We describe the first systematic evaluation of the relationship between perinatal hypoxia and excessive erythrocytosis in later life. Our findings suggest that adverse perinatal oxygenation raises the risk of excessive erythrocytosis accompanied by a modest elevation of systolic pulmonary artery pressure that is independent of increased blood viscosity.
Keywords: polycythemia, altitude, excessive erythrocytosis, developmental programming, hypoxia
Abstract
Perinatal exposures exert a profound influence on physiological function, including developmental processes vital for efficient pulmonary gas transfer throughout the lifespan. We extend the concept of developmental programming to chronic mountain sickness (CMS), a debilitating syndrome marked by polycythemia, ventilatory impairment, and pulmonary hypertension that affects ∼10% of male high-altitude residents. We hypothesized that adverse perinatal oxygenation caused abnormalities of ventilatory and/or pulmonary vascular function that increased susceptibility to CMS in adulthood. Subjects were 67 male high-altitude (3,600–4,100 m) residents aged 18–25 yr with excessive erythrocytosis (EE, Hb concentration ≥18.3 g/dl), a preclinical form of CMS, and 66 controls identified from a community-based survey (n = 981). EE subjects not only had higher Hb concentrations and erythrocyte counts, but also lower alveolar ventilation, impaired pulmonary diffusion capacity, higher systolic pulmonary artery pressure, lower pulmonary artery acceleration time, and more frequent right ventricular hypertrophy, than controls. Compared with controls, EE subjects were more often born to mothers experiencing hypertensive complications of pregnancy and hypoxia during the perinatal period, with each increasing the risk of developing EE (odds ratio = 5.25, P = 0.05 and odds ratio = 6.44, P = 0.04, respectively) after other factors known to influence EE status were taken into account. Adverse perinatal oxygenation is associated with increased susceptibility to EE accompanied by modest abnormalities of the pulmonary circulation that are independent of increased blood viscosity. The association between perinatal hypoxia and EE may be due to disrupted alveolarization and microvascular development, leading to impaired gas exchange and/or pulmonary hypertension.
NEW & NOTEWORTHY
We describe the first systematic evaluation of the relationship between perinatal hypoxia and excessive erythrocytosis in later life. Our findings suggest that adverse perinatal oxygenation raises the risk of excessive erythrocytosis accompanied by a modest elevation of systolic pulmonary artery pressure that is independent of increased blood viscosity.
developmental programming centers on the concept that environmental exposures during perinatal life influence physiological function and disease susceptibility throughout the lifespan by altering the developmental trajectory of numerous organ systems. In humans, the lung and its pulmonary circulation may be particularly vulnerable to perinatal exposures, given that developmental processes vital for efficient pulmonary gas transfer are incomplete at birth (49). Perinatal hypoxia, for instance, impairs alveolarization (34, 39) and promotes cardiopulmonary remodeling and alters vasoreactivity of the pulmonary arteries in the neonatal period (10, 21). The effects of perinatal hypoxia persist into adulthood, influencing ventilatory sensitivity (6, 36) and enhancing pulmonary vascular response to hypoxia (58). In this study we extend the concept of developmental programming to chronic mountain sickness (CMS), a condition that is induced by environmental exposure to hypoxia.
CMS is a debilitating and progressive syndrome marked by pronounced polycythemia, persistent arterial hypoxia, and ventilatory impairment without underlying cardiopulmonary disease (32, 60). A clinical diagnosis of CMS requires a Hb concentration ([Hb]) ≥19 g/dl for women or ≥21 g/dl for men that is accompanied by three or more of the following symptoms: breathlessness and/or palpitations, sleep disturbance, cyanosis, dilatation of veins, paresthesia, headache, or tinnitus (32). These cutoff values for Hb originated from data obtained in the central Andes of Peru at an altitude of 4,340 m (reviewed in Ref. 32). A large proportion of high-altitude residents develop CMS, with 8–15.6% of men being affected (43, 61) and 8.8% of postmenopausal women presenting with hematocrit levels >56% (33). Extensive complications of the pulmonary circulation, including elevated pulmonary artery pressure (PPA), pulmonary hypertension, and right ventricular (RV) hypertrophy (RVH), frequently complicate CMS (13, 37, 38, 53, 54) and, together with other severe symptoms that accompany the condition, increase the risk of major disability and mortality from RV failure and other causes. Consequently, CMS is an important public health issue for highland regions worldwide, most prominently in South America, but also in Asia and the Rocky Mountain region of North America.
Progress toward understanding the etiology of CMS has proven challenging, in part, because the condition is most frequently diagnosed in older adults in settings where the lack of medical records or diagnostic testing limits the ability to identify pathophysiological changes that precede established disease and confounds the ability to distinguish the physiological consequences of CMS from those of normal aging (31, 44). Such progress is facilitated by the recognition of a preclinical condition, “excessive erythrocytosis” (EE), which is defined by [Hb] ≥18.3 g/dl or 2 SDs above the mean [Hb] of 17.3 g/dl for men in La Paz, Bolivia; such individuals experienced a progressive decline in ventilatory function, worsening hypoxia, rising hematocrit, and increasing severity of CMS symptoms over a 4-yr period (60).
Perinatal hypoxia influences the maturation of organ systems involved in O2 transport, as evidenced by an increased incidence of restricted fetal growth, Doppler indices indicative of intrauterine hypoxia, and impaired postnatal arterial oxygenation (SaO2) (11, 28, 50). We hypothesized that perinatal hypoxia increased susceptibility to CMS and attendant abnormalities of the pulmonary circulation by impairing maturation of the lung at the level of the airways and the pulmonary vasculature. This hypothesis was supported by a pilot study (45) in which we identified lower birth weights and a higher incidence of perinatal hypoxia in a convenience sample of young men with EE (n = 12). Here we conducted a large-scale community-based survey (n = 981) to identify an unbiased sample of young highland men with clearly delineated EE and appropriate controls to evaluate 1) whether modest polycythemia was associated with impaired ventilatory function or abnormalities of the pulmonary circulation and 2) whether perinatal hypoxia increased susceptibility to EE. Understanding the mechanisms by which perinatal hypoxia influences structure and function of the lung, pulmonary circulation, and ventilatory control mechanisms has important clinical applications for identifying the environmental triggers for adult-onset cardiopulmonary disease. Moreover, interventions such as postnatal O2 supplementation and other measures that could improve the health of large numbers of the 140 million high-altitude residents worldwide are readily available (46).
METHODS
Study subjects and protocol.
Using a community-based survey, we screened 1,149 individuals at four postsecondary schools in the La Paz-El Alto metropolitan area in Bolivia to establish a representative sample of young men with EE and healthy controls of similar age, ancestry, and socioeconomic status living across a narrow high-altitude range (3,600–4,100 m) (29). Of the 1,149 individuals screened, 981 were eligible for study. Women were excluded, since polycythemia is rare in women prior to menopause (33). Additional exclusion criteria for the survey were age <18 or >25 yr, a history or evidence of cardiac or pulmonary disease on clinical examination (e.g., essential hypertension, type 1 or type 2 diabetes mellitus, or chronic bronchitis), frequent smoking (>4 cigarettes per week), or residence at <3,600 m. Survey participants completed a brief questionnaire to document age, altitude of birth, residential and medical history, and CMS symptoms of dyspnea, exercise intolerance, headache, tinnitus, cyanosis, and sleep disturbance. Initial clinical examinations, health history, self-reported symptoms, and SaO2 measurements were collected prior to our knowledge or the subjects' knowledge of EE status to reduce the potential for recall bias. Hematocrit and [Hb] were measured in triplicate using the microcentrifuge and cyanmethemoglobin method, respectively, at the time of the survey.
Survey participants were then classified as control or EE, defined as [Hb] ≥18.3 g/dl (60), and subsamples were selected at random within these two categories for participation in the complete study protocol. Of the 150 individuals selected, 133 highland men, 67 with EE and 66 healthy controls, agreed to participate. All studies were performed at the Bolivian Institute of High Altitude Biology (3,600 m) in La Paz. On the first visit, peripheral blood samples were collected from an antecubital vein by routine venipuncture for a repeat measurement of [Hb] and hematocrit and a complete blood count and to test for iron deficiency anemia using established serum ferritin and transferrin saturation cutoff values of <10 ng/ml and <15%, respectively, for highland Bolivian men (59). An in-depth interview was also conducted on the first visit to obtain a more detailed residential and health history and information regarding exposures to substances known to influence pulmonary function. Respiratory testing and cardiopulmonary evaluations were performed on subsequent visits. Medical record reviews and an in-depth interview with the subject's mother were then conducted to obtain information regarding pregnancy, delivery, and perinatal characteristics. The Colorado Multiple Institutional Review Board and Colegío Medico, its Bolivian counterpart, approved all survey and study procedures.
Systolic PPA and RVH.
Transthoracic echocardiography (GE Vivid I Ultrasound 2007) was used to estimate systolic PPA (sPPA) and to evaluate right heart structure and function. Subjects rested in the supine or lateral recumbent position for the duration of the examination. Studies began with anatomic surveys and dimensional measurements to screen for structural heart abnormalities. Tricuspid regurgitation was measured with the use of continuous-wave Doppler and averaged from three complete waveforms of good quality. Maximal tricuspid regurgitation jet flow velocity (TRmax) was used to estimate sPPA using a modified Bernoulli equation as previously reported (24, 41, 63). Estimation of sPPA by Doppler transthoracic echocardiography has been validated by right heart catheterization at sea level and at high altitude (4, 8). Tricuspid regurgitation was absent in three subjects (1 control and 2 EE); therefore, sPPA could not be calculated for these individuals. Pulsed-wave Doppler was used to evaluate pulmonary artery acceleration time (PAAT) from the onset of pulmonary flow to the peak flow velocity (23). Pulmonary vascular resistance (PVR) was calculated from the ratio of TRmax to the RV outflow tract time-velocity integral (TVI-RVOT) using the following formula: PVR = (TRmax/TVI-RVOT × 10) + 0.16 (3).
For electrocardiographic evaluations, the following were considered to be indicative of RVH: 1) peaked P waves with increased voltage in leads II and III and aVF and right precordial leads, 2) rightward axis QRS deviation, 3) a rS pattern in the right precordial leads and complexes of RS or rS type in the left precordial leads, or 4) T wave inversion over the right precordial leads. A single cardiologist-investigator (C.S.S.) performed all transthoracic echocardiography examinations to protect against interoperator effects.
Pulmonary function tests.
Forced vital capacity (FVC), forced expiratory volume at 1 s (FEV1), forced inspiratory flow, forced inspiratory vital capacity, and peak expiratory flow were determined by spirometry (model 03001, Collins), and flow-volume curves measured for forced expiratory flow at 75% of FVC (FEF75%) by whole body plethysmography (Autobox U6200, Sensor Medics). Pulmonary function testing was performed according to international standards (42), and values are reported as the best of three efforts meeting repeatability criteria established by the American Thoracic Society and the European Respiratory Society (42). The percent predicted values for spirometry were calculated using published reference equations for Mexican Americans (19). Pulmonary diffusion capacity (DlCO) was measured by the single-breath method using CO (1). Alveolar volume (Va) was calculated by measuring tracer gas (i.e., helium) dilution during the breath-hold phase of the single-breath DlCO test (1). DlCO was corrected for [Hb] and altitude (1) and expressed as a raw value and as a function of Va (DlCO/Va). Subjects performed practice efforts for all ventilatory procedures, with the exception of the single-breath DlCO test, before formal examinations were conducted to minimize the impact of learning effects.
Medical records review and maternal interview.
Perinatal data were obtained by structured interview of the subject's mother and a review of the medical records from the hospital where the subject was born concerning 1) maternal health and reproductive history, 2) pregnancy or delivery complications, 3) fetal complications, and 4) newborn characteristics and complications. Prematurity was defined as a gestational age at delivery <37 wk from the last menstrual period or as noted in the medical records. Perinatal hypoxia was defined as any one of the following: clinical diagnosis of fetal or newborn distress, birth asphyxia, bronchopneumonia, nuchal cord, severe cyanosis, meconium aspiration, resuscitation, neonatal O2 treatment, or acute neonatal respiratory infection as noted in the medical records or as obtained from the maternal interview. Hypertensive disorders of pregnancy were defined as two or more blood pressures ≥140/90 mmHg ≥6 h apart in a woman who was normotensive when nonpregnant and classified as preeclampsia if accompanied by proteinuria (>0.3 g·l protein−1·24 h−1) or gestational hypertension in the absence of proteinuria as noted in the medical records or as obtained from the maternal interview. For analysis, gestational hypertension and preeclampsia were grouped into a single category [“hypertensive pregnancy” (HTNPREG)], since both conditions can impair fetal growth (2).
Of the 133 subjects included for study, we obtained maternal and newborn information for 91 individuals (52 EE subjects and 39 controls). Recovery of the remaining 42 records was prevented by maternal death (n = 2) or the inability to contact the mother due to rural residence or no response to the request for interview (n = 40). The number of missing perinatal records was not unexpected, given that 43% of women deliver outside the health-care system in Bolivia (56). To maximize the availability of labor and delivery records, we selected schools in and around La Paz, where the proportion of hospital deliveries is higher (65%) than the national average and likely even greater in the educated segment of the population represented by our school-based samples. Age, weight, [Hb], hematocrit, SaO2, rural birth, and altitude of birth were equivalent between EE subjects and controls with and without maternal and newborn information; therefore, it is likely that the subjects with records were representative of the larger study population.
Data interpretation and analysis.
Differences between EE subjects and controls with respect to demographic variables and physiological and perinatal characteristics were determined using ANOVA or χ2 tests as appropriate. Data are reported as means ± SE or the proportion [odds ratio (OR)] and 95% confidence interval (CI). Logistic regression was used to determine variables that were significantly associated with EE status. Significant variables were introduced into multiple logistic regression models simultaneously to determine the relative contribution of pregnancy or perinatal or other physiological characteristics that were significantly associated with EE status. Age was also included as an independent variable, given its known association with CMS. Statistical analyses were performed using SPSS 19.0. Two-sided P < 0.05 was taken as evidence of significant association, and trends were considered when 0.05 < P < 0.10.
RESULTS
Individuals included in our community-based survey (n = 981) averaged 21.1 ± 2.0 yr of age, and nearly all (95%) were born at ≥2,500 m. Mean [Hb] and hematocrit values for these individuals were 16.8 ± 1.8 g/dl and 52.1 ± 3.1%, respectively. Of those surveyed, 10.4% (n = 102) had EE, with a mean [Hb] of 18.8 ± 0.5 g/dl and hematocrit of 57.5 ± 2.2% compared with 16.6 ± 1.0 g/dl and 51.5 ± 2.7% for controls.
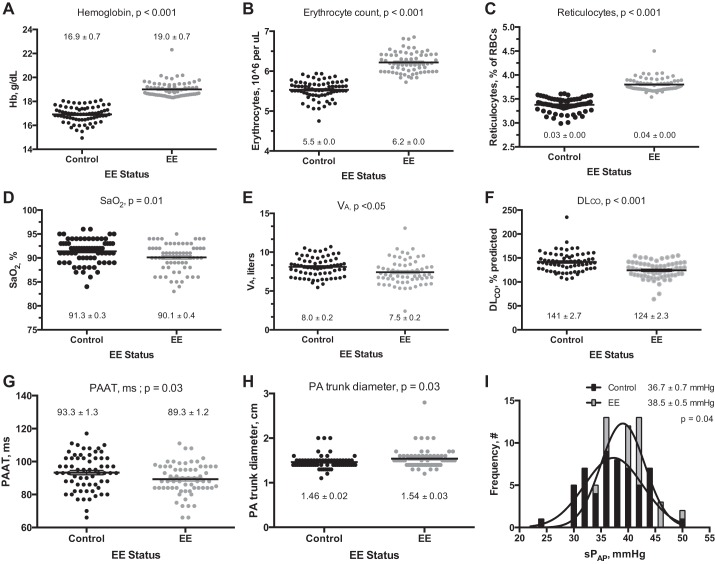
Characteristics of the 133 research subjects (67 EE subjects and 66 controls) selected at random from the community-based survey for physiological study are shown in Table 1. Age, height, weight, arterial blood pressure, and the proportion of individuals born at high altitude were equivalent between EE subjects and controls (97% and 94%, respectively; Table 1). Self-reported ancestry was also similar between groups (controls: 33% Andean, 63% Mestizo, and 4% European; EE subjects: 26% Andean, 71% Mestizo, and 3% European). Known exposure to synthetic fiber, paint, gas, or chemicals was minimal (<10%) and similar in EE subjects and controls. Smoking, alcohol, or coca use was also equivalent in EE subjects and controls (34 and 35%, 55 and 57%, and 12 and 15%, respectively). EE subjects more frequently reported experiencing headaches, cyanosis, dyspnea, or palpitations while resting or with exercise, numbness and tingling of the upper or lower extremities, and dizziness than controls (Fig. 1). Sleep disturbances, including insomnia, excess sleepiness, or interrupted sleep, and mental or physical fatigue also tended to be more common in EE subjects (Fig. 1). By definition, polycythemia was more common in EE subjects than controls; namely, [Hb], hematocrit, and erythrocyte and reticulocyte counts were elevated (Table 1, Fig. 2, A–C).
Table 1.
Subject characteristics
| Variable | Control (n = 66) | EE (n = 67) | P Value |
|---|---|---|---|
| General characteristics | |||
| Age, yr | 21.0 ± 0.3 | 21.5 ± 0.3 | 0.19 |
| Height, m | 1.68 ± 0.78 | 1.67 ± 0.50 | 0.43 |
| Weight, kg | 23.6 ± 0.31 | 22.3 ± 0.36 | <0.01 |
| Heart rate, beats/min | 58.9 ± 9.9 | 63.3 ± 10.1 | 0.01 |
| Systemic blood pressure, mmHg | |||
| Diastolic | 70.6 ± 1.3 | 70.4 ± 1.1 | 0.47 |
| Systolic | 101.3 ± 1.4 | 102.7 ± 1.1 | 0.34 |
| Hematocrit, % | 51.2 ± 0.3 | 56.9 ± 0.4 | <0.001 |
| Ventilatory function | |||
| FVC, %predicted | 118.5 ± 2.1 | 114.5 ± 2.2 | 0.20 |
| FEV1, %predicted | 122.6 ± 1.9 | 117.1 ± 2.1 | 0.06 |
| FEV1/FVC, %predicted | 88.5 ± 0.8 | 86.6 ± 0.8 | 0.08 |
| FEF75%, %predicted | 129.1 ± 3.9 | 119.3 ± 5.6 | 0.05 |
| PEF, %predicted | 123.0 ± 2.1 | 119.8 ± 3.1 | 0.39 |
| FIVC, %predicted | 112.2 ± 2.2 | 107.3 ± 2.3 | 0.13 |
Values are means ± SE; n, number of subjects. EE, excessive erythrocytosis; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF75%, forced expiratory flow at 75% of FVC; PEF, peak expiratory flow; FIVC, forced inspiratory vital capacity.
Fig. 1.
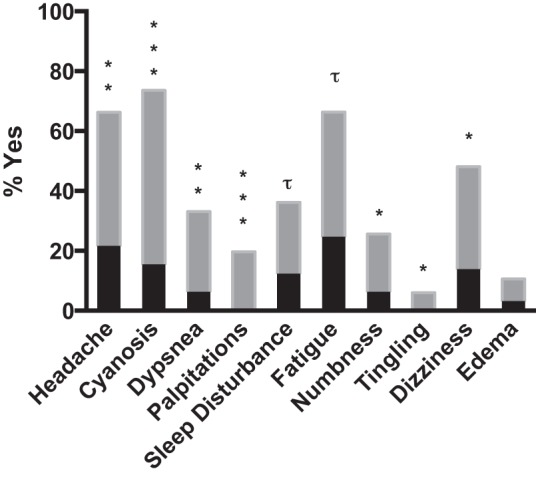
Self-reported symptoms prior to our knowledge or the subjects' knowledge of excessive erythrocytosis (EE) status. Headaches, cyanosis, dyspnea, or palpitations while resting or with exercise, numbness and tingling of the upper or lower extremities, and dizziness were reported more frequently by EE subjects (gray bars) than controls (black bars). Sleep disturbances, including insomnia, excess sleepiness, or interrupted sleep, and mental or physical fatigue also tended to be more common in EE subjects. Significant differences between controls and EE subjects for each symptom are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001; trends are indicated by τ.
Fig. 2.
Hematologic and physiological characteristics of young male highlanders with EE (n = 67) compared with healthy high-altitude controls (Control, n = 66). Black (control) and gray (EE) dots represent individual subject values; horizontal black lines within each cluster indicate the corresponding mean ± SE. As expected, Hb levels (A) and erythrocyte (B) and reticulocyte (C) counts were elevated in EE subjects. Compared with controls, EE subjects also had reduced arterial oxygenation (SaO2, D), lower alveolar volume (Va, E), lower pulmonary diffusion capacity corrected for altitude and [Hb] (DlCO, F), reduced pulmonary artery acceleration time (PAAT, G), increased pulmonary artery (PA) trunk diameter (H), and elevated pulmonary artery systolic pressure (sPPA, I).
Maternal, pregnancy, and newborn characteristics are shown in Table 2. Maternal age and parity at the time the research subject was born were similar between EE subjects and controls, and an equivalent proportion of EE subjects and controls were delivered in a hospital (67% and 55%, respectively, P = 0.27). None of the women reported smoking during pregnancy.
Table 2.
Maternal and newborn characteristics
| Control (n = 39) | EE (n = 52) | P Value | |
|---|---|---|---|
| Maternal characteristics | |||
| Age at time of delivery, yr | 27.5 ± 1.0 | 28.3 ± 1.1* | 0.60 |
| Parity, no. of live births | 4.8 ± 0.4 | 4.0 ± 0.3* | 0.11 |
| HTNPREG, %yes | 10.3 (1, 20) | 30.8 (18, 43) | < 0.05 |
| Other complications, %yes | 5.1 (0, 12) | 15.4 (6, 25) | 0.12 |
| Placenta previa | 2.6 (2, 8) | 0 (0, 0) | 0.24 |
| Premature labor | 2.6 (2, 8) | 1.9 (0, 6) | 0.84 |
| Anemia | 0 (0, 0) | 3.8 (1, 9) | 0.22 |
| Hemorrhage | 0 (0, 0) | 1.9 (0, 6) | 0.38 |
| Premature rupture of membranes | 0 (0, 0) | 1.9 (0, 6) | 0.38 |
| Delivery and newborn characteristics | |||
| Birth wt, g | 3,170 ± 12† | 3,002 ± 12† | NS |
| Perinatal hypoxia-related complications, %yes | 7.7 (0, 16) | 32.7 (20, 45) | <0.01 |
| Birth asphyxia, fetal or newborn distress | 5.1 (0, 12) | 21.2 (10, 32) | <0.05 |
| Prolonged need for O2 treatment | 2.6 (0, 8) | 1.9 (0, 6) | 0.84 |
| Bronchopneumonia | 0 (0, 0) | 3.8 (0, 9) | 0.22 |
| Nuchal cord | 0 (0, 0) | 1.9 (0, 6) | 0.38 |
| Severe cyanosis | 0 (0, 0) | 1.9 (0, 6) | 0.38 |
| Meconium aspiration | 0 (0, 0) | 1.9 (0, 6) | 0.38 |
| Acute respiratory infection | 0 (0, 0) | 1.9 (0, 6) | 0.38 |
Values are means ± SE or percentage and 95% confidence interval; n, number of subjects. HTNPREG, hypertension in pregnancy.
n = 49
n = 24.
Thirty percent of EE mothers developed HTNPREG, a rate threefold higher than that of control mothers (Table 2). Similarly, 33% of EE subjects experienced hypoxia-related complications during the perinatal period compared with 8% of controls, with birth asphyxia and fetal or newborn distress being the largest contributors to this difference (Table 2). Birth weight, corrected or uncorrected for gestational age, was similar between EE subjects and controls (Table 2). Premature delivery occurred twice as often in EE subjects as in controls, although this difference was not statistically significant (30.6% and 16.6%, respectively, P = 0.14).
Pulmonary function parameters were similar between EE subjects and controls, with only FEF75%, FEV1/FVC, and FEV1 tending to be lower in EE subjects (Table 1). SaO2 and Va were mildly reduced in EE subjects (Fig. 2, D and E). DlCO was reduced in EE subjects compared with controls whether expressed as percent predicted (Fig. 2F) or absolute value (data not shown). However, EE subjects had a higher DlCO relative to their Va (DlCO/Va) than controls (7.8 vs. 7.0, P = 0.001), indicating that their apparent diffusion limitation compared with controls was likely due to reduced Va.
PAAT was lower, pulmonary artery trunk diameter was larger, and sPPA was slightly elevated in EE subjects compared with controls (Fig. 2, G–I), but PVR was similar in controls and EE subjects (1.4 ± 0.0 and 1.4 ± 0.02, respectively, P = 0.43). As expected, PVR was inversely proportional to PAAT and positively associated with sPPA in the entire subject population and the EE subjects and controls when considered separately (Fig. 3). A higher proportion of EE subjects had sPPA ≥40 mmHg (52% vs. 37%, P = 0.02) and showed electrocardiographic evidence of RVH (72% vs. 53% P < 0.05). RV wall thickness also tended to be greater in EE subjects than controls (0.54 ± 0.01 vs. 0.57 ± 0.01 cm, P = 0.09). All other chamber, valvular, and wall dimensions were within normal limits and did not differ by EE status.
Fig. 3.
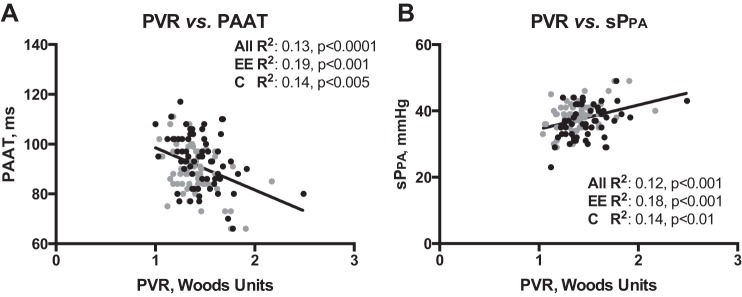
Correlations between characteristics of the pulmonary circulation [pulmonary vascular resistance (PVR), PAAT, and sPPA]. Black (control) and gray (EE) dots represent individual subject values. Pearson's correlation coefficients and significance values are shown for all subjects, EE subjects, and controls. As expected, PVR is inversely related to PAAT (A) and positively associated with sPPA (B).
Because HTNPREG, perinatal hypoxia, DlCO, sPPA, SaO2, body mass index (BMI), and Va were significantly associated with EE status [HTNPREG: exp(β) = 5.48, P = 0.01; perinatal hypoxia: exp(β) = 5.99, P = 0.01; DlCO: β = −0.039, P = 0.005; sPPA: β = 0.357, P = 0.10; SaO2: β = 0.86, P = 0.06; BMI: β = 1.22, P = 0.02; Va: β = 0.73, P = 0.05] when examined individually by logistic regression in subjects with birth-related information, we performed multiple logistic regression analyses to determine the independent contribution of each pregnancy-related factor to EE. Age was also included as an independent variable, because the incidence of CMS is known to increase with age. As shown in Table 3, HTNPREG and perinatal hypoxia significantly raised the risk of developing EE [OR = 5.25 (CI = 1.00, 27.42), P = 0.03 and OR = 6.44 (CI = 1.07, 38.94), P = 0.02, respectively] after the influences of DlCO, sPPA, age, BMI, SaO2, and Va were taken into account.
Table 3.
Hypertensive pregnancy and perinatal hypoxia are associated with EE status
|
95% CI for Exp(β) |
||||
|---|---|---|---|---|
| Dependent variable: EE | Exp(β) | Lower | Upper | P Value |
| A. Covariate | ||||
| Hypertensive pregnancy, yes | 5.25 | 1.00 | 27.42 | 0.049 |
| sPPA, mmHg | 1.11 | 0.99 | 1.24 | 0.068 |
| DlCO, %predicted | −1.02 | 1.00 | 1.04 | 0.092 |
| SaO2, % | −0.87 | 0.71 | 1.06 | 0.167 |
| Va, liters | −0.67 | 0.45 | 0.99 | 0.045 |
| Age, yr | 0.98 | 0.76 | 1.25 | 0.841 |
| BMI, kg/m2 | 1.21 | 0.99 | 1.46 | 0.056 |
| B. Covariate | ||||
| Perinatal hypoxia, yes | 6.44 | 1.07 | 38.94 | 0.042 |
| sPPA, mmHg | 1.14 | 1.02 | 1.28 | 0.017 |
| DlCO, %predicted | −1.02 | 1.00 | 1.04 | 0.124 |
| SaO2, % | −0.93 | 0.75 | 1.15 | 0.469 |
| Va, liters | −0.70 | 0.47 | 1.05 | 0.088 |
| Age, yr | 0.92 | 0.71 | 1.18 | 0.490 |
DlCO, pulmonary diffusion capacity of CO corrected for altitude and Hb; sPPA, estimated systolic pulmonary artery pressure; SaO2, arterial O2 saturation; Va, alveolar volume; BMI, body mass index; Exp(β), odds ratio for variable.
Finally, to evaluate whether the modest pulmonary vascular differences between EE subjects and controls were influenced by blood viscosity, we used Pearson's correlations to determine whether sPPA or PVR was independent of SaO2 or [Hb], since such an association would likely be due to increased blood viscosity. Neither PVR nor sPPA was associated with SaO2 or [Hb] when the EE subjects and controls were considered separately or when both groups were combined (data not shown), suggesting that the modest pulmonary circulation abnormalities between EE subjects and controls were not likely due to increased blood viscosity.
DISCUSSION
This is the first study to systematically examine the influence of perinatal hypoxia on an increase in the susceptibility to EE and its attendant pulmonary circulatory abnormalities. Our findings are novel in three respects. 1) Our observation that the modest pulmonary circulation abnormalities accompanying EE are independent of blood viscosity suggests that polycythemia itself is not a primary determinant of increased sPPA or PVR in this context. In support of this interpretation, sPPA was higher, PAAT was lower, and there was more frequent evidence of RVH in EE subjects than controls, while there was no correlation between sPPA or PVR and [Hb] or SaO2 during wakefulness. 2) We demonstrate that adverse perinatal oxygenation and HTNPREG are more common in EE subjects than controls and are significantly associated with EE status, even after other characteristics of EE (e.g., impaired DlCO and elevated sPPA) are taken into account. Specifically, HTNPREG and perinatal hypoxia increased the probability of developing EE 5.3- and 6.4-fold, respectively. 3) Our large-scale community-based survey highlights the high prevalence (10.4%) of EE in early adulthood and, therefore, the public health importance of screening for elevated Hb in young individuals and for hypoxia-related perinatal complications at high altitude. In sum, our findings suggest an association between hypoxic exposures in early life and the development of CMS during adulthood.
Conducting the study in La Paz, Bolivia was advantageous, given that >1.5 million persons live in the La Paz-El Alto metropolitan area at an altitude that is sufficient to induce polycythemia (60) and increase the incidence of hypoxia-related pregnancy complications (18, 28, 30). With respect to the latter, 13% of infants of Andean ancestry and 33% of infants of European ancestry are affected by hypoxia-induced fetal growth restriction at 3,600 m in Bolivia (28). SaO2 is also reduced from birth through the first few months of life in infants of Andean and European ancestry at 3,600 m (51). The incidence of hypertensive pregnancy complications and other hypoxia-related perinatal complications, including fetal distress and newborn respiratory distress, are also more frequent at high altitude than at sea level (30). For these reasons, conducting our study in Bolivia enabled us to identify a sufficient number of individuals who experienced hypoxia during perinatal life and to assess the relationship of these pregnancy-related complications to EE. Another prominent strength of our study was its use of a large-scale, community-based survey to minimize the selection bias introduced by convenience sampling methods.
Our observation that perinatal hypoxia increases the occurrence of EE and modest abnormalities in the pulmonary circulation is consistent with experimental animal and human studies demonstrating that O2 deficit in early life adversely affects the function and structure of the pulmonary circulation. For instance, lambs gestated at 3,500-3,800 m have greater basal PVR, higher sPPA, and enhanced postnatal pulmonary vascular contractile response to hypoxia and pharmacological agonists (10, 20, 21). Highland-gestated lambs retain a higher baseline sPPA and PVR under normoxic, low-altitude conditions, indicating that pulmonary circulation abnormalities induced by O2 deficit in early life persist in the absence of sustained hypoxia (10). In murine models, perinatal exposure to 10% O2 reduces pulmonary artery compliance, increases the hypoxic pulmonary vasoconstrictor response, decreases PAAT, and increases small pulmonary artery medial thickness (26, 57). Postnatal normalization of sPPA is also impaired in humans at high altitude, as indicated by a delayed or absent drop in sPPA (16). Basal PPA is higher in offspring of preeclamptic women than offspring of normotensive women at high altitude, regardless of birth weight (24), supporting our observation that hypertensive pregnancy increases susceptibility to EE with modest sPPA elevation.
Perinatal hypoxia is also an important determinant of the surface area available for pulmonary gas exchange (39, 40, 47). Specifically, hypoxia impairs septation of peripheral air spaces, a process that is required for the formation of the pulmonary alveoli and extends well into postnatal life (49). In rats, hypoxic (13% O2) exposure during the time frame that encompasses alveolarization (gestation to 14 days after birth) irreversibly alters septation and, thereby, increases alveolar size (9, 39). Even brief hypoxic bouts are sufficient to compromise the development of key structures involved in pulmonary gas exchange; 9 h of maternal hypoxia on the last day of pregnancy combined with 1–2 h of hypoxia after birth delays septation, slows the postnatal increase in lung volume, and accelerates alveolar wall thinning in rats (40). Our observation that Va was lower in EE subjects than controls is in line with the hypothesis that a reduced Va resulting from perinatal hypoxia raises the risk of developing EE. Moreover, smaller Va, rather than a pronounced diffusion limitation or reduced pulmonary capillary blood volume, is likely responsible for the lower DlCO evident in EE subjects than controls, since values normalized after correction for Va (15, 17). However, DlCO values are well above 100% predicted values in EE subjects and controls. Hypoxic exposure in later life has been shown to accelerate alveolar formation in animal models (9, 12, 22, 25). Therefore, it is possible that the high DlCO values we observed in all subjects are the result of rapid lung development during postnatal life and that this adaptive process may be slightly impaired in individuals who go on to develop EE. We speculate that subtle, but persistent, impairment of pulmonary gas exchange efficiency due to reduced Va contributes to the etiology of EE and that such effects are mediated, in part, by adverse perinatal oxygenation.
Counter to our expectations, neither birth weight nor the frequency of preterm delivery differed between EE subjects and controls. Previous studies indicate that prematurity increases the risk of diseases characterized by airway obstruction (62), pulmonary circulation abnormalities (5), and lower DlCO and impaired exercise capacity in later life (14, 35). We therefore anticipated that prematurity would be associated with impaired ventilatory function, elevated sPPA, and higher [Hb]. While the absolute frequency of preterm delivery was higher in the EE group, several of these cases were due to early induction of labor or cesarean section prompted by complications associated with HTNPREG (n = 4) or hemorrhage (n = 1). The lack of a statistically significant relationship between the incidence of prematurity and impaired pulmonary function may be explained, in part, by a normalization of lung function during childhood and early adulthood, as has been observed in cases of prematurity without bronchopulmonary dysplasia (48).
Perinatal hypoxia also delays peripheral chemoreceptor maturation, an effect that induces a persistent blunting of the hypoxic ventilatory response and is more pronounced in men than women (6, 27, 36, 52). Therefore, it is possible that the relationship of perinatal hypoxia to EE we observed is due, in part, to altered ventilatory control. In support of this possibility, young men with EE have sleep-disordered breathing patterns compared with controls (29). As speculated elsewhere (51), enhanced neonatal oxygenation in Tibetans compared with Andeans may be a factor in their greater hypoxic ventilatory sensitivity and protection from high-altitude polycythemia (7).
The conduct of these studies faced some limitations due to ethical constraints and logistical aspects of the health-care system in Bolivia. For ethical reasons, sPPA was estimated using well-established transthoracic echocardiographic methods, rather than directly measured by right heart catheterization (24, 37, 41, 63). Using this strategy, we identified an elevation of sPPA in EE subjects compared with controls that was statistically significant but likely to be of limited clinical relevance when considered in isolation. In this regard, it is important to emphasize that our aim was to characterize EE, rather than established CMS, which, according to the Qinghai diagnostic criteria, requires specific characteristics including [Hb] ≥21.0 g/dl and the presence of severe hypoxemia (32); such characteristics were purposefully absent in the present study population, given the early (preclinical) manifestations of the disease. Therefore, while the sPPA difference we observed was small, we consider that higher sPPA in conjunction with reduced PAAT increased pulmonary artery trunk diameter and that a tendency toward greater RV wall thickness in EE may reflect an early pathophysiological stage of CMS that could become clinically important with advancing age. Prospective studies are required to determine the clinical progression of pulmonary circulatory abnormalities in EE. An additional consideration for the interpretation of our findings relates to the criteria used for electrocardiographic evidence of RVH. Specifically, because right axis deviation and precordial T wave inversion are common in healthy adolescents and young adults prior to 20 yr of age, the high frequency of RVH we report may indicate the persistence of a juvenile electrocardiographic pattern, rather than RVH (55). However, because we have no reason to believe that the retention of juvenile electrocardiographic patterns would be more common in EE subjects than controls, our findings suggest that RVH is more frequent in EE.
The perinatal data available for these subjects were limited by the types of medical records available in a developing country such as Bolivia. We maximized the recovery of perinatal-related information by surveying segments of the population expected to have a higher proportion of hospital deliveries. Similarly, the inclusion of young adults improved the likelihood of recovering perinatal-related information, given that their births were more recent and the availability of health care and medical records in Bolivia have improved over time. One potential explanation for the higher proportion of EE subjects than controls with perinatal information may be that mothers of EE subjects were more inclined to participate, because they had a complicated pregnancy and/or they were motivated by the fact that their child had EE or was otherwise symptomatic. However, given the similarity in age, weight, [Hb], hematocrit, SaO2, residence in a rural area, and altitude of birth between EE subjects and controls with and without maternal and newborn information, we considered it likely that the EE subjects and controls with records were representative of individuals without records.
Collectively, our findings demonstrate for the first time that adverse oxygenation during perinatal life is associated with EE and attendant abnormalities of the pulmonary circulation during young adult life at high altitude. We propose that the relationship of perinatal hypoxia to polycythemia may be driven, in part, by impaired efficiency of gas exchange secondary to disrupted alveolarization, pulmonary microvascular development, and/or maturation of chemosensory pathways. Our findings highlight the importance of screening for EE in young adults and for hypoxia-related perinatal complications at high altitude. Further study using experimental animal models to manipulate the degree and magnitude of perinatal hypoxia accompanied by prospective human studies will be useful to clarify the role of O2 deficit in the onset of hypoxia-related polycythemia and to distinguish whether genetic predispositions to vascular disorders of pregnancy also predispose male offspring to vascular dysfunction and/or polycythemia.
GRANTS
This project was supported by Fogarty International Center Award R03 TW-007957 (L. G. Moore and C. G. Julian), National Heart, Lung, and Blood Institute Grant HL-079647 (L. G. Moore), and National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award UL1 TR-001082 (Child and Maternal Health Pilot, C. G. Julian). C. G. Julian is supported by National Institutes of Health Building Interdisciplinary Research Careers Women's Health Grant 5 K12 HD-057022-07.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.G.J., E.V., and L.G.M. developed the concept and designed the research; C.G.J., M.G., A.R., D.B., C.S.S., A.L., L.R., and E.V. performed the experiments; C.G.J. analyzed the data; C.G.J., E.V., and L.G.M. interpreted the results of the experiments; C.G.J. prepared the figures; C.G.J. drafted the manuscript; C.G.J., M.G., C.S.S., A.L., L.R., E.V., and L.G.M. edited and revised the manuscript; C.G.J., M.G., A.R., D.B., C.S.S., A.L., L.R., E.V., and L.G.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge all the individuals who participated in this project as research subjects. We extend our appreciation to the physicians and technical staff at the Bolivian Institute of High Altitude Biology, Sonja Jameson Van Houten, Javier Fuentes, Walter Amezaga, Padre Arego Corona, and Carmiña Mercado for assistance with the conduct of this project; to Dr. Susan Niermeyer (Neonatology Section, Department of Pediatrics, University of Colorado School of Medicine) for helpful discussion during the planning phase of this project; and to Dr. Todd Bull (Pulmonary Vascular Disease Center, University of Colorado School of Medicine) for thoughtful review of the manuscript.
REFERENCES
- 1.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor) recommendations for a standard technique—1995 update. Am J Respir Crit Care Med 152: 2185–2198, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41: 1021–1027, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Allemann Y, Sartori C, Lepori M, Pierre S, Melot C, Naeije R, Scherrer U, Maggiorini M. Echocardiographic and invasive measurements of pulmonary artery pressure correlate closely at high altitude. Am J Physiol Heart Circ Physiol 279: H2013–H2016, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Baker CD, Abman SH, Mourani PM. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Pediatr Allergy Immunol 27: 8–16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bavis RW. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir Physiol Neurobiol 149: 287–299, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Beall CM, Brittenham GM, Macuaga F, Barragan M. Variation in hemoglobin concentration among samples of high-altitude natives in the Andes and the Himalayas. Am J Hum Biol 2: 639–651, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 6: 359–365, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Blanco LN, Massaro D, Massaro GD. Alveolar size, number, and surface area: developmentally dependent response to 13% O2. Am J Physiol Lung Cell Mol Physiol 261: L370–L377, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Blood AB, Terry MH, Merritt TA, Papamatheakis DG, Blood Q, Ross JM, Power GG, Longo LD, Wilson SM. Effect of chronic perinatal hypoxia on the role of Rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol 304: R136–R146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne VA, Julian CG, Toledo-Jaldin L, Cioffi-Ragan D, Vargas E, Moore LG. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 370: 20140068, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burri PH, Weibel ER. Morphometric estimation of pulmonary diffusion capacity. II. Effect of Po2 on the growing lung, adaption of the growing rat lung to hypoxia and hyperoxia. Respir Physiol 11: 247–264, 1971. [DOI] [PubMed] [Google Scholar]
- 13.Canepa A, Chavez R, Hurtado A, Rotta A, Velasquez T. Pulmonary circulation at sea level and at high altitudes. J Appl Physiol 9: 328–336, 1956. [DOI] [PubMed] [Google Scholar]
- 14.Duke JW, Elliott JE, Laurie SS, Beasley KM, Mangum TS, Hawn JA, Gladstone IM, Lovering AT. Pulmonary gas exchange efficiency during exercise breathing normoxic and hypoxic gas in adults born very preterm with low diffusion capacity. J Appl Physiol 117: 473–481, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Farha S, Laskowski D, George D, Park MM, Tang WH, Dweik RA, Erzurum SC. Loss of alveolar membrane diffusing capacity and pulmonary capillary blood volume in pulmonary arterial hypertension. Respir Res 14: 6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamboa R, Marticorena E. Presión arterial pulmonar en recién nacidos en las grandes alturas. Arch Inst Biol Andina 4: 55–56, 1971. [PubMed] [Google Scholar]
- 17.Groepenhoff H, Overbeek MJ, Mule M, van der Plas M, Argiento P, Villafuerte FC, Beloka S, Faoro V, Macarlupu JL, Guenard H, de Bisschop C, Martinot JB, Vanderpool R, Penaloza D, Naeije R. Exercise pathophysiology in patients with chronic mountain sickness: exercise in chronic mountain sickness. Chest 142: 877–884, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Haas JD, Frongillo EJ, Stepcik C, Beard J, Hurtado L. Altitude, ethnic and sex differences in birth weight and length in Bolivia. Hum Biol 52: 459–477, 1980. [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 159: 179–187, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Herrera EA, Riquelme RA, Ebensperger G, Reyes RV, Ulloa CE, Cabello G, Krause BJ, Parer JT, Giussani DA, Llanos AJ. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 299: R1676–R1684, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsia CC, Carbayo JJ, Yan X, Bellotto DJ. Enhanced alveolar growth and remodeling in guinea pigs raised at high altitude. Respir Physiol Neurobiol 147: 105–115, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Isobe M, Yazaki Y, Takaku F, Koizumi K, Hara K, Tsuneyoshi H, Yamaguchi T, Machii K. Prediction of pulmonary arterial pressure in adults by pulsed Doppler echocardiography. Am J Cardiol 57: 316–321, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 122: 488–494, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RL Jr, Cassidy SS, Grover RF, Schutte JE, Epstein RH. Functional capacities of lungs and thorax in beagles after prolonged residence at 3,100 m. J Appl Physiol 59: 1773–1782, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Jones RD, Morice AH, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res 53: 11–17, 2004. [PubMed] [Google Scholar]
- 27.Joseph V, Soliz J, Pequignot J, Sempore B, Cottet-Emard JM, Dalmaz Y, Favier R, Spielvogel H, Pequignot JM. Gender differentiation of the chemoreflex during growth at high altitude: functional and neurochemical studies. Am J Physiol Regul Integr Comp Physiol 278: R806–R816, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Villena M, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 92: F372–F377, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julian CG, Vargas E, Gonzales M, Davila RD, Ladenburger A, Reardon L, Schoo C, Powers RW, Lee-Chiong T, Moore LG. Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis). Respir Physiol Neurobiol 186: 188–196, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young D, Villena M, Moore LG. Intrauterine growth restriction, preeclampsia and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Leon-Velarde F, Arregui A, Monge C, Ruiz y Ruiz H. Aging at high altitudes and the risk of chronic mountain sickness. J Wilderness Med 4: 183–188, 1993. [Google Scholar]
- 32.Leon-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6: 147–157, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Leon-Velarde F, Ramos MA, Hernandez JA, Deidiaquez D, Munoz LS, Gaffo A, Cordova S, Durand D, Monge C. The role of menopause in the development of chronic mountain sickness. Am J Physiol Regul Integr Comp Physiol 272: R90–R94, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Lipsett J, Tamblyn M, Madigan K, Roberts P, Cool JC, Runciman SI, McMillen IC, Robinson J, Owens JA. Restricted fetal growth and lung development: a morphometric analysis of pulmonary structure. Pediatr Pulmonol 41: 1138–1145, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Lovering AT, Laurie SS, Elliott JE, Beasley KM, Yang X, Gust CE, Mangum TS, Goodman RD, Hawn JA, Gladstone IM. Normal pulmonary gas exchange efficiency and absence of exercise-induced arterial hypoxemia in adults with bronchopulmonary dysplasia. J Appl Physiol 115: 1050–1056, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Lumbroso D, Joseph V. Impaired acclimatization to chronic hypoxia in adult male and female rats following neonatal hypoxia. Am J Physiol Regul Integr Comp Physiol 297: R421–R427, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Maignan M, Rivera-Ch M, Privat C, Leon-Velarde F, Richalet JP, Pham I. Pulmonary pressure and cardiac function in chronic mountain sickness patients. Chest 135: 499–504, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Manier G, Guenard H, Castaing Y, Varene N, Vargas E. Pulmonary gas exchange in Andean natives with excessive polycythemia—effect of hemodilution. J Appl Physiol 65: 2107–2117, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Massaro GD, Olivier J, Dzikowski C, Massaro D. Postnatal development of lung alveoli: suppression by 13% O2 and a critical period. Am J Physiol Lung Cell Mol Physiol 258: L321–L327, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Massaro GD, Olivier J, Massaro D. Short-term perinatal 10% O2 alters postnatal development of lung alveoli. Am J Physiol Lung Cell Mol Physiol 257: L221–L225, 1989. [DOI] [PubMed] [Google Scholar]
- 41.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 104: 2797–2802, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. American Thoracic Society and European Respiratory Society Task Force: standardisation of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Monge CC, Arregui A, Leon-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med 13 Suppl 1: S79–S81, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Monge CC, Leon-Velarde F, Arregui A. Increasing prevalence of excessive erythrocytosis with age among healthy high-altitude miners (Letter). N Engl J Med 321: 1271, 1989. [DOI] [PubMed] [Google Scholar]
- 45.Moore LG, Niermeyer S, Vargas E. Does chronic mountain sickness (CMS) have perinatal origins? Respir Physiol Neurobiol 158: 180–189, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life cycle perspectives. Am J Phys Anthropol 27: 25–64, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol 61: 1329–1336, 1986. [DOI] [PubMed] [Google Scholar]
- 48.Narang I, Rosenthal M, Cremonesini D, Silverman M, Bush A. Longitudinal evaluation of airway function 21 years after preterm birth. Am J Respir Crit Care Med 178: 74–80, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R, Panesar KS, Kuehni CE, Spycher BD, Williams SE, Silverman M. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med 185: 186–191, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niermeyer S, Shaffer EM, Thilo E, Corbin C, Moore LG. Arterial oxygenation and pulmonary arterial pressure in healthy neonates and infants at high altitude. J Pediatr 123: 767–772, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Niermeyer S, Vargas E, Andrade M, Moore L. Neonatal oxygenation, pulmonary hypertension and evolutionary adaptation to high altitude. Pulm Circ 5: 48–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okubo S, Mortola JP. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol Regul Integr Comp Physiol 259: R836–R841, 1990. [DOI] [PubMed] [Google Scholar]
- 53.Pei SX, Chen XJ, Si Ren BZ, Liu YH, Cheng XS, Harris EM, Anand IS, Harris PC. Chronic mountain sickness in Tibet. Q J Med 71: 555–574, 1989. [PubMed] [Google Scholar]
- 54.Penaloza D, Sime F. Chronic cor pulmonale due to loss of altitude acclimatization (chronic mountain sickness). Am J Med 50: 728–743, 1971. [DOI] [PubMed] [Google Scholar]
- 55.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, American College of Cardiology Foundation, Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. IV. The ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 119: e241–e250, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Roost M, Altamirano VC, Liljestrand J, Essen B. Priorities in emergency obstetric care in Bolivia—maternal mortality and near-miss morbidity in metropolitan La Paz. BJOG 116: 1210–1217, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Rueda-Clausen CF, Stanley JL, Thambiraj DF, Poudel R, Davidge ST, Baker PN. Effect of prenatal hypoxia in transgenic mouse models of preeclampsia and fetal growth restriction. Reprod Sci 21: 492–502, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sartori C, Allemann Y, Trueb L, Delabays A, Nicod P, Scherrer U. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet 353: 2205–2207, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Tufts DA, Haas JD, Beard JL, Spielvogel H. Distribution of hemoglobin and functional consequences of anemia in adult males at high altitude. Am J Clin Nutr 42: 1–11, 1985. [DOI] [PubMed] [Google Scholar]
- 60.Vargas E, Spielvogel H. Chronic mountain sickness, optimal hemoglobin, and heart disease. High Alt Med Biol 7: 138–149, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Vargas E, Villena M, Salinas C, Rodriguez A, Spielvogel H, Tellez M, Bellido D. Excessive polycythemia occurs in young high altitude (3600 m) residents in the absence of lung disease. In: Health & Height: Proceedings of the Fifth World Congress on Mountain Medicine and High Altitude Physiology. Barcelona: Universitat de Barcelona, 2002. [Google Scholar]
- 62.Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med 173: 890–896, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70: 657–662, 1984. [DOI] [PubMed] [Google Scholar]