Abstract
Left ventricular (LV) hypertrophy is an important physiological compensatory mechanism in response to chronic increase in hemodynamic overload. There are two different forms of LV hypertrophy, one physiological and another pathological. Aerobic exercise induces beneficial physiological LV remodeling. The molecular/cellular mechanisms for this effect are not totally known, and here we review various mechanisms including the role of microRNA (miRNA). Studies in the heart, have identified antihypertrophic miRNA-1, -133, -26, -9, -98, -29, -378, and -145 and prohypertrophic miRNA-143, -103, -130a, -146a, -21, -210, -221, -222, -27a/b, -199a/b, -208, -195, -499, -34a/b/c, -497, -23a, and -15a/b. Four miRNAs are recognized as cardiac-specific: miRNA-1, -133a/b, -208a/b, and -499 and called myomiRs. In our studies we have shown that miRNAs respond to swimming aerobic exercise by 1) decreasing cardiac fibrosis through miRNA-29 increasing and inhibiting collagen, 2) increasing angiogenesis through miRNA-126 by inhibiting negative regulators of the VEGF pathway, and 3) modulating the renin-angiotensin system through the miRNAs-27a/b and -143. Exercise training also increases cardiomyocyte growth and survival by swimming-regulated miRNA-1, -21, -27a/b, -29a/c, -30e, -99b, -100, -124, -126, -133a/b, -143, -144, -145, -208a, and -222 and running-regulated miRNA-1, -26, -27a, -133, -143, -150, and -222, which influence genes associated with the heart remodeling and angiogenesis. We conclude that there is a potential role of these miRNAs in promoting cardioprotective effects on physiological growth.
Keywords: cardiac hypertrophy, angiogenesis, swimming training, running training, microRNA
this article is part of a collection on Exercise Training in Cardiovascular Disease: Cell, Molecular, and Integrative Perspectives. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
exercise training is the most effective nonpharmacological intervention to reduce cardiovascular disease (CVD). Its prescription is recommended by the guidelines of the most important entities, such as the American College of Sport Medicine and the American Heart Association (39).
Exercise training is well known to promote beneficial adaptations in the cardiovascular system which can vary according to type, intensity, and duration of exercise (32). Exercise training induces marked beneficial systemic effects on metabolism control, skeletal muscle, cognitive function, and cardiovascular function (30, 39). Among them, the set of adaptations induced in the myocardium are collectively referred to as “athlete's heart” and includes increased cardiac mass, formations of new blood vessels, and decreased collagen content (15a, 17, 20, 23, 77, 91). Individuals with high levels of physical activity have a lower prevalence and lower death rates from CVD (32, 86). Thus exercise training has been established not only as a way to maintain a healthy lifestyle but also as an important and safe nonpharmacological prescription for prevention and treatment of CVD.
Pathological cardiac hypertrophy is associated with poor prognosis and is a hallmark of heart failure (72, 73, 103). In contrast, exercise training-induced physiological cardiac hypertrophy presents cardioprotective effects and is not related to heart failure (74). Exercise training has been described as being able to counteract structural and functional cardiac changes in CVD by contributing to the phenotypical changes of pathological into physiological cardiac hypertrophy (31, 65, 73, 74).
Despite strong evidence linking exercise training to reduction in CVD risk, much uncertainty remains with regard to the underlying mechanisms. Currently much more attention has been given to cellular and molecular mechanisms in an attempt to distinguish between pathological and physiological cardiac hypertrophy. Distinct intracellular pathways have been recognized in both situations and will be reviewed here in view of their modulation by microRNAs (miRNAs). miRNAs, small noncoding regions of the genome, are a new class of gene regulators, which have been shown to play a key role in a myriad of cellular processes, including growth, fibrosis, apoptosis, angiogenesis, and cardiac function under physiological and pathological conditions.
miRNAs are considered promising therapeutic targets for CVD (4, 15, 71, 74, 85, 90). We have found numerous miRNAs that play specific roles in regulating gene expression by exercise training (15a, 20, 21, 24, 65, 91) and confirmed by Ma et al. (58) and Martinelli et al. (61). The aim of this review is provide an overview of exercise training effects on physiological cardiac remodeling and the involvement of miRNAs in this process.
Cardiac Remodeling Induced by Exercise Training
People engaged in chronic exercise programs have improved cardiovascular function. This is observed not only in healthy subjects but mainly in those with any type of cardiovascular risk factor or disease (6, 39, 40). Even in people over 70 yr old, exercise training can lower systolic, diastolic, and median blood pressure (13). The health benefits of an active lifestyle are multifactorial and include not only biological adaptation but also changes in other social habits, such as decreases in smoking and drinking excessive amounts of alcohol (27).
Exercise training reduces the body mass index by decreasing adipocyte mass, increases insulin sensitivity as well as glucose uptake, increases muscle strength and endurance, increases antioxidant levels, and increases HDL, while decreasing LDL and total triglycerides (43, 48, 95). From the cardiovascular point of view, exercise training reduces both diastolic and systolic blood pressure, increases the left ventricular (LV) ejection fraction, decreases end-diastolic pressure, improves vascular function, and increases cardiac angiogenesis and cardiac muscle mass (33, 60, 75). Among these adaptations, our focus in this review will primarily be on the increase in cardiac muscle mass termed herein as exercise training-induced cardiac hypertrophy.
Cardiac hypertrophy.
Cardiac hypertrophy is an adaptive response of the heart to increased cardiac workload and involves a variety of mechanical, hemodynamic, and hormonal factors (70). Although any increase in heart mass is broadly defined as a hypertrophic response, there are two very different forms of ventricular hypertrophy, one physiological and another pathological (5). At the cellular level both hypertrophic processes implicate in adaptations such as increased cardiomyocyte size, enhanced protein synthesis rate, and reorganizations of the sarcomere structure; however, there are many differences that distinguish them and will be discussed below. First, they should be distinguished by the structural and functional adaptations as being physiological versus pathological remodeling; second, by the stimuli that induce the hypertrophy, which are pressure versus volume overload (19, 59, 96).
Physiological cardiac hypertrophy may be exemplified by the LV remodeling induced by exercise training, or pregnancy or during the postnatal growth of the heart from the birth to adulthood. It is also observed in other species such as in snakes that LV mass can increase even after large meals (2). The LV remodeling induced by physiological stimuli leads to preserved or even enhanced LV function, decreased collagen content, lack of fibrosis, increased angiogenesis, improved myocardial antioxidant capacity (78), and decreased mitochondrial dysfunction (7) and has been shown to prevent cardiomyocyte apoptosis (44).
Oppositely, pathological hypertrophy is associated with severe CVD illness that leads to increased risk of heart failure arrhythmia and ultimately death (41). LV remodeling induced by pathological stress leads to progressive declines in cardiac output, myocardial rarefaction, increased apoptosis, cardiomyocyte metabolism switch from fatty acid to glucose use, and increased fibrosis (35, 49).
Altogether, there are basically two ways to distinguish between physiological and pathological cardiac hypertrophy: pathological LV remodeling is accompanied by LV dysfunction (either diastolic, systolic, or both) (29) and disproportional increase between muscle mass to angiogenesis (88), whereas physiological LV remodeling preserves, or even enhances, ventricular function and there is coordinated growth of both muscle mass and angiogenesis (23, 74).
Different cardiac hypertrophy phenotypes at the molecular level.
At the molecular level, reexpression of fetal genes is used as a biomarker of pathological cardiac hypertrophy. Among them, atrial and brain natriuretic peptide, α-skeletal myosin, and α- to β-myosin heavy chain (MHC) expression ratio have been the most frequently reported (45, 51, 102). Although the best known effects of atrial and brain natriuretic peptide are natriuresis and blood pressure regulation, these small peptides also contribute to preventing cardiac hypertrophy and fibrosis in the adult heart (66). The main release factor of the atrial peptides factor is the wall strain induced by the increased workload, but other mechanisms are still being uncovered (96).
The α and β are subunits of the cardiac MHC filaments. α-MHC has the highest ATPase activity and contractile velocity, whereas the β-MHC has the lowest ATPase activity and contractile velocity (36). A decreased α- to β-MHC expression ratio has been found in pathological cardiac hypertrophy, whereas exercise training prevents this response or even increases α- to β-MHC expression ratio (10, 67).
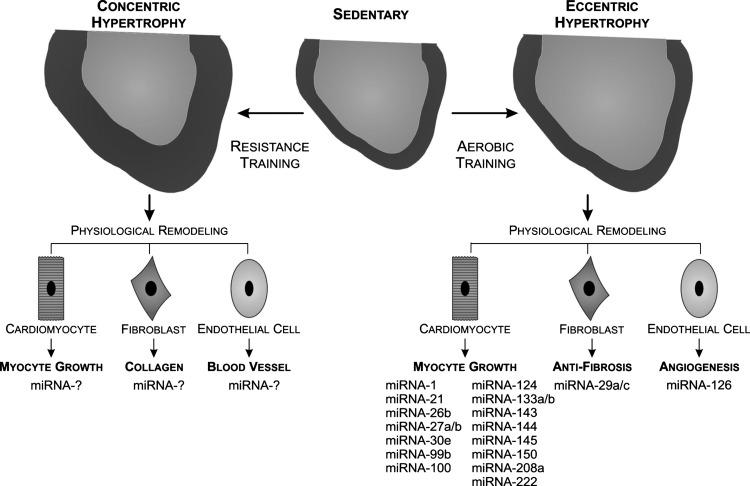
Although cardiac hypertrophy involves a variety of mechanical, hemodynamic, and hormonal factors, the main factor to determine the morphological phenotype of LV remodeling is the hemodynamic cardiac workload. Both pathological and physiological cardiac hypertrophy may be triggered by pressure or volume cardiac overload. These two different stimuli induce distinct morphological adaptations to the heart; in particular, to the LV. The LV remodeling by pressure overload is characterized by concentric hypertrophy, whereas LV remodeling by volume overload induces eccentric hypertrophy (70). At the cellular level, concentric hypertrophy is characterized by parallel addition of new sarcomeres and lateral growth of individual cardiomyocytes. This hypertrophy generally leads to increased LV wall thickness with either decreased LV chamber diameter (pathological) or no change on LV chamber diameter (physiological exercise training induced) (34). The eccentric hypertrophy due to volume overload is characterized by addition of sarcomeres in series and longitudinal cardiomyocyte growth. The phenotype of this remodeling is typically associated with LV dilatation (pathological) or proportional increase in both LV dilatation and LV wall thickness (physiological exercise training induced) (34). Figure 1 exemplifies both pressure and volume-induced physiological cardiac hypertrophy.
Fig. 1.
The schematic physiological cardiac remodeling induced by exercise training. Exercise training is characterized by a uniform profile of myocardium growth without fibrosis and cardiac dysfunction. Aerobic training promotes eccentric hypertrophy with the addition of sarcomeres in series to lengthen the cardiomyocyte and to increase the width of the cell in parallel. In contrast, resistance training promotes concentric hypertrophy with the addition of sarcomeres in parallel to an increase in cross-sectional cardiac area. Mitochondrial RNA (miRNA)-1, -21, -26b, -27a/b, -30e, -99b, -100, -124, -133a/b, -143, -144, -145, -150, -208a, and -222 are involved in cardiomyocyte growth and survival, miRNA-29a/c regulate antifibrosis process, and miRNA-126 modulates angiogenesis in response to aerobic exercise training.
At this time, it should be highlighted that different exercise training protocols predominantly change cardiac workload either by pressure or by volume overload, which leads to different cardiac hypertrophy phenotypes (57, 94) (Fig. 1).
The first observation of cardiac enlargement in trained individuals was by the Swedish clinician Henschen (42) and dates back to the 1890s, but the first description of different types of cardiac hypertrophy among athletes, resulting from different modalities, was found by Mongaroth et al. (69) and came only in 1975. Later, with the development of noninvasive and more powerful devices for cardiovascular studies, the understanding of the athlete's heart phenomenon has progressed meaningfully.
Aerobic exercises such as running or swimming that involve rhythmic contraction of large skeletal muscle mass, performed for extended periods (e.g., 30–60 min), and that are dependent on the supply of oxygen to the active muscles facilitate venous return and increase the end-diastolic volume (volume overload or increased preload) (69).
On the other hand, resistance or strength exercises, such as weightlifting, involve smaller muscle mass, but strength contraction is limited to a few repetitions (generally, <20) until exhaustion and increases systemic vascular resistance (pressure overload or increased afterload) because of isometric contraction with heavier loads. For example, systolic blood pressure higher than 250 mmHg has been found during this type of exercise (59). Finally, it should also be reminded that the magnitude of cardiac hypertrophy is much less in response to the resistance/strength exercises than aerobic exercises (92). Although we and others have used an animal model of resistance training to study cardiovascular adaptations of physiological concentric cardiac hypertrophy (1, 3, 26), data are still scarce with regard to the molecular mechanism involved. In the next section, we will focus on specific intracellular pathways involved in LV remodeling induced by aerobic exercise training.
miRNAs, Cardiac Hypertrophy, and Exercise Training
miRNA: biogenesis and gene regulation.
miRNAs comprise a novel class of endogenous, small (∼22 nucleotides in length), noncoding RNAs that play important regulatory roles in many physiological and pathological processes (71, 74, 90). There are over 2,000 miRNAs known to be encoded in the human genome, and collectively these miRNAs regulate the expression of thousands of protein-coding gene targets at posttranscriptional levels. Thus it is estimated that miRNAs regulate ∼30% of human genes (4, 47, 71, 74, 90). All miRNAs from humans and other species are included in the database miRBase (v21.0, June 2014, http://www.mirbase.org).
The biogenesis of miRNAs is accomplished through sequential enzymatic reactions. miRNAs are initially transcribed by RNA polymerase II in the nucleus to form large primary transcript (pri-miRNA) transcripts and are polyadenylated at its 3′-end and capped at its 5′-extremity (4, 47). The pri-miRNAs harbor a local hairpin structure that is then cropped by a nuclear enzyme Drosha and their cofactor Pasha (also known as DGCR8) into pre-miRNAs (∼70 nucleotides). Together, RanGTP and exportin 5 transport the pre-miRNA into the cytoplasm. Subsequently, the enzyme Dicer removes the terminal loop of the pre-miRNAs to generate the miRNA duplex (∼22 nucleotides). The duplex is loaded into the miRNA-associated multiprotein RNA-induced silencing complex, which includes the Argonaute proteins. One strand of the miRNA is preferentially retained in this complex and becomes the mature miRNA; the opposite strand, known as the passenger strand, is eliminated from the complex (4, 47, 50, 90).
Mature miRNAs can bind most commonly, but not exclusively, to 3′-untranslated regions of messenger RNAs (mRNAs) of protein-coding genes and negatively regulate their expression (4, 47, 71, 74, 90). The posttranscriptional regulation realized by the miRNAs in 3′-untranslated regions is dependent on the degree of complementarity between them and the target mRNA. Because of the fact that they have small sequences and act without the need for complete pairing, a single miRNA can regulate up to 200 mRNAs, and more than one miRNA can regulate a single mRNA (50). Thus miRNAs that bind to target mRNAs with imperfect complementarity repress target gene expression via translational silencing. In contrast, miRNAs that bind to their target mRNAs with perfect complementarily induce mRNA degradation (4, 47, 50, 71, 74, 90).
miRNAs in the heart.
miRNAs are emerging as pivotal modulators of cardiovascular development and disease (74, 90). Although several miRNAs have been described since their discovery in 1993 by Lee et al. (53), the knowledge of the molecular mechanisms involved in numerous biological functions still need to be investigated. The first evidence that miRNA plays a significant role in the development of the cardiovascular system came from a study showing that the deletion of Dicer, an enzyme key for miRNA processing, disrupted embryonic angiogenesis during mouse development (101). Later studies with specific deletion of Dicer in the heart showed misexpression of cardiac contractile proteins and profound sarcomere disarray accompanied by dilated cardiomyopathy, heart failure, and postnatal lethality (11). Thus da Costa Martins et al. (14) showed that conditional Dicer deletion in the postnatal myocardium promoted pathological cardiac remodeling and dysfunction, suggesting the important role of miRNAs in the control of cardiovascular homeostasis.
The first studies of miRNA in cardiac hypertrophy used microarray platforms to analyze the cardiac miRNA expression signature after pathological stimuli (thoracic aortic-banded mouse model and calcineurin-overexpressing transgenic mice) and indicated that miRNAs are aberrantly expressed in hypertrophic mouse hearts (12, 15, 85, 95b). Over the last decade, miRNA expression profile under either experimental or clinical conditions of cardiac hypertrophy has been revealed, showing miRNA downstream genes with hypertrophic targets. Studies have identified antihypertrophic miRNAs (miRNA-1, -133, -26, -9, -98, -29, -378, and -145) and prohypertrophic miRNAs (miRNA-143, -103, -130a, -146a, -21, -210, -221, -222, -27a/b, -199a/b, -208, -195, -499, -34a/b/c, -497, -23a, and -15a/b) in the heart (15, 18, 71, 74). Antagomir and miR-mimic approaches, knockout mice, adenoviral vector, pharmacologic inhibitors (2′-O-methyl-modified antisense oligonucleotides and locked nucleic acid), and transgenic mice regulating miRNA expression under control of the cardiac MHC-6 promoter have been used to silence or stimulate miRNAs anti- or prohypertrophic, in vitro and in vivo studies (14, 17, 68, 71). Abnormal miRNA regulation has been shown to be involved in CVD, suggesting that miRNAs might affect cardiac structure and function (15, 71, 74, 90).
Among the miRNAs described, four are recognized as cardiac-specific: miRNA-1, -133a/b, -208a/b, and -499, called myomiRs. Sayed et al. (85) showed that cardiac miRNA-1 is downregulated in hypertrophic hearts by transverse aortic constriction, and it is involved in post-mitotic muscle growth and function through a serum response factor-dependent mechanism. This downregulation is required for the release of its growth-related targets, including RasGTPase-activating protein, cyclin-dependent kinase 9, fibronectin, and Ras homolog enriched in brain. Similarly, Carè et al. (8) showed that miRNA-133 is also downregulated in hypertrophic hearts induced by transverse aortic constriction, which represses family members of the Rho kinase, Ras homolog gene family-A, and cell division control protein 42, as well as negative elongation factor complex member A, a negative regulator of RNA polymerase II. Studies have shown that Rho kinase inhibition improves LV geometry and reduces collagen deposition accompanied by improved diastolic function in transverse aortic constriction-induced cardiac hypertrophy (76). On the other hand, Van Rooij et al. (95c) showed that overexpression of miRNA-208a is required for cardiomyocyte hypertrophy, fibrosis, and expression of β-MHC in response to stress and hypothyroidism. miRNA-208 targets purine-rich element-binding protein B (Purβ), heterochromatin protein 1 (HP-1β), and transcription factors Sox6 and Sp3 related to MHC gene switching mainly by stimulating β-MHC expression (95a). In addition, overexpression of miRNA-499 also elicits cardiac hypertrophy resulting in cardiac systolic dysfunction (87). Therefore, new target genes and signaling pathways have been described to regulate cardiac hypertrophy via myomiRs (15, 71, 74). Although miRNA studies predominate in the field of cardiovascular disorders, little is known about their expression patterns or role in physiological conditions, especially exercise-regulated miRNAs.
In the same way as miRNAs, long noncoding RNAs (lncRNAs) are part of the noncoding RNAs interacting with the major pathways of cell growth, proliferation, differentiation, and survival. Recently discovered, lncRNAs have been described as regulating gene expression and may act as miRNA sponges to reduce miRNA levels (38, 97, 100). The number of noncoding RNAs encoded within the human genome is unknown; however, recent transcriptomic and bioinformatic studies suggest that there are thousands of them (37). Notably, Wang et al. (97) demonstrated that lncRNA, AK048451, which the authors called of CHRF, sequesters the miRNA-489, preventing the miRNA from acting on its target gene myeloid differentiation primary response gene 88. The authors described this as resulting from induced pathological cardiac hypertrophy in response to angiotensin II treatment. Thus, for the first time, the authors showed the participation of the lncRNA-miRNA-mRNA axis in the cardiac hypertrophy, revealing a promising area of cardiovascular research that may contribute to the understanding of physiological cardiac hypertrophy induced by exercise training.
Exercise training-regulated cardiac miRNAs.
The miRNAs are essential in different cell processes involved in the regulation of cardiovascular phenotypes, such as cardiomyocyte growth, remodeling, and vascularization (15, 25, 90). miRNAs have also been described as participating in the beneficial adaptations promoted by exercise training, mainly physiological cardiac hypertrophy (8, 15a, 20, 24, 58, 61, 91) (Fig. 1). Interestingly, in Table 1, we identified miRNAs and target genes involved in physiological cardiac remodeling induced by aerobic exercise training, both swimming and running exercises. Despite their importance, few studies have been conducted based on this concept. Carè et al. (8) conducted the first study showing the effects of high-intensity interval training (treadmill) on miRNA expression in cardiac hypertrophy. The authors showed that miRNA-1 and -133 expression were reduced in both physiological cardiac hypertrophy induced by interval training and by cardiac-specific Akt transgenic mice. We also observed that miRNA-1 and -133a/b are similarly downregulated in the eccentric cardiac hypertrophy induced by two different swimming training protocols when compared with the sedentary group (91). Irrespective of the exercise (running or swimming) and volume training (moderate and high), the expression profiles of these myomiRs were similar among the studies (8, 91). Intriguingly, as described above, these miRNAs were also reduced in pathological cardiac hypertrophy (8).
Table 1.
Effects of aerobic exercise training on miRNA expression in physiological cardiac remodeling
| Exercise Training | miRNA | Target Gene | Outcome | Reference |
|---|---|---|---|---|
| Running (interval exercise) |
↓miRNA-1 ↓miRNA-133a |
IGF-1, IGF-1R RhoA, Cdc42, NelfA |
Hypertrophy | 8, 18 |
| Swimming (continuous exercise) |
↓miRNA-1 ↓miRNA-133a/b |
— RhoA, Cdc42, NelfA |
Hypertrophy | 23, 91 |
| Swimming (continuous exercise) |
↑miRNA-29a/c | Collagen I/III | Fibrosis | 65, 91 |
| Swimmimg (continuous exercise) |
↓miRNA-208a | Purβ | Hypertrophy | 24 |
| Swimming (continuous exercise) |
↑miRNA-126 | Spred-1, PI3KR2 | Angiogenesis | 89 |
| Swimming (continuous exercise) |
↑miRNA-27a/b ↓miRNA-143 |
ECA ECA2 |
Hypertrophy | 20 |
| Wheel running (voluntary exercise) |
↓miRNA-27a ↓miRNA-143 ↓miRNA-26b ↑miRNA-150 |
GATA4* — IGF-1,* PI3K* GS3K-β,* C-MYB* |
Hypertrophy | 61 |
| Swimming (continuous exercise) |
↑miRNA-21, -144 ↑miRNA-145 ↓miRNA-124 |
PTEN TSC2 PI3K (p110α) |
Hypertrophy | 58 |
| Swimming (continuous exercise) |
↑miRNA-30e ↑miRNA-133b ↑miRNA-208a ↓miRNA-99b ↓miRNA-100 |
Bcl-2 — — IGF-1R, Akt, mTOR IGF-1R, Akt, mTOR |
Hypertrophy | 80 |
| Swimming and wheel running (continuous exercise) | ↑miRNA-222 | p27 HIPK1 HMBOX1 |
Hypertrophy | 56 |
Predicted target gene. IGF-1, insulin-like growth factor 1; IGF-1R, insulin-like growth factor-1 receptor; GATA4; GATA binding protein 4; C-MYB, cellular homolog of MYB avian myeloblastosis oncogene homolog; GS3K-β, glycogen synthase kinase-3β; PTEN, phosphatase and tensin homolog; TSC2, tuberous sclerosis complex 2; ECA and ECA2, angiotensin-converting enzymes 1 and 2; Spred-1, sprouty-related protein 1; PI3KR2, phosphoinositol-3 kinase (PI3K) regulatory subunit 2; Purβ, purine-rich element binding protein B; RhoA, Ras homologue gene family-A; Cdc42, cell division control protein 42; NelfA, negative elongation factor complex member A; Bcl-2, B-cell lymphoma 2; Akt, protein kinase B; mTOR, mammalian target of rapamycin; p27, cell-cycle inhibitor; HIPK1, protein kinase; HMBOX1, transcriptional repressor.
We also evaluated the expression of the myomiR-208a in the heart of the animals subjected to the same two swimming exercise training protocols. Unlike that found in pathological hypertrophy (95a), the data showed a reduction in cardiac miRNA-208a in the group with higher volume of exercise, parallel to an increase in the target gene Purβ compared with the sedentary group. Increased levels of Purβ inhibited β-MHC expression accompanied by increased α-MHC and improved ventricular compliance (24). Interestingly, higher levels of circulating miRNA-208a and -499 have been used as systemic biomarkers of cardiac damage in patients with CVD. In contrast, miRNA-208b and -499 levels were not changed after 24 h of a marathon run, whereas the miRNA-1, -133a, and -206 were correlated to performance parameters (maximum oxygen consumption and running speed) indicating their potential role as biomarkers of aerobic capacity (68).
In an attempt to explain the differences between pathological and physiological cardiac hypertrophy based on miRNA signatures, Lin et al. (55) identified miRNAs differentially expressed in physiological cardiac hypertrophy using transgenic mice with elevated cardiac phosphotidyinositol 3-kinase (PI3K) activity, compared with pathological hypertrophy with decreased PI3K activity and myocardial infarction. Although it was not the effect of exercise training, the authors were the first to detail a signature of miRNAs in physiological cardiac hypertrophy, demonstrating a potential role of these miRNAs in promoting cardioprotective effects on physiological growth. PI3Ks catalyze the phosphorylation of membrane lipids, known as the phosphoinositides, and thus activate a series of intracellular signaling molecules such as Akt1, which is a major downstream effector of PI3K. Akt1 is phosphorylated in physiological cardiac hypertrophy and exerts diverse beneficial functions such as inhibition of cardiomyocyte apoptosis, improvement in calcium transients, and cardiac hypertrophy (46, 62). A series of studies led by the McMullen's group (63, 64, 98) has shown the role of insulin-like growth factor 1 (IGF-1)/IGF-1 receptor (IGF-1R)/PI3K (110α) in the development of physiological cardiac hypertrophy.
IGF-1/IGF-1R intracellular signaling has been the most-studied pathway responsible for physiological cardiac hypertrophy. High-circulating levels of this factor are encountered both during postnatal development and in response to exercise training. IGF-1 is mainly produced by the liver but also by cardiomyocytes in response to exercise (28). IGF-1R is a tyrosine kinase receptor that, upon IGF-1 binding, activates the PI3K-Akt cascade. Mice with constitutively active PI3K (110α) or overexpression of IGF-1 show increased heart weight and are protected from ischemic injury and heart failure (54, 55).
Our group was the first to identify miRNAs based on miRNAs signature in cardiac hypertrophy induced by aerobic exercise training (15a, 20, 91). Soci et al. (91) showed that the expression of miRNA-29c, which targets the collagen gene, increased in parallel with cardiac hypertrophy induced by both swimming exercise training protocols (moderate and high exercise volume) correlated with a decrease in collagen I and III expression and hydroxyproline concentration relevant to the improved LV compliance and function. Thus miRNA-29 reduces collagen fibrosis in the physiologically hypertrophic heart. On the other hand, low levels of miRNA-29 were previously associated with fibrosis in myocardial infarction (95d). Recently, Melo et al. (65) showed that swimming training restored cardiac miRNA-29a and -29c levels and prevented collagen type I and III expression on the border and in the remote regions of the myocardial infarction, suggesting the cardiac effect of exercise training in myocardial-infarcted rats as a way to prevent or minimize the harmful effects in CVD.
Similarly, Liu et al. (56) identified miRNAs signature in physiological cardiac hypertrophy induced by two different types of aerobic training: voluntary wheel running and ramp swimming model. Interestingly, the authors showed that 55 miRNAs were differentially expressed by swimming, whereas 124 were differentially expressed by voluntary wheel running and 16 were similarly regulated by two types of aerobic training. miRNA-222 was chosen by to be upregulated in both models of physiological cardiac hypertrophic targeting p27 (cell-cycle inhibitor), homeodomain-interacting protein kinase 1 (protein kinase), and homeobox-containing protein 1 (transcriptional repressor) genes involved in proliferation and hypertrophy of cardiomyocytes. Curiously, the authors were the first to perform a functional study showing that the inhibition of miRNA-222 in vivo blocks cardiac hypertrophy induced by swimming. Moreover, miRNA-222 can be suggested as a potential therapeutic target against pathological cardiac remodeling since it overexpression largely protected the heart from damage caused by ischemic injury.
Angiogenesis, the growth of new blood vessels from existing vessels, is an important aspect of LV remodeling. In physiological LV remodeling, there is coordinated growth of both muscle mass and angiogenesis that is an important adaptation to enhance capacity and reserve to deliver oxygen to the myocardium (52). Da Silva et al. (15a) investigated the role of the miRNA-126 on cardiac angiogenesis induced by swimming training. Exercise training promoted an increase in the expression of miRNA-126 and repression of their target genes Spred-1 and PI3K regulatory subunit 2 related to vessel growth. Sprouty-related, EVH1 domain-containing protein 1 and PI3K regulatory subunit 2 are negative regulators of the VEGF pathway by inhibiting Raf-1/ERK 1/2 and PI3K/Akt/endothelial nitric oxide synthase (eNOS) pathways, respectively (25). Interestingly, our study revealed some of the molecular mechanisms involved in physiological cardiac remodeling in response to exercise training. VEGF is considered the most potent angiogenic factor and interacts with two specific receptors: fms-like tyrosine kinase (Flt-1 or VEGFR1) and fetal liver kinase (Flk-1 or VEGFR2). Once activated, these receptors result in a series of intracellular signaling pathways that lead to both angiogenic and vasodilator responses. One of the first effects of intracellular activation by VEGF discovered was an increase in eNOS activity and expression. VEGF mediates the activation of ezrin/calpain/PI3K/Akt cascade, which leads to eNOS Ser1179 phosphorylation and Ca2+-independent nitric oxide generation (16). VEGF can also activate AMPK activity through the Ca2+/calmodulin pathway, which, in turn, also activates eNOS to induce angiogenesis and vasodilation (93). There are no doubts about the role of VEGF as an important mediator of the exercise training-induced angiogenic response, as has been reviewed elsewhere (22, 79).
Fernandes et al. (20) showed increase of miRNA-27a and -27b in cardiac hypertrophy induced by swimming training targeting angiotensin-converting enzyme (ACE) in normotensive rats. Inactivation of the classic renin-angiotensin system (RAS) by exercise training contributed to a physiological cardiac hypertrophy by reducing the levels of ACE-ANG II axis. In contrast, we observed a decrease of miRNA-143 targeting ACE2 in the heart of rats. Activation of nonclassic RAS by exercise training counteracted the classic cardiac RAS by stimulating ACE2-ANG-(1–7) axis in physiological cardiac hypertrophy. Recently, Martinelli et al. (61) determined the profile of miRNAs in voluntary exercise (wheel running)-induced LV hypertrophy. In agreement with Fernandes et al. (20), the authors also observed a reduction in the expression of miRNA-143 with only 7 days of exercise training, but no change in expression after 35 days of training. On the other hand, the authors detected a reduction in miRNA-27a levels at 7 days of exercise training and no change in expression after 35 days of training. The different results in the studies could mainly be due to the type, intensity, and duration of exercise training used. Thus exercise training of greater intensity and long duration has been shown to promote alterations in the expression of miRNA-27a/b and -143 after periods of chronic exercise involved in the physiological cardiac remodeling.
Martinelli et al. (61) also observed a reduction in miRNA-26a expression after 7 days and increase in miRNA-150 expression after 35 days of voluntary wheel running exercise. The predicted target genes of miRNA-26b and -150 may be involved in physiological cardiac hypertrophy induced by exercise training, since they are related to survival pathways, such as IGF-1/PI3K signaling and glycogen synthase kinase-3β, respectively. Interestingly, Ma et al. (58) identified miRNAs that target the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway in swimming training-induced cardiac hypertrophy. The authors observed that exercise training increased cardiac miRNA-21 and -144 expression associated with a reduction in their target gene phosphatase and tensin homolog (negative regulator of the PI3K/Akt/mTOR pathway). In addition, an increase in miRNA-145 was accompanied by a decrease in tuberous sclerosis complex 2 after swimming training (another negative regulator of the PI3K/Akt/mTOR pathway). In contrast, exercise training decreased cardiac miRNA-124 expression associated with an increase in their target gene PI3K (p110-α) involved in physiological hypertrophy. Recently, Ramasamy et al. (80) also performed microarray miRNA in swimming training-induced cardiac hypertrophy, indicating that miRNA-30e, -133b, and -208 were significantly upregulated and miRNA-99b and -100 were significantly downregulated after real-time PCR confirmation in healthy rats. Target genes that regulate proliferation and cell death were showed, suggesting that PI3/Akt/mTOR, MAPK, and p53 signaling are involved in physiological cardiac growth.
Altogether, these data suggest that exercise training, both swimming and running, can promote physiological cardiac remodeling through regulation of specific target genes by miRNAs. These exercise training-induced adaptations might provide the additional aerobic performance required by the exercised heart.
Conclusion
Exercise training has been widely recommended as a safe and well-accepted nonpharmacological strategy to prevent CVD and even restore cardiac function. We believe that by understanding the molecular pathways behind the physiological cardiac adaptation induced by exercise training, we may find new therapeutical targets to treat CVD. Among these new targets, modulation of miRNAs seems to be a powerful therapy to reach this goal. A potential therapeutic advantage of miRNAs is that they target multiple genes involved in the same pathway process which is different compared with traditional therapies that target a single protein or gene. Furthermore, miRNA have defined target with high specificity of treatment, long-duration effect, and bioavailability, indicating miRNA therapy as a more effective strategy. On the other hand, delivery, tissue selectivity, and safety are important challenges of miRNA-based therapy to be overcome in the next years (9).
Surprisingly, little is known about the regulatory interaction networks among the multiple classes of RNAs or the mechanisms regulated by exercise-induced miRNAs on physiological cardiac remodeling. The analysis of miRNAs has made it possible to understand the development of various types of diseases. The elucidation of these processes regulated by miRNAs and identification of new target genes in the pathogenesis of CVD are very valuable strategies for prevention and treatment of the CVD. As reviewed here, very little is known about the mechanisms regulated by exercise-induced miRNAs, both by swimming and running, on physiological cardiac remodeling.
Based on our findings and reports by other investigators, the data indicate that different phenotypical changes observed in response to both swimming and running exercise training can be regulated by miRNAs and their target genes (Table 1 and Fig. 1). A set of specific miRNAs contributes to the physiological cardiac remodeling induced by aerobic exercise training (swimming regulated: miRNA-1, -21, -27a/b, -29a/c, -30e, -99b, -100, -124, -126, -133a/b, -143, -144, -145, -208a, and -222; and running-regulated: miRNA-1, -26, -27a, -133, -143, -150, and -222), suggesting a potential role of these miRNAs in promoting cardioprotective effects on physiological growth (Fig. 1). Therefore, exercise-induced miRNAs, which could be measured circulating in blood, could serve as predictors of aerobic capacity required by the hypertrophic heart. Other types of exercise training (i.e., resistance, interval and concurrent)-induced physiological cardiac hypertrophy can also be promoted under the regulation of specific miRNAs, which may also be important for the development of new therapies for CVD. Further studies are still required to evaluate cardiac miRNA signatures under pathological and physiological conditions. In addition, the functional role of miRNAs in the physiological cardiac remodeling induced by exercise training could increase our understanding of cardiac remodeling mechanisms.
GRANTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo Grant FAPESP-2010/50048-1, as well as Grant FAPESP/SPRINT 2014/50673-4 (to E. M. Oliveira and M. I. Phillips); Conselho Nacional de Desenvolvimento Científico e Tecnológico Grants CNPq-308267/2013-3, 476515/2012-2, and 485873/2012-5 and USP/PRP-NAPmiR and Pro-Infra; and National Heart, Lung, and Blood Institute Grant R01-HL077602 (to M. I. Phillips).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.F., V.G.B., and E.M.O. conception and design of research; T.F. prepared figures; T.F., V.G.B., M.I.P., and E.M.O. drafted manuscript; T.F., V.G.B., C.E.N., M.I.P., and E.M.O. approved final version of manuscript; C.E.N., M.I.P., and E.M.O. edited and revised manuscript.
REFERENCES
- 1.Alves JP, Nunes RB, Stefani GP, Dal Lago P. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure. PLoS One 9: e110317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen JB, Rourke BC, Caiozzo VJ, Bennett AF, Hicks JW. Physiology: postprandial cardiac hypertrophy in pythons. Nature 434: 37–38, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Barauna VG, Magalhaes FC, Krieger JE, Oliveira EM. AT1 receptor participates in the cardiac hypertrophy induced by resistance training in rats. Am J Physiol Regul Integr Comp Physiol 295: R381–R387, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128: 191–227, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 88: 774–787, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Campos JC, Gomes KM, Ferreira JCB. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem Toxicol 62: 107–19, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MVG, Høydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Caroli A, Cardillo MT, Galea R, Biasucci LM. Potential therapeutic role of microRNAs in ischemic heart disease. J Cardiol 61: 315–320, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Chain H, Myocardium FV, Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR. Changes in Gene Expression in the Intact Human Heart. J Clin Invest 100: 2315–2324, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA 105: 2111–2116, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol 170: 1831–1840, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cononie CC, Graves JE, Pollock ML, Phillips MI, Sumners C, Hagberg JM. Effect of exercise training on blood pressure in 70- to 79-yr-old men and women. Med Sci Sports Exerc 23: 505–511, 1991. [PubMed] [Google Scholar]
- 14.Da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, Van Oort RJ, Pinto YM, Molkentin JD, De Windt LJ. Conditional Dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 118: 1567–1576, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Da Costa Martins PA, De Windt LJ. MicroRNAs in control of cardiac hypertrophy. Cardiovasc Res 93: 563–572, 2012. [DOI] [PubMed] [Google Scholar]
- 15a.Da Silva ND, Fernandes T, Soci UP, Monteiro AW, Phillips MI, De Oliveira EM. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc 44: 1453–1462, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Dorn GW. The fuzzy logic of physiological cardiac hypertrophy. Hypertension 49: 962–970, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D, Condorelli G. Reciprocal regulation of microrna-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120: 2377–2385, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart 98: 5–10, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes T, Hashimoto NY, Magalhães FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin II, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1–7). Hypertension 58: 182–189, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes T, Magalhães FC, Roque FR, Phillips MI, Oliveira EM. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertension 59: 513–520, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes T, Nakamuta JS, Magalhães FC, Roque FR, Lavini-Ramos C, Schettert IT, Coelho V, Krieger JE, Oliveira EM. Exercise training restores the endothelial progenitor cells number and function in hypertension. J Hypertens 30: 2133–2143, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes T, Soci UP, Oliveira EM. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz J Med Biol Res 44: 836–847, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes T, Soci UP, Oliveira EM. MiRNA-208a targeting Purβ gene regulates the β-MHC content in cardiac hypertrophy induced by exercise training. Circ Res 128: A21942, 2013. [Google Scholar]
- 25.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DYR, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15: 272–284, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontes MT, Silva TL, Mota MM, Barreto AS, Rossoni LV, Santos MR. Resistance exercise acutely enhances mesenteric artery insulin-induced relaxation in healthy rats. Life Sci 94: 24–29, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Franklin BA, Cushman M. Recent advances in preventive cardiology and lifestyle medicine: a themed series. Circulation 123: 2274–2283, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Frystyk J. Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc 42: 58–66, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 58: 1733–40, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334–1359, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Garciarena CD, Pinilla OA, Nolly MB, Laguens RP, Escudero EM, Cingolani HE, Ennis IL. Endurance training in the spontaneously hypertensive rat conversion of pathological into physiological cardiac hypertrophy. Hypertension 53: 708–714, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Golbidi S, Laher I. Exercise and the cardiovascular system. Cardiol Res Pract 2012: 210852, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44: 126–131, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56: 56–64, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122: 23–35, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson TA, Bahl JJ, Markham BE, Roeske WR, Morkin E. Hormonal regulation of myosin heavy chain and alpha-actin gene expression in cultured fetal rat heart myocytes. J Biol Chem 262: 13316–13322, 1987. [PubMed] [Google Scholar]
- 37.Hangauer MJ, Vaughn IW, McManus MT. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet 9: e1003569, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 495: 384–388, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1081–1093, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol 49: 2329–2336, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human: heart structural deterioration and compensatory mechanisms. Circulation 107: 984–991, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Henschen S. Skidlauf und Skidwettlauf. Eine medizinische Sportstudie. Mitt Med Klin Upsala 2: 15, 1899. [Google Scholar]
- 43.Huang CJ, Webb HE, Zourdos MC, Acevedo EO. Cardiovascular reactivity, stress, and physical activity. Front Physiol 4: 314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang CY, Yang AL, Lin YM, Wu FN, Lin JA, Chan YS, Tsai FJ, Tsai CH, Kuo CH, Lee SD. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J Appl Physiol 112: 883–891, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA 85: 339–43, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol 303: H1001–H1010, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Kolka C. Treating diabetes with exercise—focus on the microvasculature. J Diabetes Metab 4: 308, 2013. [PMC free article] [PubMed] [Google Scholar]
- 49.Kolwicz SC, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113: 603–616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet 37: 495–500, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Kuster DW, Merkus D, Blonden LA, Kremer A, van IJcken WF, Verhoeven AJ, Duncker DJ. Gene reprogramming in exercise-induced cardiac hypertrophy in swine: a transcriptional genomics approach. J Mol Cell Cardiol 77: 168–174, 2014. [DOI] [PubMed] [Google Scholar]
- 52.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 302: H10–H23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res 84: 1007–1019, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. PI3K (p110α) protects against myocardial infarction-induced heart failure: Identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol 30: 724–732, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21: 584–595, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longhurst JC, Stebbins CL. The power athlete. Cardiol Clin 15: 413–29, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Ma Z, Qi J, Meng S, Wen B, Zhang J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur J Appl Physiol 113: 2473–2486, 2013. [DOI] [PubMed] [Google Scholar]
- 59.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol 58: 785–790, 1985. [DOI] [PubMed] [Google Scholar]
- 60.Mann N, Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation 126: 2625–2635, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinelli NC, Cohen CR, Santos KG, Castro MA, Biolo A, Frick L, Silvello D, Lopes A, Schneider S, Andrades ME, Clausell N, Matte U, Rohde LE. An analysis of the global expression of microRNAs in an experimental model of physiological left ventricular hypertrophy. PLoS One 9: e93271, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle 2: 220–223, 2003. [PubMed] [Google Scholar]
- 63.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase (p110alpha) pathway. J Biol Chem 279: 4782–4793, 2004. [DOI] [PubMed] [Google Scholar]
- 64.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase (p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100: 12355–12360, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melo SF, Fernandes T, Baraúna VG, Matos KC, Santos AA, Tucci PJ, Oliveira EM. Expression of microRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cell Physiol Biochem 33: 657–669, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Mitsui T. Notes on the peroxidase activity of human basophil leukocytes. Tokai J Exp Clin Med 13: 1–8, 1988. [PubMed] [Google Scholar]
- 67.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86: 386–390, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Mooren FC, Viereck J, Kruger K, Thum T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am J Physiol Heart Circ Physiol 306: H557–H563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med 82: 521–524, 1975. [DOI] [PubMed] [Google Scholar]
- 70.Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens 29: 1–6, 2015. [DOI] [PubMed] [Google Scholar]
- 71.Neves Das VJ, Fernandes T, Roque FR, Soci UP, Melo SF, de Oliveira EM. Exercise training in hypertension: role of microRNAs. World J Cardiol 6: 713–727, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveira EM, Krieger JE. Chronic β-adrenoceptor stimulation and cardiac hypertrophy with no induction of circulating renin. Eur J Pharmacol 520: 135–141, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira RS, Ferreira JC, Gomes ER, Paixão NA, Rolim NP, Medeiros A, Guatimosim S, Brum PC. Cardiac anti-remodelling effect of aerobic training is associated with a reduction in the calcineurin/NFAT signalling pathway in heart failure mice. J Physiol 587: 3899–3910, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ooi JY, Bernardo BC, McMullen JR. The therapeutic potential of miRNAs regulated in settings of physiological cardiac hypertrophy. Future Med Chem 6: 205–222, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens 7: 494–506, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Phrommintikul A, Tran L, Kompa A, Wang B, Adrahtas A, Cantwell D, Kelly DJ, Krum H. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am J Physiol Heart Circ Physiol 294: H1804–H1814, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation 101: 336–344, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Powers SK, Sollanek KJ, Wiggs MP, Demirel HA, Smuder AJ. Exercise-induced improvements in myocardial antioxidant capacity: the antioxidant players and cardioprotection. Free Radic Res 48: 43–51, 2014. [DOI] [PubMed] [Google Scholar]
- 79.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol 97: 1119–1128, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Ramasamy S, Velmurugan G, Shanmugha Rajan K, Ramprasath T, Kalpana K. MiRNAs with apoptosis regulating potential are differentially expressed in chronic exercise-induced physiologically hypertrophied hearts. PLoS One 10: e0121401, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 100: 416–424, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation 99: 963–972, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Shieh JT, Huang Y, Gilmore J, Srivastava D. Elevated miR-499 levels blunt the cardiac stress response. PLoS One 6: e19481, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics 43: 665–673, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol 589: 5443–5452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, Heller R. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem 285: 10638–10652, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Starnes JW, Taylor RP. Exercise-induced cardioprotection: endogenous mechanisms. Med Sci Sports Exerc 39: 1537–1543, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 56: 441–447, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95a.Van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95b.Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255–18260, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95c.Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007. [DOI] [PubMed] [Google Scholar]
- 95d.Van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105: 13027–13032, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volpe M. Natriuretic peptides and cardio-renal disease. Int J Cardiol 176: 630–639, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114: 1377–1388, 2014. [DOI] [PubMed] [Google Scholar]
- 98.Weeks KL, McMullen JR. The athlete's heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology (Bethesda) 26: 97–105, 2011. [DOI] [PubMed] [Google Scholar]
- 100.Wu C, Arora P. Long noncoding RNA-MicroRNA-mRNA: a novel tripartite axis in the regulation of cardiac hypertrophy. Circ Cardiovasc Genet 7: 729–731, 2014. [DOI] [PubMed] [Google Scholar]
- 101.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 280: 9330–9335, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Yap Bin L, Mukerjee D, Timms PM, Ashrafian H, Coghlan JG. Natriuretic peptides, respiratory disease, and the right heart. Chest 126: 1330–1336, 2004. [DOI] [PubMed] [Google Scholar]
- 103.Zile MR, Gaasch WH, Patel K, Aban IB Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail 2: 512–522, 2014. [DOI] [PubMed] [Google Scholar]