethesda, MD). Image thresholding was used to highlight cell boundaries, and the particle analysis tool was used to give a best-fit ellipse for each cell. Cell orientation either to the direction of uniaxial strain or to the longitudinal axis of the silicone tube was then determined.
Real-time quantitative RT-PCR.
Two-step RT-PCR was performed on differentiated hPSCs at various time points as we previously described (38). Total RNA was extracted by using TRIzol (Gibco, Invitrogen), per the manufacturer's instructions. All samples were verified as free of DNA contamination. The concentration of total RNA was quantified using an ultraviolet spectrophotometer. RNA (1 μg per sample) was transcribed using the reverse transcriptase MMLV (Promega, Madison, WI) and oligo(dT) primers (Promega), per the manufacturer's instructions. The specific assays used were the TaqMan Universal PCR Master Mix and Gene Expression Assay (Applied Biosystems, Foster City, CA) for COL1A1, COL3A1, COL4A1, FN1, ELN, ACTB, and GAPDH, per the manufacturer's instructions. The TaqMan PCR step was performed with an Applied Biosystems StepOne Real-Time PCR System (Applied Biosystems), in accordance with the manufacturer's instructions. The relative expressions of the genes were normalized to the amount of ACTB or GAPDH in the same cDNA by using the standard curve method provided by the manufacturer. For each primer set, the comparative computerized tomography method (Applied Biosystems) was used to calculate the amplification differences between the different samples. The relative quantitation values were entered into the Matrix2png program available at http://chibi.ubc.ca/matrix2png/index.html to generate the heat map representation of relative transcript abundance.
Statistical analysis.
Real-time RT-PCR and image analyses were performed in at least triplicate biological samples. Analyzed images were representative of independent experiments. Real-time RT-PCR analyses were also performed with triplicate readings. Statistical analyses were performed with GraphPad Prism 4.02 (GraphPad Software, La Jolla, CA). Unpaired two-tailed t-tests, one-way ANOVA analysis, and Bonferroni posttests were performed where appropriate using GraphPad Prism 4.02. Significance levels were set at *P < 0.05, **P < 0.01, and ***P < 0.001. All graphical data are reported as means ± SE.
RESULTS
Cyclic uniaxial strain and actin fiber orientation of hPSC derivatives at different stages of vSMC differentiation.
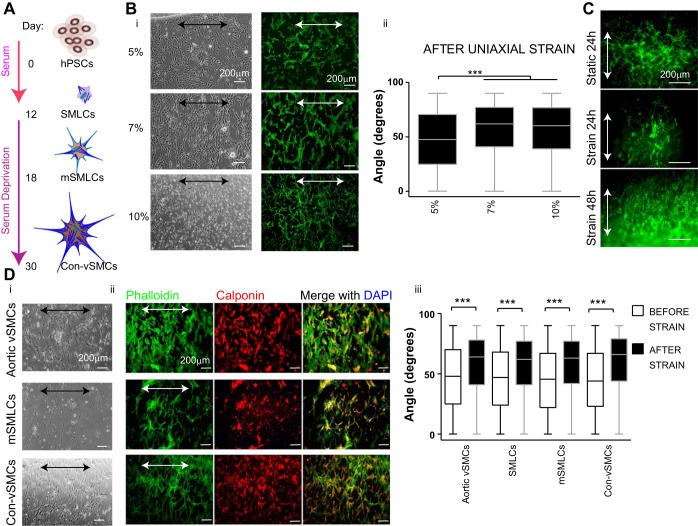
Shimizu et al. (32) have previously reported that cyclic uniaxial strain induced cell alignment of mouse ESC derivatives when elongations between 4 and 12% were used. Therefore, we used similar conditions to determine the conditions that promoted alignment in hPSC derivatives. hPSC-vSMC derivatives were generated using our established protocol (37, 38) (Fig. 1A). We began our studies with SMLCs exposed to cyclic uniaxial strain at a constant frequency of 1 Hz and various elongations including 5, 7, and 10% for 24 h (Fig. 1B, i and ii). At 5% elongation, SMLCs exhibited randomly oriented actin filaments with a mean angle of 47.11 ± 0.70° and lacked cell alignment (Fig. 1B, i and ii). In contrast, whereas SMLCs exhibited statistically significant reorientation of actin filaments perpendicular to the direction of strain with a mean angle of 56.24 ± 0.70° at 10% elongation, the cells also displayed a decrease in stress fiber assembly (Fig. 1B, i and ii). At an elongation of 7%, cell alignment was observed, and at this magnitude of elongation stress fiber assembly was not disrupted. Therefore, the elongation magnitude selected for our studies was 7%.
Fig. 1.
Vascular smooth muscle cell (vSMC) derivative organization and alignment after short-term cyclic uniaxial strain. A: schematic representation of human pluripotent stem cell (hPSC)-vSMC differentiation in vitro. B: smooth muscle-like cells (SMLCs; H9 cell line) at 5, 7, and 10% elongation. Light microscopy images, actin fiber labeled with phalloidin (green) (i) and quantification of cell orientation after 24-h uniaxial strain (ii). C: actin fiber labeled with phalloidin (green) of human embryonic stem cell (hESC) SMLCs embedded in collagen gel at 7% elongation. D: hPSC derivatives (H9 hESCs) and aortic vSMCs control cell line exposed to uniaxial strain at 7% elongation for 24 h. Light microscopy images (i), immunofluorescence images of phalloidin (green) and calponin (red; nuclei in blue) (ii), and quantification of cell orientation before (open) and after (solid) uniaxial strain (iii). Arrows indicate direction of strain. All graphical data are reported as means ± SE. ***P < 0.001. mSMLCs, mature SMLCs; Con-vSMCs, contractile vSMCs.
To determine whether these SMLCs aligned similarly in a 3D environment, cells were embedded in 3D collagen matrices and uniaxially stretched for either 24 or 48 h. After stretch, cells and stress fibers tended to align and orient in a parallel direction to that of principal strain (Fig. 1C), in contrast to the 2D stretching results. A possible explanation is that cells in 2D environments only have basal-lateral cell-matrix contacts compared with cell-matrix contacts surrounding the entire plasma membrane for cells in a 3D environment. Thus our 3D strain results may indicate that the number of cell-matrix contacts dictates how vSMCs mechanosense cyclic uniaxial strain, which affects the actin fiber reorganization.
The progress in vSMC differentiation coincides with increased cell contraction. In fact, mature Con-vSMCs functionally contract more and assemble more stress fibers than the less mature counterparts. Thus we next exposed the different hPSC-vSMC derivatives to cyclic uniaxial strain to determine its effect on stress fiber reorientation and cell alignment. We applied cyclic strain at 7% elongation to human aortic vSMC control cell line. Before the strain, the mean orientation of the aortic vSMCs was measured at 46.9 ± 0.7°. After a 24-h exposure to cyclic uniaxial strain in high-serum conditions, the aortic vSMCs exhibited a significant change in cell and actin filament reorientation to a mean angle of 57.9 ± 0.5°. These results indicate the preferred alignment in a direction perpendicular to the direction of strain or parallel to the direction of minimum strain of mature vSMCs (Fig. 1D, i–iii). A similar change in cell alignment and cell reorientation from a mean angle of 46.3 ± 0.7° to 57.0 ± 0.5° was induced in hPSC-SMLC derivatives cultured and stretched in high serum (Fig. 1D, iii). When the more mature and contractile derivatives, mSMLCs and Con-vSMCs, respectively, were stretched using cyclic uniaxial strain, both derivatives exhibited cell and actin filament alignment perpendicular to the strain. It is worthwhile to note that the Syn-vSMCs similarly aligned perpendicularly to the direction of strain to a mean angle of 60.2 ± 0.8° compared with the measured mean angle of 45.7 ± 1.1° before the exposure to strain. Additionally, all derivatives at different stages of differentiation were positive for the early vSMC marker, calponin, after strain (Fig. 1D, ii). Collectively, quantification of cell orientation before and after strain showed significant changes in the organization of all vSMC derivatives, from random to a perpendicular orientation to strain (Fig. 1D).
Effect of short-term cyclic uniaxial strain on hPSC-vSMC derivative ECM gene expression.
During embryonic development, endothelial cells in the nascent vascular networks recruit vSMC progenitors to be invested in vessel walls (23). The vSMC progenitors are exposed to strain in the vessel walls where they mature further. Additionally, during the formation of new blood vessels, hemodynamic strain from the endothelium may be felt by vSMC progenitors in various stem cell niches such as mesenchymal stem cells residing in vascularized bone marrow or neural crest stem cells migrating to differentiate into vSMCs (7). In our studies, both the mSMLCs and SMLCs represent less mature vSMC precursors. Thus, to investigate whether hPSC-vSMC derivatives exhibited mechanotransductive ECM gene regulation, we exposed cells derived from hPSCs to uniaxial strain for 24 and 48 h and initially quantified the gene expression of collagen I, fibronectin, and elastin. The H9 hESC line was used for these studies. After SMLCs were exposed to uniaxial stretch for 24 and 48 h in high-serum media, there was no significant change in the gene expression of collagen I and fibronectin ECM. However, there was a significant decrease in the elastin mRNA levels compared with static equivalents (Fig. 2A, left). On the other hand, hPSC Syn-vSMCs exposed to uniaxial strain for 24 and 48 h in high-serum conditions exhibited a significant upregulation in the expression of collagen I, fibronectin, and elastin (Fig. 2A, right). These results show that, in high-serum media, ECM genes are upregulated only in vSMCs with the synthetic phenotype in response to short-term cyclic uniaxial strain.
Fig. 2.
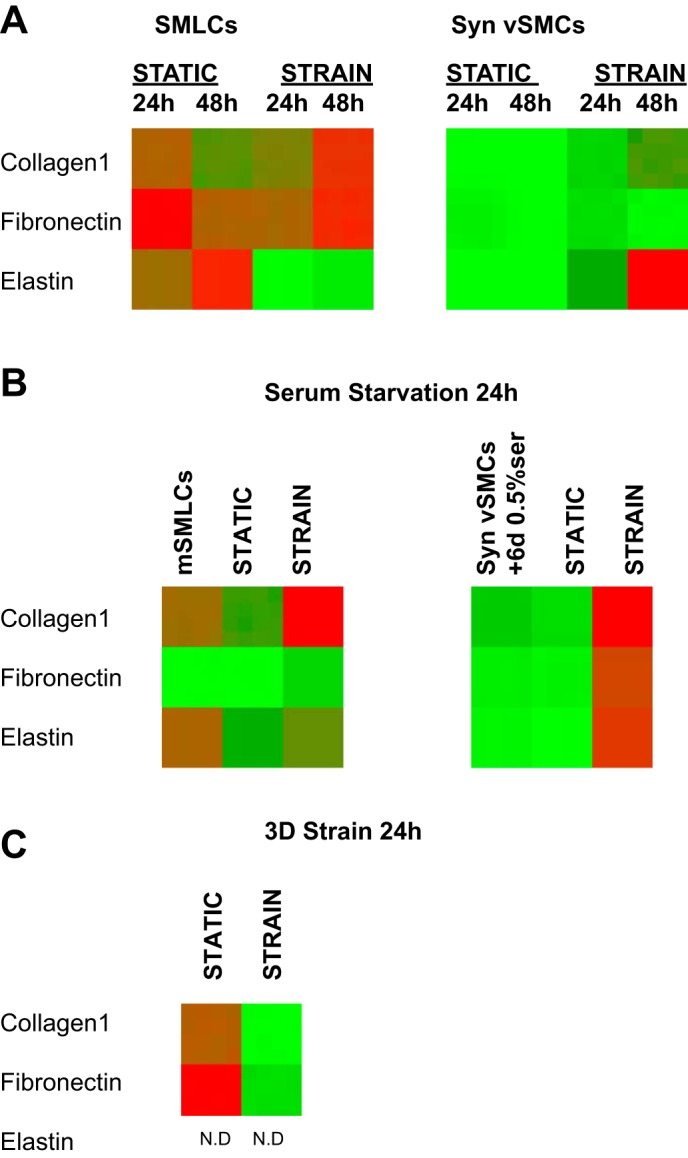
Heat map representation of relative extracellular matrix (ECM) gene expression of hPSC-vSMC derivatives after short-term cyclic uniaxial strain. A: quantitative real-time RT-PCR gene expression of collagen type I, fibronectin, and elastin of SMLCs (left) and synthetic vSMCs (Syn-vSMCs) (right) after 24 and 48 h of uniaxial strain in high-serum conditions. B: quantitative real-time RT-PCR gene expression of serum-starved SMLCs (mSMLCs) (left) and Syn-vSMCs (right) after 24 h of uniaxial strain in serum-deprived conditions. C: SMLCs embedded in collagen gels after 24 h of 3D uniaxial strain. Ct values were normalized with the GAPDH housekeeping gene to obtain relative quantitation (RQ) values. The RQ values were entered into the Matrix2png program available at http://chibi.ubc.ca/matrix2png/index.html to generate the heat map representation of relative transcript abundance. Green shows lower ECM gene expression, and red depicts higher expression. N.D., no gene expression or gene expression is below the limit of detection.
Notably, when the control human aortic vSMCs were serum starved and exposed to uniaxial strain for 24 h, there was a significant decrease in the gene expression of collagen I and elastin (data not shown). As expected, the Con-vSMCs cultured in serum-starved conditions responded similarly as the serum-starved control aortic vSMCs because these derivatives represent fully mature vSMCs. Elastin downregulation was observed in the mature Con-vSMCs cultured in serum-starved conditions after 24 h of cyclic uniaxial strain (data not shown).
The serum-starved mSMLCs also exhibited a significant upregulation in the gene expression of collagen I and fibronectin in addition to elastin, compared with static equivalents (Fig. 2B, left). To determine whether serum starvation promoted stretch-induced ECM gene regulation, both Syn-vSMCs and control aortic vSMCs were serum starved for 6 days and stretched in serum-starved conditions. Serum-deprivation effects while stretching cells could be investigated for these two cell types because, unlike other derivatives, both cell types are cultured in high-serum conditions. Switching from high-serum to serum-starved conditions allows us to study the effects of serum deprivation during stretching. After the hPSC Syn-vSMCs were serum starved for 6 days and subsequently exposed to 24 h of uniaxial strain, an increase in collagen I, fibronectin, and elastin gene expression was observed compared with static equivalents (Fig. 2B, right).
SMLCs embedded in 3D collagen gels also exhibited differences after 24 h of cyclic uniaxial strain (Fig. 2C). Whereas collagen I and fibronectin gene expression increased as a result of static culture in a 3D environment, the inclusion of stretching in a 3D environment downregulated their expression. These results differ from the SMLCs uniaxially stretched for 24 h in a 2D environment, which did not induce a significant change in the expression of collagen I and fibronectin (Fig. 2, A, left, and C), indicating that the 3D environment coupled with stretch downregulates these ECM genes. Additionally, whereas elastin decreased after 24 h of cyclic uniaxial strain in a 2D environment, it was undetected in stretched and static SMLCs in 3D samples, suggesting that the 3D environment hindered elastin gene expression in the short term (Fig. 2, A, left, and C).
Collectively, these results indicate that short-term cyclic uniaxial strain induces upregulation of ECM protein expression in Syn-vSMCs. These results also indicate that, although both serum starvation and short-term cyclic uniaxial strain combined contribute to the increased ECM gene expression of maturing hPSC vSMCs, ECM gene expression decreases for fully mature Con-vSMCs.
Long-term cyclic uniaxial strain and hPSC-vSMC derivative ECM gene expression.
To determine whether the cyclic uniaxial strain-induced ECM upregulation was a transient or long-lasting response, we exposed both SMLCs and mSMLCs to 6 days of uniaxial strain (Fig. 3A).
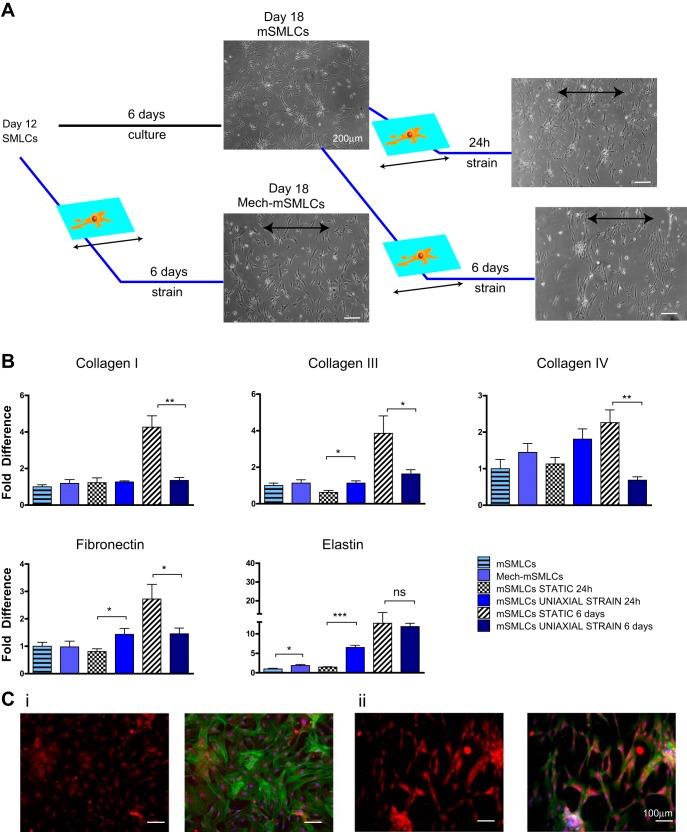
Fig. 3.
ECM gene expression of hPSC-vSMC derivatives after long-term cyclic uniaxial strain. A: schematic representation of long-term uniaxial strain experiments. Mech-mSMLCs, mechanically strained mSMLCs. B: SMLC and mSMLC collagen type I, collagen type III, collagen type IV, fibronectin, and elastin gene expression after 6 days of long-term uniaxial strain in serum-deprived media using quantitative real-time RT-PCR. C: immunofluorescence images of mSMLC elastin protein deposition after 24-h uniaxial strain: phalloidin (green), elastin (red), nuclei (blue). All graphical data are reported as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001.
We first exposed the less mature SMLCs to long-term strain. hPSC mSMLCs were used as the static equivalents of hPSC SMLCs exposed to 6 days uniaxial strain in serum-starved conditions or mechanically strained mSMLCs (Fig. 3A). Although these mechanically strained SMLCs exhibited a significant increase in elastin mRNA levels compared with mSMLCs, no significant change in collagen I, III, IV, and elastin was observed.
The more mature mSMLCs were next exposed to short-term cyclic uniaxial strain for only 24 h in serum-starved conditions. The stretched cells exhibited an increased ECM gene expression, not only in elastin, but also in collagen III and fibronectin (Fig. 3B). An increased deposition of elastin was also observed after 24 h (Fig. 3B, middle). It should be noted that, although the BC1 hiPSC mSMLCs had similar upregulation of fibronectin and elastin as their H9 hESC-mSMLCs counterparts, there was a difference in collagen I expression. Specifically, H9 hESC mSMLCs had an increase in collagen I gene expression, and the BC1 hiPSC mSMLCs did not show a significant change in collagen I after short-term cyclic uniaxial strain (Figs. 2 and 3B). This was the only notable difference in ECM expression within the different cell lines.
Lastly, to investigate the effects of long-term uniaxial strain, we compared the results of the hPSC mSMLCs stretched for 24 h to hPSC mSMLCs stretched 6 days. In contrast to the mSMLCs exposed to uniaxial strain for 24 h, after long-term uniaxial strain, the mSMLCs exhibited no change in elastin mRNA levels and a significant decrease in collagen I, III, and IV (Fig. 3B).
These results suggest that vSMCs produce more elastin in response to long-term uniaxial strain earlier in differentiation rather than when they are more mature and contractile. As such, strain-induced elastin upregulation is dependent on vSMCs initially being at a less mature state. Particularly, SMLCs exposed to long-term uniaxial strain (or mechanically strained SMLCs) expressed increased elastin, whereas the more mature mSMLCs expressed decreased elastin after exposure to long-term uniaxial strain compared with static equivalents (Fig. 3B). The results also indicate that, as vSMCs mature, they respond to long-term cyclic uniaxial strain more significantly than in less mature derivatives. Although mSMLC gene expression of all tested ECM proteins was downregulated in response to long-term uniaxial strain, there was a significant change in only one ECM protein gene expression in the less mature SMLCs in response to long-term uniaxial strain.
Long-term cyclic circumferential strain and hPSC-vSMC derivative ECM gene expression.
Although cyclic uniaxial strain closely resembles the tensile strain of blood vessels, a more physiologically relevant tensile force that takes into account the curvature of vessels is a circumferential strain. Thus we established a bioreactor that enables pressurized flow into tubular silicone constructs, which in turn allows the radial distention of the constructs (Fig. 4A).
SMLCs did not show significant change in collagen and fibronectin expression after circumferential strain, and, therefore, mSMLCs were used to study the effect of long-term circumferential strain on ECM genes. The mSMLCs were also studied because we observed that long-term tensile uniaxial strain had a significant effect on most of the ECM genes (Fig. 3B). After 24 h after seeding, silicone tubes were fully covered with hPSC mSMLCs (Fig. 4, B and C). Cell concentration on the tubes was greater than the uniaxial strain samples as a result of different seeding methods. Whereas the cells on the tubes were seeded using an orbital shaker to ensure tube coverage, cells on the 2D uniaxial strain samples were allowed to seed on a stationary surface. Orbital shaking was used to resolve difficulties in uniform cell attachment, as there were greatly reduced cells present on the silicone tubes when they were statically seeded. An elongation magnitude consistent with uniaxial strain experiments was used for the circumferential strain experiments by measuring tube distension. Radial distension was measured optically using digital calipers by tracking the diameter of the silicone tubes (6, 10). An elongation of 7% at a mean pulsatile flow of 4.30 ± 0.04 ml/min was thus used to elongate silicone tubes.
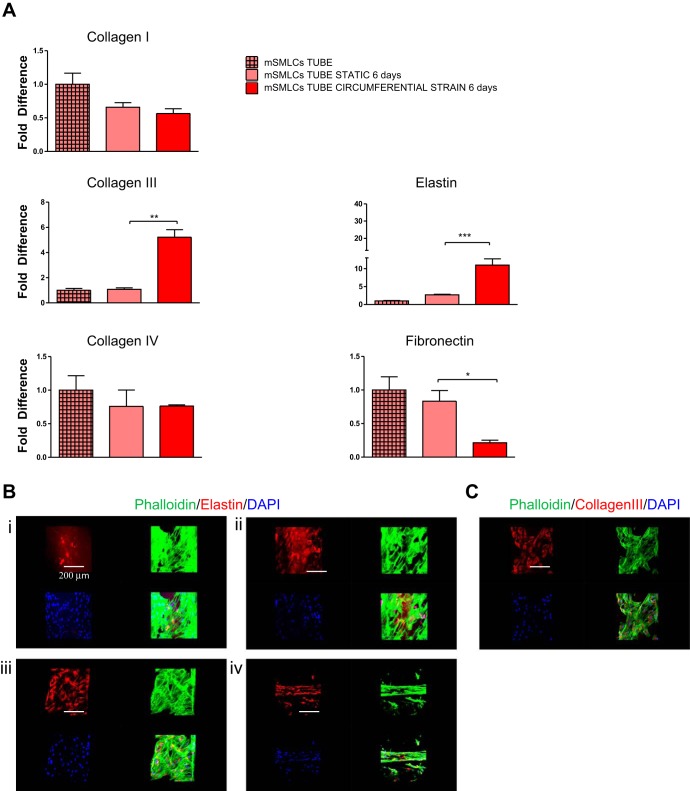
After the long-term circumferential strain of mSMLCs for 6 days, there was a significant increase in the expression of collagen III and a significant decrease in fibronectin mRNA (Fig. 5A). Elastin gene expression was also significantly upregulated after circumferential strain, and, although mSMLCs deposited small amounts of elastin compared with the mature Con-vSMCs before exposure to circumferential strain, they deposited more elastin protein after circumferential strain compared with the nonmechanically stimulated but mature Con-vSMCs (Fig. 5, A and B). These results indicate that the application of circumferential strain during vSMC differentiation results in highly increased elastin deposition. Several studies have also shown this similar increase in vSMC elastin expression after exposure of vessel constructs to cyclic loading or pulsatile flow (16, 20).
Fig. 5.
ECM gene expression after long-term cyclic circumferential strain. A: mSMLC collagen type I, collagen type III, collagen type IV, fibronectin, and elastin gene expression after 6 days of long-term circumferential strain in serum-deprived media. Gene expression was determined using quantitative real-time RT-PCR (strain conditions in red and static equivalents in light red). B: confocal images comparing mSMLC (i) and Con-vSMC (ii) elastin deposition without strain and mSMLC elastin deposition (i) and alignment (iv) after circumferential strain for 6 days. C: mSMLC collagen type III deposition after circumferential strain for 6 days. All graphical data are reported as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001.
Occasionally, stretched cells seemed to form contracted structures and exhibited alignment parallel to the direction of strain (Fig. 5B, iv). This parallel alignment may be attributed to the higher concentration of cells present in the circumferential strain samples compared with the uniaxial strain samples. The higher concentration would allow a higher number of cell-cell contacts, in which case the cells are less likely to respond individually and instead exhibit a group response. Samples exposed to circumferential strain similarly deposited significant collagen type III protein (Fig. 5C). Overall, the production of these ECM proteins by the vSMC derivatives is highly desirable for engineered vessel constructs because mechanical integrity of blood vessels is largely attributed to collagen and elastin deposition. For instance, collagen type I and III are major constituents of the interstitial connective tissue in vessels that functionally regulate vessel tensile forces by limiting vessel expansion (8). On the other hand, elastin contributes to vessel elasticity and resilience, which is necessary to accommodate vessel dilation and expansion (8). It should be noted that, whereas elastin and collagen type III expression increased after exposure to long-term circumferential strain, both ECM molecules decreased after exposure to long-term uniaxial strain, indicating that the tensile forces have quite dissimilar effects on vSMC derivatives (Figs. 3B and 5A).
DISCUSSION
The reorientation of cells perpendicular to uniaxial cyclic strain direction has been widely observed in a variety of adult cells and therefore does not seem to be cell dependent. For instance, endothelial cells (15), fibroblasts (2), and mesenchymal stem cells (21) have been reported to reorient perpendicularly to the direction of 2D uniaxial strain. Some studies attribute cell contractility, which is dependent on the number of stress fibers, for the direction of this cell reorientation (17, 19). Our studies indicate that there exists a lower elongation limit that induces cytoskeletal structure alterations in differentiating vSMCs and an upper limit in which actin stress fiber assembly is interrupted. Additionally, we found that both hPSC-vSMC differentiation stage and phenotype do not affect mechanotransductive cytoskeleton remodeling. However, vSMCs are also known to be heterogeneous, with some cells being more contractile and mature than others. Because the contractility of vSMCs is dependent on the actin and myosin machinery and uniaxial strain-induced mechanotransductive cytoskeleton remodeling, the heterogeneity of the derived vSMCs was affected by uniaxial strain. The heterogeneity of vSMCs was determined by comparing smooth muscle myosin heavy chain (SMMHC) gene expression in samples. Both short-term and long-term 2D uniaxial strain induced a reduction in SMMHC gene expression in all derived vSMCs and control aortic vSMCs compared with the static cultures, indicating a change in the heterogeneity of the vSMCs.
Although others have also reported the alignment of adult vSMCs perpendicular to the direction of strain (18, 24, 33), the 2D in vitro environment does not accurately mimic the in vivo environment in which vSMCs form concentric layers surrounded by a 3D ECM and are arranged in circumferential spirals or diagonal to the direction of strain (29). After stretching cells in 3D matrices, we speculated that the large number of cell-matrix contacts present in 3D stretched cells compared with 2D stretched cells may account for the stark differences in stretch-induced reorientation and alignment. Because cells typically interact with the surrounding matrices using integrins, it is highly likely that different uniaxial strain-induced intracellular signaling in vSMCs depends on the number of integrins responding to the external stretch of the matrix. Several other factors should be accounted for when stretching cells in 3D environments. Whereas mechanically stimulating cells that are encapsulated in 3D matrices is more representative of the in vivo physiological environment, the issue of selecting suitable materials with optimum matrix stiffness or density arises. For instance, collagen gel density has been reported to affect the direction of stress fiber orientation (35). Similarly, Foolen et al. (11) report that the existence of synergy between matrix density and Rho-kinase signaling that controls cyclic stretch induced actin stress fiber reorganization.
Because the vSMC-associated growth factors, angiotensin and PDGF, have been shown to regulate ECM proteins (12, 27, 34), these growth factors may also be involved in the strain-induced ECM gene regulation. Therefore, the observed changes in ECM in response to strain may be due to, not only integrin mechanosensing, but also the stretch-induced release of vSMC growth factors, angiotensin and PDGF. Additionally, it is likely that intercellular serum-sensitive molecules known to regulate vSMC maturation, such as serum response factor, may be modulated by a membrane-bound mechanosensor to activate ECM deposition pathways (36).
The ECM gene expression by hPSC-vSMC derivatives in response to long-term uniaxial strain also seems to mirror developing vSMCs in vivo, where most ECM gene expression is dynamic (14). For instance, in mice, there is an initial increase of ECM gene expression in the embryo followed by a brief decrease postnatally followed by a steady rise for 2 wk and a final decline to low levels in the adult (14).
Lastly, the conflicting changes in the gene expression of elastin and collagen type III observed in response to long-term uniaxial compared with circumferential strain suggest that vSMCs mechanosense the two forces differently. These results suggest that curvature effects, absent when stretching the cell uniaxially, play an important role in promoting ECM expression in the developing vSMC. Circumferential strain seems to be a later stage cue, as SMLCs did not show significant change in the expression of collagen and fibronectin compared with mSMLCs. SMLCs may therefore represent early vSMCs that exist before significant blood flow begins in a newly forming vessel and are not exposed to significant circumferential strain. Limited exposure to strain in vessels would make the SMLCs not responsive to strain. As the SMLCs settle on the wall, they may further differentiate to mSMLCs that experience more circumferential strain and therefore respond by depositing ECM. Long-term mechanical stimulation of vSMC derivatives using circumferential strain may therefore improve mechanical properties of vascular constructs more efficiently than long-term uniaxial strain by upregulating collagen and elastin.
Conclusion.
In conclusion, hPSC-derived vSMCs display mechanotransduction in response to tensile strain that affects cytoskeleton organization and ECM gene expression. Mechanical stimulation of hPSC-derived vSMCs in vessel constructs using uniaxial and circumferential strain may potentially modulate derived vSMC function and ultimately improve the construct mechanical properties needed for therapeutic purposes.
GRANTS
This work was supported by the National Institutes of Health grant R01HL107938 and National Science Foundation grant 1054415 (to S. Gerecht).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.W. and S.G. conception and design of research; M.W. and N.A. performed experiments; M.W., N.A., and S.G. analyzed data; M.W. and S.G. interpreted results of experiments; M.W. and S.G. prepared figures; M.W., N.A., and S.G. drafted manuscript; S.G. edited and revised manuscript; S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sebastian Barreto-Ortiz and Quinton Smith for critically reviewing the manuscript.
REFERENCES
- 1.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Boccafoschi F, Bosetti M, Gatti S, Cannas M. Dynamic fibroblast cultures: response to mechanical stretching. Cell Adh Migr 1: 124–128, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan-Park MB, Shen JY, Cao Y, Xiong Y, Liu Y, Rayatpisheh S, Kang GCW, Greisler HP. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J Biomed Mater Res A 88A: 1104–1121, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Hansen NF, Zhao L, Du Y, Zou C, Donovan FX, Chou BK, Zhou G, Li S, Dowey SN, Ye Z; NISC Comparative Sequencing Program, Chandrasekharappa SC, Yang H, Mullikin JC, Liu PP. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell 10: 337–344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, Zou C, Zhang YA, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res 21: 518–529, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation 17: 164–178, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eble JA, Niland S. The extracellular matrix of blood vessels. Curr Pharm Des 15: 1385–1400, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Filas BA, Knutsen AK, Taber LA, Bayly PV. A new method for measuring deformation of folding surfaces during morphogenesis. J Biomech Eng 130: 061010–061010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer MJ, Uchida S, Messlinger K. Measurement of meningeal blood vessel diameter in vivo with a plug-in for ImageJ. Microvasc Res 80: 258–266, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Foolen J, Janssen-van den Broek MW, Baaijens FP. Synergy between Rho signaling and matrix density in cyclic stretch-induced stress fiber organization. Acta Biomater 10: 1876–1885, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Ford CM, Li S, Pickering JG. Angiotensin II stimulates collagen synthesis in human vascular smooth muscle cells: involvement of the AT1 receptor, transforming growth factor-β, and tyrosine phosphorylation. Arterioscler Thromb Vasc Biol 19: 1843–1851, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Greif DM. Vascular embryology and angiogenesis. In: Vascular Medicine: A Companion to Braunwald's Heart Disease, 2nd ed., edited by Creager MA, Beckman JA, and Loscalzo J. Philadelphia, PA: W. B. Saunders, 2013, pp. 1–13. [Google Scholar]
- 15.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc Res 42: 245–254, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg B, Tranquillo R. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng 31: 937–949, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Yang C, Zhao L, Zheng Q. Stress fiber response to mechanics: a free energy dependent statistical model. Soft Matter 10: 4603–4608, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Kanda K, Matsuda T, Oka T. Two-dimensional orientational response of smooth muscle cells to cyclic stretching. ASAIO J 38: M382–M385, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA 102: 15895–15900, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 17: 979, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA 103: 16095–16100, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacNeill BD, Pomerantseva I, Lowe HC, Oesterle SN, Vacanti JP. Toward a new blood vessel. Vasc Med 7: 241–246, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res 108: 365–377, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills I, Cohen CR, Kamal K, Li G, Shin T, Du W, Sumpio BE. Strain activation of bovine aortic smooth muscle cell proliferation and alignment: study of strain dependency and the role of protein kinase A and C signaling pathways. J Cell Physiol 170: 228–234, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Na S, Trache A, Trzeciakowski J, Sun Z, Meininger GA, Humphrey JD. Time-dependent changes in smooth muscle cell stiffness and focal adhesion area in response to cyclic equibiaxial stretch. Ann Biomed Eng 36: 369–380, 2008. [DOI] [PubMed] [Google Scholar]
- 26.O'Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-β1. Hypertension 36: 319–324, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Okada Y, Katsuda S, Watanabe H, Nakanishi I. Collagen synthesis of human arterial smooth muscle cells: effects of platelet-derived growth factor, transforming growth factor-β1 and interleukin-1. Pathol Int 43: 160–167, 1993. [DOI] [PubMed] [Google Scholar]
- 27a.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, Fan Y, Wang G. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J Royal Soc Interface 11: 20130852, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodin JA. Architecture of the vessel wall. In: Comprehensive Physiology, edited by Terjung R. New York, NY: John Wiley and Sons, 2011, pp. 1–31. [Google Scholar]
- 30.Shah AM, Banerjee T, Mukherjee D. Coronary, peripheral and cerebrovascular disease: a complex relationship. Herz 33: 475–480, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Sharifpoor S, Simmons CA, Labow RS, Paul Santerre J. Functional characterization of human coronary artery smooth muscle cells under cyclic mechanical strain in a degradable polyurethane scaffold. Biomaterials 32: 4816–4829, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T, Ando J. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor β. J Appl Physiol 104: 766–772, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Standley PR, Camaratta A, Nolan BP, Purgason CT, Stanley MA. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol 283: H1907–H1914, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Tokimitsu I, Kato H, Wachi H, Tajima S. Elastin synthesis is inhibited by angiotensin II but not by platelet-derived growth factor in arterial smooth muscle cells. Biochim Biophys Acta 1207: 68–73, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Tondon A, Kaunas R. The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS One 9: e89592, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turczyńska KM, Hellstrand P, Swärd K, Albinsson S. Regulation of vascular smooth muscle mechanotransduction by microRNAs and L-type calcium channels. Commun Integr Biol 6: e22278, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vo E, Hanjaya-Putra D, Zha Y, Kusuma S, Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev 6: 237–247, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res 97: 321–330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanjare M, Kusuma S, Gerecht S. Perivascular cells in blood vessel regeneration. Biotechnol J 8: 434–447, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarbiv G, Preis M, Ben-Yosef Y, Flugelman MY. Engineering blood vessels by gene and cell therapy. Expert Opin Biol Ther 7: 1183–1191, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Zeng L, Emanueli C, Xu Q. Blood flow and stem cells in vascular disease. Cardiovasc Res 99: 251–259, 2013. [DOI] [PubMed] [Google Scholar]