H2S is a gaseous mediator that produces decreases in arterial pressure, heart rate, and at higher doses cardiac output. Systemic vascular resistance was decreased, and responses were not blocked by ATP-sensitive potassium channel, nitric oxide, cyclooxygenase, lipoxygenase, or autonomic antagonists. H2S donors may be useful in the treatment of cardiovascular diseases.
Keywords: H2S donors, KATP channels, hypotension, bradycardia, cardiac output
Abstract
Hydrogen sulfide (H2S) is an endogenous gaseous molecule formed from L-cysteine in vascular tissue. In the present study, cardiovascular responses to the H2S donors Na2S and NaHS were investigated in the anesthetized rat. The intravenous injections of Na2S and NaHS 0.03–0.5 mg/kg produced dose-related decreases in systemic arterial pressure and heart rate, and at higher doses decreases in cardiac output, pulmonary arterial pressure, and systemic vascular resistance. H2S infusion studies show that decreases in systemic arterial pressure, heart rate, cardiac output, and systemic vascular resistance are well-maintained, and responses to Na2S are reversible. Decreases in heart rate were not blocked by atropine, suggesting that the bradycardia was independent of parasympathetic activation and was mediated by an effect on the sinus node. The decreases in systemic arterial pressure were not attenuated by hexamethonium, glybenclamide, Nw-nitro-l-arginine methyl ester hydrochloride, sodium meclofenamate, ODQ, miconazole, 5-hydroxydecanoate, or tetraethylammonium, suggesting that ATP-sensitive potassium channels, nitric oxide, arachidonic acid metabolites, cyclic GMP, p450 epoxygenase metabolites, or large conductance calcium-activated potassium channels are not involved in mediating hypotensive responses to the H2S donors in the rat and that responses are not centrally mediated. The present data indicate that decreases in systemic arterial pressure in response to the H2S donors can be mediated by decreases in vascular resistance and cardiac output and that the donors have an effect on the sinus node independent of the parasympathetic system. The present data indicate that the mechanism of the peripherally mediated hypotensive response to the H2S donors is uncertain in the intact rat.
NEW & NOTEWORTHY
H2S is a gaseous mediator that produces decreases in arterial pressure, heart rate, and at higher doses cardiac output. Systemic vascular resistance was decreased, and responses were not blocked by ATP-sensitive potassium channel, nitric oxide, cyclooxygenase, lipoxygenase, or autonomic antagonists. H2S donors may be useful in the treatment of cardiovascular diseases.
gaseous modulators such as nitric oxide (NO) and carbon monoxide (CO) have been reported to play an important role in the regulation of cardiovascular function (15, 20, 21, 30). Hydrogen sulfide (H2S), which has long been known as a toxic gas, has recently been shown to have an important role in the regulation of cardiovascular function as a third endogenous gaseous transmitter (17, 18, 26). It has been hypothesized that H2S is a major physiologic endothelial derived hyperpolarizing factor (17, 18, 26). H2S is synthesized from L-cysteine by at least four enzymes with cystathionine γ-lyase (CSE) appearing to play a major role in vascular tissue (4, 7, 23, 36, 37, 39, 41, 46). Transgenic mice lacking CSE have been reported to develop hypertension similar to that observed in endothelial NO synthase (eNOS) knockout mice (46). It has been reported that hypotensive and vasorelaxant responses to H2S or H2S donors are inhibited by the ATP-sensitive potassium (KATP) channel antagonist glybenclamide and are mediated at least in part by the opening of KATP channels (9, 14, 23, 48–50). It has also been reported that there are interactions between H2S and the NO-cyclic guanylate monophosphate (cGMP) system and arachidonic acid pathways as well as effects on large conductance calcium-activated potassium channels (2, 9, 10, 18, 22, 27, 28, 42, 50).
The present study was undertaken to investigate cardiovascular responses to H2S using the H2S donors sodium sulfide (Na2S) and sodium hydrosulfide (NaHS) in the anesthetized rat. The hypothesis that decreases in arterial pressure and heart rate in response to the H2S donors are mediated by the activation of KATP channels was investigated. The results of these studies show that H2S donors decrease systemic arterial pressure and heart rate in a dose-dependent manner and at higher doses decrease cardiac output and pulmonary arterial pressure. These data indicate that decreases in systemic arterial pressures can be mediated by an effect on vascular tone and cardiac output and that systemic vasodepressor responses are not blocked by hexamethonium, glybenclamide, 5-hydroxydecanoate, Nw-nitro-l-arginine methyl ester hydrochloride (l-NAME), sodium meclofenamate, ODQ, tetraethylammonium (TEA), or miconazole, suggesting that the mechanism of action in the intact rat is uncertain.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of the Tulane University School of Medicine approved the experimental protocol used in these studies, and all procedures were conducted in accordance with institutional guidelines. For these experiments, male Sprague-Dawley rats (273–456 g) were anesthetized with an intraperitoneal injection of Inactin (thiobutabarbital; Sigma-Aldrich, St. Louis, MO) in a dose of 100 mg/kg. Supplemental doses of Inactin were given intraperitoneally as needed to maintain a uniform level of anesthesia. Body temperature was maintained with a heating lamp. The trachea was cannulated with a short segment of PE-240 tubing to maintain a patent airway, and the animals breathed room air enriched with 100% O2. The left carotid artery or left femoral artery was catheterized with PE-50 tubing for the measurement of systemic arterial pressure. Systemic arterial pressure was measured with a Namic Perceptor DT pressure transducer and a data acquisition system (Biopac MP 100A-CE; Santa Barbara, CA). Systemic arterial pressure and mean arterial pressure were obtained by electronic averaging of the pressure signal and heart rate signal. These data were continuously recorded, displayed, and stored on a Dell personal computer (PC). The left jugular vein was catheterized with PE-50 tubing for the systemic injection of drugs and fluids. The left femoral vein was also catheterized with PE-50 tubing for systemic infusion of drugs.
For the measurement of pulmonary arterial pressure, a specially designed 3-F single-lumen catheter with a curved tip and radio-opaque marker was passed from the right jugular vein into the main pulmonary artery under fluoroscopic guidance (Picker-Surveyor Fluoroscope) as described previously (19, 33–35). Pulmonary arterial pressure was measured with a Namic Perceptor DT pressure transducer and data acquisition system and stored on the PC. The cardiac output was measured by the thermodilution technique with a Cardiomax II computer (Columbus Instruments). A known volume (0.2 ml) of room temperature 0.9% saline solution was injected into the jugular vein catheter with its tip near the right atrium, and changes in blood temperature were detected by the 1.5-F thermistor microprobe catheter (Columbus Instruments) positioned in the aortic arch from the left carotid artery. The indicator dilution data were stored on the PC.
Drugs.
Na2S, NaHS, sodium nitroprusside (SNP), phentolamine, atropine sulfate, acetylcholine chloride, norepinephrine, cromakalim, l-NAME, sodium meclofenamate, and TEA, hexamethonium, and 5-hydroxydecanoate (Sigma-Aldrich) were dissolved in 0.9% NaCl, and solutions were prepared on a frequent basis. Glibenclamide (Sigma-Aldrich) was dissolved in 4 ml propylene glycol with 300 μg concentrated NaOH, 1.7 ml of 100% ethanol, and 4 ml of Tris (pH 8.4) and sonicated. ODQ (Sigma-Aldrich) was dissolved in a 10/10/80 ratio of transcretol, cremephor, and 0.9% NaCl, respectively. Miconazole (Sigma-Aldrich) was dissolved in DMSO. The doses of the antagonists used in the present study were determined from doses used in previous studies in the literature and from pilot studies in our laboratory (11, 29, 33–35, 50). The vehicles for the drugs used in the study did not alter responses to Na2S and NaHS.
Statistics.
The data are expressed as means ± SE and were analyzed using a one-way ANOVA and a Student's t-test for paired and unpaired data. A P value of less than 0.05 was used as the criterion for statistical significance.
RESULTS
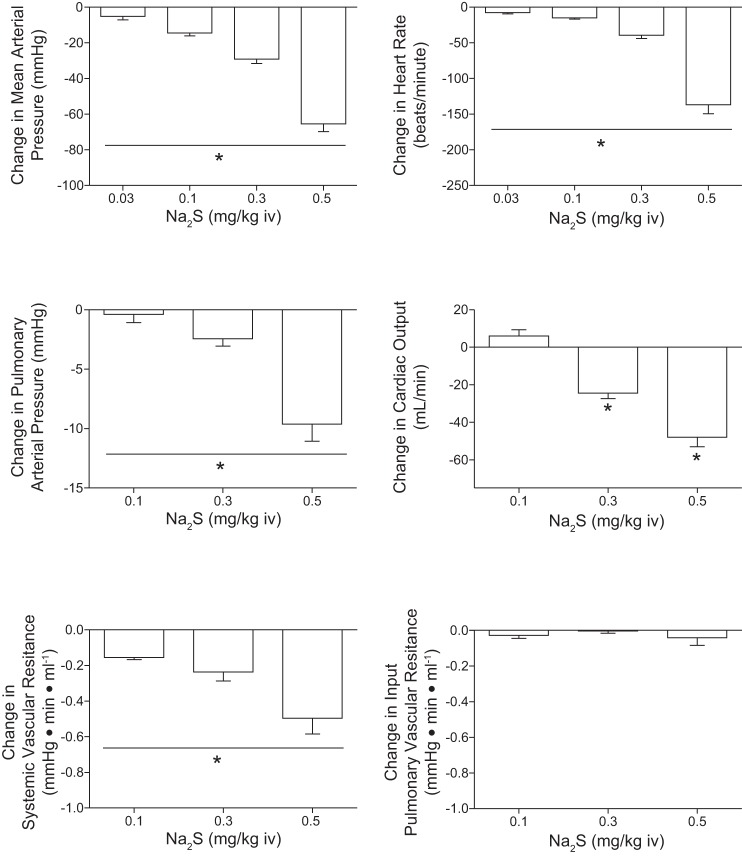
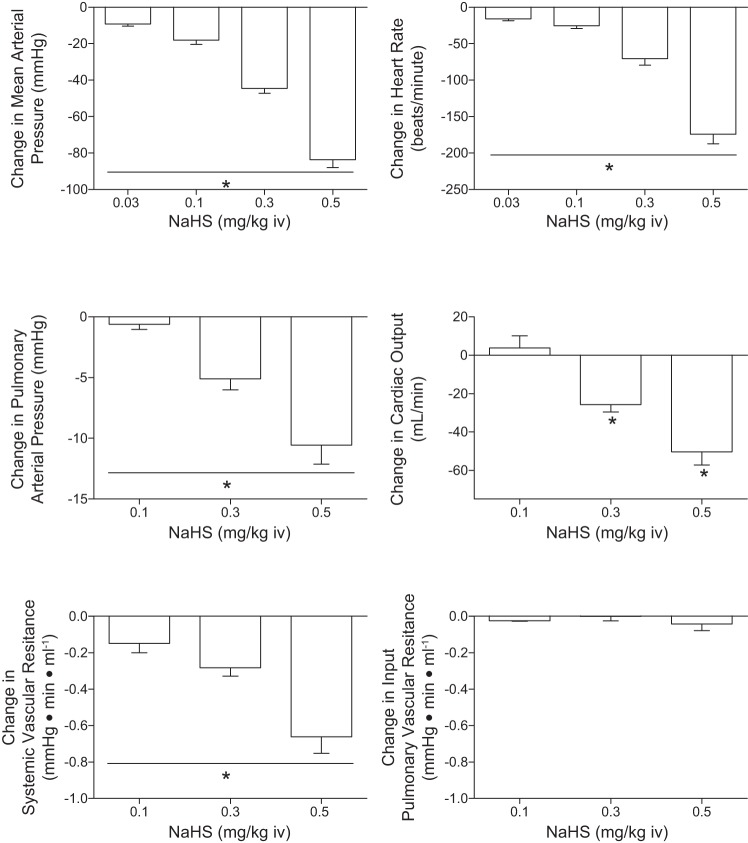
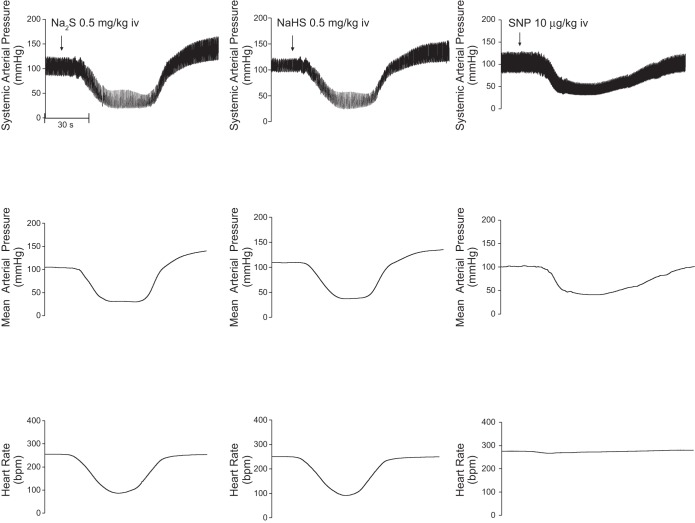
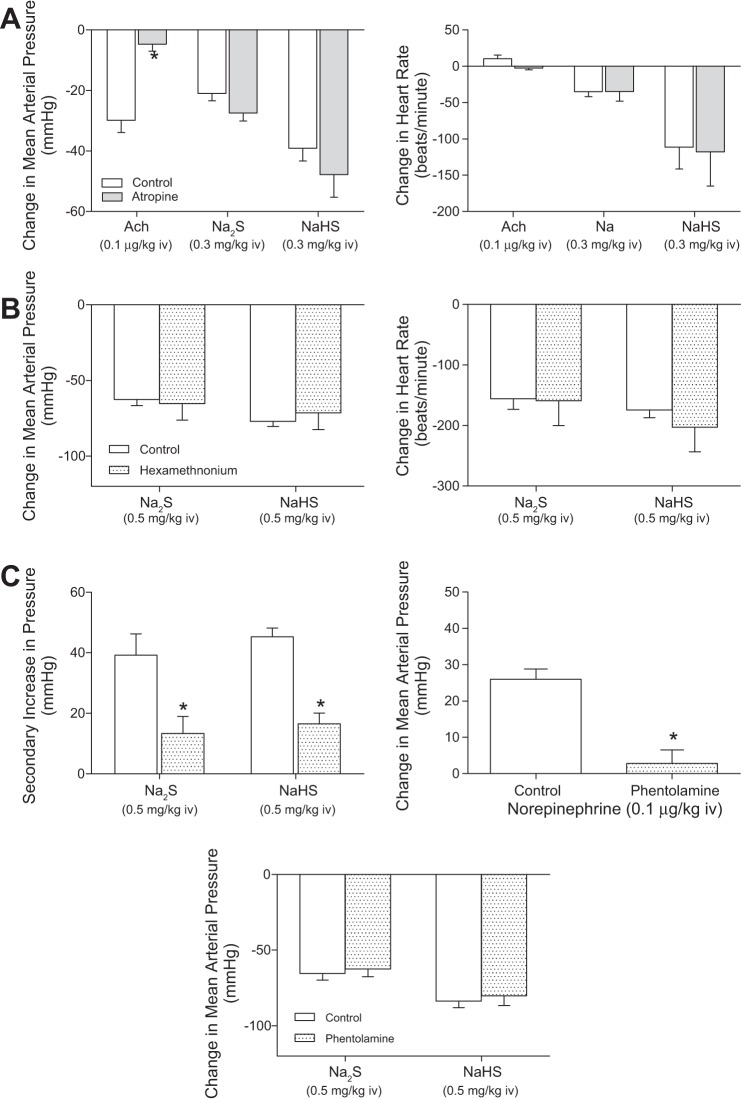
Cardiovascular responses to the H2S donors, Na2S and NaHS, were investigated in the rat, and these results are summarized in Figs. 1 and 2. The intravenous injections of Na2S and NaHS in doses of 0.03–0.5 mg/kg produced dose-related decreases in systemic arterial pressure and in heart rate (Figs. 1 and 2). The decreases in systemic arterial pressure and heart rate were rapid in onset and short in duration. At the higher doses studied, the intravenous injections of Na2S and NaHS produced significant decreases in pulmonary arterial pressure and in cardiac output (Figs. 1 and 2). Systemic vascular resistance was decreased significantly at the 0.1, 0.3, and 0.5 mg/kg doses of Na2S and NaHS (Figs. 1 and 2). Input pulmonary vascular resistance was not decreased significantly at the 0.1–0.5 mg/kg intravenous doses of the H2S donors (Figs. 1 and 2). The time course of the decreases in systemic arterial pressure, mean arterial pressure, and heart rate in response to intravenous injections of high doses of the H2S donors and to intravenous injections of the NO donor SNP are shown in Fig. 3 where it can be seen that a secondary increase in systemic arterial pressure is observed during the recovery phase of the response of the H2S donors and that SNP does not produce bradycardia (Fig. 3). The secondary increase in systemic arterial pressure during the recovery phase of the pressor response to the H2S donors was significantly attenuated after administration of 0.5 mg/kg iv of the α-receptor blocking agent phentolamine, indicating that this overshoot in systemic arterial pressure was mediated by α-adrenergic stimulation (Fig. 7C). The decreases in heart rate in response to intravenous injection of the H2S donors were not attenuated after administration of 0.5 mg/kg iv atropine, indicating that the bradycardia was not mediated by activation of the parasympathetic nervous system (Fig. 7A). After administration of the muscarinic receptor antagonist atropine, the decreases in systemic arterial pressure in response to intravenous injections of acetylcholine were decreased significantly, whereas decreases in systemic arterial pressure in response to intravenous injections of the H2S donors were not changed (Fig. 7A). The decreases in systemic arterial pressure and heart rate in response to intravenous injection of Na2S were not attenuated by treatment with phentolamine or the ganglionic blocking agent hexamethonium (Fig. 7, B and C). The data with atropine, phentolamine, and hexamethonium suggest that the decreases in arterial pressure and heart rate in response to the H2S donors are mediated by peripheral actions and that effects on the central nervous system do not play a major role. After administration of phentolamine, the pressor response to norepinephrine was significantly attenuated (Fig. 7C).
Fig. 1.
Bar graphs showing dose-related decreases in systemic arterial pressure, heart rate, pulmonary arterial pressure, cardiac output, and systemic and input pulmonary vascular resistances in responses to intravenous injections of Na2S in doses of 0.03–0.5 mg/kg. The decreases in systemic arterial pressure and heart rate in response to all doses of Na2S with the exception of the 2 lowest doses are significantly different from each other. *With bar indicates P < 0.05, ANOVA and paired comparison, Bonferroni correction; n = 9. Pulmonary vascular resistance is input pulmonary vascular resistance because left atrial pressure was not measured.
Fig. 2.
Bar graphs showing dose-related decreases in systemic arterial pressure, heart rate, pulmonary arterial pressure, cardiac output, and systemic and pulmonary vascular resistances in responses to intravenous injections of NaHS in doses of 0.03–0.5 mg/kg. The decreases in systemic arterial pressure and heart rate in response to all doses of Na2S with the exception of the 2 lowest doses are significantly different from each other. *With bar indicates P < 0.05, ANOVA and paired comparison, Bonferroni correction; n = 9. Pulmonary vascular resistance is input pulmonary vascular resistance because left atrial pressure was not measured.
Fig. 3.
Records from an experiment illustrating decreases in systemic arterial pressure, mean arterial pressure, and heart rate in response to an intravenous injection of Na2S (0.5 mg/kg), NaHS (0.5 mg/kg), and sodium nitroprusside (SNP; 10 μg/kg). Bpm, beats/min.
Fig. 7.
A: bar graphs showing decreases in mean arterial pressure and changes in heart rate in response to intravenous injections of Na2S (0.3 mg/kg), NaHS (0.3 mg/kg), and acetylcholine (0.1 μg/kg) before and after treatment with atropine (0.5 mg/kg iv; n = 8–10). B: bar graphs showing secondary increases in arterial pressure and decreases in arterial pressure in response to intravenous injection of Na2S (0.5 mg/kg) and NaHS (0.5 mg/kg) before and after treatment with phentolamine (0.5 mg/kg iv) are also shown. C: bar graph showing increases in arterial pressure in response to norepinephrine (0.1 μg/kg iv) before and after treatment with phentolamine (0.5 mg/kg iv). *P < 0.05, paired comparison; n = 11.
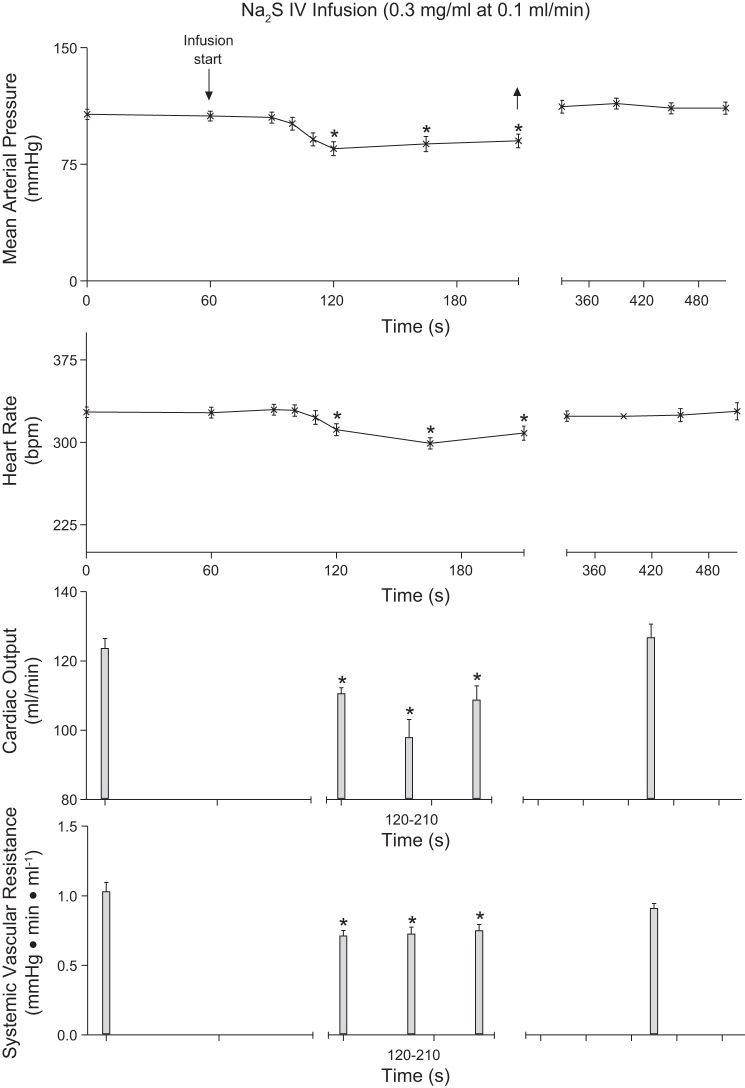
To investigate steady state responses to an H2S donor the effect of an infusion of Na2S was studied, and these data are summarized in Fig. 4. The intravenous infusion of 0.3 mg/min Na2S at a rate of 0.1 ml/min produced a significant and well-maintained decrease in systemic arterial pressure and heart rate (Fig. 4). Cardiac output and systemic vascular resistance was decreased significantly during the infusion period, and arterial pressure, heart rate, cardiac output, and systemic vascular resistance returned to control value shortly after the Na2S infusion was terminated (Fig. 4). The intravenous infusion of Na2S produced a significant decrease in plasma glucose levels from 115 ± 5 mg/dl to 83 ± 13 mg/dl.
Fig. 4.
Line graph illustrating the decreases in mean arterial pressure, and heart rate in response to an intravenous (IV) infusion of Na2S (0.3 mg/min; n = 10). Bar graphs showing decreases in cardiac output and systemic vascular resistance in response to intravenous infusion of Na2S (0.3 mg/min; n = 8) are also shown. *P < 0.05, paired comparison.
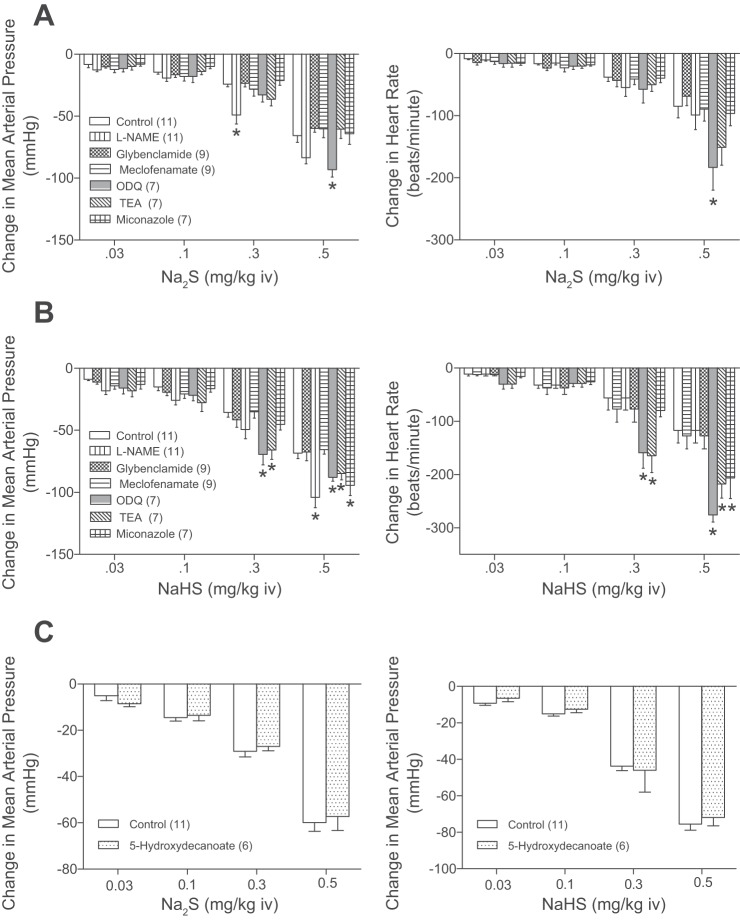
The mechanism by which the H2S donors decrease systemic arterial pressure and heart rate was investigated, and these data are summarized in Fig. 5. The decreases in systemic arterial pressure and heart rate in response to the intravenous injections of Na2S or NaHS were not significantly decreased after administration of 50 mg/kg iv l-NAME, 10 mg/kg iv glybenclamide, 5 mg/kg iv sodium meclofenamate, 2 mg/kg iv ODQ, 50 mg/kg iv miconazole, or 60 mg/kg iv TEA (Fig. 5, A and B). The decreases in systemic arterial pressure and heart rate in response to intravenous injections of Na2S and NaHS were significantly enhanced after administration of l-NAME, ODQ, TEA, and miconazole (Fig. 5, A and B). The intravenous administration of the mitochondrial KATP channel antagonist 5-hydroxydecanoate in a dose of 10 mg/kg had no effect on the decrease in systemic arterial pressure in response to the H2S donors (Fig. 5C). The effect of administration of a larger dose of glybenclamide on the decrease in systemic arterial pressure and heart rate in response to intravenous injection of 0.5 mg/kg Na2S and NaHS was investigated in seven rats and following administration of glybenclamide, in a total dose up to 20–30 mg/kg intravenously and/or intraperitoneally decreases in systemic arterial pressure and heart rate in response to the H2S donors were not attenuated.
Fig. 5.
Bar graphs showing the effect of the nitric oxide synthase (NOS) inhibitor Nw-nitro-l-arginine methyl ester hydrochloride (l-NAME; 50 mg/kg iv), the KATP channel antagonist glybenclamide (10 mg/kg iv), the cyclooxygenase inhibitor sodium meclofenamate (5 mg/kg iv), the soluble guanylate cyclase inhibitor 1H-[1, 2, 4]oxadiazolo[4, 3-a]quinoxalin-1-one (2 mg/kg iv), the Ca2+-activated K+ channel inhibitor tetraethyl ammonium (60 mg/k iv), and the P-450 epoxygenase inhibitor miconazole (50 mg/kg iv) on decreases in mean systemic arterial pressure and heart rate in response to intravenous injections of Na2S (0.03–0.5 mg/kg; A) and intravenous injections of NaHS (0.03–0.5 mg/kg; B). C: bar graphs showing the effect of the mitochondrial K+ATP channel antagonist 5-hydroxydecanoate (10 mg/kg iv) on decreases in mean system arterial pressure in response to intravenous injections of Na2S (0.03–0.5 mg/kg) and NaHS (0.03–0.5 mg/kg). *P < 0.05, paired comparison; n = 6–12 all groups. TEA, tetraethylammonium.
The administration of l-NAME produced a significant well-maintained increase in systemic arterial pressure, whereas glybenclamide, sodium meclofenamate, ODQ, miconazole, 5-hydroxydecanoate, and TEA had small inconsistent effects on systemic arterial pressure over the time course of these experiments.
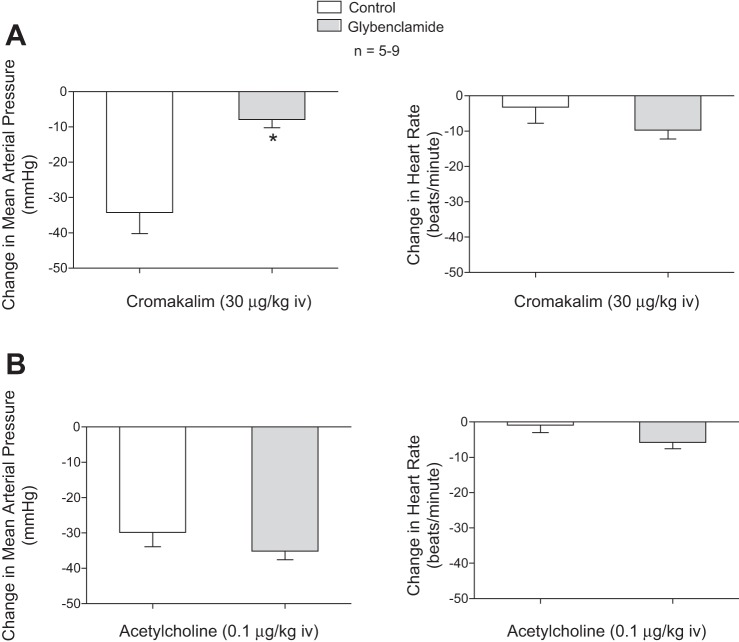
The intravenous injections of 30 μg/kg KATP channel agonist cromakalim decreased systemic arterial pressure, and the decrease in pressure in response to cromakalim was attenuated after administration of 10 mg/kg iv glybenclamide (Fig. 6). The intravenous injection of 30 μg/kg cromakalim did not produce an increase in heart rate. The intravenous administration of glybenclamide produced a significant decrease in blood glucose levels (control, 116 ± 5 mg/dl; after glybenclamide 82 ± 4 mg/dl).
Fig. 6.
Bar graphs showing the effect of the KATP channel antagonist glybenclamide (10 μg/kg iv) on the decreases in mean arterial pressure and the change in heart rate in response to intravenous injection of the KATP channel agonist cromakalim (30 μg/kg; A) and acetylcholine (0.1 μg/kg iv; B). *P < 0.05, paired comparison; n = 6.
DISCUSSION
The results of the present study show that intravenous injections of the H2S donors Na2S and NaHS decrease systemic arterial pressure in the rat and are consistent with previous results in the literature (18, 38, 46). New findings in the present study are that decreases in systemic arterial pressure in response to the H2S donors are associated with dose-dependent decreases in heart rate and at higher doses significant decreases in cardiac output. These results indicate that the hypotensive response to the H2S donors can be mediated by a decrease in systemic vascular resistance and a decrease in cardiac output or both. The decrease in heart rate in response to the H2S donors can exceed 100 beats/min and was not attenuated by atropine, indicating that the bradycardia is not mediated by an increase in parasympathetic tone. In some experiments a larger dose of atropine (2.5 mg/kg iv) had no effect on the decrease in heart rate. These data suggest that the effect of the H2S donors is mediated by an action on the sinus node and are consistent with studies showing that H2S decreases action potential amplitude and has a suppressive effect on electrical activity in the isolated rat sinus node (1, 44). The decreases in cardiac output in response to the H2S donor correlates with the decrease in heart rate and can probably be prevented or attenuated by cardiac pacing.
Decreases in systemic arterial pressure and heart rate in response to intravenous injections of the H2S donors were rapid in onset and short in duration. The decrease in heart rate in response to the H2S donors is different than the response to the NO donor SNP, which does not induce bradycardia and can increase heart rate. The recovery phase of the arterial pressure response to high doses of the H2S donors was associated with a secondary increase in systemic arterial pressure that was attenuated by the α-receptor blocking agent phentolamine, suggesting that the secondary increase in systemic arterial pressure was mediated by the activation of the adrenergic nervous system and may be a component of a reflex compensatory response. The decreases in arterial pressure and heart rate in response to intravenous injection of the H2S donors were not attenuated by treatment with atropine, phentolamine, or hexamethonium, suggesting that they were not dependent on an effect on the central nervous system (Fig. 7).
Because of the short duration of action and to investigate steady state responses to an H2S donor the effect of an intravenous infusion of Na2S was studied. The results of infusion studies show that the decreases in systemic arterial pressure, heart rate, and cardiac output were maintained during the period of the infusion, and these parameters returned to control value several minutes after the infusion was terminated. These studies also show that the H2S donor has metabolic effects in that blood glucose levels were significantly reduced. The mechanism of this hypoglycemic effect is uncertain and will be addressed in future studies.
The effect of intravenous injections of the H2S donors on the pulmonary vascular bed was investigated, and at higher doses pulmonary arterial pressure was decreased significantly. This was associated with a significant decrease in cardiac output. However, the H2S donors did not decrease input pulmonary vascular resistance. The direct effect of the H2S donors on the pulmonary vascular bed requires further study in experiments in which blood flow (cardiac output) is maintained constant and baseline tone in the pulmonary vascular bed is increased in the intact chest rat model.
The results of the present study are consistent with the observation that H2S has hypotensive activity in the rat (50). However, the present data indicate that cardiovascular responses to the H2S donors are complex. The observation that decreases in systemic and pulmonary arterial pressures can be associated with decreases in cardiac output suggests that hypotensive responses may be mediated by decreases in vascular tone, decreases in cardiac output, or a combination of effects on both determinants of systemic arterial pressure.
The mechanism by which H2S decreases systemic arterial pressure in the rat has been investigated, and the results of an early study show that decreases in systemic arterial pressure in response to intravenous injection of an H2S solution are attenuated by glybenclamide, suggesting a role for KATP channels (9, 14, 23, 48–50). The role of KATP channels in mediating hypotensive responses to H2S donors was investigated in the present study and following administration of glybenclamide in a dose that attenuated the decrease in systemic arterial pressure in response to intravenous injection of the KATP agonist cromakalim, the decreases in systemic arterial pressure and heart rate in response to Na2S and NaHS were not attenuated. The administration of glybenclamide decreased blood glucose levels, which were restored in some experiments by an intravenous infusion of glucose, and the KATP channel antagonist decreased hypotensive responses to the KATP channel agonist cromakalim. The hypotensive responses to H2S donors were not attenuated by 5-hydroxydecanoate, which blocks mitochondrial KATP channels. These data obtained in a large number of experiments suggest that the decreases in systemic arterial pressure in response to the H2S donors Na2S and NaHS are not mediated by the activation of KATP channels in the membrane of vascular smooth muscle or endothelial cells or by activation of mitochondrial KATP channels in the cardiovascular system in the intact rat. In an attempt to provide more information about KATP channels, a second or third 10 mg/kg dose of glybenclamide was administered and decreases in systemic arterial pressure and heart rate in response to the H2S donors were not attenuated. The explanation for the different results in the present study and in the previous study is uncertain, and there are studies in the literature indicating that aortic vasorelaxant responses to H2S are independent of KATP channel activation (8, 24).
It has been reported that the NO-cGMP pathway is involved in mediating or modulating vasorelaxant responses to H2S and that H2S is an endothelium-dependent vasodilator agent (10, 12, 31). The role of the NO-cGMP pathway in mediating decreases in systemic arterial pressure in response to the H2S donors was investigated in the rat. The administration of the NOS inhibitor l-NAME that increased systemic arterial pressure did not attenuate decreases in systemic arterial pressure in response to intravenous injection of Na2S and NaHS, suggesting that NO is not involved in mediating the hypotensive response in the rat. The experiments with ODQ, which inhibits the activation of sGC by NO, indicate that increases in sGC activity and cGMP levels are not playing an important role in mediating or modulating hypotensive response to the H2S donors in the rat. The observation that sodium meclofenamate or miconazole did not inhibit responses to Na2S or NaHS suggests that decreases in systemic arterial pressure in the rat are not dependent on the formation of products in the arachidonic acid, cyclooxygenase, or P450 epoxygenase pathways.
It has been reported that H2S-induced relaxation of vas deferens smooth muscle is attenuated by inhibition of large conductance calcium-activated potassium channels, which can be inhibited by TEA, a nonspecific K+ channel antagonist, and are not blocked by NOS inhibitors or by glybenclamide (29). To determine whether there was a role for large conductance calcium-activated potassium channels in mediating decreases in systemic arterial pressure in response to the H2S donors, the effect of TEA was investigated. The results of these studies show that TEA in a dose that attenuated erectile responses to the H2S donors in the rat in previous experiments did not inhibit decreases in systemic arterial pressure in response to intravenous injections of Na2S or NaHS, suggesting that a TEA-sensitive mechanism is not involved in mediating hypotensive responses to the H2S donors in the rat. However, it should be mentioned that TEA is a broad spectrum K+ channel antagonist that has actions on many tissues.
The mechanism by which the H2S donors decrease systemic arterial pressure in the rat is uncertain and subject to conjecture. Although hypotensive responses to the donors are not blocked by l-NAME or ODQ, it has been reported that H2S can be converted to polysulfides that can oxidize protein kinase G (PKG) and induce PKG dimerization, which can induce vasodilation in a NO-cGMP-independent manner (5, 6, 40, 43). However, the role of the PKG dimerization in mediating hypotensive responses to the H2S donors in the present study is uncertain.
The purpose of the present study was to investigate and characterize cardiovascular responses to the H2S donors in the anesthetized intact-chest rat. The results of these studies show that Na2S and NaHS decrease systemic arterial pressure in the rat and are in agreement with the results of an early study (50). The present study extends previous results by showing that decreases in systemic arterial pressure in response to the H2S donors are associated with a dose-dependent decrease in heart rate that is not mediated by the parasympathetic system and at higher doses the H2S donors decrease cardiac output and systemic vascular resistance. Although the mechanism of the hypotensive response to the H2S donors is not explained by the present data, the results of the present study show that decreases in systemic arterial pressure can be mediated by a decrease in systemic vascular resistance; a decrease in cardiac output, which is not central in nature; or a combination of these actions and that they are mediated by peripheral actions of the H2S donors. The observation that blood pressure responses to the H2S donors in the intact chest rat are not attenuated by glybenclamide or l-NAME does not mean that KATP channels or NO is not involved in mediating responses in some local vascular segments or regions. The present data indicate that KATP channels or NO does not play a major role in mediating the overall decreases in systemic arterial pressure in response to intravenous injections of the H2S donors in the rat and are similar to data in eNOS knockout mice where the hypotensive response to acetylcholine is not impaired but that vasodilator responses in some regional vascular segments isolated from eNOS knockout mice are attenuated (16, 25, 33).
The present data also show that H2S donor injections or infusion can produce hypoglycemia, which is not consistent with a stimulatory effect on KATP channels in the pancreatic β-cells, and this action requires further investigation (3, 47).
In summary, the results of the present study show that the H2S donors have the ability to decrease systemic arterial pressure in the rat by a peripheral effect on systemic vascular resistance and on cardiac output. These data indicate that the mechanism of the overall decrease in systemic arterial pressure in the rat is uncertain and that H2S donors decrease heart rate by a mechanism that is independent of the parasympathetic nervous system.
Limitations.
In regard to limitations in the present study, the responses to H2S were investigated using the H2S donors, Na2S and NaHS, and not H2S solutions prepared using H2S gas, which may produce different responses in the anesthetized rat. In addition, the role of KATP channels in mediating responses to H2S may depend on species and the vascular the preparation studied. Although Na2S and NaHS have a short duration of action, these H2S donors have been used as pharmacologic agents in the treatment of cardiac I/R injury (13, 32, 45). The mechanism of the putative vascular effect of the H2S donors promoting vasodilation is not clarified in the present study and needs more direct examination in better controlled experiments.
GRANTS
The present study was supported in part by National Heart, Lung, and Blood Institute Grant HL-77421.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.Y., R.C.J., E.A.P., V.G.R., J.A.E., and P.J.K. conception and design of research; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., K.W.S., T.C.P., and P.J.K. performed experiments; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., K.W.S., T.C.P., R.M., and P.J.K. analyzed data; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., K.W.S., T.C.P., R.M., and P.J.K. interpreted results of experiments; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., R.M., and P.J.K. prepared figures; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., and P.J.K. drafted manuscript; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., K.W.S., T.C.P., R.M., and P.J.K. edited and revised manuscript; D.Y., R.C.J., E.A.P., V.G.R., J.A.E., K.W.S., T.C.P., R.M., and P.J.K. approved final version of manuscript.
REFERENCES
- 1.Abramochkin DV, Moiseenko LS, Kuzmin VS. The effect of hydrogen sulfide on electrical activity of rat atrial myocardium. Bull Exp Biol Med 147: 683–686, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MY, Whiteman M, Low CM, Moore PK. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol 195: 105–112, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Burgoyne JR, Eaton P. Detecting disulfide-bound complexes and the oxidative regulation of cyclic nucleotide-dependent protein kinases by H2O2. Methods Enzymol 528: 111–128, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension 60: 1301–1308, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-Aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol 53: 94–98, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cortese-Krott MM, Fernandez BO, Kelm M, Butler AR, Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 46: 14–24, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Dabisch PA, Liles JT, Baber SR, Golwala NH, Murthy SN, Kadowitz PJ. Analysis of l-NAME-dependent and -resistant responses to acetylcholine in the rat. Am J Physiol Heart Circ Physiol 294: H688–H698, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Edwards G, Feletou M, Weston AH. Hydrogen sulfide as an endothelium-derived hyperpolarizing factor in rodent mesenteric arteries. Circ Res 110: e13–e16, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42: 539–548, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood vessels 28: 52–61, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Garland CJ, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol 105: 429–435, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313: 362–368, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Hyman AL, Hao Q, Tower A, Kadowitz PJ, Champion HC, Gumusel B, Lippton H. Novel catheterization technique for the in vivo measurement of pulmonary vascular responses in rats. Am J Physiol Heart Circ Physiol 274: H1218–H1229, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 65: 1–21, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca2+-activated potassium channels. Circ Res 108: 1439–1447, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury—Evidence for a role of KATP channels. Basic Res Cardiol 101: 53–60, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kiss L, Deitch EA, Szabo C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589–594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koeppen M, Feil R, Siegl D, Feil S, Hofmann F, Pohl U, de Wit C. cGMP-dependent protein kinase mediates NO- but not acetylcholine-induced dilations in resistance vessels in vivo. Hypertension 44: 952–955, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kubo S, Doe I, Kurokawa Y, Kawabata A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J Pharmacol Sci 104: 392–396, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation–a tale of three gases! Pharmacol Ther 123: 386–400, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51: 169–187, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zang Y, Fu S, Zhang H, Gao L, Li J. H2S relaxes vas deferens smooth muscle by modulating the large conductance Ca2+-activated K+ (BKCa) channels via a redox mechanism. J Sex Med 9: 2806–2813, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Murad F, Forstermann U, Nakane M, Schmidt H, Pollock J, Sheng H, Matsumoto T, Warner T, Mitchell J, Tracey R. The nitric oxide-cyclic GMP signal transduction pathway in vascular smooth muscle preparations and other tissues. Jpn J Pharmacol 58, Suppl 2: 150P–157P, 1992. [PubMed] [Google Scholar]
- 31.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan H, Xie X, Chen D, Zhang J, Zhou Y, Yang G. Protective and biogenesis effects of sodium hydrosulfide on brain mitochondria after cardiac arrest and resuscitation. Eur J Pharmacol 741: 74–82, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Pankey EA, Kassan M, Choi SK, Matrougui K, Nossaman BD, Hyman AL, Kadowitz PJ. Vasodilator responses to acetylcholine are not mediated by the activation of soluble guanylate cyclase or TRPV4 channels in the rat. Am J Physiol Heart Circ Physiol 306: H1495–H1506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pankey EA, Thammasiboon S, Lasker GF, Baber S, Lasky JA, Kadowitz PJ. Imatinib attenuates monocrotaline pulmonary hypertension and has potent vasodilator activity in pulmonary and systemic vascular beds in the rat. Am J Physiol Heart Circ Physiol 305: H1288–H1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pankey EA, Zsombok A, Lasker GF, Kadowitz PJ. Analysis of responses to the TRPV4 agonist GSK1016790A in the pulmonary vascular bed of the intact-chest rat. Am J Physiol Heart Circ Physiol 306: H33–H40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter PN, Grishaver MS, Jones OW. Characterization of human cystathionine beta-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine beta-synthase. Biochim Biophys Acta 364: 128–139, 1974. [DOI] [PubMed] [Google Scholar]
- 37.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Srilatha B, Adaikan PG, Li L, Moore PK. Hydrogen sulphide: a novel endogenous gasotransmitter facilitates erectile function. J Sex Med 4: 1304–1311, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: 267–277, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stubbert D, Prysyazhna O, Rudyk O, Scotcher J, Burgoyne JR, Eaton P. Protein kinase g ialpha oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 64: 1344–1351, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Uren JR, Ragin R, Chaykovsky M. Modulation of cysteine metabolism in mice–effects of propargylglycine and L-cysteine-degrading enzymes. Biochem Pharmacol 27: 2807–2814, 1978. [DOI] [PubMed] [Google Scholar]
- 42.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 343: 303–310, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Wolin MS. Novel role for protein kinase g oxidative activation in the vasodilator and antihypertensive actions of hydrogen sulfide. Hypertension 64: 1196–1197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao L, Wu YM, Zhang H, Liu YX, He RR. Hydrogen sulfide facilitates carotid sinus baroreflex in anesthetized rats. Acta Pharmacol Sin 27: 294–298, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Xie YH, Zhang N, Li LF, Zhang QZ, Xie LJ, Jiang H, Li LP, Hao N, Zhang JX. Hydrogen sulfide reduces regional myocardial ischemia injury through protection of mitochondrial function. Mol Med Rep 10: 1907–1914, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol 569: 519–531, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474–H480, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]